Abstract
In seasonally breeding, photoperiodic birds, the development of photorefractoriness is associated with decreased brain expression of gonadotropin-releasing hormone-like immunoreactivity (GnRH-li ir) and increased expression of vasoactive intestinal polypeptide-like immunoreactivity (VIP-li ir). Dissipation of photorefractoriness and reestablishment of photosensitivity are associated with increased GnRH-li ir brain production, but concurrent changes in VIP-li ir expression have not been investigated. To address this question, we compared the expression of VIP-li ir in the infundibulum (INF) of adult male dark-eyed juncos (Junco hyemalis) that were made photorefractory (PR) by prolonged exposure to long days with that of birds that were not photostimulated (PS), but had regained photosensitivity by exposure to short days for 5 (short-term-PS, ST-PS) or 13 (long-term-PS, LT-PS) consecutive months. Photosensitive males had smaller INF VIP-li ir cell bodies than PR males, but the numbers of INF VIP-li ir cells were independent of photoperiodic condition. Changes in infundibular VIP-li ir were correlated with changes in preoptic area (POA) GnRH-li expression. Specifically, photosensitive males had more and larger POA GnRH-li ir cells and more GnRH-li ir fibers in this region than PR males. Further, LT-PS males had more GnRH-li ir POA fibers and larger testes than ST-PS juncos. Thus, induction of photorefractoriness is associated with increased VIP and decreased GnRH brain expression whereas dissipation of photorefractoriness concurs with decreased VIP and increased GnRH brain expression. These results suggest a physiological role for VIP in the control of changes in GnRH expression as a function of the photosensitive condition.
Keywords: GnRH, VIP, reproduction, prolactin, seasonality, photoperiodism, photosensitivity, photorefractoriness, immunocytochemistry, preoptic area, infundibulum
Most birds breeding at middle and high latitudes reproduce only during spring and early summer, when environmental conditions are the most favorable to successful raising of young (Lack, 1968; Perrins, 1970). In these species and as originally demonstrated experimentally in dark-eyed juncos (Junco hyemalis; Rowan, 1925), photoperiod plays an essential role in the control of seasonal changes of reproductive system activity. Exposing photosensitive birds to daylength longer than approximately 12 h (defined as long days, LD), as is naturally the case during the spring, stimulates the release of hypothalamic gonadotropin-releasing hormone (GnRH) and pituitary-luteinizing hormone (LH) and follicle-stimulating hormone (FSH; Lewis and Farner, 1973; Wingfield et al., 1980; Dawson and Goldsmith, 1983; Fehrer et al., 1985; Wilson, 1985; Foster et al., 1987; McNaughton et al., 1995; Meddle and Follett, 1997). These hormones in turn induce gonadal development and the secretion of gonadal hormones that control many behavioral changes associated with the breeding period as well as the development of secondary sexual characteristics (Balthazart et al., 1979; Silverin and Viebke, 1994).
In most photoperiodic species, breeding is followed by a photorefractory period. At this time, reproductive activities are curtailed although photoperiod remains well in excess of the vernal threshold for stimulation. The insensitivity to previously stimulating photoperiod that defines photorefractoriness is independent of ocular and pineal inputs (Wilson, 1990, 1991) and is believed to originate centrally (for review, see Nicholls et al., 1988; Juss, 1993). Photorefractoriness is associated with decreased GnRH production and circulating LH levels, rapid gonadal involution, and molt (Wingfield et al., 1980; Foster et al., 1987; Kubokawa et al., 1994; Hahn and Ball, 1995; Parry et al., 1997; Cho et al., 1998). In European starlings (Sturnus vulgaris), the onset of photorefractoriness is accompanied with decreased hypothalamic expression of the GnRH precursor peptide, proGnRH-GAP (Parry et al., 1997). This decrease, which precedes a change in hypothalamic GnRH immunostaining, indicates that photorefractoriness results from a reduction in GnRH synthesis rather than release of this peptide at the median eminence level. In most species, photorefractoriness can be alleviated, i.e., photosensitivity can be restored, only by exposure to short days (SD) as would naturally occur during the fall and winter (Nicholls et al., 1988; Wilson, 1992). Restoration of photosensitivity in adults (Dawson et al., 1986; Dawson and Goldsmith, 1997), as well as acquisition of photosensitivity in juvenile birds (Goldsmith et al., 1989), is associated with an increased production of hypothalamic GnRH.
Photoinduced changes in the activity of the hypothalamo–pituitary–gonadal axis are accompanied by alterations of the secretion of the anterior pituitary hormone, prolactin. As is the case for LH, the secretion of prolactin is photoinduced (Dawson and Goldsmith, 1983; Mauro et al., 1992; Silverin and Goldsmith, 1997; Sreekumar and Sharp, 1998; for review, see Sharp et al., 1998). Photoinduced high circulating prolactin concentrations are reached during the development of photorefractoriness, when gonads are regressing (Dawson and Goldsmith, 1983; Dawson, 1997; Dawson and Sharp, 1998). Prolactin is not thought to control the transition from photosensitivity to photorefractoriness (Nicholls et al., 1988; Juss, 1993; Dawson and Sharp, 1998), but it participates in the rapid collapse of the reproductive system that characterizes photorefractoriness by inhibiting the hypothalamo–pituitary–gonadal axis at multiple levels (Buntin and Tesch, 1985; Janik and Buntin, 1985; Sharps et al., 1998).
Pituitary prolactin synthesis and release in birds are regulated primarily by vasoactive intestinal polypeptide (VIP; Cloues et al., 1990; Mauro et al., 1992; El Halawani et al., 1995; Youngren et al., 1994). A role for VIP in the reproductive system regression that occurs at the onset of photorefractoriness is suggested by the fact that male European starlings that have been actively immunized against VIP exhibit slower photoinduced regression than control males and do not molt (Dawson and Sharp, 1998). In addition, brain expression of GnRH and VIP change in opposite directions when birds become photorefractory. Specifically, preoptic area (POA) GnRH and infundibular VIP are lower and higher, respectively, in photorefractory than in photosensitive male dark-eyed juncos (Saldanha et al., 1994).
The present investigation determined whether an inverse relationship between brain GnRH and VIP expressions also exists as birds regain photosensitivity. We hypothesized that initially photorefractory male juncos exposed to SD would regain photosensitivity and restoration of photosensitivity would concur with increased brain GnRH expression and with decreased VIP expression. Our results support the idea that VIP participates in the neuroendocrine changes that take place when birds become photorefractory as well as when photorefractoriness is dissipated.
