Abstract
Chronic obstructive pulmonary disease (COPD) results in major remodeling of the distal airspaces and changes in the differentiation profile of the airway epithelium. The cellular and molecular mechanisms involved in initiation and progression of this disease are little understood. Although environmental factors, including cigarette smoke, have been directly implicated in the pathogenesis of COPD, genetic risk factors also appear to play a fundamental role in the individual's susceptibility to this disease. Lung development depends on precise coordination of signals, such as fibroblast growth factors (Fgf), Sonic Hedgehog (Shh), retinoic acid, Notch, and Tgf β. Dramatic changes in the pattern of branching and differentiation of the lung epithelium results from disruption of these signals in genetically altered mice. Recent studies, including whole-genome expression and genome-wide association analyses, suggest that some molecular regulators originally described in developmental processes may be altered in patients with COPD. Whether disturbances in the molecular and cellular events mediated by these genes during development participate in the initiation or exacerbation of COPD, needs further investigation. The role of selected pathways, including Sonic hedgehog, Notch, retinoid, and Tgf β in the developing lung and the potential association with COPD are discussed.
Keywords: lung development, COPD, emphysema, morphogenesis, lung differentiation
Chronic obstructive pulmonary disease (COPD) is a complex a multifactorial disease that encompasses chronic bronchitis and pulmonary emphysema. The molecular and cellular events that result in COPD are poorly understood and include aberrant differentiation, inflammation, structural damage in the distal lung with enlargement of alveolar spaces, and tissue remodeling. Although there is clearly a role for environmental factors in the pathogenesis of COPD, there is also accumulated evidence of a major genetic component influencing disease susceptibility and severity. For example, COPD is associated with cigarette smoke, but the disease is manifested in a relatively small fraction of the overall population of smokers (1–3).
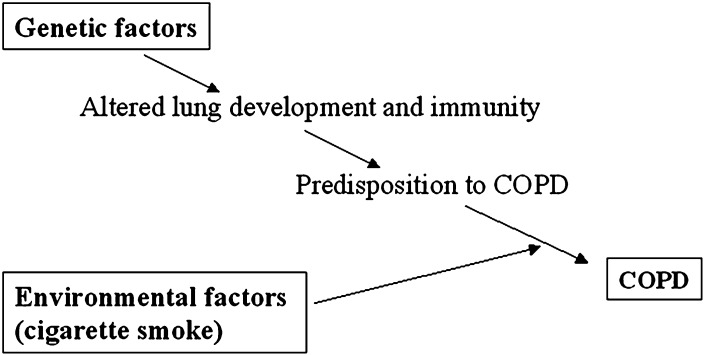
Accumulated evidence from microarray profiling, genome-wide association studies in human subjects and animal models suggests that genes originally associated with developmental processes could be implicated in COPD (Figure 1). Candidate pathways include the Sonic hedgehog, Notch, retinoid, and Tgf β. These studies raise questions as to whether the molecular or cellular events mediated by these developmental regulators are recapitulated in an aberrant fashion during injury-repair. Could subtle structural defects that arise from inappropriate expansion or differentiation of progenitor cells predispose to COPD or influence the severity of this disease? Here we discuss selected aspects of the regulation of lung development and how some of these developmental pathways could potentially influence COPD, based on information from animal models and humans.
Figure 1.
Diagram summarizing the influence of genetic and environmental factors in chronic obstructive pulmonary disease (COPD).
OVERVIEW OF LUNG DEVELOPMENT
The respiratory system arises from the primitive foregut when respiratory progenitors are specified and expanded to form the lung and the tracheal primordia. Subsequently, lung epithelial tubules grow and branch to give rise to the bronchial tree. This process is accompanied by development of the vascular structures, which arise by angiogenesis and vasculogenesis. Later in gestation, activation of specific developmental programs in airways leads epithelial progenitor cells to differentiate into a variety of phenotypes, including secretory, ciliated, neuroendocrine, and basal cells. Distal epithelial tubules differentiate into type I and type II cells and undergo sacculation to form the primitive gas-exchange region of the lung. Finally, the primitive saccules are subdivided into smaller units by a process of septation called “alveolization” (4–6). Alveolization initiates at late gestation in humans and postnatally in mice when secondary crests develop and extend to form the definitive alveoli. This increases greatly the surface area for gas exchange. Alveolization defects result in large alveoli, reminiscent of the abnormality found in emphysema, but usually with less overt destructive processes. The relationship between mild abnormal lung development and subsequent pulmonary diseases at adulthood is poorly understood and has been little explored.
