Abstract
In mammals, it is well established that circadian rhythms in physiology and behavior, including the rhythmic secretion of hormones, are regulated by a brain clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus. While SCN regulation of gonadal hormone secretion has been amply studied, the mechanisms whereby steroid hormones affect circadian functions are less well known. This is surprising considering substantial evidence that sex hormones affect many aspects of circadian responses, and that there are significant sex differences in rhythmicity. Our previous finding that “core” and “shell” regions of the SCN differ in their expression of clock genes prompted us to examine the possibility that steroid receptors are localized to a specific compartment of the brain clock, with the discovery that the androgen receptor (AR) is concentrated in the SCN core in male mice. In the present study, we compare AR expression in female and male mice using Western blots and immunochemistry. Both of these methods indicate that AR’s are more highly expressed in males than in females; gonadectomy eliminates and androgen treatment restores these sex differences. At the behavioral level, gonadectomy produces a dramatic loss of the evening activity onset bout in males, but has no such effect in females. Treatment with testosterone, or with the non-aromatizable androgen, dihydrotestosterone restores male locomotor activity and eliminates sex differences in the behavioral response. The results indicate that androgenic hormones regulate circadian responses, and suggest an SCN site of action.
Keywords: Circadian rhythms, mouse, gonadal hormones, dihydrotestosterone, testosterone, androgen receptor, locomotor activity
Introduction
In all species, appropriate timing of physiological and behavioral processes is necessary for optimal functioning in the environment. The circadian (daily) timing system ensures that incompatible biochemical and behavioral rhythms are temporally segregated within individual cells and tissues, as well as in the organism as a whole. In mammals, circadian timing is regulated by a master clock within the suprachiasmatic nucleus (SCN) of the hypothalamus, and this nucleus is necessary for the generation and maintenance of circadian rhythms (Klein, Moore, and Reppert, 1991). In proof, lesions of the SCN abolish circadian rhythms, and neural transplantation of the SCN from a donor to a lesioned host restores circadian rhythms in the host (Lehman, Silver, Gladstone, Kahn, Gibson, and Bittman, 1987; Ralph, Foster, Davis, and Menaker, 1990).
In addition to controlling daily rhythms, the SCN is also essential for the synchronization (or entrainment) of an organism’s internal circadian time to the external environmental world, integrating internal and external signals. Both environmental and internal stimuli can set the phase of SCN oscillators. The most salient environmental timing cue is the daily cycle of light and darkness (Morin and Allen, 2006). Photic input from the retina reaches the SCN via a direct projection via the retinohypothalamic tract (RHT), which terminates with greatest density within the core region of the SCN (Abrahamson and Moore, 2001; Antle and Silver, 2005). The core compartment also receives afferent input from the intergeniculate leaflet (IGL) and the raphe nucleus (RN) (Kriegsfeld, Leak, Yackulic, LeSauter, and Silver, 2004; Moga and Moore, 1997; Zhang and Rusak, 1989). As discussed below, internal cues such as hormonal signals, many of which are secreted on a circadian basis, also target the core SCN compartment.
Much experimental work done in laboratory rodents indicates that the gonadal hormones estrogen (E) and testosterone (T) modulate circadian activity rhythms (Jechura, Walsh, and Lee, 2000; Morin, 1986). Additionally, field studies demonstrate hormone-dependent effects, with some species even changing seasonally from diurnality to nocturnality (Yokota and Oishi, 1992). Though few studies have been performed in mice, they suggest that circadian period and precision, as well as the organization of daily activity bouts, are dramatically affected by gonadal hormones (Daan, Damassa, Pittendrigh, and Smith, 1975; Karatsoreos, Wang, Sasanian, and Silver, 2007). Thus, following gonadectomy (GDX) of males there is a loss of the onset component of daily activity, lengthening of free running period, a decrease in precision, a reduction in duration of daily activity bouts, and a decrease of total daily activity. In contrast, in female rats and hamsters, ovariectomy (OVX) produces an overall reduction of total daily activity levels, and hormone replacement shortens the free-running period (Albers, 1981; Morin, 1980; Morin, Fitzgerald, and Zucker, 1977; Zucker, Fitzgerald, and Morin, 1980).
Many of the circadian effects of E and T are thought to occur outside of the SCN, either on SCN afferents or efferents (de la Iglesia, Blaustein, and Bittman, 1995; de La Iglesia, Blaustein, and Bittman, 1999). This assumption has been supported, in part, by reports of sparse steroid receptor localization in the SCN (Mitra, Hoskin, Yudkovitz, Pear, Wilkinson, Hayashi, Pfaff, Ogawa, Rohrer, Schaeffer, McEwen, and Alves, 2003; Shughrue, Lane, and Merchenthaler, 1997; Shughrue and Merchenthaler, 2001). In contrast, we found that androgen receptors (AR) in male mice are highly localized to the mid-caudal region of the SCN, termed the “core” (Karatsoreos et al., 2007). In earlier studies performed when intra-SCN regional differences were unknown, this distribution of AR may have been missed, as it was thought that all SCN cells were equivalent oscillators (Reppert and Weaver, 2001). Today, there is general agreement that functional specializations map to these anatomically distinct ventral core and dorsal shell regions (reviewed in: Antle and Silver, 2005). In the present study, we explore sex differences in AR expression and in circadian locomotor activity. Because SCN AR expression in castrated males resembled that of females, we studied the effect of testosterone replacement on the androgen-dependent responses.