MATERIALS AND METHODS
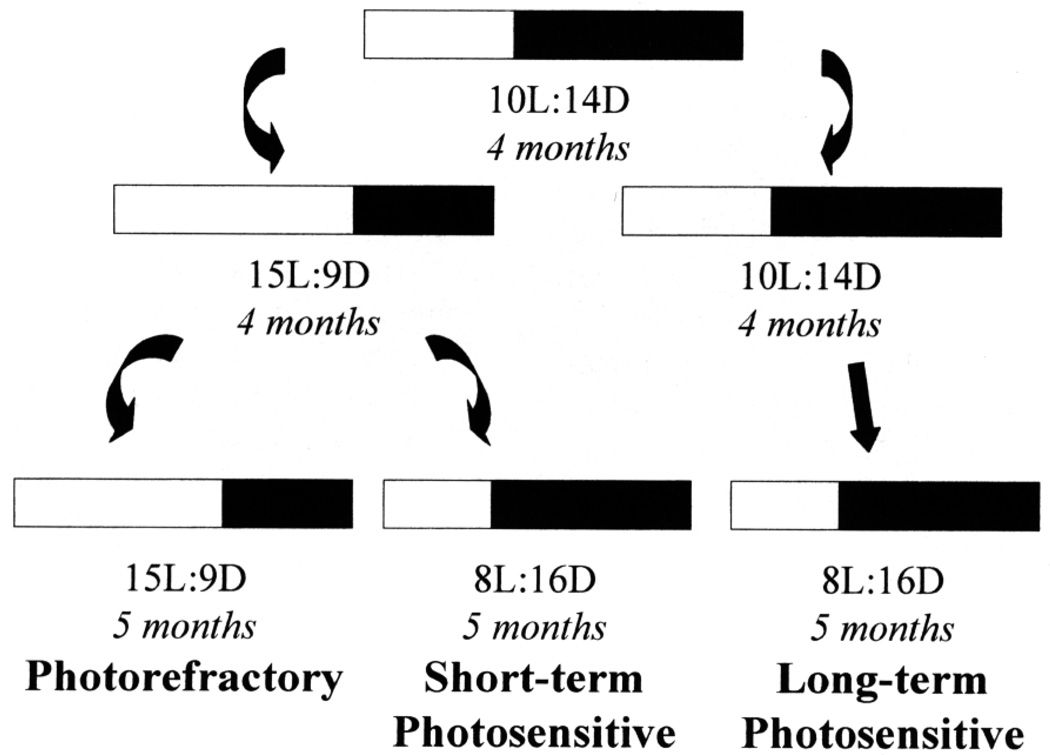
Hatching-year male dark-eyed juncos were caught from the local population in Fairbanks, Alaska (65° N, 148° W) in the fall of 1993 and transferred to the laboratory where they were housed in individual cages and exposed to SD (10 L:14 D) until January 14, 1994. At this time, birds were randomly divided into four groups. Three groups were transferred to one of the following light regimes until sacrificed on October 20, 1994: short-term photosensitive group (ST-PS; n = 5): LD (15 L:9 D) until May 6, 1994, then SD (8 L:16 D); long-term photosensitive group (LT-PS; n = 5): SD (10 L:14 D) until May 6, 1994, then SD (8 L:16 D); and photorefractory group (PR; n = 5): LD (15 L:9 D).
To confirm that the ST-PS group was photosensitive when sacrificed, a fourth group (photostimulated group; CONT-PS; n = 7) was treated as the ST-PS group until October 20, 1994. On this date (D0), CONT-PS males were transferred to LD (20 L:4 D) for 22 days and then sacrificed. One day before this transfer (D – 1) as well as approximately every 4 days thereafter, we measured the cloacal protuberance (CP, an androgen-sensitive secondary sexual characteristic: Schwabl and Farner, 1989; Deviche, 1995) width of these males to the nearest 0.1 mm with calipers. In free-living male juncos, seasonal changes in CP size correlate with changes in testis mass (Deviche et al., 1998) and we, therefore, predicted an increase in CP width following transfer from SD to LD. To determine whether the ST-PS, CONT-PS, and PR groups became photorefractory after exposure to LD starting in January, we examined them weekly for signs of body molt, starting March 3, 1994. Brains from the CONT-PS juncos are not included in the present study. While in captivity, all birds were at room temperature and received ad libitum food (Purina finch chow) and tap water supplemented with soluble vitamin (Avicon, Vet-A-Mix). The experimental design is summarized in Fig. 1.
FIG. 1.
Schematic representation of the experimental design. Male juncos were exposed to short days (10 L:14 D; SD) for 4 months, then either transferred to long days (15 L:9 D; LD) or exposed to SD (10 L:14 D, then 8 L:16 D) for another 9 months (long-term photosensitive, LT-PS). After 4 months of LD exposure, LD males either remained exposed to LD for another 5 months (photorefractory, PR) or were transferred back to SD (8 L:16 D) for the same period (short-term photosensitive, ST-PS).
At the time of sacrifice, all subjects were euthanized with an overdose of anesthetic (ketamine/xylazine solution) and perfused transcardially with 0.3 ml of heparin solution (1000 IU/ml) immediately followed with 25 ml of 0.1 M phosphate buffer (PB) and 30 ml of freshly prepared 4% paraformaldehyde solution in PB. Testes were excised and placed into 0.9% saline solution. The testicular sizes of ST-PS, LT-PS, and PR males were estimated visually without knowledge of the experimental treatment. Testes collected from CONT-PS juncos were weighed to the nearest milligram. The top of the cranium was removed and the heads were postfixed in situ for 24 h at 4°C. Brains were then dissected out and stored at 4°C in 0.1 M PB containing 0.1% sodium azide until further processed. All experimental procedures were approved by The University of Alaska Fairbanks Institutional Animal Care and Use Committee.
Tissue preparation and immunocytochemistry
Brains were individually embedded in a block of gelatin (8% gelatin following a coat of 4% gelatin). Blocks were allowed to harden at 4°C for 2–4 h and then immersed in 4% paraformaldehyde solution for 48 h at 4°C in preparation for sectioning. Coronal sections (50 µm) were cut on a vibratome from the level of the anterior parolfactory lobe to the appearance of the fourth nerve. Alternate sections were processed immunocytochemically with polyclonal antibodies raised against either GnRH (LR-1; gift of R. Benoit) or VIP (Incstar, Stillwater, MN) following a previously published and validated protocol (Saldanha et al., 1994). Studies using avian species other than juncos found that the LR-1 anti-GnRH antibody recognizes brain GnRH-I, but not GnRH-II (Silver et al., 1992; Silverman et al., 1994). Specifically, preoptic area GnRH neurons did not stain following preabsorption with GnRH-I, but were unaffected by preabsorption with GnRH-II. Furthermore, omission of the primary antiserum resulted in the absence of any reaction product.
Sections were serially exposed to 0.05% H2O2, 10% normal goat serum, primary antibody (dilution: 1:40,000 (anti-GnRH) or 1:10,000 (anti-VIP); 48 h at 4°C), 1:250 biotinylated goat anti-rabbit IgG, 1:200 avidin–biotin (Vectastain), and 0.04% diaminobenzidine activated with 0.001% H2O2. All sera were prepared in 0.3% Triton X-100 in 0.1 M PB, and three 15-min washes in 0.1% Triton X-100 in PB were performed between all incubations except immediately prior to incubation in the primary antibody solution. Following processing, sections were mounted onto gelatin-coated microscope slides, dried, dehydrated by immersion into alcohol solutions (70, 95, 95, and 100%), cleared, and coverslipped in preparation for study with a light microscope. To control for variations in processing, each immunocytochemical run included sections from one junco belonging to each experimental group.
