Abstract
Rationale: Diabetic patients have a lower incidence of acute respiratory distress syndrome (ARDS), and those who develop ARDS are less likely to die. The mechanisms that underlie this protection are unknown.
Objectives: To determine whether leptin resistance, a feature of diabetes, prevents fibroproliferation after lung injury.
Methods: We examined lung injury and fibroproliferation after the intratracheal instillation of bleomycin in wild-type and leptin-resistant (db/db) diabetic mice. We examined the effect of leptin on transforming growth factor (TGF)-β1–mediated transcription in primary normal human lung fibroblasts. Bronchoalveolar lavage fluid (BAL) samples from patients with ARDS and ventilated control subjects were obtained for measurement of leptin and active TGF-β1 levels.
Measurements and Main Results: Diabetic mice (db/db) were resistant to lung fibrosis. The db/db mice had higher levels of peroxisome proliferator–activated receptor-γ (PPARγ), an inhibitor of the transcriptional response to TGF-β1, a cytokine critical in the pathogenesis of fibroproliferative ARDS. In normal human lung fibroblasts, leptin augmented the transcription of profibrotic genes in response to TGF-β1 through a mechanism that required PPARγ. In patients with ARDS, BAL leptin levels were elevated and correlated with TGF-β1 levels. Overall, there was no significant relationship between BAL leptin levels and clinical outcomes; however, in nonobese patients, higher BAL leptin levels were associated with fewer intensive care unit– and ventilator-free days and higher mortality.
Conclusions: Leptin signaling is required for bleomycin-induced lung fibrosis. Leptin augments TGF-β1 signaling in lung fibroblasts by inhibiting PPARγ. These findings provide a mechanism for the observed protection against ARDS observed in diabetic patients.
Keywords: acute lung injury, fibrosis, lung, diabetes mellitus
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Patients with diabetes mellitus have an approximately 50% lower incidence of acute respiratory distress syndrome (ARDS) and those that develop ARDS are less likely to die. However, the mechanisms that underlie this protection are unknown. The development of TGF-β1–mediated fibroproliferation after lung injury is associated with poor clinical outcomes in patients with ARDS.
What This Study Adds to the Field
We found that leptin augments TGF-β1 signaling in lung fibroblasts and consequently promotes fibroproliferative ARDS by inhibiting peroxisome proliferator–activated receptor-γ. These findings suggest a mechanism for the observed protection against ARDS observed in patients with diabetes.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common clinical syndromes affecting almost 200,000 people per year in the United States (1). Although progress has been made in the supportive care of these patients, the mortality remains unacceptably high (∼40%). Patients with diabetes mellitus (DM) are approximately 50% (up to 62%) less likely to develop ARDS, and those with DM who develop ARDS have lower mortality rates than nondiabetic patients (2–5). The hypothesis that this protection is conferred by the immunosuppressive effects of hyperglycemia has been challenged by the results of studies suggesting that hyperglycemia exacerbates inflammation and worsens ALI (2, 3, 6).
Leptin is a 16-kD nonglycosylated protein encoded by the obese gene located on human chromosome 7 and on mouse chromosome 6 (7). Although classically considered a hormone because it regulates the balance between food intake and energy expenditure, leptin is also a member of the type I cytokine family. Type II diabetes is associated with hyperleptinemia and an acquired resistance to signaling through the leptin receptor (8, 9). Serum levels of leptin correlate with body mass index (BMI) and are increased in patients with sepsis, the most common cause of ARDS, suggesting that leptin may play a role in the pathogenesis of ARDS (10–12).
In some patients with ALI/ARDS, the activation of transforming growth factor-β1 (TGF-β1) contributes to an exuberant and persistent fibroproliferative response characterized by collagen deposition and a prolonged impairment in gas exchange (13). We and others have reported that patients with ARDS who develop an early fibroproliferative response in the lung are at increased risk for poor clinical outcomes (14–17). Investigators have reported that leptin plays an important role in the development of cirrhosis and renal fibrosis, although the mechanisms that underlie the protection seen with leptin resistance are poorly understood (18–22). We sought to determine whether leptin plays a role in the pathogenesis of fibroproliferative stage of ARDS and whether this effect is mediated via TGF-β1 signaling. Some of the results of these studies have been previously reported in the form of an abstract (23).
METHODS
Animals
The protocol for the use of mice was approved by the Animal Care and Use Committee at Northwestern University (Chicago, IL). We used 12-week-old, male, BKS.Cg-m+/+ Leprdb/J (db/db) mice (mice with leptin resistance due to defective leptin receptor) and age- and sex-matched wild-type controls from Jackson Laboratory (Bar Harbor, ME). Leptin receptor–deficient (db/db) mice are obese, hyperleptinemic, hyperglycemic, and hyperinsulinemic, and exhibit insulin resistance. As all of these features are observed in patients suffering from type II DM, these mice are used as a model for this disease (24).
Lung Histology, Collection of Bronchoalveolar Lavage Fluid, and Measurement of Leptin and Active TGF-β1 Levels
Lung histology, collection of bronchoalveolar lavage fluid, and measurement of leptin and active TGF-β1 levels were performed as previously described, using commercially available assays (25). Details are included in the online supplement.
Lung Homogenates and Immunoblotting
Lung homogenization and immunoblotting were performed as previously described (26). Details are included in the online supplement. Membranes were probed with antibodies to type I collagen (1 μg/ml) (Southern Biotechnology, Birmingham, AL), peroxisome proliferator–activated receptor-γ (PPARγ, 1 μg/ml), and actin (0.5 μg/ml) (Santa Cruz Biotech, Santa Cruz, CA).
Quantitative Assessment of Lung Collagen Content
Lung collagen was measured by a modification of a previously described method for the precipitation of lung collagen, using picrosirius red (27). Details are included in the online supplement.
Human Lung Fibroblasts
Normal human lung fibroblasts (NHLFs) (Cambrex, Charles City, IA) were grown in fibroblast growth medium-2 (FGM-2; Lonza, Inc, Allendale, NJ) supplemented with SingleQuots (Cambrex) in a humidified incubator (5% CO2) at 37°C. Quantitative RNA experiments were performed on cells before passage 5 and more than 70% confluent. The cells were incubated in serum-free medium for 24 hours before treatment with TGF-β1 and/or leptin.
