Abstract
Protein prenylation involves the addition of either a farnesyl (C15) or geranylgeranyl (C20) isoprenoid moiety onto the C-terminus of many proteins. This natural modification serves to direct a protein to the plasma membrane of the cell. A recently discovered application of prenylated peptides is that they have inherent cell-penetrating ability, and are hence termed cell penetrating prenylated peptides. These peptides are able to efficiently cross the cell membrane in an ATP independent, non-endocytotic manner and it was found that the sequence of the peptide does not affect uptake, so long as the geranylgeranyl group is still present. The present study investigates the effect of removing the fluorophore from the peptides and investigating the uptake by confocal microsopy and flow cytometry. Our results show that the fluorophore is not necessary for uptake of these peptides. This information is significant because it indicates that the prenyl group is the major determinant in allowing these peptides to enter cells; the hydrophobic fluorophore has little effect. Moreover, these studies demonstrate the utility of the Cu-catalyzed click reaction for monitoring the entry of nonfluorescent peptides into cells.
Keywords: Peptide, lipid modification, post-translational modification, prenylation, farnesyl, cell-penetrating peptide
One particularly prevalent post-translational modification is known as protein prenylation, which occurs on approximately 2% of all mammalian proteins.[1] This modification involves the addition of a C15 (farnesyl) or C20 (geranylgeranyl) isoprenoid moiety onto a cysteine residue near the C-terminus of proteins that bear a ‘CAAX’ box motif, catalyzed by either the farnesyltransferase or geranylgeranyltransferase enzyme.[2-4] In the ‘CAAX’ box, A represents any aliphatic amino acid and X represents the residue that controls farnesylation or geranylgeranylation.[5, 6] The prenylation modification serves to direct membrane association of many proteins including members of the Ras superfamily of proteins.[7] It has been previously shown that the K-Ras protein is involved in approximately 30% of all human cancers and inhibition of the farnesylation of Ras abolishes its oncogenic activity.[8-10] Hence, considerable work has been aimed at developing farnesyltransferase inhibitors, many of which are in clinical trials.[11-13]
Several important molecules for therapeutic consideration are not able to naturally traverse the cell membrane and are thus being attached to cell penetrating peptides (CPPs) to facilitate membrane crossing.[14] The majority of the current CPPs range from 5-25 amino acids and often contain several charged amino acid residues.[15-17] The drawback of most current CPPs is that they are taken up into cells by an energy-dependent endocytotic process, which leads to a majority of the cargo being transported to endosomes and eventually lysosomes for degradation.[18] Designing CPPs that are able to escape lysosomal degradation has become a high priority.
Our group has previously developed a series of peptides that are based on the C-terminus of the naturally geranylgeranylated protein CDC42. These peptides were functionalized with a fluorophore and a geranylgeranyl group and found to have intrinsic cell-penetrating ability, by a mechanism that was energy independent.[19] This study also demonstrated that without the geranylgeranyl group, the peptides were unable to enter cells; related experiments demonstrated that farnesylated peptides derived from CDC42 can also enter a wide variety of cell types.[20] A further study examining the role of the positively charged amino acids in the sequence found that these amino acids were not necessary for uptake, and that a peptide as short as two amino acids was able to penetrate cells, provided the geranylgeranyl group was present.[21] Our current study serves to elucidate the role of the 5-carboxyfluorescein (5-Fam) fluorophore on the uptake of these peptides. The benefit of this class of cell penetrating prenylated peptides is that they enter cells by a non-endocytotic, ATP independent mechanism that allows accumulation into the cytosol of cells.[19, 21] These peptides may prove useful in cell-penetrating applications in which the cargo can be easily released in the cytosol.
For this study, the peptides (Figure 1) were synthesized using standard Fmoc coupling conditions on an automated synthesizer with Rink-amide resin to afford the C-terminal amide peptides. While still on resin, the N-terminal lysine side chain (ε amine) was acetylated, followed by acylation with an alkyne-containing acid (4-pentynoic acid) on the amino group to give the resulting N-terminal alkyne. Geranylgeranylation of that peptide was performed in solution using acidic Zn(OAc)2 coupling conditions[22] to afford peptide 1. Peptide 2 was prepared in an analogous manner except that the ε-amino group of the N-terminal lysine was acylated with 5-Fam. Thus peptide 1 contains an alkyne but no fluorophore whereas peptide 2 contains both an alkyne and a fluorophore.