Data collection and statistical analysis
Expression of GnRH in the POA was ascertained via the measurement of (a) GnRH soma number, (b) GnRH somal area, and (c) GnRH fiber number. The total number of GnRH-positive soma was counted in five alternate sections through the extent of the POA, from its rostral aspect, where the tractus septomesencephalicus extends to the ventral surface of the brain, and extending caudally to the region where the third ventricle is enlarged (Stokes et al., 1974; Saldanha et al., 1994). Only soma with detectable nuclei were included in the analysis. The somal area of 50–75 neurons (approximately 10–15 per section) was measured using NIH Image Analysis software. The number of immunoreactive fibers in a section in the middle of the POA was quantified using the method of Shivers et al. (1983). Briefly, the section was aligned under high magnification and overlaid with an ocular grid and the number of intersections of immunoreactive fibers with grid segments was used as an estimate of immunoreactive fiber numbers.
VIP expression in the infundibulum (INF) was also evaluated by measurement of the number of immunoreactive soma and somal area, using the methods described for GnRH (vide supra). The number of VIP-positive soma was counted in six to eight alternate sections through the entire INF. All the neuroanatomical data were collected by an investigator who was not aware of the experimental condition of the animals to minimize potential biases in data analysis.
Neuron numbers and sizes and fiber numbers were compared across groups using analysis of variance (ANOVA) with photosensitive condition as the main variable. Post hoc Fisher least significance difference tests were used to determine specific differences between groups. For the neuron size measures, the mean cell area was computed for each bird and individual means were compared across groups. Changes in CP sizes over time in CONT-PS birds were analyzed using one-way ANOVA for repeated measures. CP sizes after transfer of these birds to LD were compared to prestimulation sizes using Dunnett’s method tests. Statistical significance level was in all cases set at α = 0.05.
RESULTS
Verification of photosensitivity and photorefractory condition
LT-PS males showed no sign of body molt at any time. Fifteen of 17 males that were photostimulated starting in January 1994 were molting on March 3, 1994, and the remaining 2 males started to molt within the following few weeks, suggesting that they became photorefractory. Thirteen males had completed molt 1 day before transfer of ST-PS and CONT-PS groups from LD to SD on May 6.
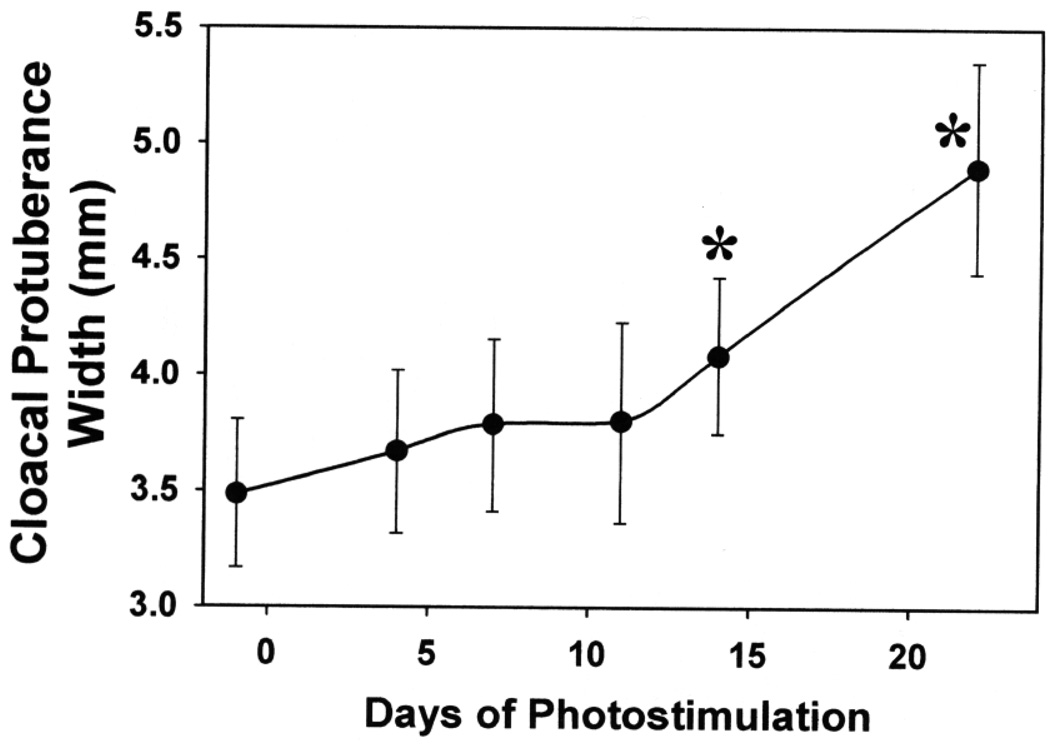
The CP widths of CONT-PS juncos gradually increased after transfer from 8 L:16 D to 20 L:4 D. A statistical difference between pre- and poststimulation sizes was reached after 14 days of exposure to LD (Fig. 2). When sacrificed, these males also had partially developed testes (mean ± sd: 112 ± 75 mg) whereas birds belonging to the three other groups had small testis sizes (median testis length: <0.5 mm; maximum: 2 mm). Thus, ST-PS (and, by extension, also LT-PS) groups were photosensitive when sacrificed. Although testis lengths were small in PR, ST-PS, and LT-PS juncos, these groups differed (Kruskall–Wallis one-way ANOVA on ranks: P < 0.001). All PR and ST-PS males had testis lengths not exceeding 0.5 mm, but LT-PS males had slightly larger testes (median and interquartile interval: 1 mm (1–1.38 mm); Student–Newman–Keuls test comparisons with PR and ST-PS groups: P < 0.05).
FIG. 2.
Changes in cloacal protuberance width (mean ± standard deviation) of adult male dark-eyed juncos (n = 7) transferred from 10 L:14 D to 15 L:9 D on Day 0. *P < 0.05, comparison with prestimulation sizes (Day −1; Dunnett’s method test).
GnRH expression
The junco hypothalamus contains numerous GnRH-expressing neurons and fibers scattered through the anterior–posterior extent of the diencephalon. Most of the neuronal staining is observed close to the midline, but some dispersed perikarya are seen more laterally. GnRH-like immunoreactive (GnRH-li ir) somas were observed in the preoptic area, nucleus of the lateral hypothalamus, paraventricular nucleus of the hypothalamus, periventricular nucleus, and the nucleus of the pallial commissure. GnRH-li ir fibers were observed at all the above loci as well as in the lateral septum (SL), infundibulum, and median eminence (ME). Since previous work on juncos had identified changes in GnRH-li ir in the POA as a function of photoperiodic condition (Saldanha et al., 1994), we quantified immunoproduct only in this area.