Methods
Animals and housing
Male and female C57BL/6J mice (Charles River Laboratories, Kingston, NY) aged 8–10wks were group housed for at least 2wks following arrival. Animals used for the behavioral experiments were placed in individual cages equipped with running wheels, as described below. All other animals were group housed. To determine the phase of the estrus cycle, vaginal smears were taken every morning (at lights on) for at least two consecutive cycles.
All animals were provided with ad libitum access to food and water, and maintained in a 12:12-h light:dark (LD) cycle (unless otherwise noted) in accordance with the guidelines of Columbia University’s Institutional Animal Care and Use Committee.
Behavioral Measurements
For assessment of wheel-running activity, C57BL/6J mice were maintained in constant darkness (DD) and housed individually in translucent plastic cages (36 × 20 × 20 cm) equipped with a running wheel (13 cm diameter) with the number of wheel revolutions recorded by a computerized data acquisition system (VitalView, Respironics, Inc, Murraysville, PA). The free-running behavior of intact (INT) mice was recorded for at least 3wks (n=14 males, 26 females). Animals were then orchidectomized (GDX, n=14) or ovariectomized (OVX, n=24), with time of surgery performed during the animal’s subjective day so as to avoid a light induced phase shift. The behavior of gonadectomized animals was monitored for 3wks, after which some of the mice were implanted with Silastic capsules containing either testosterone propionate (TP; males, n=8, females, n=9) or 5α-dihydrotestosterone (DHT; males, n=6, females, n=8), and returned to DD for an additional 3wks. The behavior of male mice was previously reported in Karatsoreos et al. (2007); for this report, a new analysis was performed on activity, period and precision to permit comparisons with the behavior of females maintained in the same housing conditions.
To evaluate sex differences and androgen effects on circadian behavior in males and females, analysis was carried out on at least 10 consecutive days of data, and at least 7 days following any manipulation. Data were analyzed using Clocklab (Actimetrics Inc., Wilmette, IL) for Matlab (The MathWorks Inc., Natick, MA). Free-running period was calculated using a fast Fourier periodogram, and is reported in hours ± SEM. Amount of daily activity was calculated as the number of wheel revolutions per day. The precision of the daily onset of activity was calculated for each individual, as previously described (Karatsoreos, 2007). Briefly, the projected activity onset time was calculated for each day from the free-running period. Precision was assessed as the difference between the actual and projected activity onset time, with smaller values reflecting more precise timing.
To quantify the re-organization of circadian activity following gonadectomy and hormone treatment, the circadian day of each animal was divided into four epochs of six hours duration. Activity offset was used to define circadian time (CT) 24, as GDX male mice show highly inaccurate early night onsets, or no detectable onsets at all. The daily activity for each animal in each of the time epochs was calculated (activity within each epoch/total daily activity), averaged over 7d, and reported as percent ± SEM.
Gonadectomy and Hormone Replacement
Mice were deeply anesthetized with ketamine (70 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.), and buprenorphine (0.5 mg/kg, s.c.) was used as an analgesic. In males, GDX was performed by abdominal incision and removal of both testes. Females were OVXd by bilateral flank incisions, and removal of both ovaries. In both cases, muscle and fascia were closed using surgical silk, and the overlying skin was sutured. For controls, animals were anesthetized but not surgically manipulated.
Steroid Implants
Implants were prepared as previously described (Lindzey, Wetsel, Couse, Stoker, Cooper, and Korach, 1998; Wersinger, Haisenleder, Lubahn, and Rissman, 1999). Briefly, silastic capsules (Dow Corning, I.D. 0.98mm, O.D. 2.16mm) were filled with crystalline TP (5mm long) or DHT (10mm long; Steraloids Inc., Newport, RI), sealed with Silastic glue and allowed to dry overnight. All capsules were primed in a 37°C 0.9% saline bath for 36h before implantation.
Western blotting
For Western blots, animals (n=6 males; 6 females) were euthanized with CO2, and their brains removed and placed in ice cold 0.9 % saline. Using a Vibratome, 400μm sections of hypothalamus were collected in ice-cold saline, and the SCN were harvested bilaterally with the aid of a dissecting microscope. To prepare SCN lysates, the tissues were homogenized by sonication in lysis buffer (1% SDS in dH2O with Roche complete, Mini, EDTA-free protease inhibitor cocktail), and then incubated in a boiling water bath for 10 min. Lysates were spun in a microfuge (13,000 rpm for 30 sec) to remove insoluble material. The protein concentration of the cleared supernatant was determined using the BCA method (Pierce, Rockford, IL). Lysates (5 μg/lane) were subjected to SDS gel electrophoresis, blotted to a nitrocellulose membrane and probed with the AR antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were visualized by chemiluminescence, according to the manufacturer's instructions (Lumiglo; Cell Signaling, Beverly, MA). Relative optical density (ROD) of the Western blots was measured using image analysis software (MCID, St. Catharines, ON). A specific band was observed at ~98 kD, the molecular weight for AR.
Perfusion and Immunochemistry
Animals were deeply anesthetized (pentobarbital: 200 mg/kg i.p.) and perfused intracardially with 50 ml saline followed by 100 ml of 4 % paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.3. Brains to be used in ICC were post-fixed for 4hr at 4 °C, and cryoprotected in 20 % sucrose in 0.1 M PB overnight, and sliced at 35μm on a cryostat.
