Abstract
Airway neural plasticity contributes to the process of airway remodeling in response to airway irritants. However, the mechanisms of neural remodeling in the airways during the early postnatal period, when responses to airway irritation may be most sensitive, have not been characterized. This study used a rat model to examine a possible mechanism of ozone (O3)-induced neural hyperresponsiveness during a critical period of developmental, postnatal day (PD) 6, that may be mediated by the neurotrophin nerve growth factor (NGF), resulting in an enhanced release of inflammatory neuropeptide substance P (SP) from airway nerves. Rat pups between PD6–PD28 were killed 24 hours after exposure to O3 (2 ppm, 3 hours) or filtered air (FA), to establish a timeline of NGF synthesis, or else they were exposed to O3 or NGF on PD6 or PD21 and re-exposed to O3 on PD28, and killed on PD29. Measurement endpoints included NGF mRNA in tracheal epithelial cells, NGF protein in bronchoalveolar lavage fluid, airway SP–nerve fiber density (NFD), and SP-positive airway neurons in vagal ganglia. Acute exposure to O3 increased NGF in bronchoalveolar lavage fluid on PD10 and PD15, and mRNA expression in epithelial cells on PD6, compared with FA controls. NGF protein and mRNA expression in the O3–PD6/O3–PD28 groups were significantly higher than in the O3–PD21/O3–PD28 and O3–PD6/FA–PD28 groups. NGF–PD6/O3–PD28 increased the SP innervation of airway smooth muscle and SP-positive sensory neurons, compared with the NGF–PD21/O3–PD28 or NGF–PD6/FA–PD28 groups. NGF enhanced sensory innervation, which may mediate acute responses or prolong sensitivity to O3 during early life. The model may be relevant in O3 responses during early childhood.
Keywords: nerve growth factor, ozone, airway, substance P, development
CLINICAL RELEVANCE.
Exposure in early life to inhaled environmental pollutants contributes to the pathogenesis of childhood asthma and other respiratory disorders. The mechanisms responsible for prenatal and early postnatal susceptibility to airway irritants are not well defined because most studies of airway sensitivity to air pollutants used adult animal models. The goal of the present investigation was to examine the role of the neurotrophin nerve growth factor as a potential signaling molecule in regulating airway neural responses to ozone exposures in early life.
Although the development of airways begins during the third week of gestation and continues through adolescence (1), the mechanisms responsible for prenatal and early postnatal susceptibility to airway irritants are not well-defined, because most studies examining airway sensitivity to air pollutants have used adult animal models. According to our recent study, one mechanism mediating airway responses to inhaled pollutants during the early postnatal period may involve the release of inflammatory neuropeptides from sensory nerves in the airways (2). The goal of the present investigation was to examine the role of the neurotrophin, nerve growth factor (NGF), as a potential signaling molecule involved in regulating airway neural responses to ozone (O3) exposures in early life. The study has potential relevance in airway disease, because children are especially sensitive to environmental pollutants (3).
Recent evidence indicates that exposures to O3 during early life (2, 4), as well as exposures to a wide range of airway irritants including cigarette smoke (5), respiratory syncytial virus (6), and antigen challenge (7), may alter the normal developmental patterns of airway neural pathways, resulting in enhanced airway smooth muscle responsiveness and inflammation. These findings suggest that normal regulatory mechanisms controlling airway neural development (particularly the production and release of neurotrophins) may be affected by exposure to O3 or other airway irritants. NGF regulates and maintains neuronal levels of inflammatory neuropeptides in sensory neurons (8). Studies of animal models indicate that neural growth and development are highly dependent on NGF during prenatal and early postnatal life (9–11). NGF promotes inflammation in a variety of tissues (12), and is implicated in the pathogenesis of symptoms in asthma (13, 14). Importantly, concentrations of NGF in serum, platelets, and plasma are significantly increased in patients with asthma (15). Furthermore, neurotrophins enhance the development of airway smooth muscle hyperresponsiveness (16, 17), and increase both sympathetic and sensory innervation of the lung (18). The role of neurotrophin expression in airway neural development may be particularly important during early life, when neurotrophin concentrations and the expression of neurotrophin receptors are both elevated, compared with older infants and adults (6, 19). NGF was shown to increase the synthesis of the tachykinin substance P (SP) in airway sensory nerves (20–22). SP is released by primary afferent C-fibers (23, 24), mediating both the airway hyperresponsiveness (25) and neurogenic inflammation (26, 27) associated with exposure to O3.
Previous work in our laboratory suggested the existence of an early postnatal critical period of susceptibility to airway irritants (2). Rat pups exposed to O3 during the proposed critical period (postnatal days [PDs] 2–6), and later re-exposed to O3 (PD28), demonstrated an increase in SP nerve fiber density (NFD) in intrapulmonary and extrapulmonary smooth muscle. This increase was not evident in rat pups initially exposed to O3 outside of the critical period (PD19–23) and re-exposed on PD28. We propose that the enhanced expression of SP induced by exposure to O3 on PD2–6 results from the release of NGF, and that the failure of exposure on PD19–23 to increase the production of SP results from the reduced production of NGF. The overall goal of the present study was to evaluate the effect of NGF expression on SP responses during the critical period, as observed in our previous study.
