Abstract
Upregulation of kynurenine (KYN) formation from tryptophan (TRY) was associated with aging in animal and human studies. TRY-KYN metabolism is affected by the activities of TRY 2,3-dioxygenase 2 (TDO) and AT P-binding cassette (ABC) transporter regulating TRY access to intracellular TDO. We studied the effects of TDO inhibitor, alpha-methyl tryptophan (aMT) and ABC transported inhibitor, 5-methyl tryptophan (5MT), on the life span of wild strain female Drosophila flies (Oregon-R). aMT and 5MT prolonged mean and maximum life span (by 27% and 43%, and 21% and 23%, resp.). The present results are the first observation of the extension of life span of Drosophila melanogaster by inhibitors of TRY-KYN metabolism, and in line with literature and previous studies on prolonged life span of TDO- and ABC-deficient female Drosophila mutants. Inhibition of TDO and ABC transporter activity might offer the new target for anti-aging interventions.
Keywords: alpha-methyl tryptophan; 5-methyl tryptophan; kynurenine; aging; tryptophan-2,3-dioxygenase; Drosophila; ABC transporter
Introduction
Tryptophan (TRY) is an amino acid participating in biosynthesis of proteins and methoxyindoles (serotonin and melatonin) (reviewed in ref. 1). TRY 2,3-dioxygenase 2 (TDO) is a rate-limiting enzyme of the major non-protein route of TRY metabolism: the cleavage of indole ring of TRY with the formation of formyl-kynurenine, and subsequently, kynurenine (KYN).2 Since TDO is intracellular enzyme,3 TRY must enter cell to be available as a substrate for KYN formation. Cellular uptake of TRY is facilitated by ATP-binding cassette (ABC) transporter.4 Thus, besides TDO, ABC transporter is a rate-limiting factor of TRY conversion into KYN.5
Animal and human studies suggested that aging is associated with upregulation of TRY-KYN metabolism. Thus, plasma KYN/TRY ratio (marker of activity of KYN formation from TRY) is increased with aging.6,7 Increased formation of KYN derivative, kynurenic acid, was observed in aged rat brain8,9 and in human serum.10 One of the mechanisms of aging-associated upregulation of TRY-KYN metabolism might be the increased production of cortisol,11 the inducer of TDO. Association between TRY-KYN metabolism and aging might be further supported by the observations of the increased rate of TRY conversion into KYN in obesity, diabetes, atherosclerosis, menopause, major depression and other aging-associated medical and psychiatric disorders (including metabolic syndrome).12,13
TRY-KYN pathway and related genes were described in Drosophila melanogaster.14 The end product of TRY-KYN pathway in Drosophila is brown eye pigment.15 TDO is the rate-limiting enzyme of KYN formation from TRY in Drosophila, as in the other species. Life spans of TDO-deficient (vermilion) 16,17 and of ABC transport impaired (white) eye mutants of Drosophila melanogaster were longer than of wild type flies.17 This data are in line with the observation of high mortality in humans with increased plasma KYN/TRY ratio at the entry of the 10-year prospective study of nonagenarians.18 Therefore, we suggested that prolongation of life span might be associated with the slow rate of KYN formation from TRY.13,17
To further evaluate this hypothesis we were interested to study the effect of TDO inhibitor, alpha-methyl tryptophan (aMT) 19 and of ABC transporter inhibitor, 5-methyltryptophan (5MT),5 on the life span of wild type Oregon flies.
Results
Effect of alpha-methyl tryptophan.
Treatment with aMT (0.46 mM) did not affect the life span of Drosophila (data not shown).
Treatment with higher concentration of aMT (18.3 mM) increased mean survival time (by 27%, p < 0.0001) and maximum life span (by 23%, p < 0.0001, two way ANOVA) (Table 1).
Table 1.
Inhibitors of tryptophan—kynurenine metabolism and life span of female Drosophila melanogaster (Oregon)
| Control (days) | aMT (days) | 5MT (days) | |
| (n = 50) | (n = 51) | (n = 58) | |
| Mean | 40.1 | 51.7* | 48.5* |
| Std. Err. | 1.4 | 0.9 | 1.0 |
| Maximum | 53 | 65* | 46* |
Concentrations of aMT (18.3 mM) and 5MT (34.5 mM); *increase in days in comparison with control group; p < 0.0001, two-way ANOVA.
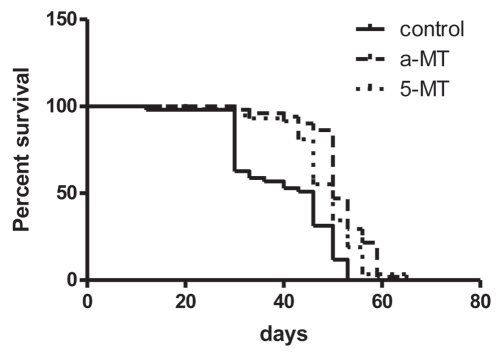
Maximum life span of female control group was 53 days. 25% (13 out of 51) flies treated with high concentrations of aMT survived longer than 53 days (up to 65 days) (Fig. 1).
