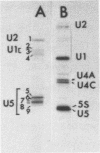
Abstract
U1, U2 and U5 RNAs were isolated from pea nuclei with antibody specific for 2,2,7-trimethylguanosine. The nucleotide sequence of the 3'-terminal halves of pea U1 and U2 snRNAs and the complete sequence of five out of the six U5 RNA variants isolated is given. The high number of U5 variants suggest they are encoded by a multigene family containing at least six different genes. Similar secondary structures could be derived for all of the pea U5 RNAs and although the degree of sequence conservation between plant and vertebrate U5 RNAs is as low as 35%, nearly identical secondary structures can be proposed for both RNA groups. All the snRNA species U1, U2 and U5 from pea share a structural domain, the so-called domain A, which is also common to all animal snRNA U1, U2, U4 and U5. Furthermore, a block of 22 consecutive nucleotides is conserved among pea and vertebrate U5 RNAs, from which 11 nucleotides constitute a hairpin-loop with a high number of posttranscriptional modifications. We propose that conservation of this hairpin-loop, together with domain A, is of prime importance for the functioning of U5 RNAs in plant and animal cells.
Full text
PDF


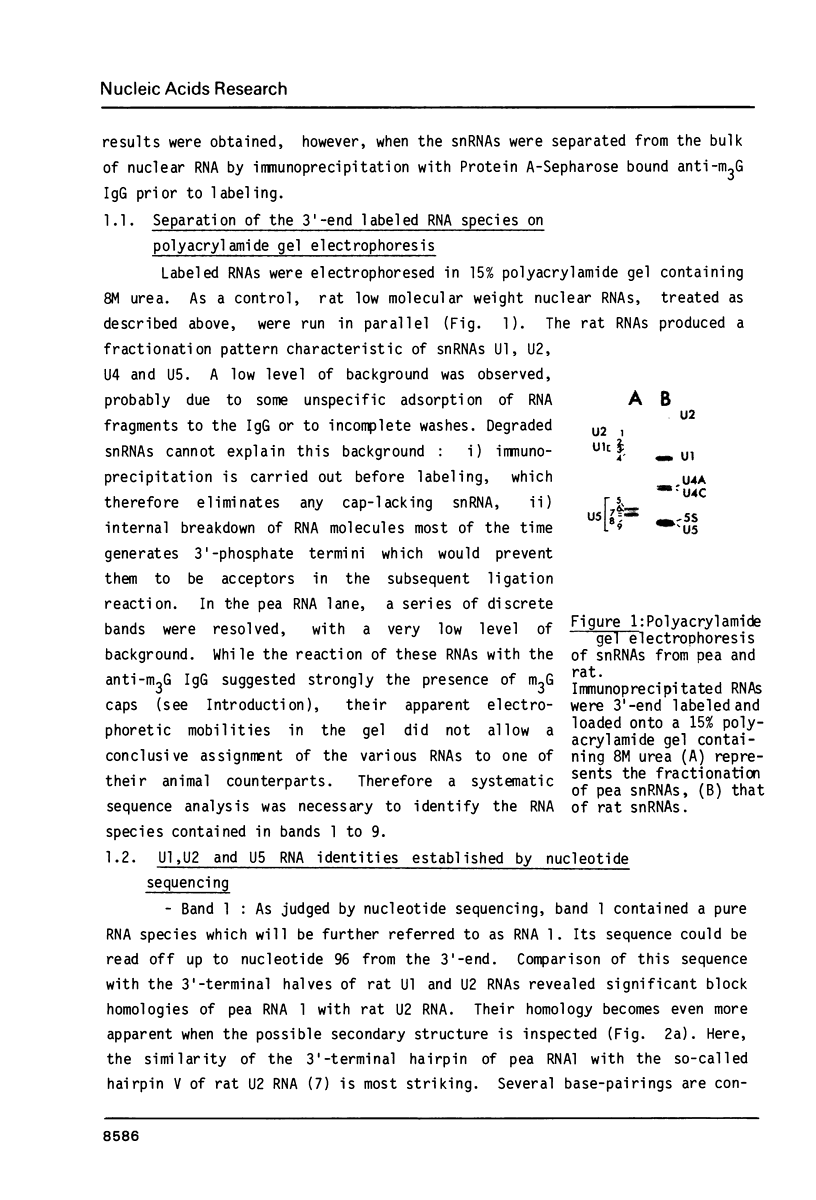
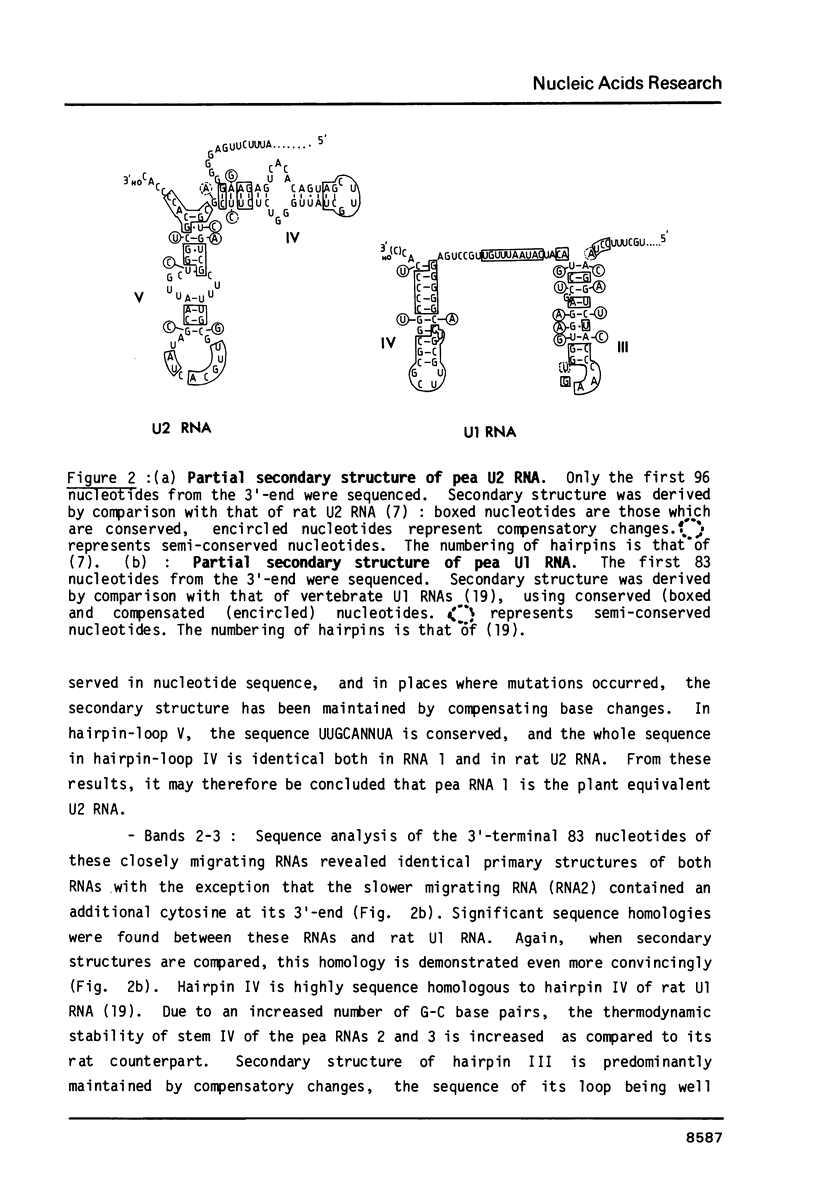


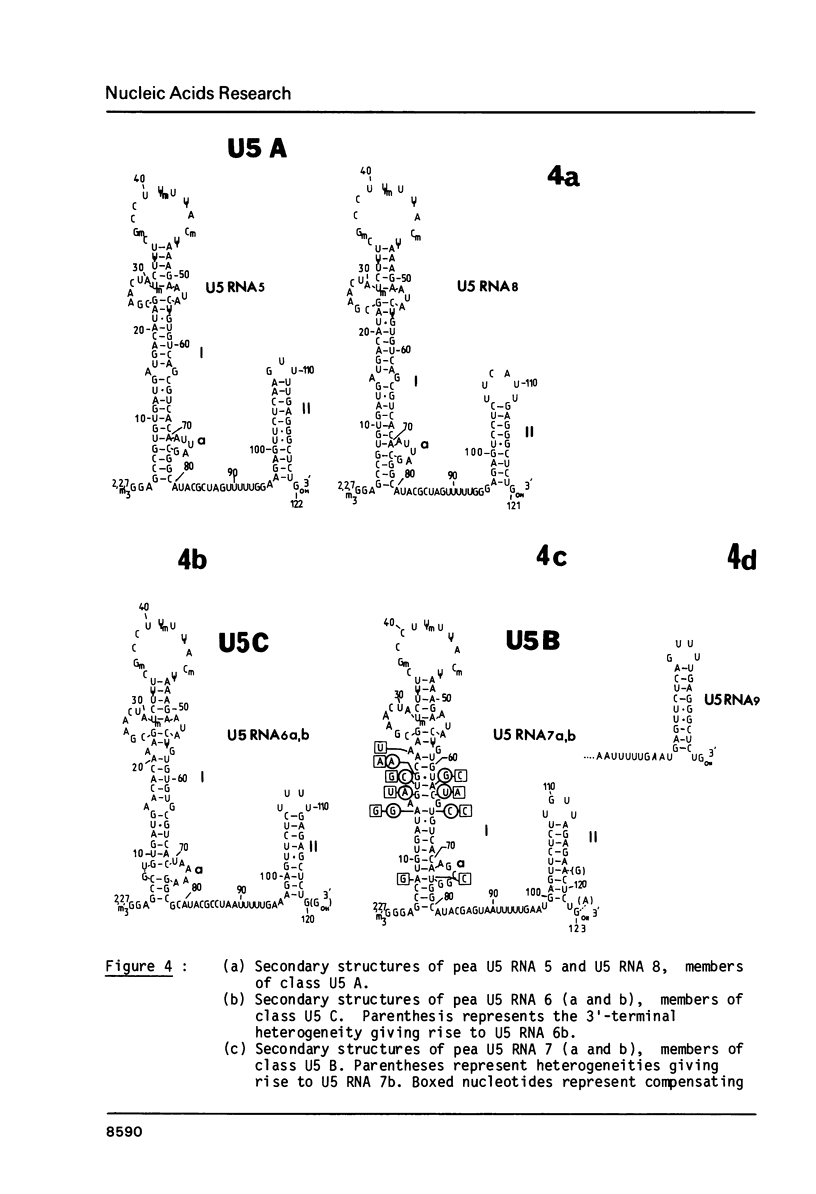




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Ebel J. P., Gallinaro H., Lazar E., Jacob M. The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res. 1981 Feb 25;9(4):841–858. doi: 10.1093/nar/9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Haendler B., Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1(10):1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Pouyet