Abstract
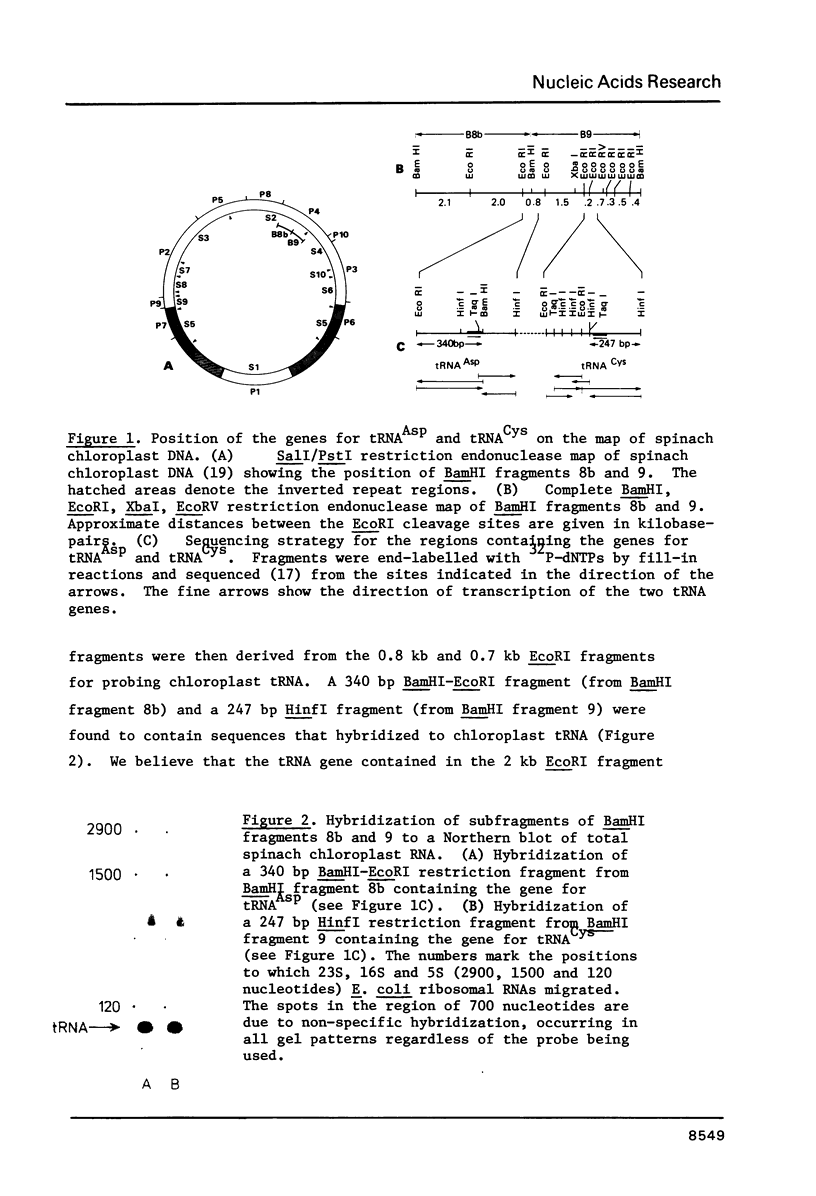
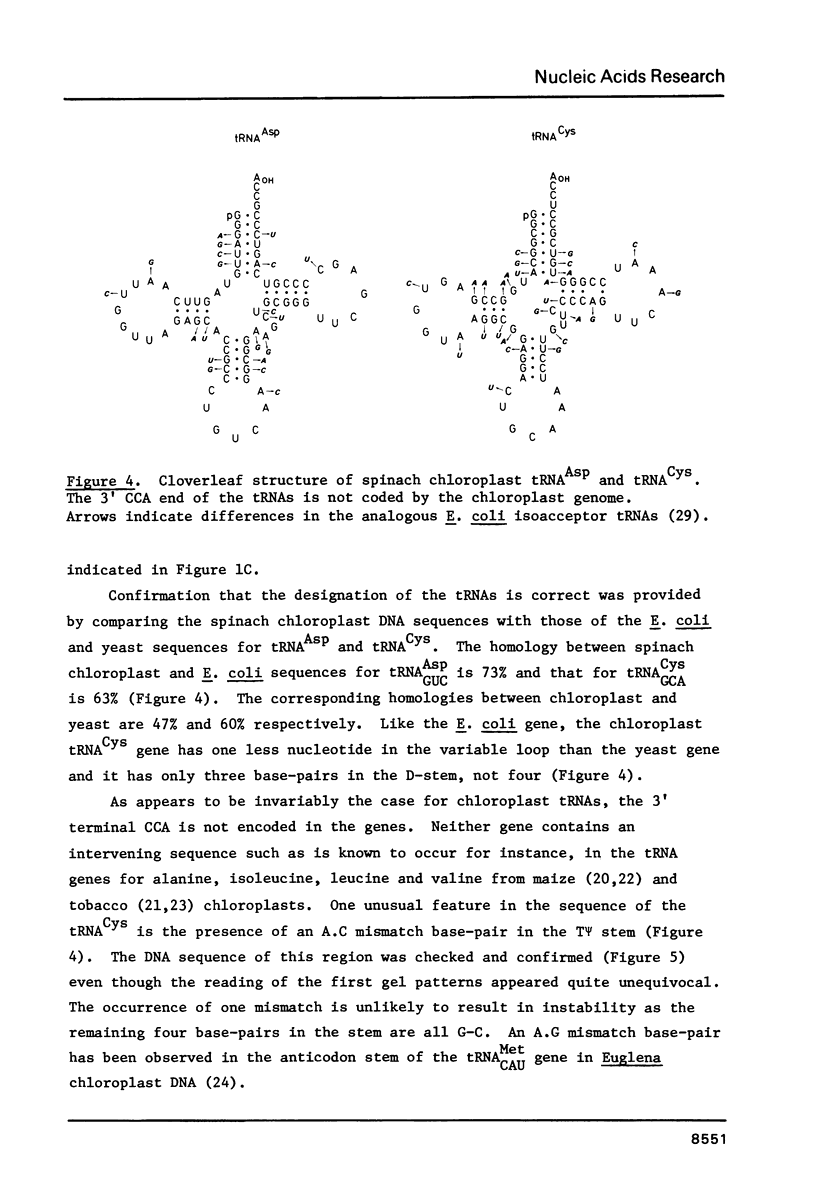
We have determined the map location and primary structure of two fragments of spinach chloroplast DNA which encompass the genes for tRNACysGCA and tRNAAspGUC. Identification of the genes for these two RNA species is based on the sequence of their anticodon triplets and on a comparison of the sequences with those of the equivalent tRNAs from Escherichia coli. Each gene occurs only once on the spinach chloroplast genome and neither contains an intervening sequence. Hybridization of the restriction fragments carrying these genes to chloroplast tRNA showed that both genes are transcribed in vivo.