GnRH expression across photosensitive states
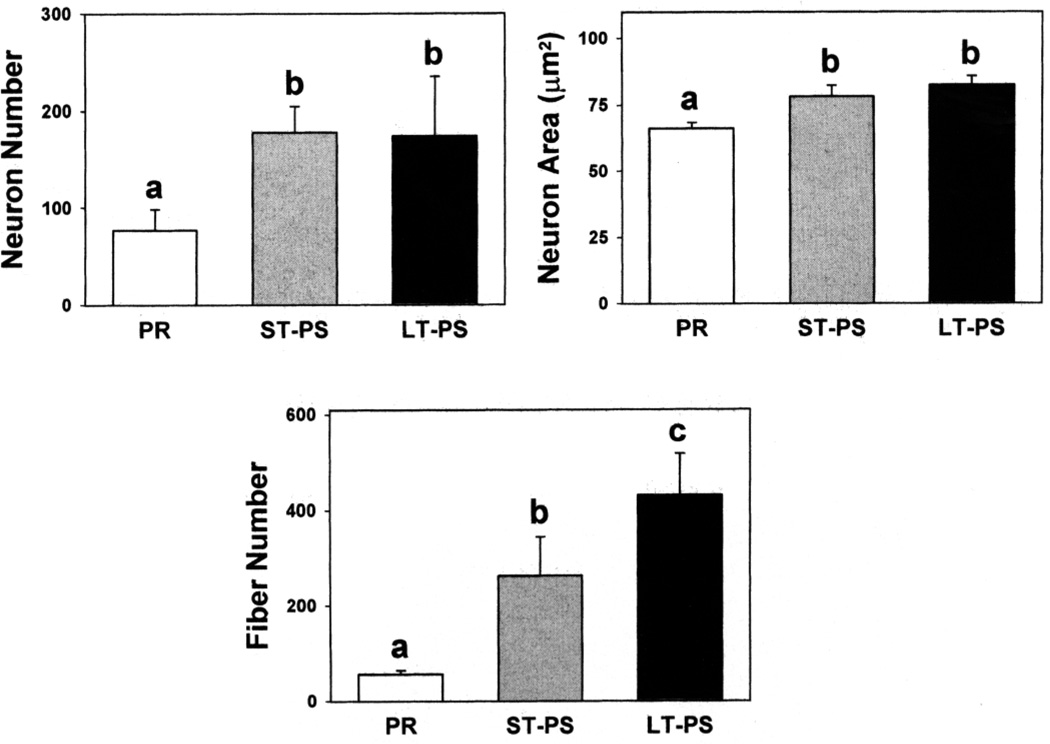
Experimental groups differed with respect to POA GnRH-li ir neuron (ANOVA: F2,14 = 10.06, P = 0.003) and fiber (id: P = 0.0001) numbers as well as neuronal areas (id: P = 0.0001; Fig. 3). PR males had fewer immunostained perikarya and fibers and smaller neuron areas than ST- and LT-PS males (Fig. 4). Although not quantified, the intensity of staining was noticeably lighter in cells and fibers of PR than in those of photosensitive birds. The two photosensitive groups had similar numbers of POA GnRH-li ir neurons and soma sizes. However, LT-PS males had more immunopositive fibers than ST-PS males.
FIG. 3.
Gonadotropin-releasing hormone-like immunoreactive neuron numbers, neuronal soma areas, and fiber numbers (means + standard deviations) in the preoptic area of photorefractory (PR), short-term photosensitive (ST-PS), and long-term photosensitive (LT-PS) adult male dark-eyed juncos (n = 5/group). For a given parameter, columns with a different superscript letter differ statistically (P < 0.05, Fisher least significant difference test).
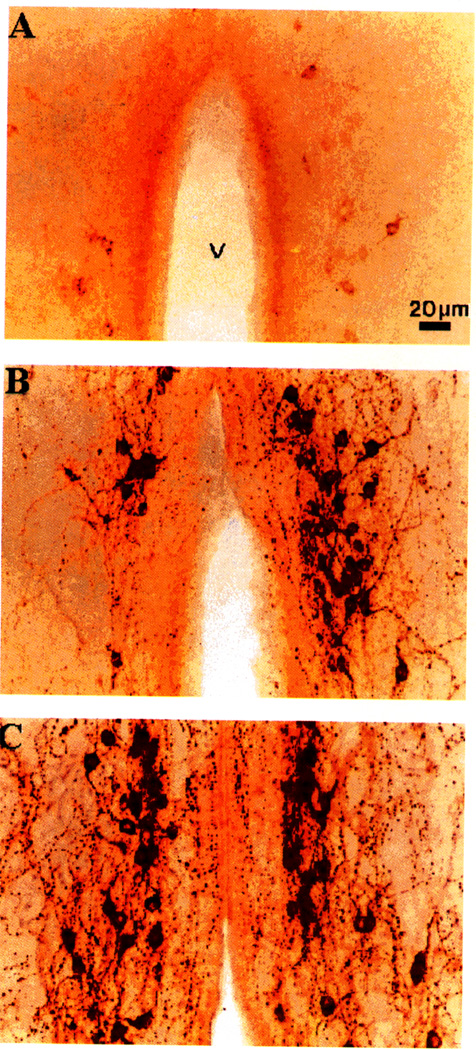
FIG. 4.
Photomicrographs depicting the distribution of gonadotropin-releasing hormone-like immunoreactivity in the preoptic area of photorefractory (A), short-term photosensitive (B), and long-term photosensitive (C) adult male dark-eyed juncos. All photomicrographs are taken at the same magnification. V, third ventricle.