Immunocytochemistry (ICC)
For hormonal regulation of AR, males (n=4 per group INT, GDX, GDX+TP) and females (n=6 per group, estrus, diestrus, OVX, and OVX+TP) were killed as above. Cycling females were killed on the morning of diestrus (low E) or the afternoon of estrus (high E). For counting of the number of AR-containing cells, DAB staining was used. Here, single label DAB-staining, free-floating sections were incubated in 1 % hydrogen peroxide, washed 3 X 10 min in phosphate buffered saline (PBS), incubated in normal goat serum for 1hr, and placed in anti-AR made in rabbit primary antibody (Santa Cruz; 1:5000) for 48 hrs. Sections were then washed 3 X 10 min in PB with 1 % Triton-X100 (PBT), incubated in biotinylated goat anti-rabbit secondary (Vector Labs, Burlingame, CA; 1: 250) for 1hr, and washed. Sections were then treated with avidin-biotin complex (ABC, Vector Labs) for 1hr, and washed. Staining was visualized with nickel chloride enhanced diaminobenzidine (DAB; Sigma Aldrich, St. Louis, MO). Sections were mounted on gel-coated glass slides, dehydrated in a graded series of alcohols (as above) and coverslipped with Permount (Fisher Scientific, Hampton, NH).
In the present work, the shell is delineated by AVP, and the core is the unstained area within the boundaries created by this marker. To compare the localization of AR in the sexes, the SCN of males (n=3) and females (n=3), double-labeled for arginine vasopressin (AVP) and AR, was used to delineate core and shell regions of the SCN. Brains were removed, and sliced on a cryostat. Free-floating sections were incubated in normal donkey serum for 1hr, and then for 48h in anti-vasopressin (AVP) made in guinea pig (Peninsula Labs/Bachem, Belmont, CA; 1:5000) and anti-AR (as above). Following the primary incubation, sections were washed 3 X 10 min with PBT, and then placed into a donkey secondary conjugated to CY2 (anti-guinea pig for) or CY3 (anti-rabbit for AR) fluorophores (1:200, Jackson ImmunoResearch, West Grove, PA) for 1hr. Sections were washed in PBS, mounted onto gel-coated slides, and dehydrated in a graded series of alcohols (50–100%). Coverslips were then applied with Krystalon (EM Science, Gibbstown, NJ), and slides allowed to dry overnight.
Quantification
Cell counts of DAB stained tissue were done to evaluate AR expression in the SCN. For each animal, two sections, representing the mid-caudal aspect of the SCN were analyzed bilaterally. An investigator blind to the experimental condition of the animal placed the SCN within a 200 × 200μm grid on an Olympus-BH2 microscope. All cells within this grid were counted to ensure that uniform measures were made across animals, and that the entire SCN was included. Each sector of the grid was analyzed by moving through the Z-plane and counting labeled nuclei. Results of the four counts for each animal were then averaged to determine the number of AR staining within the SCN of that individual. Though some cells outside of the SCN may have been included in the counts by this procedure, their numbers are diminishingly small, and could not have affected the results.
Statistical analysis
Results are reported as mean ± SEM. Western blots were analyzed using t-tests. Behavioral comparisons were made by two-way ANOVA, followed by Bonferroni post-tests. Differences in AR among hormone treatment groups were analyzed using one-way ANOVA, followed by Tukey HSD tests and were considered statistically significant at the p<0.05 level.
Results
Gonadectomy and Androgen Replacement alter Circadian Behavior in a Sex Dependent Manner
The effects of gonadectomy and androgen replacement on circadian patterns of locomotor activity in female and male mice are shown for individual animals in Figure 1, with the quantification given in Figure 2. In males, GDX alters the period, precision, activity duration, and total amount of activity. Compared with INT males, period is lengthened (p<0.05), precision is greatly reduced (p<0.001), activity duration is reduced (p<0.001), and total amount daily of activity is lower (p<0.001). Treatment with TP restores all of these responses to normal, while treatment with DHT restores all but total amount of daily activity (p>0.05). However, DHT treatment does increase total daily activity above levels observed in GDX males (p<0.01).
Figure 1.
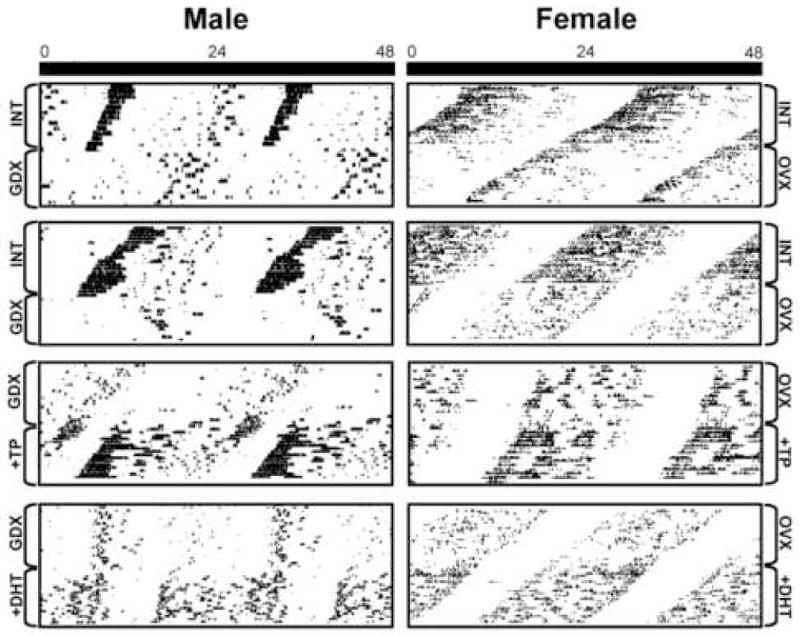
Actograms show free running locomotor activity of male (left) and female (right) mice housed in constant darkness. The horizontal axis is 48 hours and the vertical axis is consecutive days. Each box shows an individual animal before and after an experimental manipulation. The top two panels show intact, then GDX/OVX male and female mice. The bottom two panels show individual GDX/OVX animals that are then treated with either TP and DHT, respectively.