Quantitative Real-time Reverse Transcription PCR
Quantitative real-time reverse transcription PCR assays were performed as previously described (28). All values were normalized to mitochondrial ribosomal protein RPL19. Specific primer sequences, RNA protocols, and normalization procedures are described in the online supplement.
Human Study Population
Subjects were recruited from the medical intensive care unit at Northwestern Memorial Hospital (Chicago, IL). The protocol was approved by the Institutional Review Board of Northwestern University and has been previously described (28). Details are provided in the online supplement.
Collection of Bronchoalveolar Lavage Fluid
Bronchoalveolar lavage (BAL) fluid was collected within 48 hours of intubation and stored as previously described (28). Details are provided in the online supplement.
Statistical Analysis
Data are expressed as means ± SEM unless otherwise specified. Differences between groups were analyzed by one-way analysis of variance (ANOVA). When ANOVA indicated a significant difference, we explored individual differences with the Student t test, using the Bonferroni correction for multiple comparisons. Direct comparisons between two treatment groups were performed with the unpaired Student t test or the nonparametric Mann-Whitney test when the data sets were not normally distributed (Prism 4; Graphpad Software, Inc., San Diego, CA). As human BAL fluid leptin and TGF-β1 levels were not normally distributed, we used the Spearman coefficient for the correlation analysis (SPSS for Windows 11.5; SPSS Inc., Chicago IL). Statistical significance in all experiments was defined as P < 0.05.
RESULTS
Leptin-resistant Mice Are Protected from Bleomycin-induced Pulmonary Fibrosis
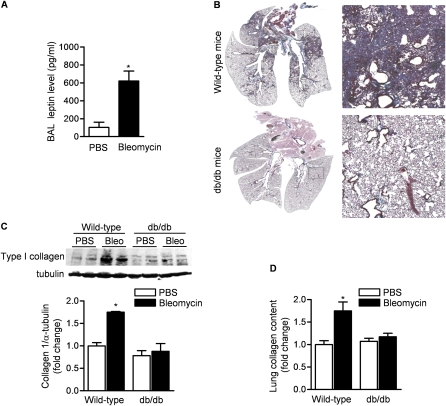
The intratracheal instillation of bleomycin in mice results in lung injury, which peaks 3 to 5 days later and is followed by fibroblast proliferation, collagen deposition, and pulmonary fibrosis, which is evident at 21 days (25, 29). To address the role of leptin in the regulation of fibroproliferative ARDS, we treated db/db and wild-type control mice with intratracheal bleomycin (0.075 unit/mouse) in sterile saline or with saline (control). We found that BAL fluid levels of leptin were increased sixfold in bleomycin-treated compared with phosphate-buffered saline (PBS)-treated wild-type mice (Figure 1A). Twenty-one days after the instillation of bleomycin, we observed severe fibrosis in wild-type mice as assessed by Masson trichrome staining (Figure 1B; and see Figure E1 in the online supplement). By contrast, db/db mice did not exhibit fibrosis. Total lung collagen, as evaluated by immunoblotting whole lung homogenates with an antibody that recognizes collagen I (Figure 1C) and picrosirius red collagen precipitation (Figure 1D), was significantly higher in wild-type mice than db/db mice.
Figure 1.
Mice with leptin resistance are protected against bleomycin-induced pulmonary fibrosis. (A) Bronchoalveolar lavage (BAL) fluid levels of leptin in wild-type mice 5 days after intratracheal instillation of bleomycin (0.075 unit) or phosphate-buffered saline (PBS). (B) Masson's trichrome staining for collagen in lungs from mice (wild-type and db/db) 21 days after intratracheal instillation of bleomycin or PBS. Both low-power images were captured with Neurolucida software (MBF Bioscience, Williston, VT) (original magnification: ×5) and high-power field views (original magnification, ×200) are shown. Total collagen content in lungs at 21 days after bleomycin treatment was assessed by (C) collagen I immunoblotting and (D) picrosirius red collagen precipitation (*P < 0.05, bleomycin vs. PBS treatment; n = 8 in each treatment group from two independent experiments).
Protection against Bleomycin-induced Pulmonary Fibrosis in Mice Is Independent of Bleomycin-induced Lung Injury
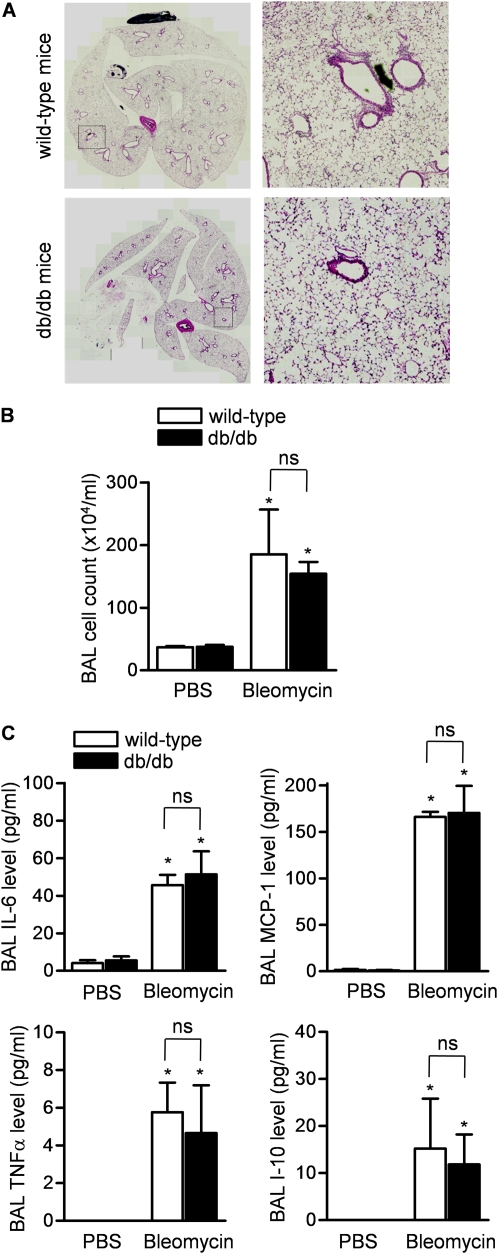
We evaluated the severity of bleomycin-induced lung injury in wild-type and db/db mice 5 days after the intratracheal administration of bleomycin. Examination of hematoxylin and eosin–stained lung sections from wild-type and db/db mice did not reveal differences in lung injury severity (Figure 2A). The bleomycin-induced increase in numbers of inflammatory cells (Figure 2B) and levels of proinflammatory cytokines (Figure 2C) in the BAL fluid were similar in wild-type and db/db mice.
