Abstract
Activin and inhibin are important local modulators of theca cell steroidogenesis in the ovary. Using a serum-free primary theca cell culture system, this study investigated the effects of inhibin on theca cell androgen production and expression of steroidogenic enzymes. Androstenedione secretion from theca cells cultured in media containing activin, inhibin and follistatin was assessed by RIA over 144 h. Activin (1–100 ng/ml) suppressed androstenedione production. Inhibin (1–100 ng/ml) blocked the suppressive effects of added activin, but increased androstenedione production when added alone, suggesting it was blocking endogenous activin produced by theca cells. Addition of SB-431542 (activin receptor inhibitor) and follistatin (500 ng/ml) increased androstenedione production, supporting this concept. Infection of theca cells with adenoviruses expressing inhibitory Smad6 or 7 increased androstenedione secretion, confirming that the suppressive effects of activin required activation of the Smad2/3 pathway. Activin decreased the expression levels of steroidogenic acute regulatory protein (STAR), whereas STAR expression was increased by inhibin and SB-431542, alone and in combination. CYP11A was unaffected. The expression of CYP17 encoding 17α-hydroxylase was unaffected by activin but increased by inhibin and SB-431542, and when added in combination the effect was further enhanced. The expression of 3β-hydroxysteroid dehydrogenase (3β-HSD) was significantly decreased by activin, while inhibin alone and in combination with SB-431542 both potently increased the expression of 3β-HSD. In conclusion, activin suppressed theca cell androstenedione production by decreasing the expression of STAR and 3β-HSD. Inhibin and other blockers of activin action reversed this effect, supporting the concept that endogenous thecal activin modulates androgen production in theca cells.
Introduction
Female fertility relies on a tightly controlled balance of hormonal signals and cellular interactions that result in the successful production of a mature oocyte for fertilization. Of these hormones, estradiol is a vital component required for oocyte maturation (Tesarik & Mendoza 1995). It is synthesized in granulosa cells from androgen precursors produced by theca cells that surround developing antral follicles, and is produced in increasing quantities by the growing follicle as development reaches the preovulatory stage (Young & McNeilly 2010). Before the expression of aromatase in granulosa cells, follicles are exposed to varying levels of androgens derived from thecal cells largely under the control of LH.
In vitro, testosterone inhibits meiotic maturation of the oocyte and subsequent embryo development (Laufer et al. 1984, Anderiesz & Trounson 1995). This negative effect of androgens would potentially be neutralized in vivo by expression of aromatase in the cumulus granulosa cells surrounding the oocyte in the preovulatory follicle, thus reducing exposure to these potentially damaging effects of excess testosterone and androgens.
In rodents, androgens not only can induce follicular atresia (Hillier & Ross 1979, Daniel & Armstrong 1986, Billig et al. 1993) but also have been shown to promote follicular development (Murray et al. 1998) and upregulate FSH-receptor expression in granulosa cells in culture (Tetsuka & Hillier 1997). In primates, intermittent exposure to androgens in normal female monkeys resulted in accelerated early stages of ovarian follicle development with reduced granulosa cell atresia and increased thecal cell mass, and enhanced granulosa cell FSH-receptor expression (Vendola et al. 1998, Weil et al. 1999). In contrast, chronic exposure led to intermittent or absent menstrual cycles (Billiar et al. 1985, Faiman et al. 1988). Thus, excess androgen production at an inappropriate time could directly affect the rates of follicle activation and lead to premature loss of primordial follicles.
In polycystic ovary syndrome (PCOS), follicle development is stalled, and a major characteristic is the presence of excess androgens that are produced by an intrinsic ability of thecal cells to produce increased levels of androgens (Gilling-Smith et al. 1994, 1997, Nelson et al. 1999, 2001, Nelson-Degrave et al. 2005). It has been suggested that androgen-induced expression of insulin-like growth factor 1 (IGF1) and its receptor in early growing follicles may predispose thecal cells to produce more androgens (Vendola et al. 1999), while MEK/ERK phosphorylation has been shown to be decreased in thecal cells from patients with PCOS compared with normal subjects (Nelson-Degrave et al. 2005). This excess androgen itself has been implicated in the failure of follicles to continue to grow in PCOS.