Figure 2.
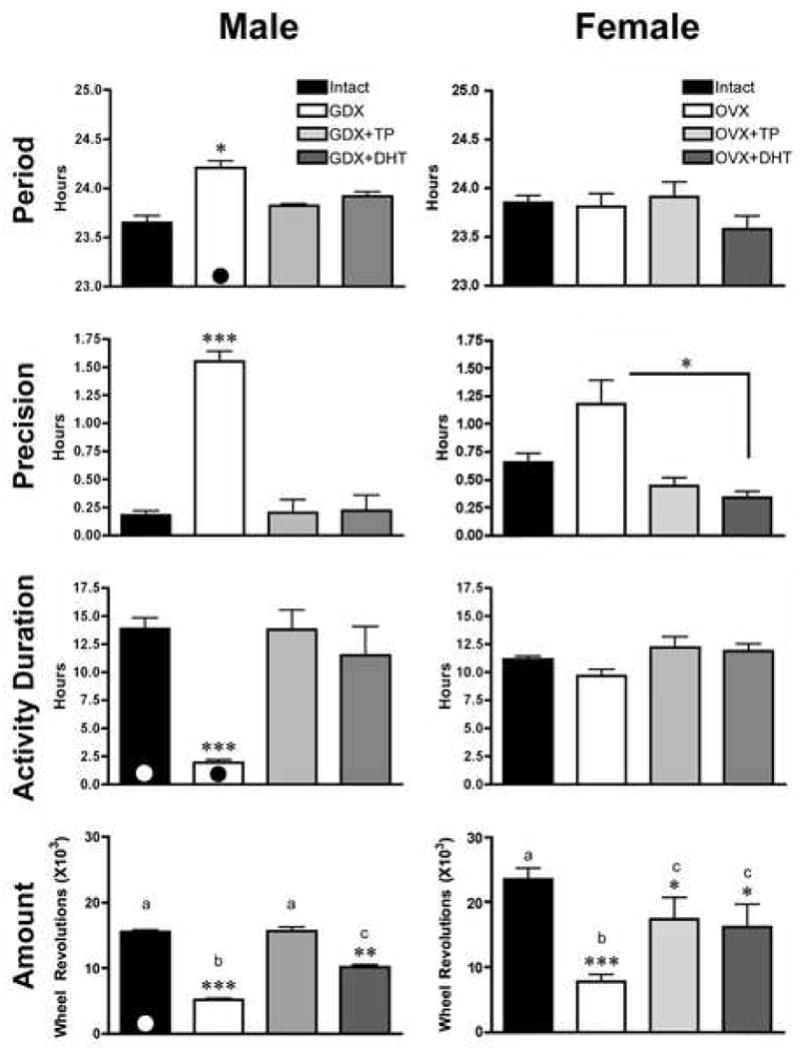
The bar graphs show quantification of the effects of GDX/OVX and hormone replacement in male (left column) and female (right column) mice, as well as sex differences on several behavioral measures. Each of the measures (Period, Precision, Activity Duration and Amount of activity) are presented for each sex. For Amount of activity, histograms sharing common letters are not statistically different from each other. The presence of a black or white circle at the bottom of the bar indicates a measure in which the sexes differ significantly (p<0.05) from each other. *p<0.05, **p<0.01, ***p<0.001.
In females, there is no effect of OVX on period or activity duration. However, there is a significant reduction in both precision and total amount of daily activity, compared to INT females (p<0.05 and p<0.001, respectively). Precision is restored to that of intact animals with either TP or DHT treatment. Amount of daily activity is increased following treatment with either TP or DHT over that of OVX animals (p<0.05), though neither hormone treatment restores activity levels to that of INT animals (p>0.05).
Comparison of males and females reveals sex differences in the effects of GDX and androgen replacement (Figure 3A–D). Amount of daily activity is higher in females than in males (p<0.01). However, this sex difference disappears following gonadectomy (p>0.05). TP or DHT treatment of castrates increases overall activity levels in both sexes, and a sex difference is no longer observed (p>0.05). Activity duration is also different in the sexes, with intact males having longer activity duration than females (p<0.05). However, upon GDX, activity duration is much shorter in males than in females (p<0.001). As for activity, replacement with either TP or DHT eliminates the sex differences in activity duration observed in INT animals. Precision tends to be lower in females than in males (p=0.06), and this sex difference is eliminated following GDX, or GDX and androgen replacement. Period of free-running activity did not differ between INT males and females. However, in males the period lengthened following GDX, while no effect was seen in females (p<0.05). There were no sex differences after GDX animals were treated with TP or DHT.
Figure 3.