Figure 2.
Protection against bleomycin-induced pulmonary fibrosis is independent of bleomycin-induced lung injury in mice. (A) Hematoxylin and eosin–stained lungs from mice (wild-type and db/db) 5 days after the intratracheal administration of bleomycin. Both low-power images were captured with MBF Neurolucida (original magnification: ×5) and high-power field views (original magnification: ×200) are shown. (B) Cell count and (C) levels of proinflammatory cytokines/chemokines in the bronchoalveolar lavage (BAL) fluid from mice (wild-type and db/db) 5 days after the intratracheal administration of bleomycin. (*P < 0.05, bleomycin vs. phosphate-buffered saline [PBS] treatment; n = 5 in each treatment group). MCP-1 = monocyte chemotactic protein-1; TNF-α = tumor necrosis factor-α; ns = not significant.
Leptin Signaling Affects Bleomycin-induced TGF-β1 Activation Downstream of Up-regulation of Integrin αvβ6
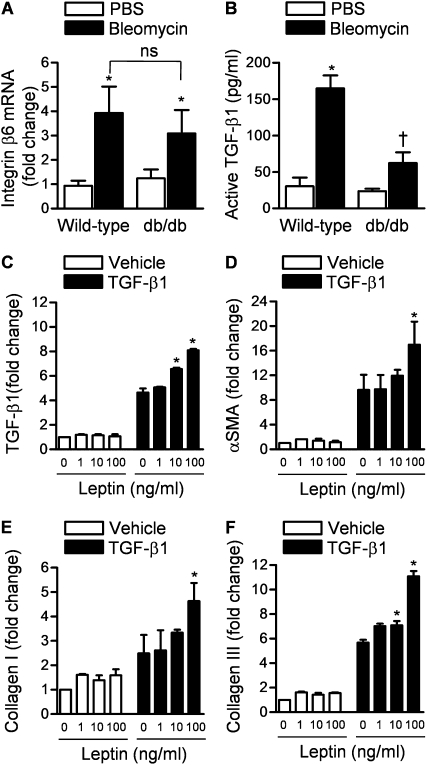
Activation of TGF-β1 requires increased epithelial expression of integrin αvβ6, which interacts with latent TGF-β1 complex in the lung interstitium to release active TGF-β1 (30). We observed by quantitative real-time reverse transcription PCR (qRT-PCR) a similar induction in the levels of integrin β6 mRNA in lung homogenates of wild-type and db/db mice 5 days after treatment with saline or bleomycin (Figure 3A). We then measured active TGF-β1 levels in freshly isolated BAL fluid samples from wild-type and db/db mice 5 days after the intratracheal instillation of bleomycin or PBS. The intratracheal administration of bleomycin led to a fivefold rise in the BAL fluid level of active TGF-β1 in wild-type mice, whereas the bleomycin-induced increase in active TGF-β1 in db/db mice was significantly attenuated (Figure 3B).
Figure 3.
Leptin signaling affects bleomycin-induced transforming growth factor (TGF)-β1 activation downstream of up-regulation of integrin αvβ6. (A) Real-time quantitative mRNA levels of integrin αvβ6 (corrected to keratin mRNA) (*P < 0.05, bleomycin vs. phosphate-buffered saline [PBS]; ns = not significant). (B) Bronchoalveolar lavage (BAL) fluid levels of TGF-β1 in wild-type and db/db mice 5 days after intratracheal instillation of bleomycin or PBS (P < 0.05: *bleomycin vs. PBS, †db/db plus bleomycin vs. wild-type plus bleomycin). (C) Normal human lung fibroblasts were treated with either vehicle or TGF-β1 (5 ng/ml) in the presence of various concentrations of leptin and TGF-β1 mRNA was measured 24 hours later by quantitative real-time reverse transcription PCR (qRT-PCR). (D–F) Normal human lung fibroblasts were treated with either vehicle or TGF-β1 (5 ng/ml) in the presence of various concentrations of leptin, and α-smooth muscle actin (α-SMA), collagen I, and collagen III mRNAs were measured 24 hours later (qRT-PCR). (*P < 0.05 leptin vs. PBS) (n ≥ 4 in each treatment group from three independent experiments).
Increased expression of integrin αvβ6 spatially restricts the activation of TGF-β1 to regions of epithelial injury. However, active TGF-β1 induces its own transcription and release from resident lung fibroblasts in an autocrine loop that can amplify the fibrotic response (31, 32). To test the hypothesis that signaling through the leptin receptor might be involved in this amplification, we treated normal human lung fibroblasts (NHLFs) with TGF-β1 and measured TGF-β1 transcription in the presence or absence of leptin. Leptin alone did not alter TGF-β1 mRNA but it augmented the induction of TGF-β1 in response to active TGF-β1 (Figure 3C).
Leptin Augments TGF-β1–induced Transcription of Profibrotic Genes in Normal Human Lung Fibroblasts
The finding that leptin augmented the autocrine release of TGF-β1 in NHLFs suggested that leptin may positively regulate the TGF-β1–induced transcription of other profibrotic genes. To test this hypothesis, we treated NHLFs with vehicle or TGF-β1 in the absence or presence of various concentrations of leptin in vitro and 24 hours later measured the induction of profibrotic genes, including α-smooth muscle actin (α-SMA), collagen I, and collagen III, by qRT-PCR. Whereas leptin treatment by itself did not induce the transcription of profibrotic genes, the addition of leptin to TGF-β1 augmented their TGF-β1–mediated transcription (Figures 3D−3F). The augmentation mediated by leptin at the highest dose was on average twofold higher than that induced by TGF-β1 alone. Treatment with SB431542 (10 μM), an inhibitor of the ubiquitously expressed TGF-β1 receptor ALK5 (activin receptor–like kinase-5) (33), completely inhibited the stimulatory effects of TGF-β1 on profibrotic genes in the presence and absence of leptin (Figure E2).