MATERIALS AND METHODS
Age-Related Changes in the Production of NGF and Responses to O3
In previous studies, we demonstrated increased sensory innervation and enhanced responsiveness in the airways of newborn rats exposed to O3 during an early postnatal critical period and then re-exposed later, compared with rat pups exposed outside the postnatal critical period and re-exposed later (2). In the present study, we propose that NGF plays a role in regulating these airway responses during the early critical period of development. Thus, we first designed experiments to establish a timeline of NGF production in rats exposed to filtered air (FA) or O3. According to our hypothesis in this set of experiments, acute exposure to O3 alters the normal production of NGF during early postnatal periods. Rat pups were exposed to FA or O3 on PD6, PD10, PD15, PD21, or PD28. Lung lavage was performed 24 hours after exposure to measure NGF protein concentrations using ELISA, and airways were removed 12 hours after exposure to measure NGF mRNA expression using RT-PCR. In addition, cytospins were prepared from lung lavage fluid, and neutrophil counts were performed to verify airway inflammatory responses after exposure to O3.
NGF Production during the Critical Period
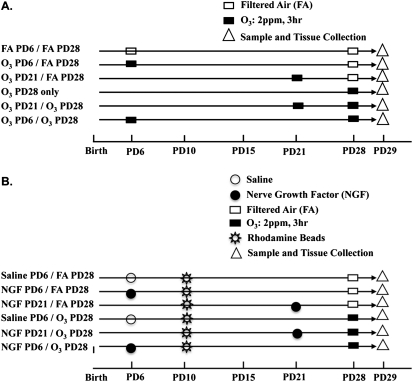
The second series of studies served to test the hypothesis that an initial exposure to O3 during early life causes an enhanced NGF response to a second exposure to O3 later in life. The experimental protocol included exposure to O3 or FA on PD6 (an age within the putative critical period) or on PD21 (an age beyond the critical period). The production of NGF in each exposure group was compared after a second exposure to O3 or FA on PD28. As additional controls, we also included a group that received an initial exposure to O3 on PD6 and FA on PD28, a group exposed to O3 only on PD28, and an FA–PD6/FA–PD28 group. The experimental protocols with the appropriate control groups for the second series of experiments are shown in the model outlined in Figure 1A. Lung lavages (to measure NGF protein) and airway removal (to measure NGF mRNA) were performed 24 hours and 12 hours after the final exposure on PD28, respectively.
Figure 1.
Diagrams outline (A) second and (B) third parts of experimental protocol used to evaluate nerve growth factor (NGF) during the critical period. PD, postnatal day.
NGF Treatment in the Critical Period
The third series of studies served to test the hypothesis that NGF directly regulates sensory airway innervation during the critical period of development (on PD6). If NGF is involved in modulating irritant-induced responses during this early postnatal period, then direct treatment with NGF on PD6 and O3 on PD28 should cause similar changes in sensory airway innervation. The protocol included the instillation of NGF or saline into the airways on PD6 or PD21. SP-NFD in airway smooth muscle and the SP content in jugular and nodose airway neurons in each exposure group were compared after exposure to O3 on PD28. As additional controls, we also included groups that received NGF on PD6 and FA on PD28, a group that received saline on PD6 and O3 on PD28, and a group that received saline on PD6 and FA on PD28. The protocols with the appropriate control groups for the third series of experiments are shown in the model outlined in Figure 1B.
RESULTS
Age-Related Responses to O3 Exposure in NGF Protein and Messages
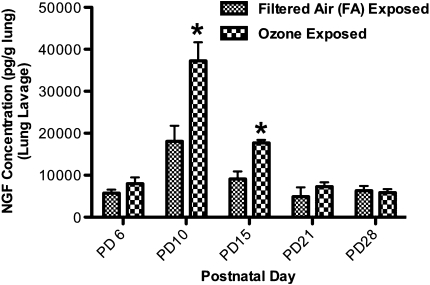
NGF responses 24 hours after exposure to O3 (2 ppm, 3 hours) were examined throughout the first month of postnatal life, to establish a timeline of NGF production. In lung lavages, exposure to O3 caused a significant increase in NGF protein concentrations on both PD10 and PD15, compared with rat pups exposed to FA (Figure 2). Concentrations of NGF increased from 18,083.00 ± 3,689.13 pg/g lung in control samples to 37,254.00 ± 4,418.12 pg/g lung after exposure to O3 on PD10, and from 9,099.00 ± 1,639.93 pg/g lung to 17,703.00 ± 704.10 pg/g lung on PD15. Concentrations of NGF did not significantly increase on PD6, PD21, and PD28 after exposure to O3, compared with control values.
Figure 2.
