Abstract
We generated FM7a and CyO balancer chromosomes bearing a Tubby1 (Tb1) dominant transgene. Flies heterozygous for these FM7a and CyO derivatives exhibit a phenotype undistinguishable from that elicited by the Tb1 mutation associated with the TM6B balancer. We tested two of these Tb-bearing balancers (FM7-TbA and CyO-TbA) for more than 30 generations and found that the Tb1 transgene they carry is stable. Thus, these new Tb-tagged balancers are particularly useful for balancing lethal mutations and distinguish homozygous mutant larvae from their heterozygous siblings.
Keywords: balancer chromosomes, Tubby, Drosophila melanogaster
One of the great advantages of Drosophila melanogaster as model organism is the availability of balancer chromosomes. These chromosomes suppress recombination with their homologues, allowing the maintenance of lethal and sterile mutants as balanced heterozygotes. All balancers carry dominant markers that are visible in adult flies, but only a subset have markers that unambiguously distinguish homozygous mutant larvae from their heterozygous siblings. The latter balancers include those that express high levels of the GFP protein under the indirect control, via the UAS/GAL4 system, of either the Kruppel (Kr),1 or hsp70,2 promoter. In addition, there are direct-drive balancers that express GFP under the control of the actin A5c promoter3 or YFP under the control of Deformed (Dfd HZ2.7rev) or glass (GMR) enhancer elements.4 Each of the GFP- or YFP-expressing balancers has specific advantages, but all share a common drawback: These balancers require the use of a dissecting microscope equipped with an UV light source, which for reliable fluorescence detection is preferably used in the dark.
The TM6B balancer5 carries the Tb1 dominant mutation, which results in squat larvae and pupae. We have been using this balancer for many years to unambiguously distinguish homozygous mitotic mutants dying at late larval stages from their heterozygous siblings (reviewed in ref. 6). This balancer proved particularly useful when mutant larvae are rare and one has to examine several vials (or bottles) to find third instar larvae suitable for dissection and cytological analysis. To generate new tools for easy detection of larvae homozygous for lethal mutations on the X or the second chromosome, we decided to generate Tb-marked versions of FM7a7 and CyO,8 respectively.
We have previously demonstrated that the Tubby phenotype results from the deletion in the Tb1 chromosome of DNA encoding amino acids 167–190 of the TwdlA cuticle protein.9 A genomic fragment spanning the TwdlATb1 transcription unit, as well as 1 kb of DNA upstream, was inserted into a modified polylinker sequence in the pCaSpeR vector. Using standard methods, we coinjected DNA with a Δ2–3 transposase source into w118 embryos, selected for w+ eye color, and generated balanced stocks. All such stocks (n = 10) exhibited the squat larval and pupal phenotype characteristic of the Tb1 mutation. One such stock, referred to hereafter as P{Tb1}/CyO, was used as the starting point for mobilizing Tb1 onto X and 2nd chromosome balancers.
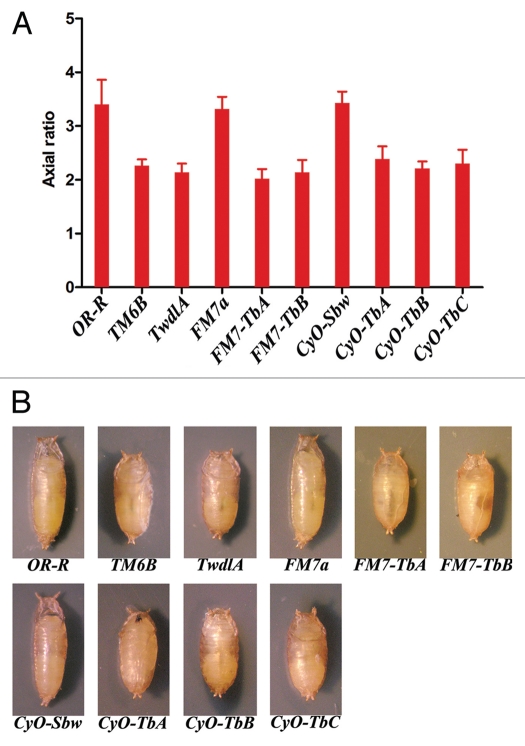
To generate FM7a-P{Tb1} we crossed FM7a; P{Tb1}/CyO; TMS/+ males to w/w females (TMS is a Δ2–3-bearing third chromosome balancer expressing the P-element transposase described in ref. 10); from approximately 1,000 progeny we recovered two FM7 chromosomes that co-segregated with Tb (we propose to name these balancers FM7-TbA and FM7-TbB). To generate a second chromosome balancer with a P{Tb1} insertion we used a CyO balancer bearing the additional markers S and bw1 (designated as CyO-Sbw in FlyBase). We crossed w/w; P{Tb1}/CyO-Sbw; TMS/+ females to w; CyO/Sco males and recovered three CyO-Sbw-P{Tb1} chromosomes from approximately 1,000 progeny (we propose to name these balancers CyO-TbA, CyO-TbB and CyO-TbC). To assess the utility of these FM7a and CyO Tb-bearing balancers, we compared their Tb phenotype with the Tb1 mutant phenotype associated with TM6B.5 To quantify the squat phenotype elicited by the balancers we measured the axial ratio11 of larvae and pupae (AR, length/width) heterozygous for each balancer. It has been previously shown that the AR does not depend on larval and pupal size and provides a reliable measure of the Tb phenotype.9 As shown in Figure 1, the ARs observed in FM7-TbA, FM7-TbB, CyO-TbA, CyO-TbB, CyO-TbC and TM6B heterozygotes are fully comparable and significantly different from those of wild-type or non-Tb-bearing larvae and pupae. We thus conclude that the FM7a and CyO Tb-tagged chromosomes are highly suitable to distinguish larvae and pupae that bear the balancers from those that are homozygous for the balanced chromosome.
