Abstract
To develop a molecular pattern that might help in understanding carcinogenesis of postcricoid carcinoma (PCC) on top of Plummer-Vinson syndrome (PVS) in a prospective controlled study. Twenty-four patients with PVS were diagnosed and followed up over a 4 year period, during which eight of them showed malignant change to PCC. Twenty volunteers free of neoplastic diseases were included as a control group. In the two groups, DNA extraction from mononuclear peripheral blood cells, and analysis of loss of heterozygosity (LOH) and microsatellite instability (MSI) using six paired simple tandem repeats (STRs) primers were done. The molecular weight of each STRs locus was scored and statistical correlations were performed. LOH occurred in 55.6 and 72.9% of PVS and PCC cases compared to 25% of control group. At loci D17S695, D9S753 and D9S171, LOH occurred in 54.2, 66.7, and 70.8% of PVS cases; and in 62.5% of PCC cases for each locus compared to 15, 25 and 45% of control cases. D3S1286 and CFS1-R displayed the highest frequency of LOH in PCC (100% for each) while recorded in 58.3 and 33.3% in PVS compared to 30 and 0% in control cases. Certain genetic events tend to occur as early and late events in malignant change of PVS to PCC. Detection of these events may help in understanding carcinogenesis and in early detection of malignancy. CFS1-R is the most informative marker of tumor progression.
Keywords: Plummer Vinson syndrome, Postcricoid carcinoma, Microsatellites instability
Introduction
Plummer-Vinson syndrome is associated with iron deficiency anaemia, glossitis, dysphagia and occurs almost in females. A number of proposed etiologies had been reported including: iron deficiency, general nutritional deficiencies and gastric lesion [1]; autoimmunity and thyroiditis [2]; and genetically predetermined gastric atrophy [3]. The reported incidence of upper aero-digestive tract carcinoma in PVS was between 4 and 16% and almost occurred in postcricoid region [4]. Other documented sites included the oral cavity, esophagus, stomach and pyriform fossa [5–7].
STRs are informative markers with known propensity for MSI and LOH [8]. PCR amplification of these repeats provides a rapid method of assessment of LOH and facilitates mapping of tumor suppressor genes [9, 10]. These specific genetic events could be detected simply in the saliva and serum of patients with malignancy, thus paves the way for early cancer detection and tumor surveillance [11, 12]. LOH is defined as a reduction in intensity of an allele of DNA of a patient to more than 40% compared to a retained allele in the control [13]. MSI is defined as an alteration of the length of an allele in one or more microsatellites for a particular sample. It is determined by the presence of new bands or bands shift that were not present in PCR products of the corresponding normal DNA.
The aim of the current study is to develop a molecular pattern that may help in understanding carcinogenesis of PCC on top of PVS and in early detection of malignancy.
Patient and Methods
A prospective design was followed for the study that included all patients with PVS presented to the ENT Department at Sohag University Hospital. In addition, 20 volunteers free of neoplastic diseases were included as a control group.
Patients with PVS were subjected to:
History taking.
General and E.N.T examination.
Laboratory investigations including Hb level, serum iron, iron binding capacity.
Plain lateral X-ray film on the neck and barium swallow in those with progressive dysphagia.
Direct endoscopic examination and biopsy taking for histopathological examination.
Blood sample (5 ml) collected in EDTA was obtained from the study and control groups.
DNA Extraction
DNA samples were isolated from the mononuclear peripheral blood cells of both groups, using Promega Wizard Genomic DNA Purification Kit A1120 (Promega, WI, USA).