THE HEDGEHOG PATHWAY, A POTENTIAL CANDIDATE TARGET IN COPD
Two independent genome-wide association studies in subjects from the Framingham Heart Study and from a homogenous case-control cohort from Norway have identified single nucleotide polymorphisms (SNPs) in chromosome 4 in individuals with airflow obstruction, based on pulmonary function parameters. These SNPs were mapped to an intergenic region near the Hedgehog interacting protein (Hhip) locus (7, 8). Although SNP is not synonymous with loss of function, its finding is suggestive of pathway implication that warrants further expression and functional studies. Hhip is a critical regulator of the Hedgehog (Hh) pathway, which has been implicated in development, repair, and cancer in multiple tissues (9). Components of this pathway include the ligands Sonic (Shh), Indian (Ihh), and Desert (Dhh) Hedgehog, which signal through Patched (Ptc) and Smoothened (Smo) receptors and Gli transcription factors (10). Hhip sequesters Hh ligands and inhibits signaling by preventing Hh ligands from binding to Ptc. Thus, Hhip deficiency leads to hyperactive Hh signaling in target tissues. How does Hhip and Hh signaling influence lung development? Loss of Hhip function in Hhip null mice leads to lethality at birth due to respiratory distress. Branching morphogenesis is greatly impaired, and the lungs are hypoplastic (9). The defect ultimately results from hyperactive Shh inhibiting the expression of Fgf10, a fibroblast growth factor expressed in the mesenchyme, which is critical for epithelial morphogenesis.
There is evidence that tight regulation of Fgf10 expression is necessary for proper expansion and patterning of progenitor cells in a number of developing organs (11–13). Lungs do not form in Fgf10 null mice (14). Decreased Fgf10 levels in hypomorphic mice generated by an allelic series approach result in inhibition of branching and markedly hypoplastic lungs (13). In turn, excessive Fgf10 also results in disruption of branching and severe hypoplasia, in this case due to uncontrolled epithelial growth. A number of studies show that Shh at the bud tips helps to control the spatial pattern and levels of Fgf10. By preventing the widespread expression of Fgf10, Shh contributes to controlling bud size and shape (15). One of the mechanisms proposed for this regulation involves Shh induction of its own regulator, Hhip, in the lung mesenchyme. Hhip1 inhibits Shh signaling by ligand sequestration and thus releases Shh-mediated repression of Fgf10. Insufficient levels of Hhip results in reduced Fgf10 levels, insufficient growth, and lung hypoplasia (5, 9). The idea that COPD could be associated with inappropriate growth or structural defects in small airways makes Hhip an attractive candidate developmental gene implicated in COPD.
There is evidence that altered Hh signaling has a profound impact on airway smooth muscle differentiation. Airway smooth muscle originates in part from progenitor cells located in the distal lung mesenchyme. These cells undergo a program of differentiation that initially depends on epithelial signals in distal lung buds, including Shh. Activation of Shh signaling in the distal lung mesenchyme initiates a myogenic program that continues as these cells migrate to more proximal regions (16, 17). The program is completed when these progenitor cells reach proximal airways. Shh promotes smooth muscle differentiation by induction of myocardin and α-smooth muscle actin (18, 19).
In principle, mild deficits in Hh-mediated processes, such as airway branching or smooth muscle cell differentiation, could have an impact later in adult life, presumably on how the lung responds to environmental stimuli. Whether there is altered Hh signaling in target tissues in the subjects identified by the studies mentioned above has not been demonstrated.