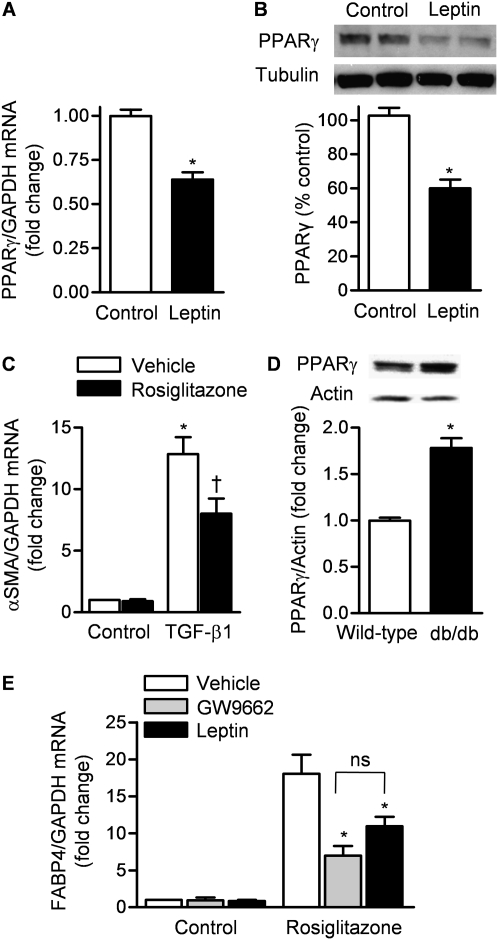
Leptin Decreases Expression and Activity of TGF-β1 Suppressor PPARγ in Normal Human Lung Fibroblasts
Activation of PPARγ has been shown to provide protection against organ fibrosis in the lung, kidney, and skin by acting as a corepressor of Smad-dependent gene transcription (34, 35). We found lower levels of PPARγ mRNA and protein in NHLFs treated with leptin (100 ng/ml) compared with vehicle 24 hours after their administration (Figures 4A and 4B) and treatment with the PPARγ agonist rosiglitazone suppressed the TGF-β1–induced transcription of α-SMA (Figure 4C). To verify the importance of leptin signaling in PPARγ expression, we immunoblotted total lung homogenates from wild-type and db/db mice, using an antibody that recognizes PPARγ. The protein abundance of PPARγ was increased almost twofold in db/db mice compared with wild-type mice (Figure 4D).
Figure 4.
Leptin decreases the expression and activity of the transforming growth factor (TGF)-β1 suppressor, peroxisome proliferator–activated receptor-γ (PPARγ). (A) mRNA (quantitative real-time reverse transcription PCR [qRT-PCR]) and (B) protein (immunoblot) levels of PPARγ in normal human lung fibroblasts 24 hours after treatment with leptin (100 ng/ml) or vehicle (*P < 0.05, leptin vs. control treatment). GAPDH = glyceraldehyde-3-phosphate dehydrogenase. (C) α-Smooth muscle actin (α-SMA) mRNA (qRT-PCR) in normal human lung fibroblasts 24 hours after treatment with TGF-β1 (5 ng/ml) or vehicle (control) in the presence or absence of rosiglitazone (50 μM) (a PPARγ agonist) (P < 0.05: *TGF-β1 vs. control, †TGF-β1 plus vehicle vs. TGF-β1 plus rosiglitazone). (D) PPARγ protein levels (immunoblot) in mouse lungs from untreated wild-type and db/db mice (P < 0.05: *wild-type vs. db/db). (E) mRNA (qRT-PCR) for fatty acid–binding protein-4 (FABP4) in normal human lung fibroblasts (NHLFs) treated with rosiglitazone (50 μM) in the presence or absence of GW9662 (a PPARγ antagonist) (10 μM) and leptin (100 ng/ml) (P < 0.05: *GW9662 vs. vehicle, *leptin vs. Vehicle; ns = not significant) (n ≥ 4 in each treatment group from three independent experiments).
To determine how leptin affects the PPARγ response, we treated NHLFs with rosiglitazone (a PPARγ agonist) or control vehicle and then treated them with PBS (control), GW9662 (a selective inhibitor of PPARγ), or leptin (100 ng/ml) and determined mRNA levels of fatty acid–binding protein-4 (FABP4), a transcriptional target gene of PPARγ (qRT-PCR) (36). The rosiglitazone-induced increase in FABP4 mRNA was suppressed by both GW9662 and leptin (Figure 4E).
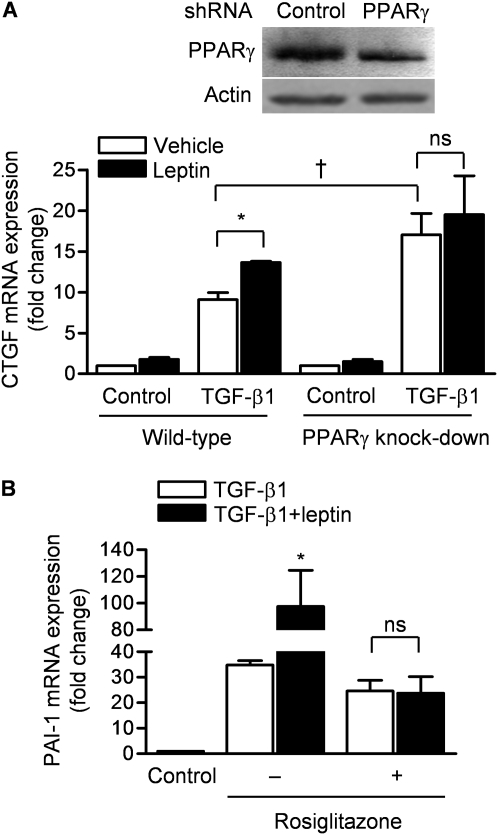
PPARγ Is Required and Sufficient for Leptin-mediated Augmentation of TGF-β1 Transcription in Normal Human Lung Fibroblasts
To determine whether PPARγ is required for the leptin-induced augmentation of TGF-β1 transcriptional activity, we used a lentiviral short hairpin RNA (shRNA) to generate NHLFs harboring a stable knockdown of PPARγ. In these cells, the levels of PPARγ were about 50% of those observed in control transfected cells (Figure 5A). We treated control and PPARγ knockdown NHLFs with TGF-β1 in the absence or presence of leptin (100 ng/ml) and 24 hours later measured mRNA levels of CTGF, a transcriptional target of TGF-β1 (qRT-PCR). Compared with control transfected cells, NHLFs in which PPARγ expression was knocked down showed an enhanced increase in CTGF mRNA in response to TGF-β1. The leptin-induced augmentation of TGF-β1 transcriptional activity in control transfected cells was prevented in the PPARγ knockdown cells (Figure 5A). To determine whether PPARγ was sufficient to prevent the leptin-mediated augmentation of TGF-β1, we treated wild-type NHLFs with rosiglitazone in the presence or absence of leptin and measured the expression of plasminogen activator inhibitor (PAI)-1, another transcriptional target of TGF-β1. Treatment with rosiglitazone completely prevented the leptin-induced augmentation of PAI-1 transcription (Figure 5B).