Effect of exposure to ozone (O3) on NGF protein concentration in lung lavage during early postnatal days (PDs). The concentration of NGF protein was measured 24 hours after exposure to O3 (2 ppm, 3 hours) or filtered air (FA) on PD6, PD10, PD15, PD21, or PD28. Concentrations of NGF were measured to determine if exposure to O3 would transiently increase NGF in rat airways during the early postnatal period, compared with animals exposed to FA. NGF protein was measured in pg/g of lung tissue, using ELISA. All values are means ± SE, n = 6 for each group. *P < 0.05, significant in FA groups.
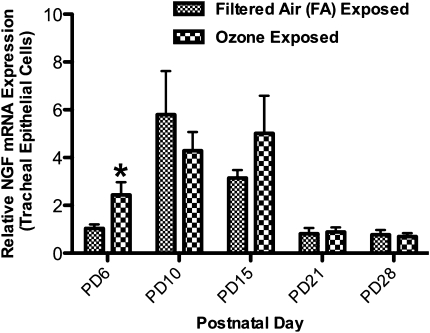
The relative expression of NGF mRNA in tracheal epithelial cells significantly increased from 1.00 ± 0.16 to 2.40 ± 0.53 on PD6 in rat pups exposed to FA and O3, respectively (Figure 3). The expression of NGF mRNA in animals exposed at all other postnatal ages did not differ from control levels.
Figure 3.
Effect of exposure to O3 on relative expression of NGF mRNA in tracheal epithelial cells during early PDs. NGF mRNA was measured 12 hours after exposure to O3 (2 ppm, 3 hours) or FA on PD6, PD10, PD15, PD21, or PD28. The expression of NGF mRNA was measured to determine if exposure to O3 would transiently increase the NGF message in rat airways during early postnatal periods, compared with animals exposed to FA. NGF was measured using real-time PCR relative to the expression of β-actin mRNA. Values are means ± SE, n = 6 for each group. *P < 0.05, significant in FA groups.
A significant increase in neutrophils was evident 24 hours after exposure to O3 at all postnatal ages in comparison with control levels, verifying airway inflammation in all exposed animals (data not shown).
Production of NGF during the Critical Period
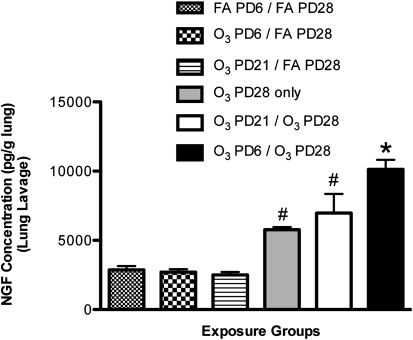
Concentrations of NGF protein in lung lavages of the O3–PD6/O3–PD28 group significantly increased, versus all other exposure groups (Figure 4). Importantly, the concentrations of NGF in this group significantly increased compared with the O3–PD21/O3–PD28 exposure group. Concentrations of NGF were 10,130.11 ± 694.01 pg/g lung in the O3–PD6/O3–PD28 group, and 6,972.53 ± 1,387.31 pg/g lung in the O3–PD21/O3–PD28 group. Concentrations of NGF in the O3–PD28–only group (5,773.63 ± 185.76 pg/g lung) and the O3–PD21/O3–PD28 group (6,972.53 ± 1387.31 pg/g lung) were significantly elevated compared with all three FA controls: the FA–PD6/FA–PD28 group (2,874.73 ± 268.19 pg/g lung), the O3–PD6/FA–PD28 group (2,701.19 ± 212.39 pg/g lung), and the O3–PD21/FA–PD28 group (2,505.22 ± 217.34 pg/g lung). However, these increases were not significantly different from each other, indicating that the PD28 ozone response was not affected by the exposure on PD21.
Figure 4.
Effect of exposure to O3 on NGF protein concentration in lung lavage during a critical period of development. NGF protein was measured to determine if exposure to O3 (2 ppm, 3 hours) during a critical period (PD6) would result in prolonged changes of NGF concentrations upon a second exposure to O3 (PD28), compared with control samples and exposures outside the critical period (PD21). Values are means ± SE, n = 6 for each group. *P < 0.05, significant in all groups. #P < 0.05, significant in FA control groups.
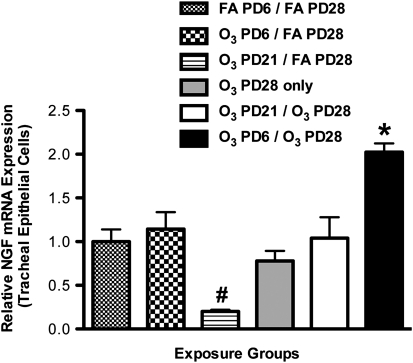
The relative expression of NGF mRNA in tracheal epithelial cells was significantly elevated in the O3–PD6/O3–PD28 group, versus all other exposure groups (Figure 5). Specifically, the expression of NGF mRNA in the O3–PD6/O3–PD28 group (2.02 ± 0.10%) was significantly higher than in the O3–PD6/FA–PD28 group (1.14 ± 0.19%) and the O3–PD21/O3–PD28 group (1.04 ± 0.24%). Furthermore, the expression of NGF in the O3–PD21/O3–PD28 group (1.04 ± 0.24%) was not significantly different from that in the FA–PD6/FA–PD28 group (1.00 ± 0.14%) and the O3–PD28–only group (0.78 ± 0.11%). In addition, the relative expression of NGF mRNA in the O3–PD21/FA–PD28 group was significantly decreased, versus all other groups.