SMOC2, AIRWAY DEVELOPMENT, AND COPD
In a study by Wilk and colleagues (20), a quantitative trait locus in chromosome 6 that influences FEV1 measurements has been identified by linkage analysis in subjects from the Framingham Heart Study. In a subsequent report, these authors showed in the NHLBI Framingham Heart Study and the NHLBI Family Heart Study populations a strong association of airflow obstruction with SNPs and haplotypes in the intronic region of SMOC2 (SPARC-related modular calcium binding 2), which lies on 6q27. SMOC2 is a matricellular protein expressed in several tissues, including the adult lung and aorta (21). SMOC2 contains a Kazal domain that codes for a serine protease inhibitor, as in α1-antitrypsin. Although its function is unclear, there is evidence of SMOC2 up-regulation in the vascular wall of injured aortas, which suggests a role in tissue remodeling. In the embryonic murine lung, Smoc2 signals are present in the developing smooth muscle of pulmonary artery and airways (PL, JL, WVC, and CV, unpublished observations). Smoc2 is a transcriptional target of activated aryl-hydrocarbon receptor, which mediates the adverse effects of environmental aryl-hydrocarbon exposure (22, 23). SMOC2 expression in COPD has not been reported. Whether disruption of SMOC2 could affect smooth muscle growth, differentiation, or homeostasis or influence the airway response to environmental factors in diseases such as COPD remains to be explored.
NOTCH, A KEY PATHWAY IN LUNG DEVELOPMENT, AND ITS POTENTIAL ROLE IN COPD
Lung exposure to environmental agents, such as cigarette smoke, triggers maladaptive responses that alter the differentiation profile of the airway epithelium. The altered balance of ciliated and secretory cells, particularly the increase in mucous (goblet) cells, is a hallmark of the chronic bronchitis in patients with COPD. Although IL-13 and SPDEF (SAM-pointed domain-containing ETS transcription factor) are among the known regulators of secretory cell differentiation (24), there is an emerging role for Notch in this process. Notch is a major regulator of cell fate and progenitor cell stemness in multiple developing organs. Notch signaling results from cell–cell contact via interactions of Notch receptors (in mammalians, Notch 1–4) with ligands (Delta-like 1, 3, and 4, and Jagged 1, 2), leading to activation of Hes and Hey, bHLH transcriptional targets of Notch signaling (25).
A recent study by Tilley and colleagues (2009) shows that Notch pathway components are widely expressed in epithelial cells from bronchial brushings and biopsies of the adult lung. These authors show that Notch components, such as Notch3, Dll1, Hes, and Hey genes, are down-regulated in samples from adult smokers and smokers with COPD (26). Although it is uncertain whether these changes translate into decreased Notch signaling, they raise the possibility that the Notch pathway may contribute to the aberrant differentiation profile of the airways in these patients. How could Notch influence airway epithelial differentiation? In the developing mouse lung, Notch is critical for the normal balance of differentiated cell fates in the airway epithelium. Conditional epithelial disruption of Pofut1, an O-fucosyltransferase essential for Notch-ligand binding, or Rbpjk, the transcriptional effector of canonical Notch signaling (25), reveals a similar dramatic lung phenotype. In both mutants there is complete ablation of the secretory Clara cell lineage, and the airways become overpopulated with ciliated cells and neuroendocrine cells (27). Conversely, transgenic mice expressing a constitutively activated form of Notch1 in the lung epithelium show a reduced number of ciliated cells and an increase in mucin-producing cells (28). These studies point to a potential mechanism in which different thresholds of activation of Notch signaling may determine whether a cell will become a secretory (Clara cell or mucous) or a nonsecretory (ciliated or neuroendocrine) cell. This mechanism is likely to be in place not only during development but also in aberrant responses of the mature epithelium to environmental agents that results in airway epithelial metaplasia.
RETINOIC ACID FROM BUDS TO ALVEOLI: A ROLE IN LUNG REPAIR AND COPD?
Evidence of an association between serum vitamin A level and lung function in patients with COPD suggest an involvement of retinoic acid (RA) signaling in lung homeostasis (29). A study by Massaro and Massaro showing that exogenous RA can induce alveolar regeneration in adult rats with elastase-induced emphysema sparked tremendous enthusiasm in using RA as a potential therapy to treat patients with COPD (30). RA, the active metabolite of vitamin A, has an essential role in numerous developing and regenerating biological models, including limb, skin, and central nervous system (31). Several studies have established RA signaling as a critical regulator of early lung development. Analysis of a RA reporter mouse model reveals that RA activity is at its highest during the initiation of lung morphogenesis (32). Thus, it is not surprising that maternal deficiency of vitamin A results in dramatic abnormalities in the respiratory system, including tracheoesophageal fistula, lung hypoplasia, and lung agenesis (33). Similar phenotypes are seen in mice lacking a combination of retinoic receptors (RAR-RXR) (34), and no lungs form in mice deficient in the RA-synthesizing enzyme RALDH2 (retinaldehyde dehydrogenase) (35, 36). The mechanism by which RA influences these early events has been recently dissected and involves RA regulation of multiple pathways. For example, the lung bud agenesis seen in RA-deficient embryos results, at least in part, from hyperactivation of the Tgf β pathway, leading to inhibition of Fgf10 at the prospective sites of lung formation (37). These studies show that RA is not required to specify lung progenitors but is critical to expand an initial progenitor cell pool to form the lung primordium (35).