Figure 1. The sequences of the peptides used in this study.
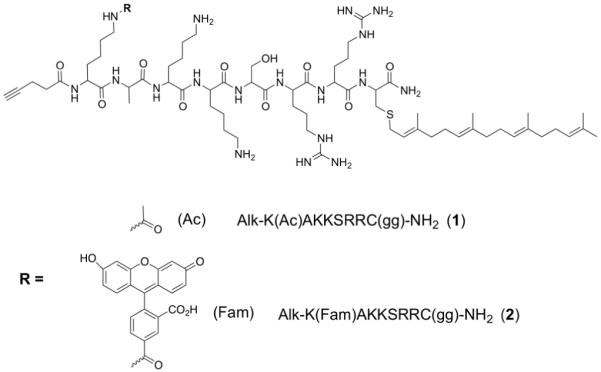
The ability of both of these peptides to enter cells was first established using confocal laser scanning microscopy (CLSM). Because peptide 2 contains the 5-Fam fluorophore, visualization after uptake in HeLa cells is easily accomplished after a 2 h incubation of the peptide at 1 μM (Figure 2B). To investigate whether the 5-Fam fluorophore is necessary for uptake, peptide 1 was synthesized lacking the fluorophore. Following cellular incubation of 1 at 1 μM for 2 h, HeLa cells were rinsed three times with PBS and fixed with 4% paraformaldehyde, followed by permeabilization with 0.1 % Triton X-100. A copper catalyzed click reaction was then conducted using tetramethylrhodamine azide (TAMRA-N3) to form a covalent triazole linkage between the peptide-alkyne and the tetramethylrhodamine (TAMRA) fluorophore; when 1 enters cells, it can be visualized through the TAMRA fluorophore using CLSM (Figure 2C). Thus, it can be seen that the peptide lacking a fluorophore is still able to efficiently cross the membrane of HeLa cells. While control experiments in which HeLa cells were treated with TAMRA-N3 (without prior peptide treatment) manifested no background labeling (Figure 2A), we wanted to compare the localization pattern obtained by direct visualization of 5-Fam with that observed from the TAMRA-N3 labeling. Incubation of 2 with HeLa cells and subsequent click reaction to TAMRA-N3 resulted in strong co-localized fluorescence in the cells (yellow color, Figure 2D); this observation also confirms that little background reaction occurs in the TAMRA-N3 labeling process since the red TAMRA fluorophore is only present where the green 5-Fam of the peptide is observed. The Mander’s overlap coefficient of the cells is 0.901 when the nucleus is included in the analysis and 0.960 when it is excluded, indicating there is significant overlap of green and red fluorescence, even when the nucleus is considered (a Mander’s coefficient of 1 indicates perfect overlap[23]). It is important to note that it appears there is some nuclear labeling when HeLa cells are incubated with 1. To explore this issue, we incubated peptide 1 with HeLa cells, followed by a subsequent click reaction to either TAMRA-N3 or 5-Fam-PEG-N3. This will help establish if the nuclear localization is an artifact of the fixation or of the fluorophore used. Regardless of the fluorophore used, nuclear staining is observed when incubating HeLa cells with 1 (Figure 3). This effect is absent when performing the click reaction on cells that were not treated with alkyne peptide (see Figure 2A), and is also absent in cells treated with 2 (see Figure 2B). This suggests that peptide 1 that lacks the fluorophore partitions differently within cells and is able to enter the nucleus whereas peptide 2 does not; however, it should be noted that the amount of nuclear localization is small compared to the amount of peptide distributed throughout the remainder of the cell. Overall, the localization patterns of 1 and 2 are quite similar.
Figure 2.
