Abstract
We describe a novel thermosensitive escape behavior in Drosophila larvae and a simple assay to accurately define the response temperature. When a larva is placed in a droplet of water that is subsequently heated, a stereotypical escape response is robustly elicited at 29°C. Larvae defective for the painless TRP receptor, or blocked in the function of class IV multi-dendritic sensory dendrites respond to this stimulus at reproducibly higher temperature (34°C). The escape response has novel behavioral components and a lower temperature threshold in comparison with the responses to touch with a hot needle. Furthermore the assay minimizes operator bias that is present in current tests of thermosensitive nociception and generates a precise determination of temperature at the point of response. This response is highly reproducible and directly applicable to genetic and neural circuit analysis of a simple escape behavior.
Keywords: nociception, nocifensive, TRP receptor, Drosophila larvae
Animals detect and respond to temperature changes in their environment. Responses can be choice based to achieve an optimum environment for physiological function, or escape reflexes as temperature reaches noxious levels. Both types of response can be observed in Drosophila.1,2 Recent descriptions of temperature responses in Drosophila have uncovered mechanisms and pathways involved in optimal temperature detection in flies3 that appear to be shared in nociceptive perception in mammals.4 Mutations in a number of Transient Receptor Potential (TRP) receptors have been identified. Behavioral analysis of TRP receptor mutants and the expression patterns of the TRP proteins have helped to identify components of the underlying neurocircuitry.1,3,5–7
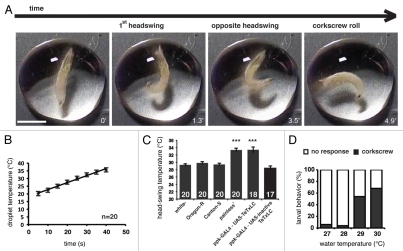
We describe here a robust behavioral escape response from larvae to rising temperature. The behavior closely resembles previously described nocifensive responses elicited by touch with a heated probe set at 39–41°C producing a corkscrewing avoidance of the stimulus.1 A later modification of this stimulus application employed a temperature regulated thermal probe.8 For both studies, duration and force of contact between probe and larvae is a function of the testers' judgement and dexterity. In an attempt to remove this potential operator variability, we applied a thermal stimulus to larvae by immersion in a water droplet and subsequent heating of the water to noxious levels. In practical terms, we placed a larva in 30 µl of water at room temperature on a petri dish lid. The lid was then transferred to a hotplate surface set to 70°C. Care was taken to ensure that the petri dish lid consistently came into close contact with the hotplate. As our baseline, we used a fine temperature probe (a thermocouple) to determine the rate of temperature increase in the water droplet so that we have an exact determination of the temperature of the droplet at any time-point (Fig. 1B). Upon transferral of the petri dish holding the droplet and larva to the hot plate, we recorded the time at which the larva initiated the characteristic nocifensive corkscrew roll. We use our time/temperature relationship curve to infer the temperature in the droplet of water when the escape response is activated. In our assay, the escape response in wild-type larvae was evoked at 28–29°C (Fig. 1C). We determined the escape response temperature for larvae of our lab stock of w1118, and two wild-type stocks, Canton-S and Oregon-R. The three wild-type stocks all responded at a temperature just above 29°C (29.3°C, 29.8°C and 29.4°C respectively). In larvae mutant for the TRP receptor painless (pain1), or functionally blocked by the expression of tetanus toxin light chain10 in the class IV da sensory neurons, the response occurred at 33–34°C (Fig. 1C). Control animals expressing inactive tetanus toxin light chain respond at 28.1°C. This suggests that the painless TRP receptor may be involved in responses to temperature around 29°C in this behavioral test.
Figure 1.
Drosophila larvae immersed in water initiate an escape response when a temperature of 29°C is reached. (A) Third instar larvae respond with a characteristic lateral head thrash followed by a whole body corkscrewing motion when the surrounding water temperature rises to 29°C (see also Movie S1). (B) A determination of the relationship between time and temperature in a 30 µl drop of water on a petri dish placed on a 70°C hotplate determined with a thermocouple. (C) Wild-type and control (ppkGAL4-UA S-inactive TeTxLC) larvae respond with an escape behaviour at 29°C, larvae mutant for the TRP receptor painless (pain1) or defective for function of the Class IV multi-dendritic neurons (ppkGAL-UA S-TeTxLC) have a delayed response at 33°C (***p < 0.001 significantly different from wild-types, Student's t-test). Numbers within bars = n, error bars are SEM. (D) Wild-type and control larvae directly immersed in water of increasing temperatures respond at temperatures of 29°C and above. Fresh larvae were used for each immersion. n = 100 for each temperature.
In addition to the corkscrew roll escape response, we observed another aspect to the behavior preceding the roll where the larva thrashes the mouthparts laterally once to each side (Fig. 1A and Sup. Movie S1). This response invariably precedes the corkscrew roll. We suggest that this aspect of the behavior might be a rapid test of the surrounding medium for lower temperatures for a potential route of escape. To further differentiate between a response to absolute temperature, and an anticipatory response to further increasing heat, we devised a ‘dipping’ assay where we rapidly immersed larvae in water baths with set temperatures using a cell strainer for tissue culture (Fig. 1D). Sudden exposure of larvae to heat in this manner elicited a response in the majority of larvae at temperatures at 29°C and above, identical to the response in a heated water droplet, suggesting the response we see in the heated droplet is not anticipatory to increasing heat. Our results together describe a robust behavioral response to temperature that allows a precise determination of thermal sensory acuity for an invertebrate.
Comparing the response we describe with the ‘hot needle’ escape behavior,1,8 there is a striking difference in the activation temperature. We observe an elicitation of the escape response at 29°C while the response to a ‘hot needle’ stimulus is activated at 42°C. We suggest that the response may be a function of consolidation where the majority of body wall thermosensitive neurons are activated simultaneously in the immersion test. In contrast, the focal application of heat used in the “hot needle” response would potentially activate only a few local sensory neurons. The parallels between the two behaviors suggest that the response is nociceptive and interestingly resembles the behavior elicited when we stab larvae with tungsten needles near the mouth-hooks during standard larval dissection.
The optimal temperature preference for Drosophila melanogaster is 24°C.2 When flies are raised at temperatures above 28.5°C, male sterility is observed suggesting that this temperature is deleterious to optimal reproductive fitness and may cause developmental damage.9 Temperatures approaching and above this point would therefore require detection and avoidance in the long-term.
The behavior we describe is robust and the assay we employ simple, scalable and amenable to genetic and neural circuitry analysis. In addition to lending itself to the analysis of thermoception and escape responses, these characteristics also make the assay ideal for undergraduate teaching practicals and projects.
Acknowledgments
Matthew Oswald was funded by an MRC (UK) studentship, work in the Sean T. Sweeney lab was funded by an MRC (UK) project grant (G0400580). We thank Ryan West for support, Dan Tracey for fly stocks, Chris Elliott for help with imaging and Stephen Goodwin and Gareth Evans for comments on the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Tracey WD, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 2.Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci USA. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallio M, Ofstad TA, MacPherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144:614–621. doi: 10.1016/j.cell.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M, et al. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci USA. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genetics. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 8.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociception sensitization in Drosophila larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northrop JH. Concerning the hereditary adaptation of organisms to higher temperature. J Gen Physiol. 1920;2:313–318. doi: 10.1085/jgp.2.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.