The idea that RA could regulate alveolization came from evidence that pulmonary lipofibroblasts, which store retinoids and make elastin, are present in the regions undergoing secondary septae formation during alveolization (39). Moreover, RA mediates up-regulation of elastin in vitro (38). Studies in RAR mutant mice suggest that RA can act as a positive or a negative regulator of alveolization depending on the RAR type. RARβ-null mice septate earlier and faster than control mice, whereas RARγ null have decreased alveolar number and an increase in alveolar volume (40, 41).
Since the publication of the original study of Massaro and Massaro, other groups have shown that RA is able to fully or partially rescue emphysema-like lesions in different models (42–44). However, there are also studies that failed to show benefits of RA in emphysema (45–49). The reason for the conflicting findings is unclear. The difference in RA pharmacokinetics between strains and species may play a role (50).
The FORTE study (Feasibility of Retinoids for the Treatment of Emphysema) was a multicenter trial conducted to investigate the effect of pharmacologic dosing of RA on lung function of patients with moderate to severe COPD. Although it was shown that the RA therapy was well tolerated, there were no significant improvements in lung functions or quality of life. This study was limited by the lack of a true placebo group and the small sample size (51). RARγ agonists are currently in phase II clinical trials in adults with emphysema induced by α1-antitrypsin deficiency and in patients with smoking-related emphysema (50). The outcome of these studies should be available in a few years.
ABNORMALITIES IN GENES INVOLVED IN TGF-β–SMAD SIGNALING ARE ASSOCIATED WITH EMPHYSEMA
Altered TGF-β signaling has been implicated in the pathogenesis and exacerbation of emphysema. TGF-βs consist of a multifamily group of growth factors that signal through serine-thereonine kinase receptors to regulate fundamental biological processes (52). Three TGF-β subfamily ligands exist in mammals, TGF-β1, TGF-β2, and TGF-β3, which bind to heteromeric complexes of transmembrane TGF-β type II and type I receptors (TβRII and TβRI) and activate downstream Smad2 and/or Smad3 proteins (53, 54). These activated Smads form complexes with Smad4 and translocate into the nucleus to induce or repress TGF-β–target gene expression, including TGF-β1 ligand and Smad7 (54). In turn, Smad7 can inhibit Smad2/3 activation. Integrin β6, latent TGF-β binding proteins (LTBPs), and thrombospondin are involved in regulating the release of TGF-β active peptide, while betaglycan/endoglin or decorin influence the affinity of TGF-β receptor binding.
Genetic polymorphisms of TGF-β1 or TβRII or the β-glycan gene have been associated with emphysema in smokers (55–59). Decreased expression of TGF-β1, TβRI, and Smad3 has been reported in lung tissue specimens from patients with stage II COPD compared with normal control subjects (60). Moreover, cultured pulmonary fibroblasts from patients with emphysema show reduced response to TGF-β1 stimulation accompanied by increased levels of inhibitory Smad7 (Smad2/3 inhibitor) and reduced Smad3 activation, although TGF-β ligand production is increased (61). Reduced Smad3 and increased Smad7 expression have been also reported in cultured fibroblasts in another group of patients with COPD; these changes in expression were further exacerbated by the addition of cigarette smoke extract (62). Furthermore, the potential role of altered TGF-β signaling in the pathogenesis of emphysema has been suggested from several studies in animal models. For example, loss of integrin αvβ6-mediated TGF-β activation leads to matrix metalloproteinase 12–dependent emphysema in integrin β6 null mutant mice (63). Together these observations suggest that reduced TGF-β-Smad3–mediated signaling may play a role in the pathogenesis of pulmonary emphysema in some patients with COPD. Increased TGF-β ligand expression or activity may be associated with emphysema in other cases (64); overexpression of TGF-β1 in mice results in emphysematous lungs and in fibrosis (65).