In spite of these studies, the modulation of androgen secretion by thecal cells from small antral stages as follicles develop into a preovulatory follicle during normal reproductive cycles is still unclear (Young & McNeilly 2010). Previous studies have shown that both BMPs (Glister et al. 2005, Campbell et al. 2006) and activin (Hillier et al. 1991a) can reduce androgen secretion from primary cultures in vitro. Furthermore, it was shown that inhibin alone could ‘stimulate’ androgen production and also neutralize the effects of added activin by human theca cells (Hillier et al. 1991b). However, there is no evidence that inhibin has a receptor coupled to any second messenger signaling pathway, but instead binds to betaglycan and the type 2 activin receptor to block the effects of activins (Lewis et al. 2000). Given that this is the case, the real effects of inhibin would then be to block the intrinsic inhibitory effects of activins that presumably are being produced by the thecal cells themselves. In this study, we have sought to clarify the role of inhibin on thecal cell androgen production by normal thecal cells maintained in primary cultures in conditions that prevent luteinization, thus mimicking the situation during normal follicle development. Using adenoviral vectors, follistatin and a specific chemical activin receptor blocker (SB-431542), we have shown that inhibin acts only as an activin inhibitor, rather than ‘stimulating’ androgen production. Instead inhibin removes the activin-induced brake on androgen production.
Materials and methods
Primary theca cell culture
Ovaries were obtained from sheep throughout the year and transferred to the laboratory in Medium 199 containing 20 mmol/l HEPES, 100 kIU/l penicillin, 0·1 ng/ml streptomycin and 1 mg/l amphotericin (Fungizone; all supplied by Sigma–Aldrich). Small follicles (<3·5 mm in diameter) were dissected from ovaries in Dulbecco's PBS without calcium or magnesium, with particular attention given to removal of all extraneous stromal tissues surrounding the thecal layers. Follicles were then hemisected, and washed vigorously using a 1 ml syringe, flushing repeatedly to separate granulosa cells from the thecal cells as described previously (Campbell et al. 1996). The thecal cells were then dispersed in an enzyme mixture containing 10 ml PBS, 5 g/l collagenase, 1 g/l hyaluronidase, 1 g/l protease and 0·001% donor calf serum (vol/vol) for ∼10 min at 37 °C with gentle agitation. The reaction was stopped by addition of 2 ml FCS, and cells were then washed by centrifugation at 800 g for 5 min and resuspended in culture media (DMEM-F12 with 100 kIU/l penicillin, 0·1 μg/l streptomycin, 3 mmol/l l-glutamine, 0·1% BSA (w/vol), 2·5 mg/l transferrin, 4 μg/l selenium, 10 ng/ml bovine insulin and 10 ng/ml LR3 IGF1). Ovine LH (code #AFP 8614B-NHPP-NIDDK supplied by Dr A Parlow, NHPP, Harbor-UCLA, Torrance, CA, USA) was also added to all culture media at 0·1 ng/ml ovine LH, unless otherwise stated. The cell pellets were then resuspended in culture media, and after a further wash, the number and viability of the cells were estimated using Trypan Blue exclusion. Cell viability was routinely more than 95%.
Cells were plated in 96-well plates at 75 000 cells in a total of 200 μl media/well. Various concentrations and combinations of ligand and chemical treatments were added to the media in quadruplicate, and the exact details are given in the ‘Results’ section. Activin A (code #338-AC) and follistatin (code #669FO/CF) were obtained from R & D Systems (Abingdon, Oxon, UK), inhibin A was from NIBSC (code #91/624, Hertfordshire, UK) and SB-431542 was supplied by Sigma–Aldrich. Cells were cultured under standard culture conditions consisting of a humidified atmosphere with 5% CO2 at 37 °C. For hormone analysis, cells were cultured for up to 6 days and the media changed every 48 h and stored at −20 °C for analysis at a later stage. At the end of the culture period, the cell viability was determined by Neutral Red dye uptake as described elsewhere (Campbell et al. 1996).