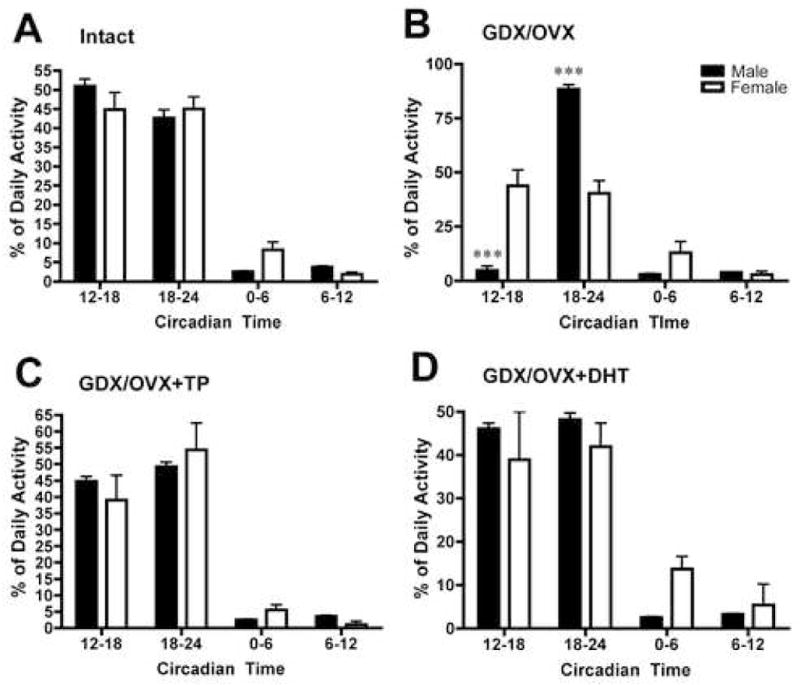
Analysis of % of Daily Activity levels in each of four epochs, each lasting six hours, in male and female (A) intact, (B) GDX, (C) GDX+TP, (D) GDX+DHT mice. The offset of activity was taken as circadian time 24 (see methods for further explanation). ***p<0.001
Daily Activity is Re-organized following GDX in Males but not Females
Comparisons of the temporal organization of locomotor activity in free-running animals in each of 6 hr time epochs in INT, GDX and hormone replaced animals is shown in Figure 3. INT females and males show similar partitioning of daily behavior (Figure 3A). However, following GDX, male behavior was dramatically re-organized, with almost all of the activity restricted to the late night (Figure 3A vs. 3B). No such effect was seen in females. Following GDX/OVX, males and females differ significantly from each other (males vs. female, p<0.001). TP or DHT restored pre-castration temporal organization in males (Figure 3B vs. 3C), with no sex differences seen in animals given similar hormone treatment (Figure 3C and 3D, p>0.05).
Sex Differences in SCN AR
Western blots of SCN indicate a two-fold sex difference in total amount of AR protein in the SCN of INT males and females (Figure 4; p<0.001), with higher levels observed in males.
Figure 4.
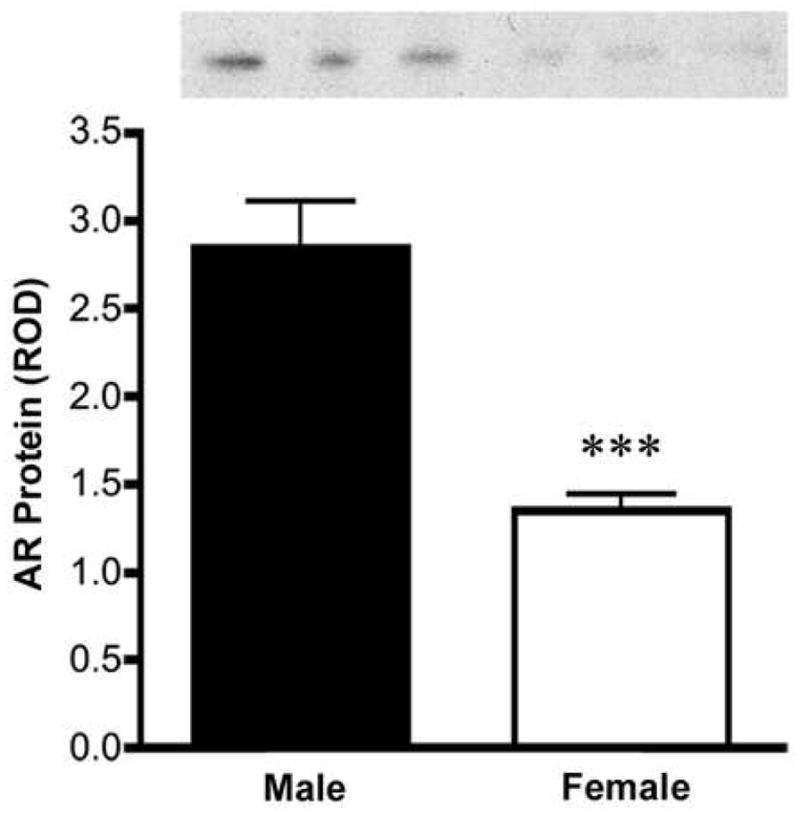
Western blot analysis of AR protein levels in male and female SCN punches indicate that males have about two-fold more AR protein in the SCN than do females, ***p<0.001.
Expression and Hormonal regulation of SCN AR
Using double label immunofluorescence for AVP (a marker of the SCN shell) and AR, we examined the pattern of SCN AR expression in males and females (Figure 5A). In both sexes, AR-ir is localized to the core region of the SCN, though the female SCN has fewer AR-containing cells than does the male. Single label immunochemistry (Figure 5B) indicates that GDX reduces AR expression in males, while OVX does not affect levels of AR in females. However, treatment with TP increases AR staining in both sexes.
Figure 5.