Figure 5.
Effect of exposure to ozone (O3) on relative expression of NGF mRNA in tracheal epithelial cells during a critical period of development. NGF was measured to determine if exposure to O3 (2 ppm, 3 hours) during a critical period (PD6) would result in prolonged changes of NGF message upon a second exposure to O3 (PD28), compared with control samples and exposures outside the critical period (PD21). NGF mRNA was measured by real-time PCR relative to expression of β-actin mRNA. Values are means ± SE, n = 6 for each group. *P < 0.05, significant in all groups. #P < 0.05, significant in all groups.
NGF Treatment during the Critical Period
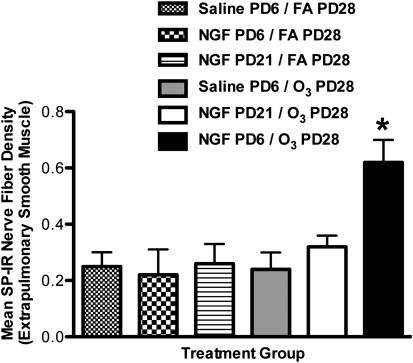
In our NGF instillation studies, significant differences in SP innervation were evident only in extrapulmonary smooth muscle (Figure 6). The density of SP-immunoreactive (IR) nerve fibers in the extrapulmonary smooth muscle of the NGF–PD6/O3–PD28 group was significantly elevated, versus all other groups. Importantly, a significant increase was evident only in the NGF–PD6/O3–PD28 group (0.56 ± 0.13%) compared with the NGF–PD6/FA–PD28 group (0.22 ± 0.09%) and the saline–PD6/O3–PD28 group (0.24 ± 0.06%). In addition, the NGF–PD21/O3–PD28 group (0.32 ± 0.09%) did not exhibit a significant increase in the percentage of SP-NFD, compared with the FA–PD6/FA–PD28 group (0.25 ± 0.05%) and the NGF–PD21/FA–PD28 group (0.27 ± 0.07%).
Figure 6.
Effect of NGF tracheal instillation on substance P (SP) nerve fiber density in extrapulmonary airway smooth muscle during a critical period of development. The percent area of SP-immunoreactive (IR) nerve fibers was measured in the extrapulmonary smooth muscle to determine if NGF tracheal instillation during a critical period (PD6) would increase sensory innervation upon exposure to O3 (2 ppm, 3 hours) on PD28, compared with control samples and NGF treatment outside the critical period (PD21). Values are means ± SE, n = 5 for all groups. *P < 0.05, significant in all groups.
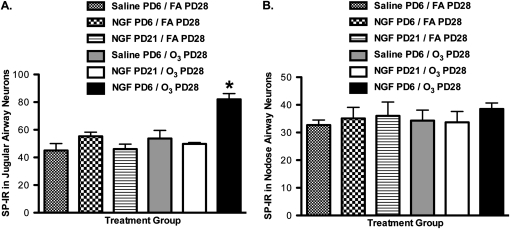
Increased SP-positive airway neurons (labeled with rhodamine beads) were only observed in the jugular ganglia (Figure 7A). SP-positive neurons in the jugular ganglia of the NGF–PD6/O3–PD28 group were significantly elevated, versus all other groups. Notably, the significant increase was evident only in the NGF–PD6/O3–PD28 group (81.30% ± 0.52%), compared with the NGF–PD6/FA–PD28 group (55.30% ± 3.00%) and the saline–PD6/O3–PD28 group (53.72% ± 5.80%). In addition, the NGF–PD21/O3–PD28 group did not exhibit a significant increase in SP-positive neurons (49.21% ± 1.01%), compared with the FA–PD6/FA–PD28 group (47.80% ± 5.40%) and the NGF–PD21/FA–PD28 group (48.51% ± 0.36%). In the nodose ganglia, no significant differences in SP-positive neurons were evident in any experimental group (Figure 7B).
Figure 7.
Effect of NGF tracheal installation on SP-IR airway-labeled neurons during a critical period of development. Percent SP-IR was measured in jugular (A) and nodose (B) neurons innervating the airways, to determine if tracheal instillation of NGF during a critical period (PD6) would enhance SP neuronal content upon exposure to O3 (2 ppm, 3 hours) on PD28, compared with control samples and NGF treatment outside the critical period (PD21). Values are means ± SE, n = 4 for all groups. *P < 0.05, significant in all groups.