HeLa cells incubated with peptides 1 or 2 at 1 μM for 2 h. Panel A) Click reaction performed on cells that were not treated with an alkyne peptide, showing no background labeling by TAMRA-N3. Panel B) Peptide 2 visualized with green 5-Fam fluorescence. Panel C) Peptide 1 monitored by click reaction with TAMRA-N3 on fixed cells. Panel D) Peptide 2 monitored by click reaction with TAMRA-N3 on fixed cells and green 5-Fam fluorescence; the yellow color indicates co-localization of 5-Fam and TAMRA fluorophores, now present on the same peptide. The size bar represents a distance of 25 μm.
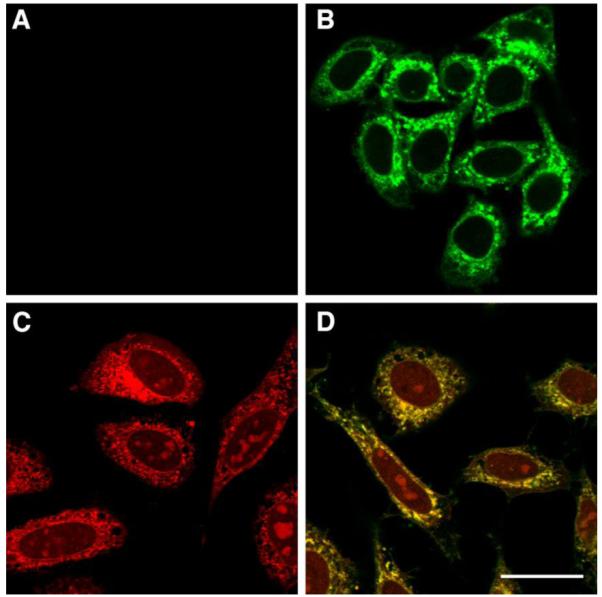
Figure 3.
HeLa cells incubated with peptide 1 at 1 μM for 2 h. Panel A) Peptide 1 visualized by click reaction with 5-Fam-PEG-N3 on fixed cells. Panel B) Peptide 1 visualized by click reaction with TAMRA-N3 on fixed cells. Regardless of the fluorophore used, nuclear staining is observed in both cases. This effect is not seen in cells that are subjected to the click reaction but not treated with peptide; this indicates that peptide 1 has a small amount of nuclear localization. The size bar represents a distance of 25 μm.
Having visually established that peptide 1 can enter HeLa cells, we next sought to quantify the differences in uptake between 1 and 2. Accordingly, a reagent containing 5-Fam linked to an azide moiety was synthesized for this purpose. This reagent was designed so that the fluorescence of the two peptides could be more directly compared since both peptides would be labeled by the same fluorophore. A succinimidyl ester of 5-Fam was reacted with 11-Azido-3,6,9-trioxaundecan-1-amine in the presence of diisopropylethylamine overnight at room temperature. Following purification by reverse phase HPLC, 5-Fam-PEG-N3 was isolated and used as the subsequent azide for click chemistry. HeLa cells were incubated with 1 or 2 at various concentrations for 1 h. Both sets of cells were rinsed, fixed and permeabilized. Cells treated with peptide 1 were subjected to the click reaction with 5-Fam-PEG-N3 for 1 h followed by several rinses, and subsequent flow cytometry analysis. Cells treated with peptide 2 were analyzed directly by flow cytometry without click reaction. Based on those experiments, peptide 1 appears to enter the cells to a lesser extent (2-3-fold) than 2 (Figure 4) at all concentrations tested. This could indicate that 1 is indeed not as efficient at penetrating the cellular membrane compared to 2 since it lacks the fluorophore. Alternatively, this result could be an artifact of the analysis; the click reaction may not quantitatively label all of the available alkyne-functionalized peptide during the course of the reaction. It is also plausible that if 1 enters the cells and localizes to an internal membrane (endoplasmic reticulum localization is natural for geranylgeranylated proteins[24]) that the N-terminal alkyne becomes buried in the membrane and is inaccessible for the click reaction. These latter two possibilities would both lead to an artificially lower mean fluorescence value. Regardless of which explanation is correct for the reduced fluorescence of cells treated with 1 versus 2, the important conclusion remains – peptide 1 lacking a fluorophore is able to efficiently enter HeLa cells. This further substantiates the importance of the hydrophobic geranylgeranyl group for peptide uptake; without that moiety, the peptide is unable to enter cells.[19]
Figure 4.