VIP expression
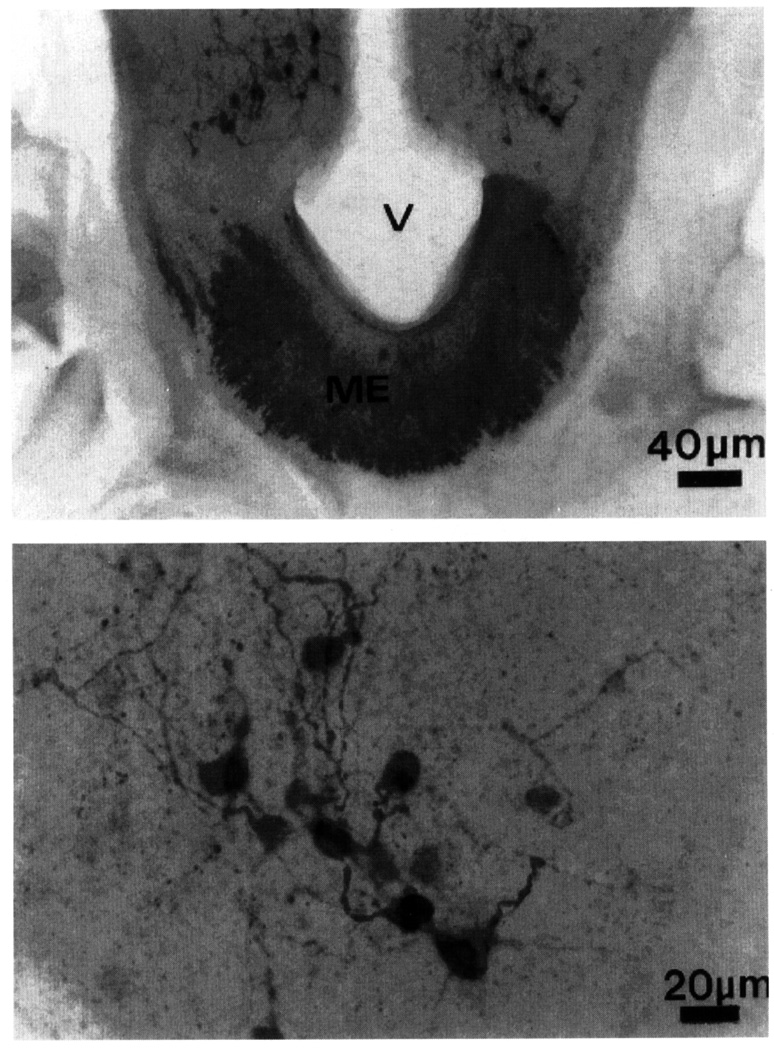
The expression of VIP is surprisingly sparse in the junco diencephalon. Although ir fibers course along the midline toward the ME, VIP-li ir soma are observed only in the infundibulum (Fig. 5). These cells are numerous and clustered around the third ventricle. Another group of VIP-li ir cells is seen more rostrally within the telencephalic SL. Since changes in VIP-li ir have been described across photosensitive states in the infundibular, but not the septal, group of VIP neurons (Saldanha et al., 1994), we measured ir signal only in the infundibulum.
FIG. 5.
Low- (top) and high- (bottom) power magnification photomicrographs showing the distribution of vasoactive intestinal polypeptide-like immunoreactive cell bodies and fibers in the infundibulum and the median eminence (ME) of an adult male dark-eyed junco. V, third ventricle.
VIP expression across photosensitive states
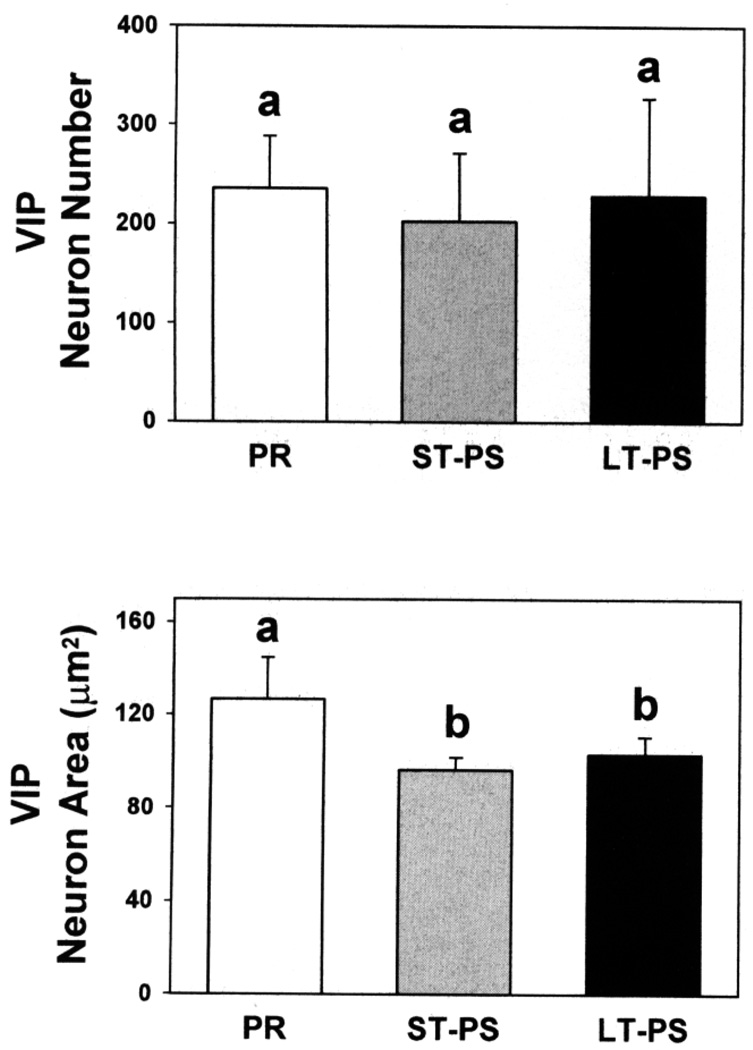
The numbers of INF VIP-li ir cells did not differ among groups (Fig. 6), but there were differences in soma sizes (ANOVA: F2,14 = 28.00, P = 0.0001). PR birds had larger VIP-li ir soma than ST- or LT-PS groups. There was no difference between ST- and LT-PS males (Fig. 6).
FIG. 6.
Vasoactive intestinal polypeptide-like immunoreactive neuron numbers and neuronal soma areas (means + standard deviations) in the infundibulum of photorefractory (PR), short-term photosensitive (ST-PS), and long-term photosensitive (LT-PS) adult male dark-eyed juncos (n = 5/group). For a given parameter, columns with a different superscript letter differ statistically (P < 0.05, Fisher least significant difference test).
DISCUSSION
Previous studies on birds identified marked changes in POA GnRH synthesis (Parry et al., 1997) and content (Dawson et al., 1986; Millam et al., 1995; Dawson and Goldsmith, 1997) as a function of the reproductive condition. Generally, birds that are undergoing gonadal regression or are photorefractory have less hypothalamic GnRH than do photosensitive subjects (Foster et al., 1987; Saldanha et al., 1994; Cho et al., 1998). The present investigation confirms and extends these findings. Photosensitive males were exposed to LD until they became photorefractory, as indicated by body molt (Nicholls et al., 1988; Dawson, 1997). At this point, they were either held on LD to maintain refractoriness or transferred to SD to restore photosensitivity and then sacrificed. Restoration of photosensitive condition in ST-PS males at the time of sacrifice was confirmed by the results obtained for CONT-PS males. As expected, the reproductive system (testis sizes, cloacal protuberance widths) of these males recrudesced within weeks of photostimulation. The results demonstrate that restoration of photosensitivity correlates with an increased number and size of GnRH-containing neurons as well as with increased GnRH-containing nerve fiber numbers in the POA. These changes took place in birds held on SD and, therefore, did not require photostimulation. Similarly, Dawson and Goldsmith (1997) found that POA GnRH concentration increases after transfer of LD-exposed photorefractory European starlings to SD. It is unknown how long photorefractory juncos must be exposed to SD for hypothalamic GnRH expression to increase. It is likely that considerably less than the 5 months used in this study is necessary because GnRH levels in hypothalami of starlings were elevated after transfer from LD to SD for as little as 10 days (Dawson and Goldsmith, 1997).