Figure 5.
Leptin-mediated augmentation of transforming growth factor (TGF)-β1 transcription in lung fibroblasts requires peroxisome proliferator–activated receptor-γ (PPARγ). (A) Normal human lung fibroblasts (NHLFs) were stably transfected (lentivirus) with control short hairpin RNA (shRNA) or an shRNA against PPARγ and cell lysates were immunoblotted for PPARγ (top). These cells were treated with medium and TGF-β1 (5 ng/ml) in the absence or presence of leptin (100 ng/ml) and 24 hours later connective tissue growth factor (CTGF) mRNA expression was measured (quantitative real-time reverse transcription PCR [qRT-PCR]) (P < 0.05: *leptin vs. vehicle, †wild-type plus TGF-β1 plus vehicle vs. PPARγ knockdown plus TGF-β1 plus vehicle; ns = not significant). (B) NHLFs were treated with TGF-β1 (5 ng/ml), leptin (100 ng/ml), and/or rosiglitazone (50 μM) and 24 hours later plasminogen activator inhibitor (PAI)-1 mRNA was measured (qRT-PCR) (*P < 0.05, TGF-β1 vs. TGF-β1 plus leptin) (n = 4 in each treatment group from two independent experiments).
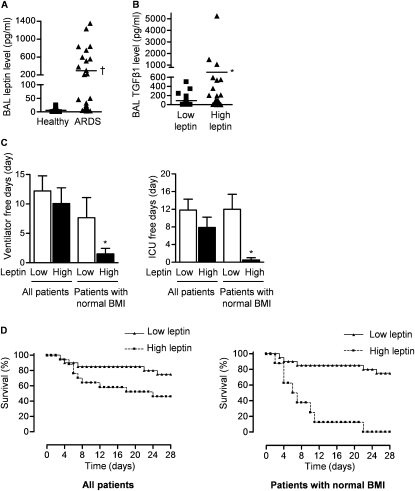
Increased Levels of BAL Fluid Leptin and TGF-β1 Levels Are Associated with Adverse Outcomes in Patients with ARDS
To evaluate the role of leptin signaling in human ARDS, we measured leptin and TGF-β1 levels in BAL fluid obtained from patients with ALI/ARDS or ventilated control patients without lung disease within 72 hours of intubation. Table 1 summarizes the demographics and physiology of these patients. The BAL fluid levels of leptin were sixfold higher in nonobese patients with ALI/ARDS compared with the control patients (Figure 6A). There was a significant correlation between BAL fluid levels of leptin and TGF-β1 (r = 0.522, P < 0.001) when all patients with ALI/ARDS were included in the analysis. This association was stronger in the cohort of patients with ARDS with normal BMI (r = 0.637, P < 0.0001). None of the control patients had leptin levels greater than 100 pg/ml, which was used as the cutoff value to define patients with high leptin (≥100 pg/ml) and low leptin (<100 pg/ml) levels. Patients with ALI/ARDS and high leptin levels had higher BAL fluid levels of active TGF-β1 (Figure 6B).
TABLE 1.
PATIENT DEMOGRAPHICS AND PHYSIOLOGY
| Characteristic | Subjects with ALI/ARDS (n = 36) |
|---|---|
| Age, yr | 53 ± 18 |
| Sex | |
| Male | 21 (58%) |
| Female | 15 (42%) |
| BMI, kg/m2 | 25.9 ± 6.2 |
| Patients with BMI ≥ 30 kg/m2 | 10 (28%) |
| Patients with BMI < 30 kg/m2 | 26 (72%) |
| Diabetes mellitus | 6 (16.7%) |
| PaO2/FiO2 ratio | 127 ± 53 |
| PaCO2 (mm Hg) | 44 ± 14 |
| APACHE II score | 25 ± 9 |
| Risk factors for ALI/ARDS | |
| Pneumonia | 15 (42%) |
| Extrapulmonary sepsis | 14 (39%) |
| Other | 7 (19%) |
Definition of abbreviations: ALI/ARDS = acute lung injury/acute respiratory distress syndrome; APACHE = Acute Physiology and Chronic Health Evaluation; BMI = body mass index; FiO2 = fraction of inspired oxygen.
Values are presented as means ± SD.
Figure 6.
Alveolar levels of leptin and transforming growth factor (TGF)-β1 correlate in patients with acute respiratory distress syndrome (ARDS). (A) Bronchoalveolar lavage (BAL) fluid levels of leptin in healthy intubated control patients and all patients with ARDS. (B) BAL fluid levels of TGF-β1 (all patients) and (C) clinical outcomes (ventilator-free days, intensive care unit [ICU]–free days) and (D) survival of patients with ARDS with low (<100 pg/ml) and high (>100 pg/ml) levels of leptin. (P < 0.05: †ARDS vs. healthy control subjects, *high leptin vs. low leptin) (n = 36 patients with ARDS and n = 15 healthy intubated patients). BMI = body mass index.
Overall, there was no difference in clinical outcomes between patients with ARDS with low and high lung leptin levels (Figures 6C and 6D). Obesity correlates with serum leptin levels in humans and animals and is associated with hyperleptinemia secondary to acquired leptin resistance (8, 9). To exclude the effects of BMI on leptin levels, we evaluated the relationship of BAL leptin levels with clinical outcomes in patients with a normal BMI (BMI < 30 kg/m2) (37). In the subgroup of patients with ARDS and a normal BMI, higher BAL levels of leptin were associated with fewer ventilator- and intensive care unit–free days (Figure 6C) and a higher mortality (Figure 6D).