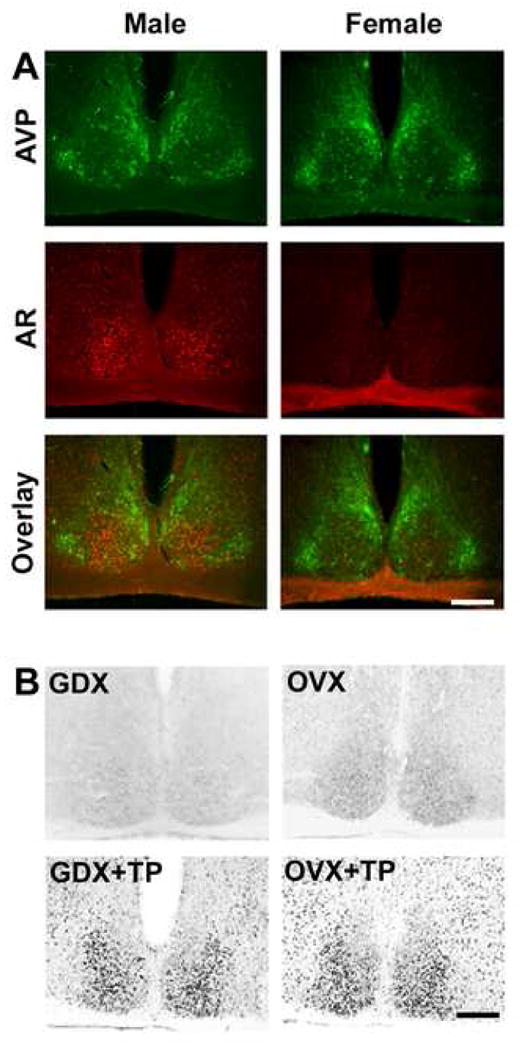
(A) Photomicrographs show fluorescent double-labeled AR- (red) and AVP-ir (green) in SCN of intact male and female mice. Note that AR-ir is localized to the core SCN region lacking AVP-ir. Upper panel shows AVP, middle panel shows AR, and lower panel shows overlay. (B) Photomicrographs show AR-ir in GDX/OVX (upper panel) and GDX/OVX+TP (lower panel) treated male and female mice.
Given the effects of GDX and androgen treatment on behavior, we explored whether AR was modulated by hormones in males and cycling females. Figure 6A shows quantification of AR-ir in gonadally intact animals, and demonstrates that the number of AR cells is higher in intact males than estrus (E) or diestrus (D) females (p<0.001), while the latter did not differ from each other (p>0.05). Upon replacement of GDX males and OVX females with TP, SCN AR staining increases, though TP treated males show higher levels of AR staining than TP treated females (p<0.001).
Figure 6.
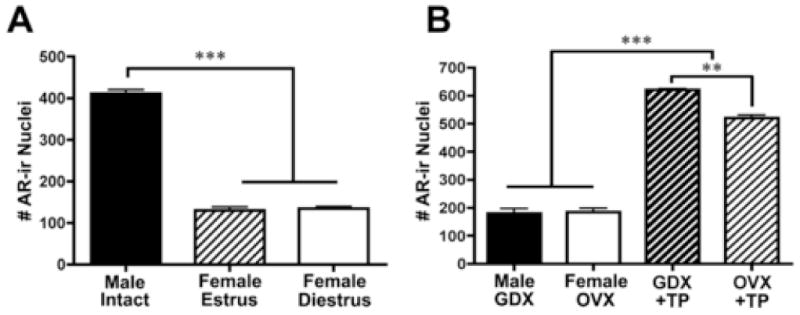
Histograms show number of AR-ir cells (A) in intact males and estrus and diestrus females and (B) GDX male and OVX female and TP-treated mice. **p<0.01, ***p<0.001.
Discussion
A major finding described here is the sex differences in AR density in the core SCN region. This is noteworthy as cells of the core region of the SCN differ from the rest of the nucleus in their morphology, afferent and efferent connections, pattern of rhythmic and stimulus-induced gene expression, and peptidergic phenotypes (reviewed in: Antle and Silver, 2005; Van den Pol, 1980; van den Pol and Tsujimoto, 1985). Neurons within separate SCN sub-regions serve distinctly separate functions in the overall organization of the circadian clock. Upon exposure to a light pulse, an increase in the expression of the immediate early gene FOS, and the circadian clock genes Per1 and Per2 is observed. Directly retinorecipient cells lying in the core SCN rapidly respond to photic stimulation whereas shell cells respond with a longer latency (Hamada, LeSauter, Venuti, and Silver, 2001; Karatsoreos, Yan, LeSauter, and Silver, 2004; Silver, Romero, Besmer, Leak, Nunez, and LeSauter, 1996; Yan and Silver, 2002; Yan and Silver, 2004). The coordinated interaction of these functionally distinct cells is integral to the coherent functioning of the brain clock (Yan, Karatsoreos, LeSauter, Welsh, Kay, Foley, and Silver, In Press). One view of integration of function between these SCN regions is the “gate-oscillator model (Antle, Foley, Foley, and Silver, 2003; Antle, Foley, Foley, and Silver, 2007). Here, we hypothesize that cells in the core area act as a “gate”, sometimes open and sometimes closed. The biological basis for this suggestion derives from the fact that light phase shifts circadian rhythms during the night or subjective night, but not during the day or subjective day. When the signal from activated gate cells reaches shell oscillators, it acts to limit the phase dispersion of the SCN’s independent cell-based oscillators. Given that the core SCN region receives neural afferent inputs, including those from the RHT and the IGL (Abrahamson and Moore, 2001; Moore, 1996; Moore and Silver, 1998), the present demonstration of sex differences in density of AR localized to the core SCN, points to a convergence of endocrine and neural input to this SCN compartment. This sets the stage for the male-female differences in integration of environmental and internal cues modulating circadian rhythmicity.
The present results provide several insights into the neural mechanisms that mediate sex differences in the circadian system. First, AR expression is highly localized to the core ventrolateral region of the SCN in both males and females, with significantly greater expression in males. These results were confirmed using two different methods, namely Western blots and immunocytochemical analyses. The sex differences seen in intact animals disappear following gonadectomy, and treatment of gonadectomized mice with TP eliminates the sex differences. Surprisingly, in males the response to the same dose of hormone produces a greater AR response than in females, indicating a sex difference in the adult response to hormones.