Microsatellite, LOH, and MSI Analysis
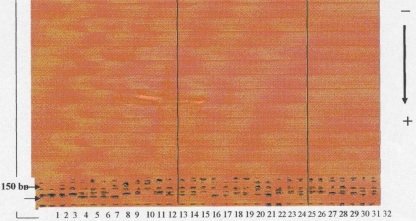
Genomic DNA (100 ng) was amplified with 50 pmol of six paired STRs primers named D3S1286, D9S171, D9S753, D17S654, D17S695 and CFS1-R; obtained from Promega, Madison, WI, USA. PCR amplification applied according to Partridge et al. [14]. PCR products for each sample were pooled (3 ml each) and cooled to 4°C and stop solution (95% formamide, 0.8% dextran blue and 25 mM EDTA, PH 9) was added. Samples were heat denatured at 95°C for 3 min, then rapidly cooled on ice prior to loading onto the gels. The gels were made up of Hydrolink 9 (Long ranger TM, AT Biochem Ltd) in 0.6 × TBE (0.06 M Tris-base, 0.05 mM boric acid, 0.6 mM EDTA) containing 7 M urea and run in the same buffer at constant power (55 w) and constant temperature (50°C) for 14 h at a rate of limiting voltage of 1200 V on a manual (500 mm long) DNA Sequencer (Bio-Rad, USA). The gels were stained with Promega’s DNA Silver Staining System Kits according to the technical manual instructions. Then, gels were captured and scored using computerized Densitometer (Bio-Rad, USA) supplied with Gene Scan Software (1996). The molecular weight of each STRs locus was scored. Both LOH (deletion of one allele) and MSI (alteration in the position of one allele by one or more bp) were recorded in the study and control groups (Figs. 1, 2).
Fig. 1.
A photomicrograph showing silver stained SDS–polyacrylamide gel electrophoresis (PAGE) of the six STRs primers with amplified samples in PVS, PCC and control groups. The internal standard (200–50 bp) is shown on the left of the slide
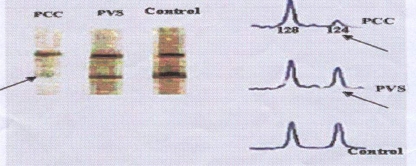
Fig. 2.
LOH for STRs detected in PCC, PVS and control cases. Arrows show a deletion and low intensity of STR locus (124 bp) in PCC and PVS respectively compared to control
Statistical Analysis
Linkage analysis of STRs, LOH, and MSI were carried out using Gene Pop Software (Version 3.1, 1997) program. All correlations were performed using a two-tailed Fisher’s exact and Qui-square t-tests.
Results
Over a 4 year period, 24 patients with PVS were included and followed up. They presented with dysphagia, manifestations of iron deficiency anemia, angular stomatitis and glazed tongue. During the follow up period, eight of them showed malignant change to PCC proved by histopathological examination.
STRs mutations were examined in six regions (3pter-3p24.2, 5q33.3-q34, 9p21, 9q21.1-22.3, 17p and 17p12) of chromosomes 3, 5, 9 and 17 in PVS, PCC and control groups.
Table 1 demonstrated the LOH frequencies of six polymorphic microsatellite markers in control, PVS and PCC cases. LOH occurred in 55.6 and 72.9% of PVS and PCC cases compared to 25% of control group.
Table 1.
LOH frequencies of six polymorphic microsatellite markers in control, PVS and PCC
| Marker No | Name | Chromosome location | Control | PVS | PCC |
|---|---|---|---|---|---|
| 1 | D3S1286 | 3pter-3p24.2 | 6/20 (30%) | 14/24 (58.3%) | 8/8 (100%) |
| 2 | D9S171 | 9p21 | 9/20 (45%) | 17/24 (70.8%) | 5/8 (62.5%) |
| 3 | D9S753 | 9q21.1-22.3 | 5/20 (25%) | 16/24 (66.7%) | 5/8 (62.5%) |
| 4 | D17S654 | 17p | 7/20 (35%) | 12/24 (50%) | 4/8 (50%) |
| 5 | D17S695 | 17p12 | 3/20 (15%) | 13/24 (54.2%) | 5/8 (62.5%) |
| 6 | CFS1-R | 5q33.3-q34 | 0/20 (0%) | 8/24 (33.3%) | 8/8 (100%) |
| Total | 30/120 (25%) | 80/144 (55.6%) | 34/48 (72.9%) |
LOH in D17S654 at 17p occurred in 50% of both PVS and PCC cases. The most frequent microsatellite markers with LOH in PVS cases were detected at loci D9S171 (70.8% of cases) and D9S753 (66.7% of cases).