DISCUSSION
Leptin is a peptide hormone that acts in the brain to reduce hunger and increase energy expenditure (8). However, the functional long form of the leptin receptor, Ob-Rb, is ubiquitously distributed in almost all tissues including the lung, where its functions have been less well studied (38). The majority of obese patients with type II diabetes exhibit chronic elevations of leptin and demonstrate resistance to leptin signaling (8, 9). This population is resistant to the development of ALI/ARDS and has lower mortality when it develops (1–3). We and others have shown that markers of fibrosis in the BAL fluid are predictors of outcome in patients with ARDS and the resolution of fibrosis coincides with clinical improvement (14, 16, 17, 28). As leptin plays an essential role in murine models of liver (18, 39) and kidney (22) fibrosis, we hypothesized that leptin might contribute to the development of fibroproliferative response during ALI/ARDS. Consistent with this hypothesis, we observed that leptin receptor–deficient mice are resistant to bleomycin-induced fibrosis and found a positive correlation between BAL levels of leptin and active TGF-β1 in patients with ARDS. In nonobese patients with ARDS, higher levels of leptin in the BAL fluid were associated with fewer ventilator- and intensive care unit–free days and higher mortality.
The long form of the leptin receptor resembles the gp130 family of cytokine receptors and leptin has been reported to function as an immunomodulator (40–42). Leptin enhances phagocytosis in macrophages and induces the transcription and secretion of proinflammatory cytokines such as IL-6 from inflammatory cells (dendritic cells and monocytes), adipocytes, microglia, and endometrial and gastrointestinal epithelial cells (43–47). The stimulatory effect of leptin on IL-6 release appears to be mediated by nuclear factor-κB (46, 47). Consistent with these findings, we and others have shown that mice with leptin resistance (db/db) and leptin deficiency (ob/ob) have less lung injury and improved survival in a murine model of ALI/ARDS induced by exposure to hyperoxia (11, 12). Using the same methods employed in those reports, we observed a similar degree of acute inflammation in the lungs of wild-type and db/db mice after the instillation of bleomycin. Furthermore, the bleomycin-induced transcription of integrin β6 was similar in wild-type and db/db mice. As this integrin has been shown to be required for the activation of TGF-β1 and the development of bleomycin-induced fibrosis downstream of the acute inflammatory response, these results suggest that the protection conferred against fibrosis by the loss of the leptin receptor is independent of an effect on bleomycin-induced lung injury (25, 30). This is further supported by our in vitro finding that leptin augments TGF-β1–mediated transcription in normal human lung fibroblasts. However, we cannot exclude the possibility that we failed to detect a small but important difference in bleomycin-induced lung inflammation, which may have contributed to the observed protection.
PPARγ is a nuclear hormone receptor and transcription factor that is essential for normal adipogenesis and glucose homeostasis (48). Activation of PPARγ can be induced by endogenous lipids and eicosanoids or by the thiazolidinedione class of antidiabetic drugs such as rosiglitazone (48). The activation of PPARγ has been shown to attenuate fibrosis in the bleomycin model of lung fibrosis and other murine models of tissue fibrosis, where it inhibits TGF-β1 signaling by interfering with the binding of activated Smads to their genomic DNA consensus sequences (35, 49–53). We found that the administration of leptin to normal human lung fibroblasts reduced both the protein abundance and activity of PPARγ. In cells harboring a stable knockdown of PPARγ and in cells treated with rosiglitazone to augment PPARγ, the leptin-induced augmentation of TGF-β1–mediated transcription was lost. These results suggest that leptin augments TGF-β1–mediated transcription by reducing the abundance and activity of PPARγ. Consistent with this mechanism, the levels of PPARγ were higher in leptin receptor–deficient mice.
BAL levels of leptin were significantly higher in patients with ARDS than in control ventilated patients and positively correlated with the levels of active TGF-β1. These levels were not predictive of clinical outcomes in unselected patients with ARDS. Patients with type II DM develop acquired leptin resistance and elevated leptin levels, which correlate with BMI. We therefore performed a separate analysis of the relationship between BAL fluid leptin levels and clinical outcomes in patients without obesity as defined by the World Health Organization (BMI < 30). In these patients, higher levels of leptin correlated with poor clinical outcomes. Although our study was not designed to evaluate leptin as a prognostic indicator, these findings support our hypothesis that signaling through the leptin receptor might play a pathophysiological role in the development and progression of acute lung injury.
As obesity is associated with leptin resistance even in the absence of type II DM, our results suggest that the risk reduction seen in patients with DM may also be present in obese patients. Unfortunately, BMI has not been prospectively evaluated as a potential risk factor in many of the large cohorts examining ARDS outcomes (1, 54). Investigators who have examined this association retrospectively have reported conflicting results. In 1,291 patients with ARDS, Dossett and colleagues reported that the 30% who were obese had a lower severity-adjusted rate of ARDS (odds ratio, 0.36) but similar mortality and longer lengths of stay when compared with nonobese patients (55). In contrast, in two retrospective analyses of prospectively collected cohorts of patients at risk for ARDS, Gong and colleagues and Anzueto and colleagues found that an elevated BMI was positively associated with the development of ARDS but was not associated with increased mortality or other adverse outcomes (56, 57). In 902 mechanically ventilated patients with ALI, O'Brien and colleagues found that BMI was not independently associated with improved or worsened outcomes (58). Our results and these highlight the need for further prospective epidemiological studies examining the influence of obesity, insulin and leptin levels, and insulin and leptin resistance on the development of and outcomes after ALI and ARDS.
In this investigation and our previous report, we have focused on the role played by leptin resistance in the protection observed in patients with type II DM against the development of lung injury and fibroproliferation. Type II DM is a complex disease that is primarily characterized by hyperglycemia and hyperinsulinemia (insulin resistance), which develop spontaneously in leptin receptor–deficient mice. There is evidence that leptin and insulin modulate each other's action in target organs and both have a direct and independent role in the regulation of blood glucose levels (59–65). It is therefore likely that hyperinsulinemia, insulin resistance, or other hormonal and metabolic consequences of type II DM play a role in the protection we observe in leptin receptor–deficient mice.
The experimental model we used to study fibroproliferative response during ARDS has some limitations. Pathologically, ARDS in humans has three phases including (1) an early exudative phase of edema and inflammation, (2) a proliferative phase with alveolar epithelial cell hyperplasia and myofibroblast proliferation, and (3) a fibrotic phase with collagen deposition and progressive lung fibrosis (25). The fibroproliferative response, if excessive, impairs gas exchange and is associated with increased morbidity and mortality. Although the intratracheal instillation of bleomycin model mimics the pathology of human ARDS, as it initially causes lung injury followed by fibroblast proliferation, collagen deposition, and pulmonary fibrosis (25, 29), it leads to severe lung fibrosis, which is not seen in most cases of ARDS. Therefore, the clinical implication of our findings may be limited to patients with severe ARDS complicated by excessive fibroproliferative response and fibrosis.