In contrast to the present finding, previous work reported sparse AR expression in the brain clock in several species (Kashon, Arbogast, and Sisk, 1996; Rees and Michael, 1982; Wu, Nathanielsz, and McDonald, 1995; Zhou, Blaustein, and De Vries, 1994), including humans (Fernandez-Guasti, Kruijver, Fodor, and Swaab, 2000; Kruijver and Swaab, 2002). It is possible that the pattern of dense and localized AR expression in the core is unique to the mouse. Alternatively, it may reflect the fact that many prior studies were done before the heterogeneity of the SCN was appreciated, and this core region was missed, as it lies in a fairly caudal position within the SCN of mouse and hamster (Hamada et al., 2001; Karatsoreos et al., 2004; Silver et al., 1996).
Consistent with the present results, the modulatory effects of gonadal hormones on circadian behavior and physiology have been reported in a number of studies, though most have focused on estrogenic effects (Albers, 1981; Gerall, Napoli, and Cooper, 1973; Turek, Losee-Olson, Swann, Horwath, Van Cauter, and Milette, 1987). In constant conditions, the period of activity is shorter in ovariectomized hamsters and rats after treatment with estrogen (Albers, 1981; Morin, 1980; Zucker et al., 1980). In animals housed in LD cycles, an interesting “scalloping” effect is seen, with shorter latency onset of activity in estrus hamsters and rats (Albers, 1981; Morin et al., 1977). The present study was done in DD, thus we cannot evaluate scalloping in mice. That said, it seems unlikely that scalloping is the explanation for sex differences in precision, as the male female sex difference in precision (~29 minutes) is much greater than the hamster hormone-mediated scalloping effect (~7 minutes).
The precise effects of steroids on the SCN are not well known. T can be reduced to 5alpha-DHT, an androgen with higher biological activity at brain androgen receptors than testosterone, or aromatized to estrogens (Martini, 1982). 5alpha-reduced androgens cannot be metabolized to estrogens but may be metabolized into other androgens, such as 3″-androstanediol. The SCN appears to be a minor target for estrogen, as few estrogen receptors (ERs) have been demonstrated in this nucleus (Mitra et al., 2003; Shughrue et al., 1997; Shughrue and Merchenthaler, 2001), though the SCN projects to regions containing ERs, and ER-containing cells project to the SCN (de la Iglesia et al., 1995; de la Iglesia et al., 1999). Furthermore, the estrogen synthesizing enzyme, aromatase, is not localized to the adult SCN, but rather to the retino-hypothalamic tract (Horvath and Wikler, 1999). Given our results indicating that DHT paralleled the effect of T, we conclude that aromatization of estrogen is not a necessary mediating mechanism of gonadal hormone effects on period, precision, and activity duration reported in this paper. It is well established that period and precision are properties of the circadian clock itself, rather than extra-SCN effects (Pittendrigh and Daan, 1976).
The possible function of a sex differences in AR in the SCN is a topic on which we can only speculate. It is believed that direct SCN projections to neuroendocrine cells are associated with the generation of steroid-induced LH surges. SCN efferents contact GnRH neurons (Van der Beek, Horvath, Wiegant, Van den Hurk, and Buijs, 1997), and such contacts increase around the time of puberty (Kriegsfeld, Silver, Gore, and Crews, 2002). Given that SCN lesions disrupt these LH surges (reviewed in: de la Iglesia and Schwartz, 2006; Kriegsfeld and Silver, 2006), one possibility is that the sex differences in SCN AR reflect male-female differences in GnRH regulation.
Another interesting possibility is that androgen action on circadian rhythms, perhaps through SCN AR’s, enable male and female mice to inhabit overlapping temporal niches in their daily activity. Thus, as shown here, in the absence of androgens males and females have very different temporal patterns of activity, as gonadectomized males have severely reduced activity at the start of the night (Figure 1,2). This could reduce the possibility of their meeting in the wild. In contrast, intact, and androgen-replaced males run at the start of the night, as do females. This temporal pattern of activity has been well characterized in a microanalysis of running behavior (Eikelboom and Mills, 1988). Thus, the androgenic hormones acting on SCN AR receptors may allow males and females to display similar temporal behavior patterns. It has been suggested that there are instances where in the absence of hormones, the sexes would differ in some manner that are not advantageous (De Vries and Boyle, 1998). In such a case they suggest that hormones may serve to make the sexes more similar. This is a clever idea, and the present study provides one example of such an instance.
Acknowledgments
The authors would like to thank Miss Alice Wang for assistance in data collection and analyses, and would also like to thank Mr. David Vernon and Miss Catherine Insel for assistance in the preparation of the manuscript and figures.