DISCUSSION
This study provides evidence that adverse airway responses occurring during early postnatal exposures to O3 are mediated through the release of NGF and its effect on SP concentrations in the airway. In a previous study, we demonstrated that exposure to O3 in rat pups on PD2–6 resulted in increased SP innervation in airway smooth muscle, and in airway hyperreactivity on PD29 after a re-exposure to O3 on PD28 (2). However, the increased SP innervation and hyperresponsiveness were not evident when the initial exposure occurred on PD19–23, suggesting that the early exposure (PD2–6) represented a critical window of susceptibility to O3 exposure. In the present investigation, we tested the involvement of NGF as a possible mediator of the early-life critical window by measuring the relative amounts of NGF in BAL fluid and mRNA in tracheal epithelial cells after re-exposure on PD28 in rat pups initially exposed on PD6 or PD21. The NGF protein concentrations in BAL fluid and the NGF message in epithelial cells had both increased after re-exposure in the PD6–O3–exposed group, but not in the group exposed on PD21. Afterward, NGF was intratracheally instilled on PD6 or PD21 to mimic the O3-induced release of NGF. In rat pups that received NGF on PD6 and were then exposed to O3 on PD28, the SP innervation of extrapulmonary airway smooth muscle and SP concentrations in airway neurons of the jugular (but not nodose) ganglia had both increased. Conversely, after NGF instillation on PD21 and exposure to O3 on PD28, neither SP airway innervation nor SP concentrations in the jugular ganglia had increased.
Overall, these findings are consistent with the results in our previous study, showing that the early postnatal period, generally up to approximately PD15, may represent a critical window of susceptibility. Here, we demonstrated an important feature of the critical period, namely, that exposure during the critical period prolongs the period of susceptibility to subsequent exposure to O3 (Figures 4 and 5). These results suggest the presence of a critical period of development, during which younger animals are more susceptible to the long-term effects of airway irritants. An important distinction should be made between acute hyperresponsiveness, involving immediate responses to O3, and the prolonged responses that represent a process of continued or prolonged sensitivity to a subsequent exposure. The experiments described in this study show that early-life treatment with NGF can exert an effect on responses in later life. However, the possible mechanisms for this prolonged level of sensitivity have not been defined. Two findings of the present study suggest a possible basis for the critical-period effect. First, we observed that NGF message and protein are increased by exposure to O3 only during the early postnatal period. Second, SP airway innervation and SP concentrations in airway neurons increased only when NGF was instilled on PD6 and not on PD21. These finding suggest that the enhanced responsiveness on PD28 after exposure to O3 or NGF on PD6 may result from increased inflammation driven by an NGF-induced production of SP that does not occur on PD21. Previous studies (18, 28) demonstrated that NGF upregulates the expression of SP in airway nerves, and that SP increases irritant-induced vascular permeability by binding to endothelial cell neurokinin-1 receptors (29). In addition, tropomyosin-receptor kinase A (trkA) receptors are expressed in the nodose ganglia of neonatal but not adult rats, suggesting developmental differences in the mechanisms of NGF signal transduction (30).
Several other explanations may help clarify early-life susceptibility to O3 exposure. For instance, the minute ventilation after exposure to O3 observed in adult rats is depressed or absent in 2-week-old rat pups, leading to increased injury in pups (31). Alternately, antioxidant systems involving superoxide dismutase, catalase, and glutathione peroxidase are not fully developed in early postnatal periods, and could reduce mechanisms that normally counteract oxidant injury in the airways of adults (32). However, the experimental design of the present study tested the differential effects of NGF instillation on PD6 and PD21, with the finding that increased airway innervation after exposure to O3 on PD28 only occurred in the group that received NGF on PD6. Thus, although enhanced airway responses to O3 exposure in early life may be related to underdeveloped airway defense mechanisms, the release of NGF during such inflammatory events could cause a heightened neural response to NGF and an enhanced production of inflammatory neuropeptides such as SP.