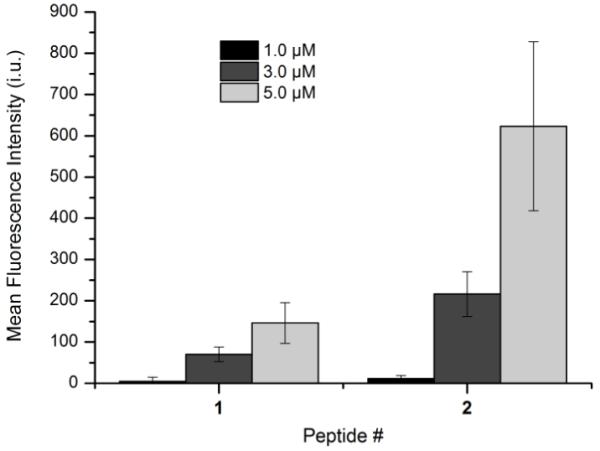
Flow cytometry analysis of peptides 1 and 2 incubated for 1 h at various concentrations. Each bar represents the geometric mean fluorescence of 10,000 cells, with the background fluorescence subtracted from each sample. Each experiment was performed in at least triplicate, expressed as the geometric mean ± standard deviation.
In previous work, we have shown that peptide sequences containing a geranylgeranyl group are efficiently taken into cells in an energy-independent manner, regardless of positive charge in the sequence. The findings reported here demonstrate that the presence of the hydrophobic fluorophore 5-Fam in such peptides has minimal effect on their cell penetrating ability, further underscoring the importance of the isoprenoid moiety. This information may be useful for applications utilizing cell-penetrating peptides to deliver cargo across membranes. Using the smallest molecule for a CPP has the benefit of facile synthesis as well as minimum disturbance of the cell during cargo delivery. The non-endocytotic mechanism that functions in the uptake of these peptides may also prove to be useful since it avoids potential endosomal localization/degradation of cargo. For instance, Medintz and coworkers attached a CPP to a quantum dot and a fluorescent protein to study the internalization of cargo, finding that microinjection of the conjugate into cells was necessary to avoid endosomal uptake.[25] Finally, we note the novel method for visualizing cell penetrating peptides described herein. To date, most studies of cell penetrating peptides and related materials have employed fluorophores linked to the compounds themselves to report on cellular entry. The incorporation of bulky, hydrophobic fluorescent groups inevitably perturbs the chemical and physical properties of the molecules under study and hence complicates structure/function analysis. For example, the addition of the 5-Fam fluorophore to a lysine residue alters the calculated partition coefficient (CLogP) by 3 units, illustrating a large change in the hydrophobic properties of the parent molecule (Figure S3). The method reported here provides a simple solution to this problem. The incorporation of a small alkyne-containing moiety into the peptide results in minimal alteration of the properties of the parent peptide. The CLogP value of Lys(5-Fam) with either an acetylated N-terminus or an N-terminal alkyne differs by only approximately 0.5 units, indicating the addition of the alkyne is a rather benign change to the hydrophobicity of the peptide (see Figure S4). However, the presence of the alkyne allows for facile visualization via click-mediated fluorescent labeling when desired. Furthermore, the alkyne group may be used in a variety of click reactions beyond fluorophore attachment. This approach should be quite useful for a variety of studies of cell penetrating molecules.