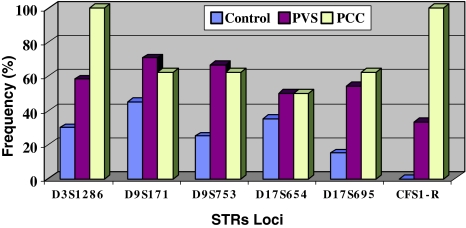
Among the markers analyzed, D3S1286 at the short arm of chromosome 3 (3pter-3p24.2), and CFS1-R at the long arm at chromosome 5 (5q33.3-q34) displayed the highest frequency of LOH in PCC patients where occurred in 100% of cases. CFS1-R displayed the least frequency of LOH in PVS (33.3% of cases) and in control cases (0% of cases). Figure 3 demonstrated the graphic presentations of the frequency indices of the six STRs loci in control, PVS and PCC patients.
Fig. 3.
The frequency indices of 0036 STRs loci in controls, PVS, and PCC patients
Discussion
In the current study, Microsatellite analysis was done on DNA samples isolated from the mononuclear peripheral blood cells. Nawroz et al., found that 29% of patients with primary head and neck squamous cell carcinoma had one or more microsatellite alterations in serum precisely matching those in the primary tumors [10].
LOH and MSI had been implicated as a possible mean for early detection of head and neck malignancy [11, 12]. In our cases, these microsatellite alterations had been detected in the precancerous lesion, PVS and in PCC developed on top of it. Sundaresan et al., found LOH at loci on short arm of chromosome 3 in samples of invasive squamous cell carcinoma of the lung and adjacent samples of bronchial dysplasia [15].
In the present study, LOH in D17S654 at 17p occurred in 50% of both PVS and PCC cases, compared to 35% of control cases. MSI and LOH at loci D17S695, D9S753 and D9S171 showed a high incidence in PVS cases (54.2, 66.7, and 70.8%) and PCC cases (62.5% for each) compared to control one (15, 25 and 45%). These findings suggest that LOH and MSI in these loci could be early events in carcinogenesis of PVS to PCC. D3S1286 at the short arm of chromosome 3 (3pter-3p24.2) and CFS1-R at the long arm of chromosomes 5 (5q33.3-q34) displayed the highest frequency of LOH in PCC (100% for each) while recorded in 58.3 and 33.3% in PVS cases compared to 30 and 0% in control cases. These results suggest that LOH and MSI in these loci could be late events in carcinogenesis. Progression of microsatellite alterations had been reported by other researchers. Califano et al. [16], studied 87 lesions of the head and neck and found that the spectrum of chromosomal loss progressively increased at each histopathological step from benign hyperplasia to dysplasia to carcinoma in situ to invasive cancer.
Nassar and Ibrahim reported that the high incidence of PCC in Egypt could not be explained by the predisposition caused by PVS since no one case with this tumor gave a history related to this syndrome [17]. In contrast, the present study revealed the occurrence of PCC on top of PVS in 8/24 = 33.3% of cases with progression of microsatellite alterations. The high incidence of PCC on top of PVS in our cases compared to that reported by Paterson [4], can be explained by the delayed presentation of our PVS cases.
Conclusion
Microsatellite analysis can be done non-invasively on a blood sample.
Certain genetic events tend to occur as early and late events in malignant changes of PVS to PCC. The detection of these changes may help in understanding carcinogenesis and in early detection of malignant changes, thus pave the way for rational and effective treatment. CFS1-R on chromosome 5 is the most informative marker of tumor progression from normal (0%) to PVS (33.3%) to PCC (%100).
Recommendation
Cytogenic alteration study in PVS and PCC is recommended to complete the genetic instability study.
To study the correlation between genetic instability and severity of iron deficiency in PVS and other cases of iron deficiency than PVS is recommended.
Conflict of intrest
None.