Photorefractory and photosensitive SD-exposed juncos have an inactive pituitary–gonadal axis (but see below) as assessed by plasma LH concentrations and gonadal sizes (Saldanha et al., 1994). These birds, however, differ in that photosensitive birds have more hypothalamic GnRH than photorefractory birds. Inactivity of the pituitary–gonadal axis in photosensitive, but nonphotostimulated, birds presumably results from a lack of release rather than synthesis of the peptide and dissipation of photorefractoriness in juncos, as in other species (Dawson and Goldsmith, 1997), is therefore probably associated with increased production without concurrent release of GnRH. It should, however, be noted that photosensitive males that were exposed to SD for 5 months had similar POA GnRH-containing neuron numbers and sizes, but fewer GnRH-li ir nerve fibers and smaller testes than males exposed to SD for 13 consecutive months. Thus, prolonged (i.e., more than 5 months) exposure to SD may have been associated with some release of GnRH that induced a modest, but detectable activation of the pituitary–gonadal axis. Other investigations support the hypothesis that changes in hypothalamo–pituitary–gonadal axis activity can occur in SD-exposed birds that are regaining or have regained photosensitivity. For example, pituitary stores of FSH in starlings increase after prolonged exposure to SD, indicating a release of GnRH from the median eminence (Dawson et al., 1986). Further, several species show a subtle, but real pituitary–gonadal axis stimulation when regaining photosensitivity under SD (canary, Serinus canarius, Nicholls and Storey, 1976; rook, Corvus frugilegus, Lincoln et al., 1980; European starling, Dawson, 1983). Finally, dissipation of photorefractoriness is a progressive rather than an all-or-none process (Boulakoud and Goldsmith, 1995; Dawson and Goldsmith, 1997). For example, initially photorefractory Harris’ sparrows (Zonotrichia querula) require 13 weeks of SD exposure to achieve full photosensitivity (Wilson, 1992) as measured by testicular growth rate after transfer to LD. Similarly, in castrated male Japanese quail (Coturnix coturnix japonica), 5 weeks of SD exposure are necessary to fully restore LH response to photostimulation (Follett and Pearce-Kelly, 1990). Although juncos exposed to SD for 5 months were photosensitive, complete dissipation of photorefractoriness in this species may require additional time.
In turkeys (Meleagris gallopavo; Mauro et al., 1992) and dark-eyed juncos (Saldanha et al., 1994) development of photorefractoriness is associated with high hypothalamic VIP expression. The present study extends these findings, demonstrating that reestablishment of photosensitivity is associated with decreased area of infundibular VIP-containing neurons, but no change in numbers of detectable neurons. In contrast, juncos that were refractory as a result of prolonged LD exposure had more VIP-li ir neurons than photosensitive birds that were or were not photostimulated (Saldanha et al., 1994). Further research is necessary before it can be concluded with certainty that the changes in VIP-containing neuron characteristics that occur as a function of the photoperiodic condition represent alterations of the peptide synthesis. Nevertheless, the results suggest that brain GnRH and VIP expressions change in opposite directions when birds become photorefractory as well as when they regain photosensitivity.
VIP is the main hypothalamic neuropeptide controlling prolactin release in birds (Lea et al., 1991; El Halawani et al., 1995). Plasma concentrations of prolactin are low in photosensitive birds kept on SD, increase during photostimulation, and are highest at the onset of photorefractoriness (Goldsmith, 1985; Nicholls et al., 1988; Dawson, 1997). This hormone inhibits the reproductive system (Buntin and Tesch, 1985; Janik and Buntin, 1985; Juss, 1993). Thus, elevated circulating prolactin concentrations when birds become photorefractory and shortly thereafter probably contribute to the rapid involution of reproductive tissues that takes place at this time (Dawson and Sharp, 1998). VIP may, therefore, participate in the inactivation of the hypothalamo–pituitary–gonadal axis that characterizes the onset of photorefractoriness indirectly by stimulating prolactin secretion.
VIP receptors are present in various diencephalic regions of the pigeon (Columba livia) brain including areas rich in GnRH cells and fibers such as the lateral septum, POA, and median eminence (Hof et al., 1991). Further, there is a close association between VIP-expressing terminals and GnRH-expressing dendrites in the lateral septum of this species (Kiyoshi et al., 1998). A similar association between VIP-li ir axons, VIP receptors, and GnRH-li ir dendrites may exist in the junco as suggested by previous studies on this species showing an overlap between VIP- and GnRH-expressing elements in the septum and POA (Saldanha et al., 1994). This association may underlie a mechanism whereby elevated VIP may inhibit GnRH during photorefractoriness, and a decrease in this inhibition may permit hypothalamo–pituitary–gonadal axis activity during the reestablishment of photosensitivity.
Low infundibular VIP content in birds that have regained photosensitivity as a result of SD exposure may also be of physiological significance when these birds are exposed to LD. When transferred from SD to LD, photosensitive birds can respond by a rapid (within 1 day) activation of their hypothalamo–hypophyseal system including GnRH release (Perera and Follett, 1992) and elevated plasma LH levels (Follett et al., 1975; Meddle and Follett, 1997). The rapid transduction of photic information into a neuroendocrine response may be facilitated by the fact that hypothalamic VIP content is then relatively low.
In conclusion, it appears that VIP may have an important role in the regulation of seasonal cycles and may be part of the neuroendocrine processes that mediate transition from one condition of photosensitivity to another. Further research is warranted to identify and localize avian brain VIP receptors in photoperiodic species and to define the mechanism responsible for the anti-gonadotropic activity of this neuropeptide in birds. Additional research is also necessary to evaluate the functional relationships between GnRH and VIP at different stages of the reproductive cycle.
ACKNOWLEDGMENTS
The authors thank Dr. Cynthia Gulledge for assistance and Renee Crain for comments on an early draft of the manuscript. This work was partly supported by National Scientific Foundation Award BNS-9121258 (P.D.) and IBN 9511300 (R.S.).