J., Ebel J. P., Edwards K., Kössel H. Primary and secondary structures of Escherichia coli MRE 600 23S ribosomal RNA. Comparison with models of secondary structure for maize chloroplast 23S rRNA and for large portions of mouse and human 16S mitochondrial rRNAs. Nucleic Acids Res. 1981 Sep 11;9(17):4303–4324. doi: 10.1093/nar/9.17.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann P., Reuter R., Rinke J., Appel B., Bald R., Lührmann R. 5'-terminal caps of snRNAs are accessible for reaction with 2,2,7-trimethylguanosine-specific antibody in intact snRNPs. J Biol Chem. 1983 Mar 10;258(5):2745–2747. [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinaro H., Lazar E., Jacob M., Krol A., Branlant C. Small RNAs in HnRNP fibrils and their possible function in splicing. Mol Biol Rep. 1981 May 22;7(1-3):31–39. doi: 10.1007/BF00778730. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Hu J. C., Dahlberg J. E. Structural features required for the binding of tRNATrp to avian myeloblastosis virus reverse transcriptase. Nucleic Acids Res. 1983 Jul 25;11(14):4823–4833. doi: 10.1093/nar/11.14.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Branlant C., Lazar E., Gallinaro H., Jacob M. Primary and secondary structures of chicken, rat and man nuclear U4 RNAs. Homologies with U1 and U5 RNAs. Nucleic Acids Res. 1981 Jun 25;9(12):2699–2716. doi: 10.1093/nar/9.12.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol A., Gallinaro H., Lazar E., Jacob M., Branlant C. The nuclear 5S RNAs from chicken, rat and man. U5 RNAs are encoded by multiple genes. Nucleic Acids Res. 1981 Feb 25;9(4):769–787. doi: 10.1093/nar/9.