References
- 1.Chen TS, Chen PS. Rise and fall of the Plummer-Vinson syndrome. J Gastroenterol Hepatol. 1994;9:654–658. doi: 10.1111/j.1440-1746.1994.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 2.Chrisholm M, Ardran GM, Callander ST, Wright R. Iron deficiency and autoimmunity in postcricoid webs. Q J Med. 1971;40:421–433. [PubMed] [Google Scholar]
- 3.Jacobs A, Kilpatrick GS. The Patterson-Kelly syndrome. Br Med J. 1964;2:79–82. doi: 10.1136/bmj.2.5401.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paterson DR. A clinical type of dysphagia. J Laryngol. 1919;24:289–291. [PMC free article] [PubMed] [Google Scholar]
- 5.Shamma’a MH, Benedict EB. Esophageal webs; a report of 58 cases and an attempt at classification. N Engl J Med. 1958;259:378–384. doi: 10.1056/NEJM195808212590805. [DOI] [PubMed] [Google Scholar]
- 6.Chisolm M, Ardan GM, Callendar ST, Wright R. A follow-up study of patients with post-cricoid webs. Q J Med. 1971;40:409–420. [PubMed] [Google Scholar]
- 7.Chisholm M. The association between webs, iron, and post-cricoid carcinoma. Postgrad Med J. 1974;50:215–219. doi: 10.1136/pgmj.50.582.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge M, Emilion G, Pateromichelakis S, Phillips E, Langdon J. Location of candidate tumour suppressor gene loci at chromosome 3p, 8p, and 9p for oral squamous cell carcinomas. Int J Cancer. 1999;83:318–325. doi: 10.1002/(SICI)1097-0215(19991029)83:3<318::AID-IJC6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Ruppert JM, Tokino K, Sidransky D. Evidence for two bladder cancer suppressor loci on human chromosome 9. Cancer Res. 1993;53:5093–5095. [PubMed] [Google Scholar]
- 10.Nawroz H, Koch W, Anker P, Stroun M, Sidransky D. Microsatellite alterations in serum DNA of head and neck cancer patients. Nat Med. 1996;2:1035–1037. doi: 10.1038/nm0996-1035. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Farrington SM, Petersen GM, Hamilton SR, Parsons R, Papadopoulos N, Fujiwara T, Jen J, Kinzler KW, Wyllie AH, Vogelstein B, Dunlop MG. Genetic instability occurs in the majority of young patients with colorectal cancer. Nat Med. 1995;1:348–352. doi: 10.1038/nm0495-348. [DOI] [PubMed] [Google Scholar]
- 12.Spafford MF, Koch WM, Reed AL, Califano JA, Xu LH, Eisenberger CF, Yip L, Leong PL, Wu L, Liu SX, Jeronimo C, Westra WH, et al. Detection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by micrrosatellite analysis. Clin Cancer Res. 2001;7:607–612. [PubMed] [Google Scholar]
- 13.Starostik P, Greiner A, Schultz A, Zettl A, Peters K, Rosenwald A, Kolve M, Müller-Hermelink HK. Genetic aberrations common in gastric high-grade large B-cell lymphoma. Blood. 2000;95:1180–1187. [PubMed] [Google Scholar]
- 14.Partridge M, Kiguwa S, Langdol JD. Frequent deletion of chromosome 3p in oral squamous cell carcinoma. Eur J Cancer Oral Oncol. 1994;30B:248–251. doi: 10.1016/0964-1955(94)90006-X. [DOI] [PubMed] [Google Scholar]
- 15.Sundaresan V, Ganly P, Hasleton P, Rudd R, Sinha G, Bleehen NM, Rabbitts P. p52 and chromosome 3 abnormalities, characteristic of malignant lung tumours, are detectable in preinvasive lesions of the bronchus. Oncogene. 1992;7:1989–1997. [PubMed] [Google Scholar]
- 16.Califano J, Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D. Genetic progression model for head and cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 17.Nassar M, Ibrahim SA. A 5-year experience with hypopharyngeal carcinoma in Ain Shams University Hospitals. Inter Cong Ser. 2003;1240:1007–1014. doi: 10.1016/S0531-5131(03)00711-8. [DOI] [Google Scholar]