TGF-β SIGNALING IS ESSENTIAL FOR LUNG AND IMMUNE SYSTEM DEVELOPMENT
Disruption of TGF-β signaling during development results in abnormalities in the respiratory tract and the immune system. Mice lacking TGF-β1 develop severe pulmonary inflammation, whereas TGF-β2 null mutant mice die immediately after birth with collapsed conducting airway (66, 67). TGF-β3 null mutant mice display cleft palate, retarded lung development, and neonatal lethality (68, 69). The null mutation of LTBP-3 or LTBP-4 causes profound defects in elastin fiber structure and lung alveolization, similar to the phenotypic changes observed in Smad3 knockout mouse lung (70–72). In addition, blockade of TGF-β signaling in epithelial cells versus mesenchymal cells of the developing mouse lung shows a cell-specific regulatory impact on lung branching morphogenesis and alveolization in vivo. Selective blockade of endogenous TGF-β signaling in embryonic lung mesenchymal cells results in retarded lung branching after midgestation, whereas abrogation of epithelial cell-specific TGF-β signaling causes mild abnormality in postnatal lung alveolization, but does not appear to have a significant impact on prenatal lung development (73). However, overexpression of TGF-β1 in the embryonic lung epithelium also results in the arrest of lung growth and epithelial cell differentiation and in inhibition of pulmonary vasculogenesis (74, 75). Thus, appropriate TGF-β signaling activity is essential for normal lung development.
TGF-β has an important role in the development of the innate and adaptive immune system, including multiple inhibitory effects on T/B cell proliferation, differentiation, and activation (76). The dominant role of TGF-β in the development and function of the immune system is to induce tolerance and to contain and resolve inflammation. In the absence of proinflammatory signals from the innate immune system, activated TGF-β signaling combined with antigen stimulation promotes the development of induced regulatory T cells, which exerts immune suppressive function (77). Studies on patients and animal models suggest that defective TGF-β signaling is involved in many systemic immune diseases, including autoimmune diseases (e.g., systemic lupus erythematosus, rheumatoid arthritis, type I diabetes, multiple sclerosis), inflammatory diseases (e.g., atherosclerosis, asthma, inflammatory bowel disease), infection, and tumor growth (76). However, TGF-β also has proinflammatory effects by promoting Th17 cell differentiation when IL-6 is available in the milieu of inflammation.
ABNORMAL LUNG DEVELOPMENT AND IMMUNITY BY DYSREGULATED TGF-β SIGNALING MAY PREDISPOSE TO EMPHYSEMA
Analysis of Smad3 null mutant mice indicates that Smad3 deficiency impairs early alveolization and immunity, and may contribute as a developmental antecedent to the pathogenesis of centrilobular emphysema (72). Short-term exposure to cigarette smoke during postnatal alveolization exacerbates the alveolar destruction seen in Smad3 null mutants, whereas it has no significant effect on the lung of wild-type littermates. Mice with selective deletion of TGF-β signaling in lung epithelial cells or in the immune system do not develop emphysema-like pathology at early adulthood. However, blockade of TGF-β signaling in the lung epithelium and immune system results in hypoplastic lungs and subsequent alveolar destruction (WS, DW, unpublished observation). This suggests a possible mechanism by which genetic deficiency of TGF-β signaling contributes to the predisposition to emphysema by disturbing lung structural development and the lung inflammatory response. Thus, cigarette smoke, particularly second-hand smoke exposure at childhood, may be a major risk factor in the subjects that are genetically predisposed to emphysema.
CONCLUSIONS
A wealth of information on the molecular regulation of murine lung development has been accumulated over the past decades. The data from these studies in mouse models have unveiled important mechanisms by which growth factors, transcription factors, and matrix components influence developmental processes in the lung. Some of these studies show alterations in developmental pathways in animal models of disease that may be relevant to human lung conditions, including COPD. However, interspecies differences should be considered when translating the information from murine models to humans. For example, there are important interspecies differences in the abundance and distribution of specific cell types in the airways that may influence the response of the lung to particular types of injury. Moreover, some mouse genetic models may be unable to reproduce the spontaneous disease phenotype seen in humans, as in the case of cystic fibrosis. Nevertheless, overall the data from animal models offer invaluable insights into the potential involvement of developmental genes in disease pathogenesis.