Figure 1.
Survival time of Drosophila melanogaster (Oregon) treated with alpha-methyl (aMT) and 5-methyl (5MT) tryptophan (p < 0.0001, Logrank test).
Effect of 5-methyl tryptophan.
Treatment with 5MT (2.4 mM) did not affect the life span of Drosophila (data not shown).
Treatment with higher concentration of 5MT (34.5 mM) increased mean survival time (by 21%) and maximum life span (by 23%) of flies (p < 0.0001, two way ANOVA) (Table 1).
Maximum life span of female control group was 53 days. 19% (11 out of 58) female flies treated with high concentrations of 5MT survived longer than 53 days (up to 65 days) (Fig. 1).
Discussion
This is the first (as far as we know) observation of the prolongation of life span in Drosophila by TDO inhibitor, aMT, and by ABC transporter inhibitor, 5MT. The effect appeared to be a dose-dependent: prolongation of life spam was observed only with high but not low concentrations of aMT and 5MT. Intrathorasic administration of aMT inhibited TDO in domestic flies,19 and TRY-KYN metabolism is similar in domestic flies and Drosophila melanogaster: in both species the end product of TRY-KYN pathway is brown eye pigment, ommochrome. Therefore, aMT effect on life span is, most likely, related to inhibition of TDO. However, the direct assessment of TDO activity is needed to proof this suggestion. The present results are in line with the previous observations of prolonged life span of TDO-deficient eye color mutants (vermilion) of Drosophila.16,17
Aging might be affected by the manipulations of the upstream biochemical pathways of TRY metabolism. Thus, reducing the TRY content of the diet extended maximum life span in mammals.20 Since TRY is required for the synthesis of proteins and methoxyindoles (serotonin, N-acetylserotonin and melatonin),1 dietary reductions of TRY might not be advisable. It is noteworthy that TRY participation in protein biosynthesis is not affected by inhibition of ABC transporter.5
TRY has to enter the cell to interact with intracellular TDO.3 TRY shares the same ABC transporter with guanine, the initial substrate for formation of the red eye pigment of Drosophila.21 Since the eye color of wild stock Drosophila depends on a combination of red and brown pigments, mutants with impaired ABC transporter of both guanine and TRY have no eye pigment, and hence, have white colored eyes.22 Prolonged life span was observed in white mutants of Drosophila.17 ABC transporter is encoded by white gene, and inhibition of ABC transporter by 5MT was observed in in vitro experiments.5 Since TRY is transported by ABC transporter, administration of 5MT might prolong life span by inhibiting TRY-KYN metabolism. However, ABC transporter might be involved in transport of KYN,5 3-hydroxy KYN,4 and cGMP.23 Further studies with the assessment of the effect of 5MT on the content of TRY, KYN, and its metabolites are needed to explain the effect of 5MT on life span.
In conclusion, prolongation of life span was observed in wild stock flies treated by the inhibitors of TDO and of ABC transporter. Both treatments supposed to limit TRY conversion into KYN. This data are in agreement with previously observed extension of life span in Drosophila mutants with deficient TDO16,17 or impaired ABC transporter,17 and with neuroprotective effect of genetic inhibition of TDO.24 Importantly, in difference with genetic mutations, pharmacological interventions increased not only mean survival time but maximum life span as well. Inhibition of TRY conversion into KYN might be a target for anti-aging intervention.
Future studies might explore the effect of potential inhibitors of TDO and ABC transporter among tryptophan derivatives25,26 on the life span of Drosophila and other species.
Methods
Female wild-type stock Oregon of Drosophila melanogaster from the collection of V.N. Karazin Kharkiv National University was used in the experiments and maintained at 23°C on a standard Drosophila medium consisting of sugar, yeast, agar and semolina. Two concentrations of aMT (alpha-DL-methyl tryptophan) (0.46 mM or 18.3 mM) or 5MT (5-methyl-DL-tryptophan) (2.4 mM and 34.5 mM) (Sigma Aldrich Chemical Co., USA) were added to nutrition medium of experimental groups. To examine life span, 1-day-old adult flies were collected and then regularly transferred to fresh medium every 3–4 days. The number of dead flies was recorded at the time of transfer. The study was carried out between April and June.
Statistics.
The data were analyzed using two ways ANOVA and Logrank test.