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Luhrmann R., Appel B., Bringmann P., Rinke J., Reuter R., Rothe S., Bald R. Isolation and characterization of rabbit anti-m3 2,2,7G antibodies. Nucleic Acids Res. 1982 Nov 25;10(22):7103–7113. doi: 10.1093/nar/10.22.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Human U1 loci: genes for human U1 RNA have dramatically similar genomic environments. Cell. 1982 May;29(1):257–264. doi: 10.1016/0092-8674(82)90110-6. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Steitz J. A. Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res. 1981 Dec 11;9(23):6351–6368. doi: 10.1093/nar/9.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar R. N., Lo A. C., Wildeman A. G., Sitz T. O. Effect of 2'-O-methylation on the structure of mammalian 5.8S rRNAs and the 5.8S-28S rRNA junction. Nucleic Acids Res. 1983 Sep 10;11(17):5989–6001. doi: 10.1093/nar/11.17.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Rich A. Structural domains of transfer RNA molecules. Science. 1976 Nov 19;194(4267):796–806. doi: 10.1126/science.790568. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Ebel J. P., Ehresmann C. A general secondary-structure model for procaryotic and eucaryotic RNAs from the small ribosomal subunits. Eur J Biochem. 1981 Dec;120(3):487–495. doi: 10.1111/j.1432-1033.1981.tb05727.x. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Denison R. A. Either gene amplification or gene conversion may maintain the homogeneity of the multigene family encoding human U1 small nuclear RNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1141–1149. doi: 10.1101/sqb.1983.047.01.129. [DOI] [PubMed] [Google Scholar]