REFERENCES
- Balthazart J, Massa R, Negri-Cesi P. Photoperiodic control of testosterone metabolism, plasma gonadotrophins, cloacal gland growth, and reproductive behavior in the Japanese Quail. Gen. Comp. Endocrinol. 1979;39:222–235. doi: 10.1016/0016-6480(79)90227-2. [DOI] [PubMed] [Google Scholar]
- Boulakoud MS, Goldsmith AR. The effect of duration of exposure to short days on the gonadal response to long days in male starlings (Sturnus vulgaris) J. Reprod. Fertil. 1995;104:215–217. doi: 10.1530/jrf.0.1040215. [DOI] [PubMed] [Google Scholar]
- Buntin JD, Tesch D. Effects of intracranial prolactin administration on maintenance of incubation readiness, ingestive behavior, and gonadal condition in Ring Doves. Horm. Behav. 1985;19:188–203. doi: 10.1016/0018-506x(85)90018-2. [DOI] [PubMed] [Google Scholar]
- Cho RN, Hahn TP, MacDougall-Shackleton S, Ball GF. Seasonal variation in brain GnRH in free-living breeding and photorefractory house finches (Carpodacus mexicanus) Gen. Camp. Endocrinol. 1998;109:244–250. doi: 10.1006/gcen.1997.7027. [DOI] [PubMed] [Google Scholar]
- Cloues R, Ramos C, Silver R. VIP-like immunoreactivity during reproduction in doves: Influence of experience and number of offspring. Horm. Behav. 1990;24:215–231. doi: 10.1016/0018-506x(90)90006-j. [DOI] [PubMed] [Google Scholar]
- Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen. Comp. Endocrinol. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. Plasma-luteinizing hormone and prolactin during circannual rhythms of gonadal maturation and molt in male and female European starlings. J. Biol. Rhythms. 1997;12:371–377. doi: 10.1177/074873049701200409. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith AR. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. J. Endocrinol. 1983;97:253–260. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith AR. Changes in gonadotrophin-releasing hormone (GnRH-I) in the pre-optic area and median eminence of starlings (Sturnus vulgaris) during the recovery of photosensitivity and during photostimulation. J. Reprod. Fertil. 1997;111:1–6. doi: 10.1530/jrf.0.1110001. [DOI] [PubMed] [Google Scholar]
- Dawson A, Sharp PJ. The role of prolactin in the development of reproductive photorefractoriness and postnuptial molt in the European starling (Sturnus vulgaris) Endocrinology. 1998;139:485–490. doi: 10.1210/endo.139.2.5701. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith AR, Nicholls TJ, Follett BK. Endocrine changes associated with the termination of photorefractoriness by short daylengths and thyroidectomy in starlings (Sturnus vulgaris) J. Endocrinol. 1986;110:73–79. doi: 10.1677/joe.0.1100073. [DOI] [PubMed] [Google Scholar]
- Deviche P. Androgen regulation of avian premigratory hyperphagia and fattening: From ecophysiology to neuroendocrinology. Am. Zool. 1995;35:234–245. [Google Scholar]
- Deviche P, Meddle SL, Wingfield J. Age-related differences in reproductive physiology of adult male dark-eyed juncos (Junco hyemalis) during the breeding period. West, Reg. Meeting on Comp. Endocrinol.; Flagstaff, AZ. 1998. Abstract. [Google Scholar]
- El Halawani ME, Youngren OM, Rozenboim I, Pitts GR, Silsby JL, Phillips RE. Serotonergic stimulation of prolactin secretion is inhibited by vasoactive intestinal peptide immunoneutralization in the turkey. Gen. Comp. Endocrinol. 1995;99:69–74. doi: 10.1006/gcen.1995.1086. [DOI] [PubMed] [Google Scholar]
- Fehrer SC, Silsby JL, Behnke EJ, El Halawani ME. Hypothalamic and serum factors influence on prolactin and luteinizing hormone release by the pituitary gland of the young turkey (Meteagris gallopavo) Gen. Comp. Endocrinol. 1985;59:73–81. doi: 10.1016/0016-6480(85)90420-4. [DOI] [PubMed] [Google Scholar]
- Follett BK, Farner DS, Mattocks PW. Luteinizing hormone in the plasma of white-crowned sparrows (Zonotrichia leucophrys gambelii) during artificial photostimulation. Gen. Comp. Endocrinol. 1975;26:126–134. doi: 10.1016/0016-6480(75)90223-3. [DOI] [PubMed] [Google Scholar]
- Follett BK, Pearce-Kelly A. Photoperiodic control of the termination of reproduction in Japanese quail (Coturnix coturnix japonica) Proc. R. Soc. London. B Biol. Sci. 1990;242:225–230. doi: 10.1098/rspb.1990.0128. [DOI] [PubMed] [Google Scholar]
- Foster RG, Plowman G, Goldsmith AR, Follett BK. Immunohistochemical demonstration of marked changes in the LHRH system of photosensitive and photorefractory European starlings (Sturnus vulgaris) J. Endocrinol. 1987;115:211–220. doi: 10.1677/joe.0.1150211. [DOI] [PubMed] [Google Scholar]
- Goldsmith AR. Prolactin in avian reproduction: Incubation and the control of seasonal breeding. In: MacLeod RM, Scapagnini U, Thorner MO, editors. Prolactin: Basic and Clinical Correlates. Padua, Italy: Springer-Verlag; 1985. pp. 411–425. [Google Scholar]
- Goldsmith AR, Ivings WE, Pearce-Kelly AS, Parry DM, Plowman G, Nicholls TJ, Follett BK. Photoperiodic control of the development of the LHRH neurosecretory system of European starlings (Sturnus vulgaris) during puberty and the onset of photorefractoriness. J. Endocrinol. 1989;122:255–268. doi: 10.1677/joe.0.1220255. [DOI] [PubMed] [Google Scholar]
- Hahn TP, Ball GF. Changes in brain GnRH associated with photorefractoriness in house sparrows (Passer domesticus) Gen. Comp. Endocrinol. 1995;99:349–363. doi: 10.1006/gcen.1995.1119. [DOI] [PubMed] [Google Scholar]
- Hof PR, Dietl MM, Martin J-L, Bouras C, Palacious M, Magistretti PJ. Vasoactive intestinal polypeptide binding sites and fibers in the brain of the pigeon Columbia livia: An autoradiographic and immunohistochemical study. J. Comp. Neurol. 1991;305:393–411. doi: 10.1002/cne.903050304. [DOI] [PubMed] [Google Scholar]
- Janik DS, Buntin JD. Behavioural and physiological effects of prolactin in incubating ring doves. J. Endocrinol. 1985;105:201–209. doi: 10.1677/joe.0.1050201. [DOI] [PubMed] [Google Scholar]
- Juss TS. Neuroendocrine and neural changes associated with the photoperiodic control of reproduction. In: Sharp PJ, editor. Avian Endocrinology. Bristol, UK: Society for Endocrinology; 1993. pp. 47–60. [Google Scholar]
- Kiyoshi K, Kondoh M, Hirunagi K, Korf H. Confocal laser scanning and electron-microscopic analyses of the relationship between VIP-like and GnRH-like immunoreactive neurons in the lateral septal-preoptic area of the pigeon. Cell Tissue Res. 1998;293:39–46. doi: 10.1007/s004410051096. [DOI] [PubMed] [Google Scholar]
- Kubokawa K, Ishii S, Wingfield JC. Effect of day length in luteinizmg hormone (β-subunit mRNA and subsequent gonadal growth in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Gen. Comp. Endocrinol. 1994;95:42–51. doi: 10.1006/gcen.1994.1100. [DOI] [PubMed] [Google Scholar]