The present study focuses on NGF as a mediator of the critical-period response. NGF is a neurotrophin known to regulate neuronal growth and proliferation in sensory and sympathetic neurons. In the respiratory system, mice that overexpress NGF in bronchiolar Clara cells exhibit increased sensory and sympathetic innervation in their airways (28). In adult animal models, the increased synthesis or release of NGF enhances the expression of SP in sensory neurons innervating the nasal cavity after exposures to toluene diisocyanate (TDI) (22), and in the airways after allergen sensitization and challenge (33). In a recent study using exposure protocols similar to those in the present study, the expression of NGF in mice was increased by exposure to cigarette smoke during the first 10 postnatal days, followed by re-exposure on PD59, but the expression of NGF was not increased when exposure occurred for the same length of time beginning on PD21 (5). Concentrations of NGF and the expression of neurotrophin receptors trkA and p75 in whole-lung extracts declined between 2 and 12 weeks, but NGF was increased approximately threefold by a respiratory syncytial virus (RSV) infection in 2-week-old pups (6). In the same study, concentrations of NGF were increased in 12-week-old pups after RSV, but the magnitude of the increase was substantially lower. Our present findings that NGF protein and message were elevated by acute exposure to O3 on PD6, PD10, and PD15, but not on PD21 or PD28 (Figures 2 and 3), suggest that the NGF response is unique to the early postnatal period (up to PD15), and thus may be another potential mechanism for prolonging susceptibility to O3 exposure. The inability to demonstrate increased message on PD10 and PD15 during the early postnatal period, although the concentration of protein was elevated, probably results from differences in the timing of NGF gene transcription and translation that occur at different postnatal ages during the early postnatal period (34). Although tracheal epithelial cells are known to produce NGF (35, 36), other cells in the airway such as immune cells and fibroblasts also produce NGF. The epithelial cell population used to measure mRNA in the present studies comprised 96.6% pure epithelial cells, and our results provide an accurate representation of epithelial cell response without contributions from inflammatory cells. On the other hand, data regarding protein were obtained using whole-lung lavages, and the increase in NGF may have originated from other sources, including inflammatory cells that infiltrate the airway after exposure to O3.
In addition to its effects on neuronal growth and proliferation, NGF is known to stimulate SP message transcription and translation. This occurs through binding to trkA receptors on the neuronal cell membrane, vesicular transport through the axon to the nerve cell body, and initiation of signaling cascades leading to the activation of the preprotachykinin (PPT) gene (37–40). In the present study, increased concentrations of SP in the airway neurons of the jugular ganglia (i.e., an increase in number of SP-positive neurons) and the increased SP innervation of extrapulmonary airway smooth muscle (i.e., increased nerve fiber density) were consistent with this process (Figures 6 and 7). We interpret the increase in smooth muscle SP nerve fiber density as an increase in the level of SP protein in nerve terminals that already exists in the airway. This interpretation is at variance with the possibility that NGF stimulates axonal branching. Our conclusion is based on the previous finding that although SP-NFD increases, the total nerve fiber density measured by the neuron-specific marker protein gene produce 9.5 (PGP 9.5) does not increase (2). Thus, some nerve terminals in the smooth muscle are branches of sensory neurons that either do not normally produce SP or produce it at concentrations below the sensitivity of our immunocytochemical assay. However, after exposure to O3 and the upregulation by NGF of the PPT gene, the production of SP in neurons is increased, and both the nerve cell body and their axonal branches in airway smooth muscle contain sufficient SP to be detected and measured. The exact mechanisms of airway neuronal growth and the production of SP during the critical period remain to be confirmed, because previous studies only examined acute responses and not the process of early-life exposure followed by re-exposure at a later age.
Airway sensory neurons originate in mixed embryonic backgrounds and respond to various neurotrophic factors. Neurons in the jugular ganglion are derived from the neural crest, and are dependent on NGF for their development and survival. Neurons in the nodose ganglion are derived from epibranchial placodes, and are not dependent on NGF (41, 42). The difference in NGF requirements may explain why we observed changes in SP-positive neurons in the jugular ganglion and not in the nodose ganglion after the instillation of NGF on PD6 and exposure to O3 on PD28. Alternately, jugular ganglion neurons are found to project many more fibers to the epithelial compartment of the tracheal airway wall than do nodose ganglion neurons (43). In the present study, we used rhodamine-labeled beads specifically to label airway neurons innervating the tracheal airway epithelium. Therefore, if more jugular fibers innervate the tracheal epithelial airway, this allows NGF to interact with more jugular than nodose nerve endings, which may also explain the increase of SP-positive neurons in the jugular ganglion. Nodose neurons may project lower down the bronchial tree, and upon similar NGF treatments would cause increases in the number of SP-positive nodose neurons. However, further retrograde tracers are needed to confirm this possibility.
Previous studies in our laboratory showed that expression levels of SP are elevated after exposure to O3 in nerves innervating the airway epithelium. This expression is dependent on age at exposure, and follows the critical-period paradigm. However, an exposure at 2 ppm for 3 hours is not normally experienced by human infants or children, although fairly high concentrations of O3 for acute or subchronic exposure periods were used extensively as models for O3-induced airway responses (44–46). In fact, the response is likely related to the inflammatory events produced by this level of O3 exposure and not O3 per se. The O3-induced injury observed in the present study, especially at the concentration of 2 ppm, probably represents generalized inflammatory injury, as opposed to a specific O3-related effect. Similar responses would be expected with a host of inflammatory agents, including allergens (33), viruses (47), or irritants such as TDI (22).
These studies have potential relevance in understanding long-term human responses to inhaled irritant exposures during early childhood. Children who live in areas with elevated ground-level O3 show an increased risk of developing asthma and manifesting symptoms of asthma when environmental O3 levels are elevated. The present study suggests that NGF is important in prolonging sensitivity to O3 exposures during early life that would result in increased sensitivity upon a subsequent exposure. The findings show that NGF released during the critical period increases the potential for tachykinin synthesis in airway sensory nerves by exposure to O3 at a later time. However, additional experiments are needed to show that blocking the initial increase in NGF reduces the prolonged O3-induced responsiveness in SP airway nerves. At present, the lack of selective NGF antagonists precludes the performance of these important experiments. The cellular and molecular mechanisms that would confer prolonged sensitivity have not yet been identified.