Supported by NIH grants NHLBI-P01 HL47049 (W.V.C.) and NIH/NHLBI 2RO-1 HL067129–05 (W.V.C., F.C.).
Conflict of Interest Statement: W.S. received lecture fees from AstraZeneca ($1,001– $5,000) and received grant support from the NIH ($100,001 or more) and from Philip Morris External Research Program ($50,001–$100,000) (January 1, 2008 to July 30, 2008). F.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.V.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Siafakas NM, Tzortzaki EG. Few smokers develop COPD. Why? Respir Med 2002;96:615–624. [DOI] [PubMed] [Google Scholar]
- 2.Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc 2008;5:475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburton D, Gauldie J, Bellusci S, Shi W. Lung development and susceptibility to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:668–672. [DOI] [PubMed] [Google Scholar]
- 4.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 2000;92:55–81. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 2006;133:1611–1624. [DOI] [PubMed] [Google Scholar]
- 6.Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev 2007;87:219–244. [DOI] [PubMed] [Google Scholar]
- 7.Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, Feng S, Hersh CP, Bakke P, Gulsvik A, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 2009;5:e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilk JB, Chen TH, Gottlieb DJ, Walter RE, Nagle MW, Brandler BJ, Myers RH, Borecki IB, Silverman EK, Weiss ST, et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet 2009;5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev 2003;17:342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet 2000;67:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 2001;128:5109–5117. [DOI] [PubMed] [Google Scholar]
- 12.Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 signaling controls stomach morphogenesis. Dev Biol 2007;303:295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, et al. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 2007;307:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev 1998;12:3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 1998;8:1083–1086. [DOI] [PubMed] [Google Scholar]
- 16.Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, Thiery JP, Bellusci S. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development 2005;132:2157–2166. [DOI] [PubMed] [Google Scholar]
- 17.Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol 2003;258:169–184. [DOI] [PubMed] [Google Scholar]
- 18.White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development 2006;133:1507–1517. [DOI] [PubMed] [Google Scholar]
- 19.Yi L, Domyan ET, Lewandoski M, Sun X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn 2009;238:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilk JB, DeStefano AL, Joost O, Myers RH, Cupples LA, Slater K, Atwood LD, Heard-Costa NL, Herbert A, O'Connor GT, et al. Linkage and association with pulmonary function measures on chromosome 6q27 in the Framingham Heart Study. Hum Mol Genet 2003;12:2745–2751. [DOI] [PubMed] [Google Scholar]
- 21.Vannahme C, Gosling S, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem J 2003;373:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Lu J, Cardoso WV, Vaziri C. The SPARC-related factor SMOC-2 promotes growth factor-induced cyclin D1 expression and DNA synthesis via integrin-linked kinase. Mol Biol Cell 2008;19:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Pazin DE, Merson RR, Albrecht KH, Vaziri C. The developmentally-regulated Smoc2 gene is repressed by Aryl-hydrocarbon receptor (Ahr) signaling. Gene 2009;433:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park KS, Korfhagen TR, Bruno MD, Kitzmiller JA, Wan H, Wert SE, Khurana Hershey GK, Chen G, Whitsett JA. SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 2007;117:978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009;137:216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O'Connor TP, Crystal RG. Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;179:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 2009;136:2297–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 2009;136:1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morabia A, Menkes MJ, Comstock GW, Tockman MS. Serum retinol and airway obstruction. Am J Epidemiol 1990;132:77–82. [DOI] [PubMed] [Google Scholar]