- Lack D. Ecological Adaptations for Breeding in Birds. London: Methuen; 1968. [Google Scholar]
- Lea RW, Talbot RT, Sharp PJ. Passive immunization against chicken vasoactive intestinal polypeptide suppresses plasma prolactin and crop sac development in incubating ring doves. Horm. Behav. 1991;25:283–294. doi: 10.1016/0018-506x(91)90002-y. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Farner DS. Temperature modulation of photoperiodically induced vernal phenomena in white-crowned sparrows (Zonotrichia leucophrys) Condor. 1973;75:279–286. [Google Scholar]
- Lincoln GA, Racey PA, Sharp PJ, Klandorf H. Endocrine changes associated with spring and autumn territoriality of the rook, Corvus frugilegus. J. Zool. 1980;190:137–153. [Google Scholar]
- Mauro LJ, Youngren OM, Proudman JA, Phillips RE, El Halawani ME. Effects of reproductive status, ovariectomy, and photoperiod on vasoactive intestinal peptide in the female turkey hypothalamus. Gen. Comp. Endocrinol. 1992;87:481–493. doi: 10.1016/0016-6480(92)90056-p. [DOI] [PubMed] [Google Scholar]
- McNaughton FJ, Dawson A, Goldsmith AR. A comparison of the responses to gonadotrophin-releasing hormone of adult and juvenile, and photosensitive and photorefractory European starlings, Sturnus vulgaris. Gen. Comp. Endocrinol. 1995;97:135–144. doi: 10.1006/gcen.1995.1013. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Follett BK. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J. Neurosci. 1997;17:8909–8918. doi: 10.1523/JNEUROSCI.17-22-08909.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millam JR, Craig-Veit CB, Faris PL. Concentration of chicken gonadotropin-releasing hormones I and II in microdissected areas of turkey hen brain during the reproductive cycle. Domestic Animal Endocrinol. 1995;12:1–11. doi: 10.1016/0739-7240(94)00004-k. [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Storey CR. The effects of castration on plasma LH levels in photosensitive and photorefractory canaries (Serinus canaries) Gen. Comp. Endocrinol. 1976;29:170–174. doi: 10.1016/0016-6480(76)90019-8. [DOI] [PubMed] [Google Scholar]
- Parry DM, Goldsmith AR, Millar RP, Glennie LM. Immunocytochemical localization of GnRH precursor in the hypothalamus of European starlings during sexual maturation and photorefractoriness. J. Neuroendocrinol. 1997;9:235–243. doi: 10.1046/j.1365-2826.1997.00575.x. [DOI] [PubMed] [Google Scholar]
- Perera AD, Follett BK. Photoperiodic induction in vitro: The dynamics of gonadotropin-releasing hormone release from hypothalamic explants of the Japanese quail. Endocrinology. 1992;131:2898–2908. doi: 10.1210/endo.131.6.1446626. [DOI] [PubMed] [Google Scholar]
- Perrins CM. The timing of birds’ breeding seasons. Ibis. 1970;112:242–255. [Google Scholar]
- Rowan W. Relation of light to bird migration and developmental changes. Nature. 1925;115:494–495. [Google Scholar]
- Saldanha CJ, Deviche PJ, Silver R. Increased VIP and decreased GnRH expression in photorefractory dark-eyed juncos (Junco hyemalis) Gen. Comp. Endocrinol. 1994;93:128–136. doi: 10.1006/gcen.1994.1015. [DOI] [PubMed] [Google Scholar]
- Schwabl H, Farner DS. Endocrine and environmental control of vernal migration in male white-crowned sparrows, Zonotrichia leucophrys gambelii. Physiol. Zool. 1989;62:1–10. [Google Scholar]
- Sharp PJ, Dawson A, Lea RW. Control of luteinizing hormone and prolactin secretion in birds. Comp. Biochem. Physiol. C. 1998;119C:275–282. doi: 10.1016/s0742-8413(98)00016-4. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Harlan RE, Morrell JL, Pfaff DW. Immunocytochemical localization of luteinizing hormone-releasing hormone in male and female brains. Quantitative studies on the effect of gonadal steroids. Neuroendocrinology. 1983;36:1–12. doi: 10.1159/000123522. [DOI] [PubMed] [Google Scholar]
- Silver R, Ramos CL, Silverman A-J. Sex behavior triggers appearance of non-neural cells containing gonadotropin-releasing hormone in doves. J. Neuroendocrinol. 1992;4:207–210. doi: 10.1111/j.1365-2826.1992.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Silverin B, Goldsmith A. Natural and photoperiodically induced changes in plasma prolactin levels in male great tits. Gen. Comp. Endocrinol. 1997;105:145–154. doi: 10.1006/gcen.1996.6817. [DOI] [PubMed] [Google Scholar]
- Silverin B, Viebke PA. Low temperatures affect the photoperiodically induced LH and testicular cycles differently in closely related species of tits (Parus spp.) Horm. Behav. 1994;28:199–206. doi: 10.1006/hbeh.1994.1017. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Millar RR, King JA, Zhuang X, Silver R. Mast cells with gonadotropin-releasing hormone-like immunoreactivity in the brain of doves. Proc. Natl. Acad. Sci. USA. 1994;26:3695–3699. doi: 10.1073/pnas.91.9.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar KR, Sharp PJ. Effect of photostimulation on concentrations of plasma prolactin in castrated bantams (Gallus domesticus) J. Neuroendocrinol. 1998;10:147–154. doi: 10.1046/j.1365-2826.1998.00187.x. [DOI] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary Serinus canaria, in stereotaxic coordinates. J. Comp. Neurol. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Wilson FE. Androgen feedback-dependent and -independent control of photoinduced LH secretion in male tree sparrows (Spizella arborea) J. Endocrinol. 1985;105:141–152. doi: 10.1677/joe.0.1050141. [DOI] [PubMed] [Google Scholar]
- Wilson FE. Extraocular control of seasonal reproduction in female tree sparrows (Spizella arborea) Gen. Comp. Endocrinol. 1990;77:397–402. doi: 10.1016/0016-6480(90)90229-f. [DOI] [PubMed] [Google Scholar]
- Wilson FE. Neither retinal nor pineal photoreceptors mediate photoperiodic control of seasonal reproduction in American tree sparrows (Spizella arborea) J. Exp. Zool. 1991;259:117–127. [Google Scholar]
- Wilson FE. Photorefractory Harris’ sparrows (Zonotrichia querula) exposed to a winter-like daylength gradually regain photosensitivity after a lag. Gen. Comp. Endocrinol. 1992;87:402–409. doi: 10.1016/0016-6480(92)90047-n. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Follett BK, Matt KS, Farner DS. Effect of day length on plasma FSH and LH in castrated and intact white-crowned sparrows. Gen. Comp. Endocrinol. 1980;42:464–470. doi: 10.1016/0016-6480(80)90212-9. [DOI] [PubMed] [Google Scholar]
- Youngren OM, Silsby JL, Rozenboim I, Phillips RE, El Halawani ME. Active immunization with vasoactive intestinal peptide prevents the secretion of prolactin induced by electrical stimulation of the turkey hypothalamus. Gen. Comp. Endocrinol. 1994;95:330–336. doi: 10.1006/gcen.1994.1130. [DOI] [PubMed] [Google Scholar]