Supplementary Material
Acknowledgements
The authors would like to thank Professor George Barany for use of his automated peptide synthesizer and lyophilizer. We would also like to acknowledge the assistance of the Flow Cytometry Core Facility of the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598. This work was supported by the National Institutes of Health (GM 58442).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lamango NS, Koikov L, Duverna R, Abonyo BO, Hubbard JB. 2007:37–68. [Google Scholar]
- [2].Benetka W, Koranda M, Eisenhaber F. Monatsh. Chem. 2006;137:1241–1281. [Google Scholar]
- [3].Bergo MO, Wahlstrom AM, Fong LG, Young SG. Methods Enzymol. 2008;438:367–389. doi: 10.1016/S0076-6879(07)38026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nguyen UTT, Goody RS, Alexandrov K. ChemBioChem. 2010;11:1194–1201. doi: 10.1002/cbic.200900727. [DOI] [PubMed] [Google Scholar]
- [5].Lane KT, Beese LS. J. Lipid Res. 2006;47:681–699. doi: 10.1194/jlr.R600002-JLR200. [DOI] [PubMed] [Google Scholar]
- [6].Long SB, Beese LS. 2001:37–48. [Google Scholar]
- [7].Casey PJ. J. Lipid Res. 1992;33:1731–40. [PubMed] [Google Scholar]
- [8].Ayral-Kaloustian S, Salaski EJ. Curr. Med. Chem. 2002;9:1003–1032. doi: 10.2174/0929867024606687. [DOI] [PubMed] [Google Scholar]
- [9].Cortes J. Clin Lymphoma. 2003;4(Suppl 1):S30–5. doi: 10.3816/clm.2003.s.006. [DOI] [PubMed] [Google Scholar]
- [10].Ohkanda J, Knowles DB, Blaskovich MA, Sebti SM, Hamilton AD. Curr Top Med Chem. 2002;2(3):303–23. doi: 10.2174/1568026023394281. [DOI] [PubMed] [Google Scholar]
- [11].Blum R, Kloog Y. Drug Resist. Updates. 2005;8:369–380. doi: 10.1016/j.drup.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [12].Karp JE. Basic Clin. Oncol. 2008;35:491–512. [Google Scholar]
- [13].Zhu K, Hamilton AD, Sebti SM. Curr. Opin. Invest. Drugs (Thomson Curr. Drugs) 2003;4:1428–1435. [PubMed] [Google Scholar]
- [14].Brasseur R, Divita G. Biochim. Biophys. Acta, Biomembr. 2010;1798:2177–2181. doi: 10.1016/j.bbamem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- [15].Hansen M, Eriste E, Langel U. The internalization mechanisms and bioactivity of the cell-penetrating peptides. Wiley-VCH Verlag GmbH & Co. KGaA; 2009. pp. 125–143. [Google Scholar]
- [16].Lindgren M, Langel U. Methods Mol. Biol. (N. Y., NY, U. S.) 2011;683:3–19. doi: 10.1007/978-1-60761-919-2_1. [DOI] [PubMed] [Google Scholar]
- [17].Tuennemann G, Cardoso MC. IUL Biotechnol. Ser. 2010;9:329–362. [Google Scholar]
- [18].Raeaegel H, Saeaelik P, Hansen M, Langel U, Pooga M. J. Controlled Release. 2009;139:108–117. doi: 10.1016/j.jconrel.2009.06.028. [DOI] [PubMed] [Google Scholar]
- [19].Wollack JW, Zeliadt NA, Mullen DG, Amundson G, Geier S, Falkum S, Wattenberg EV, Barany G, Distefano MD. J. Am. Chem. Soc. 2009;131:7293–7303. doi: 10.1021/ja805174z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ochocki JD, Igbavboa U, Wood WG, Wattenberg EV, Distefano MD. Chem. Biol. Drug Des. 2010;76:107–115. doi: 10.1111/j.1747-0285.2010.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wollack JW, Zeliadt NA, Ochocki JD, Mullen DG, Barany G, Wattenberg EV, Distefano MD. Bioorg. Med. Chem. Lett. 2010;20:161–163. doi: 10.1016/j.bmcl.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xue CB, Becker JM, Naider F. Tetrahedron Lett. 1992;33:1435–8. [Google Scholar]
- [23].Adler J, Parmryd I. Cytometry A. 2010;77:733–42. doi: 10.1002/cyto.a.20896. [DOI] [PubMed] [Google Scholar]
- [24].Bracha-Drori K, Shichrur K, Lubetzky TC, Yalovsky S. Plant Physiol. 2008;148:119–131. doi: 10.1104/pp.108.120477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Medintz IL, Pons T, Delehanty JB, Susumu K, Brunel FM, Dawson PE, Mattoussi H. Bioconjugate Chem. 2008;19:1785–1795. doi: 10.1021/bc800089r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