In conclusion, we report that lung leptin levels are increased in patients and mice with acute lung injury. Signaling through the leptin receptor is required for bleomycin-induced lung fibrosis in mice. Leptin exerts a profibrogenic effect in primary human lung fibroblasts by augmenting the transcriptional activity of TGF-β1 via suppression of the antifibrotic activity of PPARγ. The loss of leptin signaling inhibits the bleomycin-induced activation of TGF-β1 and the TGF-β1–mediated transcription of profibrotic genes in part by augmenting the expression and activity of PPARγ. In nonobese patients with ARDS, elevated levels of leptin in the lung are associated with increased levels of active TGF-β1 and the development of adverse clinical outcomes. Given that variable leptin resistance is observed in patients with type II DM, these results provide a potential mechanism explaining the unexpected protection against ALI/ARDS observed in this population and suggest that therapeutic strategies that inhibit leptin signaling might be effective in selected patients with ALI/ARDS.
Supplementary Material
Supported by ES015024 (G.M.M.), the American Lung Association (G.M.M.), ES013995 (G.R.S.B.), HL071643 (G.M.M., G.R.S.B., and N.S.C.), a Northwestern University Clinical and Translational Sciences Institute (NUCATS) CTI pilot award (UL1 RR025741 [NCCR]; G.M.M.), a Northwestern Memorial Foundation Dixon Young Investigator grant (G.M.M.), and a Scleroderma Research Foundation grant (J.V.).
Author contributions: M.J., G.R.S.B., A.K.G., J.V., N.S.C., and G.M.M. were involved in the conception and design of experiments, analyzed the data, and wrote the manuscript. A.L., D.U., S.E.R., A.K.G., A.G., S.E.C., K.M., H.K.D., S.S., and K.A.R. performed the experiments. M.J. and H.K.D. obtained informed consent and collected human samples.
Originally Published in Press as DOI: 10.1164/rccm.201009-1409OC on February 11, 2011
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Author Disclosure: M.J. has received advisory board fees from Genentech and the Cystic Fibrosis Foundation; he has received industry-sponsored grants from Millennium Pharmaceuticals. G.R.S.B., A.L., D.U., S.E.R., A.K.G., A.G., S.E.C., K.M., H.K.D., S.S., J.V., K.A.R., N.S.C., and G.M.M. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Moss M, Guidot DM, Steinberg KP, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE. Diabetic patients have a decreased incidence of acute respiratory distress syndrome. Crit Care Med 2000;28:2187–2192. [DOI] [PubMed] [Google Scholar]
- 3.Frank JA, Nuckton TJ, Matthay MA. Diabetes mellitus: a negative predictor for the development of acute respiratory distress syndrome from septic shock. Crit Care Med 2000;28:2645–2646. [DOI] [PubMed] [Google Scholar]
- 4.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H III, Hoth JJ, Mikkelsen ME, Gentile NT, et al.; on behalf of the U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 2010;183:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 2005;33:1191–1198. [DOI] [PubMed] [Google Scholar]
- 6.Honiden S, Gong MN. Diabetes, insulin, and development of acute lung injury. Crit Care Med 2009;37:2455–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JM. Modern science versus the stigma of obesity. Nat Med 2004;10:563–569. [DOI] [PubMed] [Google Scholar]
- 9.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995;1:1155–1161. [DOI] [PubMed] [Google Scholar]
- 10.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol 2000;68:437–446. [PubMed] [Google Scholar]
- 11.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 2007;175:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barazzone-Argiroffo C, Muzzin P, Donati YR, Kan CD, Aubert ML, Piguet PF. Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol Lung Cell Mol Physiol 2001;281:L1150–L1156. [DOI] [PubMed] [Google Scholar]
- 13.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 14.Budinger GR, Chandel NS, Donnelly HK, Eisenbart J, Oberoi M, Jain M. Active transforming growth factor-β1 activates the procollagen I promoter in patients with acute lung injury. Intensive Care Med 2005;31:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandel NS, Budinger GR, Mutlu GM, Varga J, Synenki L, Donnelly HK, Zirk A, Eisenbart J, Jovanovic B, Jain M. Keratinocyte growth factor expression is suppressed in early acute lung injury/acute respiratory distress syndrome by Smad and c-Abl pathways. Crit Care Med 2009;37:1678–1684. [DOI] [PubMed] [Google Scholar]
- 16.Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome: association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med 1995;122:17–23. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006;354:1671–1684. [DOI] [PubMed] [Google Scholar]
- 18.Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, Lang T, Fukuda T, Yamashina S, Kitamura T, et al. Leptin receptor–mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology 2002;122:1399–1410. [DOI] [PubMed] [Google Scholar]
- 19.Elinav E, Ali M, Bruck R, Brazowski E, Phillips A, Shapira Y, Katz M, Solomon G, Halpern Z, Gertler A. Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology 2009;49:278–286. [DOI] [PubMed] [Google Scholar]
- 20.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology 2002;35:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J 2004;18:1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA. Leptin stimulates proliferation and TGF-β expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [see comments]. Kidney Int 1999;56:860–872. [DOI] [PubMed] [Google Scholar]
- 23.Budinger GRJ, Bellmeyer M, Urich A, Soberanes D, Chiarella S, Rivera SE, Chandel NS, Mutlu GM. Leptin resistance protects against fibroproliferaive ARDS via loss of augmentation of TGFβ1 and preservation of PPARγ activity [abstract]. Am J Respir Crit Care Med 2010;181:A3771. [Google Scholar]
- 24.Leibel RL, Chung WK, Chua SC Jr. The molecular genetics of rodent single gene obesities. J Biol Chem 1997;272:31937–31940. [DOI] [PubMed] [Google Scholar]
- 25.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, et al. Proapoptotic Bid is required for pulmonary fibrosis. Proc Natl Acad Sci USA 2006;103:4604–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soberanes S, Urich D, Baker CM, Burgess Z, Chiarella SE, Bell EL, Ghio AJ, De Vizcaya-Ruiz A, Liu J, Ridge KM, et al. Mitochondrial complex III–generated oxidants activate ASK1 and JNK to induce alveolar epithelial cell death following exposure to particulate matter air pollution. J Biol Chem 2009;284:2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whittaker P, Kloner RA, Boughner DR, Pickering JG. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol 1994;89:397–410. [DOI] [PubMed] [Google Scholar]