- 30.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med 1997;3:675–677. [DOI] [PubMed] [Google Scholar]
- 31.Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev Dyn 2003;226:237–244. [DOI] [PubMed] [Google Scholar]
- 32.Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development 2000;127:3057–3067. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency: effects of restoration of vitamin A at various times during gestation. Am J Anat 1953;92:189–217. [DOI] [PubMed] [Google Scholar]
- 34.Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II): multiple abnormalities at various stages of organogenesis in RAR double mutants. Development 1994;120:2749–2771. [DOI] [PubMed] [Google Scholar]
- 35.Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev Biol 2004;273:402–415. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Dolle P, Cardoso WV, Niederreither K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev Biol 2006;297:433–445. [DOI] [PubMed] [Google Scholar]
- 37.Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 2007;134:2969–2979. [DOI] [PubMed] [Google Scholar]
- 38.McGowan SE, Doro MM, Jackson SK. Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am J Physiol 1997;273:L410–L416. [DOI] [PubMed] [Google Scholar]
- 39.Burri PH. The postnatal growth of the rat lung: 3. Morphology. Anat Rec 1974;180:77–98. [DOI] [PubMed] [Google Scholar]
- 40.Massaro GD, Massaro D, Chan WY, Clerch LB, Ghyselinck N, Chambon P, Chandraratna RA. Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics 2000;4:51–57. [DOI] [PubMed] [Google Scholar]
- 41.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 2000;23:162–167. [DOI] [PubMed] [Google Scholar]
- 42.Maden M. Retinoids have differing efficacies on alveolar regeneration in a dexamethasone-treated mouse. Am J Respir Cell Mol Biol 2006;35:260–267. [DOI] [PubMed] [Google Scholar]
- 43.Belloni PN, Garvin L, Mao CP, Bailey-Healy I, Leaffer D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest 2000;117:235S–241S. [DOI] [PubMed] [Google Scholar]
- 44.Tepper J, Pfeiffer J, Aldrich M, Tumas D, Kern J, Hoffman E, McLennan G, Hyde D. Can retinoic acid ameliorate the physiologic and morphologic effects of elastase instillation in the rat? Chest 2000;117:242S–244S. [DOI] [PubMed] [Google Scholar]
- 45.Fujita M, Ye Q, Ouchi H, Nakashima N, Hamada N, Hagimoto N, Kuwano K, Mason RJ, Nakanishi Y. Retinoic acid fails to reverse emphysema in adult mouse models. Thorax 2004;59:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucey EC, Goldstein RH, Breuer R, Rexer BN, Ong DE, Snider GL. Retinoic acid does not affect alveolar septation in adult FVB mice with elastase-induced emphysema. Respiration 2003;70:200–205. [DOI] [PubMed] [Google Scholar]
- 47.March TH, Bowen LE, Finch GL, Nikula KJ, Wayne BJ, Hobbs CH. Effects of strain and treatment with inhaled aII-trans-retinoic acid on cigarette smoke-induced pulmonary emphysema in mice. COPD 2005;2:289–302. [PubMed] [Google Scholar]
- 48.March TH, Cossey PY, Esparza DC, Dix KJ, McDonald JD, Bowen LE. Inhalation administration of all-trans-retinoic acid for treatment of elastase-induced pulmonary emphysema in Fischer 344 rats. Exp Lung Res 2004;30:383–404. [DOI] [PubMed] [Google Scholar]
- 49.Srinivasan G, Bruce EN, Houtz PK, Bruce MC. Dexamethasone-induced changes in lung function are not prevented by concomitant treatment with retinoic acid. Am J Physiol Lung Cell Mol Physiol 2002;283:L275–L287. [DOI] [PubMed] [Google Scholar]
- 50.Hind M, Gilthorpe A, Stinchcombe S, Maden M. Retinoid induction of alveolar regeneration: from mice to man? Thorax 2009;64:451–457. [DOI] [PubMed] [Google Scholar]
- 51.Roth MD, Connett JE, D'Armiento JM, Foronjy RF, Friedman PJ, Goldin JG, Louis TA, Mao JT, Muindi JR, O'Connor GT, et al. Feasibility of retinoids for the treatment of emphysema study. Chest 2006;130:1334–1345. [DOI] [PubMed] [Google Scholar]
- 52.Sporn MB, Roberts AB. 1991. The transforming growth factor bs. In: Sporn MB, Roberts AB, editors Peptide growth factors and their receptor: handbook of experimental pharmacology. Springer-Verlag, Heidelberg, Germany. pp. 419–472.