Full text
PDF





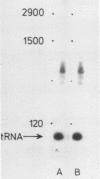
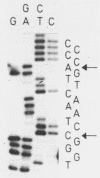
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calagan J. L., Pirtle R. M., Pirtle I. L., Kashdan M. A., Vreman H. J., Dudock B. S. Homology between chloroplast and prokaryotic initiator tRNA. Nucleotide sequence of spinach chloroplast methionine initiator tRNA. J Biol Chem. 1980 Oct 25;255(20):9981–9984. [PubMed] [Google Scholar]
- Canaday J., Guillemaut P., Gloeckler R., Weil J. H. The nucleotide sequence of spinach chloroplast tryptophan transfer RNA. Nucleic Acids Res. 1981 Jan 10;9(1):47–53. doi: 10.1093/nar/9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deno H., Kato A., Shinozaki K., Sugiura M. Nucleotide sequences of tobacco chloroplast genes for elongator tRNAMet and tRNAVal (UAC): the tRNAVal (UAC) gene contains a long intron. Nucleic Acids Res. 1982 Dec 11;10(23):7511–7520. doi: 10.1093/nar/10.23.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- Francis M., Kashdan M., Sprouse H., Otis L., Dudock B. Nucleotide sequence of a spinach chloroplast proline tRNA. Nucleic Acids Res. 1982 Apr 24;10(8):2755–2758. doi: 10.1093/nar/10.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemaut P., Weil J. H. The nucleotide sequence of the maize and spinach chloroplast isoleucine transfer RNA encoded in the 16S to 23S rDNA spacer. Nucleic Acids Res. 1982 Mar 11;10(5):1653–1659. doi: 10.1093/nar/10.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R. G., Whitfeld P. R., Bottomley W. Construction of a SalI/PstI restriction map of spinach chloroplast DNA using low-gelling-temperature-agarose electrophoresis. Gene. 1980 Jan;8(2):179–191. doi: 10.1016/0378-1119(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Hollingsworth M. J., Hallick R. B. Euglena gracilis chloroplast transfer RNA transcription units. Nucleotide sequence analysis of a tRNATyr-tRNAHis-tRNAMet-tRNATrp-tRNAGlu-tRNAGly gene cluster. J Biol Chem. 1982 Nov 10;257(21):12795–12799. [PubMed] [Google Scholar]
- Kashdan M. A., Dudock B. S. Structure of a spinach chloroplast threonine tRNA gene. J Biol Chem. 1982 Feb 10;257(3):1114–1116. [PubMed] [Google Scholar]
- Kashdan M. A., Pirtle R. M., Pirtle I. L., Calagan J. L., Vreman H. J., Dudock B. S. Nucleotide sequence of a spinach chloroplast threonine tRNA. J Biol Chem. 1980 Sep 25;255(18):8831–8835. [PubMed] [Google Scholar]
- Koch W., Edwards K., Kössel H. Sequencing of the 16S-23S spacer in a ribosomal RNA operon of Zea mays chloroplast DNA reveals two split tRNA genes. Cell. 1981 Jul;25(1):203–213. doi: 10.1016/0092-8674(81)90245-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Rushlow K. E., Dodd J. R., Hallick R. B. Euglena gracilis chloroplast ribosomal RNA transcription units. II. Nucleotide sequence homology between the 16 S--23 S ribosomal RNA spacer and the 16 S ribosomal RNA leader regions. J Biol Chem. 1980 Nov 25;255(22):10997–11003. [PubMed] [Google Scholar]
- Pirtle R., Calagan J., Pirtle I., Kashdan M., Vreman H., Dudock B. The nucleotide sequence of spinach chloroplast methionine elongator tRNA. Nucleic Acids Res. 1981 Jan 10;9(1):183–188. doi: 10.1093/nar/9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r1–55. [PMC free article] [PubMed] [Google Scholar]
- Sprouse H. M., Kashdan M., Otis L., Dudock B. Nucleotide sequence of a spinach chloroplast valine tRNA. Nucleic Acids Res. 1981 Jun 11;9(11):2543–2547. doi: 10.1093/nar/9.11.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz A., Gubbins E. J., Bogorad L. The anticodon of the maize chloroplast gene for tRNA Leu UAA is split by a large intron. Nucleic Acids Res. 1982 May 25;10(10):3027–3037. doi: 10.1093/nar/10.10.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaiwa F., Sugiura M. Nucleotide sequence of the 16S - 23S spacer region in an rRNA gene cluster from tobacco chloroplast DNA. Nucleic Acids Res. 1982 Apr 24;10(8):2665–2676. doi: 10.1093/nar/10.8.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld P. R., Herrmann R. G., Bottomley W. Mapping of the ribosomal RNA genes on spinach chloroplast DNA. Nucleic Acids Res. 1978 Jun;5(6):1741–1751. doi: 10.1093/nar/5.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Structures of the genes for the beta and epsilon subunits of spinach chloroplast ATPase indicate a dicistronic mRNA and an overlapping translation stop/start signal. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6260–6264. doi: 10.1073/pnas.79.20.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Perrot B., Bottomley W., Whitfeld P. R. The structure of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981 Jul 24;9(14):3251–3270. doi: 10.1093/nar/9.14.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]