Supplementary Material
This work was supported by National Institutes of Health grant RO1HL80566.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0345OC on November 12, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Harding R, Pinkerton KE, Plopper CG, editors. The lung: development, aging, and the environment. San Diego: Elsevier Academic Press; 2004.
- 2.Hunter DD, Wu Z, Dey RD. Sensory neural responses to ozone exposure during early postnatal development in rat airways. Am J Respir Cell Mol Biol 2010;43:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuna-Dibbert B, Krzyzanowski M, Schneider J. Effects of air pollution on children's health and development: a review of the evidence. Bonn: WHO Regional Office for Europe; 2005.
- 4.Larsen GL, Loader J, Nguyen DD, Fratelli BA. Mechanisms determining cholinergic neural responses in airways of young and mature rabbits. Pediatr Pulmonol 2004;38:97–106. [DOI] [PubMed] [Google Scholar]
- 5.Wu ZX, Hunter DD, Kish VL, Benders KM, Batchelor TP, Dey RD. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ Health Perspect 2009;117:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus–infected lungs. Am J Physiol Lung Cell Mol Physiol 2002;283:L494–L502. [DOI] [PubMed] [Google Scholar]
- 7.Kajekar R, Pieczarka EM, Smiley-Jewell SM, Schelegle ES, Fanucchi MV, Plopper CG. Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir Physiol Neurobiol 2007;155:55–63. [DOI] [PubMed] [Google Scholar]
- 8.Kessler JA, Black IB. Nerve growth factor stimulates the development of substance P in sensory ganglia. Proc Natl Acad Sci USA 1980;77:649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forgie A, Kuehnel F, Wyatt S, Davies AM. In vivo survival requirement of a subset of nodose ganglion neurons for nerve growth factor. Eur J Neurosci 2000;12:670–676. [DOI] [PubMed] [Google Scholar]
- 10.Buj-Bello A, Pinon LG, Davies AM. The survival of NGF-dependent but not BDNF-dependent cranial sensory neurons is promoted by several different neurotrophins early in their development. Development 1994;120:1573–1580. [DOI] [PubMed] [Google Scholar]
- 11.Kessler JA, Black IB. The effects of nerve growth factor (NGF) and antiserum to NGF on the development of embryonic sympathetic neurons in vivo. Brain Res 1980;189:157–168. [DOI] [PubMed] [Google Scholar]
- 12.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994;62:327–331. [DOI] [PubMed] [Google Scholar]
- 13.Nassenstein C, Kerzel S, Braun A. Neurotrophins and neurotrophin receptors in allergic asthma. Prog Brain Res 2004;146:347–367. [DOI] [PubMed] [Google Scholar]
- 14.Frossard N, Freund V, Advenier C. Nerve growth factor and its receptors in asthma and inflammation. Eur J Pharmacol 2004;500:453–465. [DOI] [PubMed] [Google Scholar]
- 15.Bonini S, Lambiase A, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc Natl Acad Sci USA 1996;93:10955–10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennedich Kahn L, Gustafsson LE, Olgart Hoglund C. Brain-derived neurotrophic factor enhances histamine-induced airway responses and changes levels of exhaled nitric oxide in guinea pigs in vivo. Eur J Pharmacol 2008;595:78–83. [DOI] [PubMed] [Google Scholar]
- 17.Renz H. The role of neurotrophins in bronchial asthma. Eur J Pharmacol 2001;429:231–237. [DOI] [PubMed] [Google Scholar]
- 18.Hoyle GW, Graham RM, Finkelstein JB, Nguyen KPT, Gozal D, Friedman B. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 1998;18:149–157. [DOI] [PubMed] [Google Scholar]
- 19.Larsen GL, Fratelli C, Loader J, Kang JK, Dakhama A. Neuropeptide release from airways of young and fully-grown rabbits. Pediatr Pulmonol 2006;41:1242–1249. [DOI] [PubMed] [Google Scholar]
- 20.Otten U, Goedert M, Mayer N, Lembeck F. Requirement of nerve growth factor for development of substance P–containing sensory neurones. Nature 1980;287:158–159. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz JP, Pearson J, Johnson EM. Effect of exposure to anti-NGF on sensory neurons of adult rats and guinea pigs. Brain Res 1982;244:378–381. [DOI] [PubMed] [Google Scholar]
- 22.Wilfong ER, Dey RD. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am J Respir Cell Mol Biol 2004;30:793–800. [DOI] [PubMed] [Google Scholar]
- 23.Budai D, Larson AA. Role of substance P in the modulation of C-fiber–evoked responses of spinal dorsal horn neurons. Brain Res 1996;710:197–203. [DOI] [PubMed] [Google Scholar]
- 24.Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol 2001;125:145–154. [DOI] [PubMed] [Google Scholar]