- 53.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 54.Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol 2000;12:235–243. [DOI] [PubMed] [Google Scholar]
- 55.Wu L, Chau J, Young RP, Pokorny V, Mills GD, Hopkins R, McLean L, Black PN. Transforming growth factor-beta1 genotype and susceptibility to chronic obstructive pulmonary disease. Thorax 2004;59:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Celedon JC, Lange C, Raby BA, Litonjua AA, Palmer LJ, Demeo DL, Reilly JJ, Kwiatkowski DJ, Chapman HA, Laird N, et al. The transforming growth factor-beta1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD). Hum Mol Genet 2004;13:1649–1656. [DOI] [PubMed] [Google Scholar]
- 57.Baraldo S, Bazzan E, Turato G, Calabrese F, Beghe B, Papi A, Maestrelli P, Fabbri LM, Zuin R, Saetta M. Decreased expression of TGF-beta type II receptor in bronchial glands of smokers with COPD. Thorax 2005;60:998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hersh CP, Hansel NN, Barnes KC, Lomas DA, Pillai SG, Coxson HO, Mathias RA, Rafaels NM, Wise RA, Connett JE, Klanderman BJ, Jacobson FL, Gill R, Litonjua AA, Sparrow D, Reilly JJ, Silverman EK. Transforming growth factor beta receptor-3 is associated with pulmonary emphysema. Am J Respir Cell Mol Biol 2009;41:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito M, Hanaoka M, Droma Y, Hatayama O, Sato E, Katsuyama Y, Fujimoto K, Ota M. The association of transforming growth factor beta 1 gene polymorphisms with the emphysema phenotype of COPD in Japanese. Intern Med 2008;47:1387–1394. [DOI] [PubMed] [Google Scholar]
- 60.Zandvoort A, Postma DS, Jonker MR, Noordhoek JA, Vos JT, van der Geld YM, Timens W. Altered expression of the Smad signalling pathway: implications for COPD pathogenesis. Eur Respir J 2006;28:533–541. [DOI] [PubMed] [Google Scholar]
- 61.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, Kawasaki S, Ahn Y, Fredriksson K, Skold CM, et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med 2008;178:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zandvoort A, Postma DS, Jonker MR, Noordhoek JA, Vos JT, Timens W. Smad gene expression in pulmonary fibroblasts: indications for defective ECM repair in COPD. Respir Res 2008;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 2003;422:169–173. [DOI] [PubMed] [Google Scholar]
- 64.Churg A, Tai H, Coulthard T, Wang R, Wright JL. Cigarette smoke drives small airway remodeling by induction of growth factors in the airway wall. Am J Respir Crit Care Med 2006;174:1327–1334. [DOI] [PubMed] [Google Scholar]
- 65.Lee CG, Cho S, Homer RJ, Elias JA. Genetic control of transforming growth factor-beta1-induced emphysema and fibrosis in the murine lung. Proc Am Thorac Soc 2006;3:476–477. [DOI] [PubMed] [Google Scholar]
- 66.Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science 1994;264:1936–1938. [DOI] [PubMed] [Google Scholar]
- 67.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 1997;124:2659–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 1995;11:415–421. [DOI] [PubMed] [Google Scholar]
- 69.Shi W, Heisterkamp N, Groffen J, Zhao J, Warburton D, Kaartinen V. TGF-beta3-null mutation does not abrogate fetal lung maturation in vivo by glucocorticoids. Am J Physiol 1999;277:L1205–L1213. [DOI] [PubMed] [Google Scholar]
- 70.Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, et al. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev 2002;16:2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, Dabovic B, Rifkin DB. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol 2005;167:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen H, Sun J, Buckley S, Chen C, Warburton D, Wang XF, Shi W. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol 2005;288:L683–L691. [DOI] [PubMed] [Google Scholar]
- 73.Chen H, Zhuang F, Liu YH, Xu B, Del MP, Deng W, Chai Y, Kolb M, Gauldie J, Warburton D, et al. TGF-{beta} receptor II in epithelia versus mesenchyme plays distinct role in developing lung. Eur Respir J 2008;32:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou L, Dey CR, Wert SE, Whitsett JA. Arrested lung morphogenesis in transgenic mice bearing an SP-C-TGF-beta 1 chimeric gene. Dev Biol 1996;175:227–238. [DOI] [PubMed] [Google Scholar]
- 75.Zeng X, Gray M, Stahlman MT, Whitsett JA. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev Dyn 2001;221:289–301. [DOI] [PubMed] [Google Scholar]
- 76.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99–146. [DOI] [PubMed] [Google Scholar]
- 77.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol 2009;21:274–280. [DOI] [PubMed] [Google Scholar]