Acknowledgments
Authors greatly appreciate invaluable statistical expertise of Robin Ruthazer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Oxenkrug GF. Genetic and hormonal regulation of the kynurenine pathway of tryptophan metabolism: new target for clinical intervention in vascular dementia, depression and aging. Ann NY Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- 2.Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol. 2004;4:12–17. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Kudo Y, Boyd CA, Sargent IL, Redman CW. Tryptophan degradation by human placental indoleamine-2,3-dioxygenase regulates lymphocyte proliferation. J Physiol. 2001;535:207–215. doi: 10.1111/j.1469-7793.2001.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackenzie SM, Brooker MR, Gill TR, Cox GB, Howells AJ, Ewart GD. Mutations in the white gene of Drosophila melanogaster affecting ABC transporters that determine eye coloration. Biochim Biophys Acta. 1999;1419:173–185. doi: 10.1016/s0005-2736(99)00064-4. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan DT, Bell LA, Paton DR, Sullivan MC. Genetic and functional analysis of tryptophan transport in Malpighian tubules of Drosophila. Biochem Genet. 1980;18:1109–1130. doi: 10.1007/BF00484342. [DOI] [PubMed] [Google Scholar]
- 6.Frick B, Schroecksnadel K, Neurauter G. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Capuron L, Schroecksnadel S, Féart C, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role inneuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Moroni F, Russi P, Carlá V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci Lett. 1988;94:145–150. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- 9.Gramsbergen JB, Schmidt W, Turski WA, Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- 10.Urbañska EM, Luchowski P, Luchowska E. Serum kynurenic acid positively correlates with cardiovascular disease risk factor, homocysteine: a study in stroke patients. Pharm Rep. 2006;58:507–511. [PubMed] [Google Scholar]
- 11.Oxenkrug GF, Pomara N, McIntyre I, Branconnier R, Stanley M, Gershon S. Aging and cortisol resistance to suppression by dexamethasone: a positive correlation. Psychiatry Research. 1983;10:125–130. doi: 10.1016/0165-1781(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 12.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders and dysregulation of tryptophan-kynurenine pathway metabolism. Ann NY Acad Sci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- 13.Oxenkrug GF. Interferongamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated medical and psychiatric disorders. J Neural Transm. 2011;118:75–85. doi: 10.1007/s00702-010-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savvateeva-Popova EV, Popov AV, Heinemann T, Riederer P. Drosophila mutants of the kynurenine pathway as a model for ageing studies. Adv Exp Med Biol. 2003;527:713–722. doi: 10.1007/978-1-4615-0135-0_84. [DOI] [PubMed] [Google Scholar]
- 15.Tearle R. Tissue specific effects of ommochrome pathway mutations in Drosophila melanogaster. Genet Res. 1991;57:257–266. doi: 10.1017/s0016672300029402. [DOI] [PubMed] [Google Scholar]
- 16.Kamyshev MG. Longevity and its relation to the locomotor activity in tryptophan-xanthommatin metabolic pathway mutant of Drosophila. Dokl Acad Nauk USSR. 1980;253:1476–1480. [Google Scholar]
- 17.Oxenkrug GF. The extended life span of Drosophila melanogaster eye-color (white and vermilion) mutants with impaired formation of kynurenine. J Neural Transm. 2010;117:23–26. doi: 10.1007/s00702-009-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pertovaara M, Raitala A, Lehtimäki T. Indoleamine-2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Lancaster GA, Sourkes TL. Effect of alpha-methyldl-tryptophan on tryptophan metabolism of Musca Domestica L. Comp. Biochem Physiol. 1969;28:1435–1441. doi: 10.1016/0010-406x(69)90581-7. [DOI] [PubMed] [Google Scholar]
- 20.de Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech Ageing Dev. 1986;36:161–171. doi: 10.1016/0047-6374(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 21.Borycz J, Borycz JA, Kubów A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol. 2008;211:3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- 22.Beadle GW, Ephrussi B. Development of eye colors in Drosophila: transplantation experiments with suppressor of vermilion. Proc Natl Acad Sci USA. 1936;22:536–540. doi: 10.1073/pnas.22.9.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans JM, Day JP, Cabrero P, Dow JA, Davies SA. A new role for a classical gene: white transports cyclic GMP. J Exp Biol. 2008;211:890–899. doi: 10.1242/jeb.014837. [DOI] [PubMed] [Google Scholar]
- 24.Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, et al. The kyn-urenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease. Curr Biol. 2001;21:961–966. doi: 10.1016/j.cub.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lester G. Genetic control of amino acid permeability in Neurospora crassa. J Bacteriol. 1966;91:677–684. doi: 10.1128/jb.91.2.677-684.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oxenkrug GF, Bachurin SO, Prakhie IV, Zefirov N. Quinone reductase 2 and antidepressant effect of melatonin derivatives. Ann NY Acad Sci. 2010;1199:121–124. doi: 10.1111/j.1749-6632.2009.05354.x. [DOI] [PubMed] [Google Scholar]