Figure 1.
Quantitation of the squat phenotype of pupae heterozygous for different Tb-bearing chromosomes or balancers; OR-R is a wild-type Oregon R stock; TwdlA contains a single copy of the TwdlATb1 transgene; FM7a and CyO-Sbw are the balancer chromosomes used to construct the Tb-bearing derivatives indicated as FM7-TbA, FM7-TbB, CyO-TbA, CyO-TbB and CyO-TbC. (A) Axial ratios (ARs; length/width, ±standard deviation) determined from digital photographs of at least 40 pupae of each genotype (B) examples of pupae heterozygous for different Tb mutations and transgenes.
We generated the two FM7a and the three CyO Tb-bearing balancers in October 2009 and March 2010, respectively. Within two months from the time of their generation, one balancer of each type (FM7-TbA and CyO-TbA) was used to rebalance 40 chromosomes bearing different lethal mutations associated with either the X or the second chromosome. These stocks were reexamined in March 2011 (after more than 30 generations) and none of them had lost the Tb marker from the balancer. We thus conclude that the Tb1 transgene is very stable and that the FM7-TbA and CyO-TbA balancers are suitable for the establishment and long-term maintenance of balanced stocks.
Using inverse PCR (an EcoRI fragment was ligated and amplified using the 5′-CAGCTCCATAGTTATAGCCGC and CGTTAAGTGGATGTCTTCTTG-3′ primers), we also determined the insertion sites of the Tb1 transgenes within the FM7-TbA and CyO-TbA balancers. The insertion in FM7-TbA mapped at 17C in the intergenic space between CG15047 and beadex; accordingly, this balancer is homozygous-viable. The Tb1 transgene of CyO-TbA was inserted into the Cytochrome P450 reductase (Cpr) gene in region 26C3. We did not determine whether this insertion inactivates the Cpr gene.
In summary, we have generated Tb-marked versions of FM7a and CyO. These chromosomes carry a stable Tb1 transgene that has the same expressivity as the original Tb1 mutation. We believe that the use of the FM7-TbA and CyO-TbA balancers will facilitate a number of experimental strategies, allowing researchers to readily distinguish homozygous larvae and pupae from their heterozygous siblings simply by looking through the vial or the bottle used to grow the flies. In addition, the availability of balancers with different dominant larval markers such as Tb or GFP will allow construction of stocks carrying two lethal mutations balanced on Tb- and GFP-bearing balancers, respectively. This will be particularly helpful for selection and analysis of single and double mutants from the same culture. The FM7-TbA and CyO-TbA balancers can be obtained from the Bloomington Drosophila Stock Center at Indiana University (http://flystocks.bio.indiana.edu).
References
- 1.Casso D, Ramirez-Weber FA, Kornberg TB. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech Develop. 1999;88:229–232. doi: 10.1016/s0925-4773(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph T, Lu B, Westphal T, Szidonya J, Eissenberg J, Reuter G. New type of CyO and TM3 green balancers. Dros Inf Serv. 1999;82:99–100. [Google Scholar]
- 3.Reichhart JM, Ferrandon D. Green balancers. Dros Inf Serv. 1998;81:201–202. [Google Scholar]
- 4.Le T, Liang Z, Patel H, Yu MH, Sivasubramaniam G, Slovitt M, et al. A new family of Drosophila balancer chromosomes with a w−dfd-GMR yellow fluorescent protein marker. Genetics. 2006;174:2255–2257. doi: 10.1534/genetics.106.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craymer L. TM6B: Third Multiple Six, B structure. Dros Inf Serv. 1984;60:234. [Google Scholar]
- 6.Gatti M, Baker BS. Genes controlling essential cell cycle functions in Drosophila melanogaster. Genes Dev. 1989;3:438–453. doi: 10.1101/gad.3.4.438. [DOI] [PubMed] [Google Scholar]
- 7.Merriam JR. FM7: First multiple seven. Dros Inf Serv. 1968;43:64. [Google Scholar]
- 8.Oster II. A new crossing-over suppressor in chromosome 2 effective in the presence of heterologous inversions. Dros Inf Serv. 1956;30:145. [Google Scholar]
- 9.Guan X, Middlebrooks BW, Alexander S, Wasserman SA. Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc Natl Acad Sci USA. 2006;103:16794–16799. doi: 10.1073/pnas.0607616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsley DL, Zimm . The Genome of Drosophila melanogaster. Academic Press, Inc.; 1992. pp. 1–1133. [Google Scholar]
- 11.Letsou A, Alexander S, Orth K, Wasserman SA. Genetic and molecular characterization of tube, a Drosophila gene maternally required for embryonic dorsoventral polarity. Proc Natl Acad Sci USA. 1991;88:810–814. doi: 10.1073/pnas.88.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]