This work was supported by a Japan Society for the Promotion of Science fellowship to E.I., a Natural Sciences and Engineering Research Council of Canada Pre-doctoral Fellowship to I.K., and a grant from the National Institutes of Health (#37919) to R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916(1–2):172–91. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241(1):R62–6. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- Antle MC, Foley DK, Foley NC, Silver R. Gates and oscillators: a network model of the brain clock. J Biol Rhythms. 2003;18(4):339–50. doi: 10.1177/0748730403253840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Foley NC, Foley DK, Silver R. Gates and oscillators II: a network model of the brain clock. J Biol Rhythms. 2007;22(1):14–25. doi: 10.1177/0748730406296319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28(3):145–51. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus) Proc Natl Acad Sci U S A. 1975;72(9):3744–7. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport. 1995;6(13):1715–22. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- de La Iglesia HO, Blaustein JD, Bittman EL. Oestrogen receptor-alpha-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol. 1999;11(7):481–90. doi: 10.1046/j.1365-2826.1999.00341.x. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147(3):1148–53. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92(2):205–13. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43(5):625–30. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425(3):422–35. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Gerall AA, Napoli AM, Cooper UC. Daily and hourly estrous running in intact, spayed and estrone implanted rats. Physiol Behav. 1973;10(2):225–9. doi: 10.1016/0031-9384(73)90302-8. [DOI] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21(19):7742–50. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Wikler KC. Aromatase in developing sensory systems of the rat brain. J Neuroendocrinol. 1999;11(2):77–84. doi: 10.1046/j.1365-2826.1999.00285.x. [DOI] [PubMed] [Google Scholar]
- Jechura TJ, Walsh JM, Lee TM. Testicular hormones modulate circadian rhythms of the diurnal rodent, Octodon degus. Horm Behav. 2000;38(4):243–9. doi: 10.1006/hbeh.2000.1624. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Wang A, Sasanian J, Silver R. A Role for Androgens in Regulating Circadian Behavior and the Suprachiasmatic Nucleus. Endocrinology. 2007 doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, Silver R. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J Neurosci. 2004;24(1):68–75. doi: 10.1523/JNEUROSCI.1666-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashon ML, Arbogast JA, Sisk CL. Distribution and hormonal regulation of androgen receptor immunoreactivity in the forebrain of the male European ferret. J Comp Neurol. 1996;376(4):567–86. doi: 10.1002/(SICI)1096-9861(19961223)376:4<567::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. Oxford University Press; New York, NY: 1991. [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468(3):361–79. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49(5):557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R, Gore AC, Crews D. Vasoactive intestinal polypeptide contacts on gonadotropin-releasing hormone neurones increase following puberty in female rats. J Neuroendocrinol. 2002;14(9):685–90. doi: 10.1046/j.1365-2826.2002.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75(5):296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7(6):1626–38. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology. 1998;139(10):4092–101. doi: 10.1210/endo.139.10.6253. [DOI] [PubMed] [Google Scholar]
- Martini L. The 5alpha-reduction of testosterone in the neuroendocrine structures. Biochemical and physiological implications. Endocr Rev. 1982;3(1):1–25. doi: 10.1210/edrv-3-1-1. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389(3):508–34. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Moore RY. Entrainment pathways and the functional organization of the circadian system. Prog Brain Res. 1996;111:103–19. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int. 1998;15(5):475–87. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- Morin LP. Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav. 1980;24(4):741–9. doi: 10.1016/0031-9384(80)90406-0. [DOI] [PubMed] [Google Scholar]
- Morin LP. A concept of physiological time: rhythms in behavior and reproductive physiology. Ann N Y Acad Sci. 1986;474:331–51. doi: 10.1111/j.1749-6632.1986.tb28023.x. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Rev. 2006;51 (1):1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196(4287):305–7. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. The Stability and Lability of Spontaneous Frequency. Journal of Comparative Physiology. 1976;106:223–252. [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Rees HD, Michael RP. Brain cells of the male rhesus monkey accumulate 3H-testosterone or its metabolites. J Comp Neurol. 1982;206(3):273–7. doi: 10.1002/cne.902060307. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport. 1996;7(6):1224–8. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- Turek FW, Losee-Olson S, Swann JM, Horwath K, Van Cauter E, Milette JJ. Circadian and seasonal control of neuroendocrine-gonadal activity. J Steroid Biochem. 1987;27(1–3):573–9. doi: 10.1016/0022-4731(87)90356-6. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15(4):1049–86. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384(4):569–79. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF. Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine. 1999;11(2):137–43. doi: 10.1385/ENDO:11:2:137. [DOI] [PubMed] [Google Scholar]
- Wu SS, Nathanielsz PW, McDonald TJ. Immunocytochemical distribution of androgen receptors in the hypothalamus and pituitary of the fetal baboon in late gestation. Brain Res Dev Brain Res. 1995;84(2):278–81. doi: 10.1016/0165-3806(94)00184-2. [DOI] [PubMed] [Google Scholar]
- Yan L, Karatsoreos IN, LeSauter J, Welsh DK, Kay S, Foley DK, Silver R. Exploring Spatiotemporal Organization of SCN Circuits. Symposium on Quantitative Biology. 72 doi: 10.1101/sqb.2007.72.037. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci. 2002;16(8):1531–40. doi: 10.1046/j.1460-9568.2002.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R. Resetting the brain clock: time course and localization of mPER1 and mPER2 protein expression in suprachiasmatic nuclei during phase shifts. Eur J Neurosci. 2004;19(4):1105–9. doi: 10.1111/j.1460-9568.2004.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Oishi T. Seasonal change in the locomotor activity rhythm of the medaka, Oryzias latipes. Int J Biometeorol. 1992;36(1):39–44. doi: 10.1007/BF01208733. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Rusak B. Photic sensitivity of geniculate neurons that project to the suprachiasmatic nuclei or the contralateral geniculate. Brain Res. 1989;504(1):161–4. doi: 10.1016/0006-8993(89)91617-x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Blaustein JD, De Vries GJ. Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology. 1994;134(6):2622–7. doi: 10.1210/endo.134.6.8194487. [DOI] [PubMed] [Google Scholar]
- Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of the circadian system in the golden hamster. Am J Physiol. 1980;238(1):R97–101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]