- 28.Synenki L, Chandel NS, Budinger GR, Donnelly HK, Topin J, Eisenbart J, Jovanovic B, Jain M. Bronchoalveolar lavage fluid from patients with acute lung injury/acute respiratory distress syndrome induces myofibroblast differentiation. Crit Care Med 2007;35:842–848. [DOI] [PubMed] [Google Scholar]
- 29.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol 2005;33:9–13. [DOI] [PubMed] [Google Scholar]
- 30.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 31.Pardoux C, Derynck R. Jnk regulates expression and autocrine signaling of TGF-β1. Mol Cell 2004;15:170–171. [DOI] [PubMed] [Google Scholar]
- 32.Ventura JJ, Kennedy NJ, Flavell RA, Davis RJ. Jnk regulates autocrine expression of TGF-β1. Mol Cell 2004;15:269–278. [DOI] [PubMed] [Google Scholar]
- 33.Massague J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 2000;103:295–309. [DOI] [PubMed] [Google Scholar]
- 34.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARγ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2005;288:L1146–L1153. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Melichian DS, Chang E, Warner-Blankenship M, Ghosh AK, Varga J. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am J Pathol 2009;174:519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 1992;358:771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 38.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J 2006;393:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang M, Potter JJ, Mezey E. Leptin enhances the effect of transforming growth factor β in increasing type I collagen formation. Biochem Biophys Res Commun 2002;297:906–911. [DOI] [PubMed] [Google Scholar]
- 40.Tartaglia LA. The leptin receptor. J Biol Chem 1997;272:6093–6096. [DOI] [PubMed] [Google Scholar]
- 41.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998;394:897–901. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 2000;199:15–24. [DOI] [PubMed] [Google Scholar]
- 43.Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apcmin/+ colon epithelial cell line. Carcinogenesis 2006;27:1507–1515. [DOI] [PubMed] [Google Scholar]
- 44.Zarkesh-Esfahani H, Pockley G, Metcalfe RA, Bidlingmaier M, Wu Z, Ajami A, Weetman AP, Strasburger CJ, Ross RJ. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol 2001;167:4593–4599. [DOI] [PubMed] [Google Scholar]
- 45.Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol 2005;174:6820–6828. [DOI] [PubMed] [Google Scholar]
- 46.Tang CH, Lu DY, Yang RS, Tsai HY, Kao MC, Fu WM, Chen YF. Leptin-induced IL-6 production is mediated by leptin receptor, insulin receptor substrate-1, phosphatidylinositol 3-kinase, Akt, NF-κB, and p300 pathway in microglia. J Immunol 2007;179:1292–1302. [DOI] [PubMed] [Google Scholar]
- 47.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, et al. Leptin regulates proinflammatory immune responses. FASEB J 1998;12:57–65. [PubMed] [Google Scholar]
- 48.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 2008;77:289–312. [DOI] [PubMed] [Google Scholar]
- 49.Ma LJ, Marcantoni C, Linton MF, Fazio S, Fogo AB. Peroxisome proliferator–activated receptor-γ agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int 2001;59:1899–1910. [DOI] [PubMed] [Google Scholar]
- 50.Kawai T, Masaki T, Doi S, Arakawa T, Yokoyama Y, Doi T, Kohno N, Yorioka N. PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab Invest 2009;89:47–58. [DOI] [PubMed] [Google Scholar]
- 51.Milam JE, Keshamouni VG, Phan SH, Hu B, Gangireddy SR, Hogaboam CM, Standiford TJ, Thannickal VJ, Reddy RC. PPAR-γ agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L891–L901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michalik L, Wahli W. Involvement of PPAR nuclear receptors in tissue injury and wound repair. J Clin Invest 2006;116:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh AK, Bhattacharyya S, Lakos G, Chen SJ, Mori Y, Varga J. Disruption of transforming growth factor β signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator–activated receptor γ. Arthritis Rheum 2004;50:1305–1318. [DOI] [PubMed] [Google Scholar]
- 54.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:293–301. [DOI] [PubMed] [Google Scholar]
- 55.Dossett LA, Heffernan D, Lightfoot M, Collier B, Diaz JJ, Sawyer RG, May AK. Obesity and pulmonary complications in critically injured adults. Chest 2008;134:974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax 2010;65:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anzueto A, Frutos-Vivar F, Esteban A, Bensalami N, Marks D, Raymondos K, Apezteguia C, Arabi Y, Hurtado J, Gonzalez M, et al. Influence of body mass index on outcome of the mechanically ventilated patients. Thorax 2011;66:66–73. [DOI] [PubMed] [Google Scholar]
- 58.O'Brien JM Jr, Welsh CH, Fish RH, Ancukiewicz M, Kramer AM. Excess body weight is not independently associated with outcome in mechanically ventilated patients with acute lung injury. Ann Intern Med 2004;140:338–345. [DOI] [PubMed] [Google Scholar]
- 59.Dagogo-Jack S, Fanelli C, Paramore D, Brothers J, Landt M. Plasma leptin and insulin relationships in obese and nonobese humans. Diabetes 1996;45:695–698. [DOI] [PubMed] [Google Scholar]
- 60.Hennige AM, Stefan N, Kapp K, Lehmann R, Weigert C, Beck A, Moeschel K, Mushack J, Schleicher E, Haring HU. Leptin down-regulates insulin action through phosphorylation of serine-318 in insulin receptor substrate 1. FASEB J 2006;20:1206–1208. [DOI] [PubMed] [Google Scholar]
- 61.Askari H, Tykodi G, Liu J, Dagogo-Jack S. Fasting plasma leptin level is a surrogate measure of insulin sensitivity. J Clin Endocrinol Metab 2010;95:3836–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 2010;11:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA 2010;107:4813–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen B, Novick D, Rubinstein M. Modulation of insulin activities by leptin. Science 1996;274:1185–1188. [DOI] [PubMed] [Google Scholar]
- 65.Kitabchi AE, Umpierrez GE. Changes in serum leptin in lean and obese subjects with acute hyperglycemic crises. J Clin Endocrinol Metab 2003;88:2593–2596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.