- 25.Wu ZX, Dey RD. Nerve growth factor–enhanced airway responsiveness involves substance P in ferret intrinsic airway neurons. Am J Physiol Lung Cell Mol Physiol 2006;291:L111–L118. [DOI] [PubMed] [Google Scholar]
- 26.Hsiue TR, Garland A, Ray DW, Hershenson MB, Leff AR, Solway J. Endogenous sensory neuropeptide release enhances nonspecific airway responsiveness in guinea pigs. Am Rev Respir Dis 1992;146:148–153. [DOI] [PubMed] [Google Scholar]
- 27.Donkin JJ, Turner RJ, Hassan I, Vink R. Substance P in traumatic brain injury. Prog Brain Res 2007;161:97–109. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DD, Myers AC, Undem BJ. Nerve growth factor–induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 2000;161:1985–1990. [DOI] [PubMed] [Google Scholar]
- 29.McDonald DM, Mitchell RA, Gabella G, Haskell A. Neurogenic inflammation in the rat trachea: II. Identity and distribution of nerves mediating the increase in vascular permeability. JNeurocytol 1988;17:605–628. [DOI] [PubMed] [Google Scholar]
- 30.Zhuo H, Helke CJ. Presence and localization of neurotrophin receptor tyrosine kinase (trkA, trkB, trkC) mRNAs in visceral afferent neurons of the nodose and petrosal ganglia. Brain Res Mol Brain Res 1996;38:63–70. [DOI] [PubMed] [Google Scholar]
- 31.Shore SA, Abraham JH, Schwartzman IN, Murthy GG, Laporte JD. Ventilatory responses to ozone are reduced in immature rats. J Appl Physiol 2000;88:2023–2030. [DOI] [PubMed] [Google Scholar]
- 32.Fanucchi MV. Development of antioxidant and xenobiotic metabolizing enzyme systems. In: Harding R, Pinkerton K, Plopper C, editors. The lung: development, aging and the environment. San Diego: Elsevier Academic Press; 2004. pp. 177–185.
- 33.Fischer A, McGregor GP, Saria A, Philippin B, Kummer W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J Clin Invest 1996;98:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrell-Jacks LD, Dey RD, Hunter DD. Altered development of airway sensory neurons in rats following acute ozone exposures in early postnatal life. Am J Respir Crit Care Med 2010;181:A6425. [Google Scholar]
- 35.Fox AJ, Patel HJ, Barnes PJ, Belvisi MG. Release of nerve growth factory by human pulmonary epithelial cells: role in airway inflammatory diseases. Eur J Pharmacol 2001;424:159–162. [DOI] [PubMed] [Google Scholar]
- 36.Frossard N, Naline E, Olgart Hoglund C, Georges O, Advenier C. Nerve growth factor is released by IL-1beta and induces hyperresponsiveness of the human isolated bronchus. Eur Respir J 2005;26:15–20. [DOI] [PubMed] [Google Scholar]
- 37.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. P38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002;36:57–68. [DOI] [PubMed] [Google Scholar]
- 38.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets 2008;7:46–62. [DOI] [PubMed] [Google Scholar]
- 39.Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci USA 2007;104:13666–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 2003;39:57–68. [DOI] [PubMed] [Google Scholar]
- 41.Baker CVH. The embryology of vagal sensory neurons. In: Undem BJ, Weinreich D, editors. Advances in vagal afferent neurobiology. Boca Raton: Taylor and Francis; 2005. pp. 3–26.
- 42.Lindsay RM, Barde YA, Davies AM, Rohrer H. Differences and similarities in the neurotrophic growth factor requirements of sensory neurons derived from neural crest and neural placode. J Cell Sci Suppl 1985;3:115–129. [DOI] [PubMed] [Google Scholar]
- 43.Hunter DD, Undem BJ. Identification and substance P content of vagal afferent neurons innervating the epithelium of the guinea pig trachea. Am J Respir Crit Care Med 1999;159:1943–1948. [DOI] [PubMed] [Google Scholar]
- 44.Sterner-Kock A, Kock M, Braun R, Hyde DM. Ozone-induced epithelial injury in the ferret is similar to nonhuman primates. Am J Respir Crit Care Med 2000;162:1152–1156. [DOI] [PubMed] [Google Scholar]
- 45.Harkema JR, Wagner JG. Epithelial and inflammatory responses in the airways of laboratory rats coexposed to ozone and biogenic substances: enhancement of toxicant-induced airway injury. Exp Toxicol Pathol 2005;57:129–141. [DOI] [PubMed] [Google Scholar]
- 46.Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 2005;288:L390–L397. [DOI] [PubMed] [Google Scholar]
- 47.Carr MJ, Hunter DD, Jacoby DB, Undem BJ. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am J Respir Crit Care Med 2002;165:1071–1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.