Adenovirus transfection
Recombinant adenoviruses were constructed from pcDNA3–Smad6, and pcDNA3–Smad7 expression plasmids obtained from Dr P Ten Dijke (The Netherlands Cancer Institute, Amsterdam, The Netherlands), as described previously (Fujii et al. 1999, Nakao et al. 1999), and were a generous gift from Dr Aristidis Moustakas (Ludwig Institute for Cancer Research, Uppsala, Sweden). Thecal cells were isolated and dissociated enzymatically as previously described and were plated in culture media under standard culture conditions at 75 000 cells/well and incubated overnight to allow cell attachment. The following day, all media were removed, infection of recombinant adenoviruses was performed at concentrations of 10, 50 and 100 plaque-forming units (pfu)/cell overnight, and then the following day, the cells were resuspended in 200 μl culture media±appropriate ligands/treatments. The culture media were then changed every 48 h up to 144 h, and stored at −20 °C for RIA.
Steroid assays
Concentrations of androstenedione were determined from non-extracted cell culture media by a previously described RIA method (Campbell et al. 1998). The sensitivity of the androstenedione assay was ∼5 pg/ml, and the inter- and intra-assay variation was <15%.
Quantitative RT-PCR
Thecal cell cultures for RNA collection were established as described above, but were plated at ∼750 000 cells/well in six-well plates and left to attach overnight. Treatments were added in fresh media the following day and left to incubate under standard culture conditions. After 24 h, another dose of ligand was added to the same media, and then again at 48 h. After the final dose was added at the 48 h time point, cells were incubated for a further 1 h, then media removed and stored at −20 °C for RIA, and cells lysed and RNA extracted using the Qiagen RNeasy Micro RNA extraction kit. RNA concentration and purity (A260/A280 ratio) were measured using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific). RNA was stored at −80 °C until cDNA was synthesized from 200 ng total RNA per reaction using Superscript VILO cDNA synthesis kit (Invitrogen) in a 20 μl reaction.
Primer sets for expression analysis were designed to amplify short regions of the target genes crossing an intron/exon boundary and are listed in Table 1. The primers were pre-validated using conventional PCR and the product was sequenced to confirm authenticity. Quantitative RT-PCR (qRT-PCR) was carried out using Power SYBR Green (Applied Biosystems), whereby the primer efficiency was determined by generating standard curves. A 10 μl final reaction volume was prepared using 1 μl synthesized cDNA, 2× Power SYBR Green PCR Master Mix, 5 μM primer pairs, and nuclease-free water. The qRT-PCR cycling program consisted of a denaturing step (95 °C for 10 min), annealing and extension step (95 °C for 15 s and 60 °C for 1 min) repeated 40 times, and a dissociation step (95 °C, 60 °C and 95 °C for 15 s each), using a real-time thermal cycler from Applied Biosystems (ABI-7500). Each sample was measured in duplicate, and negative controls included a reaction using cDNA prepared leaving out reverse transcriptase and a reaction substituting cDNA with nuclease-free water. The relative expression level of each target gene to GAPDH was quantified by the ΔΔCt method. Data were presented as average±s.e.m. and the statistical analysis was performed using the Student's t-test. P values <0·05 were regarded as significant, and levels of significance were indicated on graphs for each gene analyzed.
Table 1.
Forward and reverse primer sequences for quantitative RT-PCR and respective amplicon size
| Gene (accession) | Nucleotide sequence (5′–3′) | Product size (bp) |
|---|---|---|
| GAPDH (NM_001034034) | 229 | |
| Forward | GGCGTGAACCACGAGAAGTATAA | |
| Reverse | AAGCAGGGATGATGTTCTGG | |
| STAR (NM_001009243) | 194 | |
| Forward | GCATCCTCAAAGACCAGGAG | |
| Reverse | CTTGACACTGGGGTTCCACT | |
| CYP17 (NM_001009483) | 215 | |
| Forward | AGACATATTCCCTGCGCTGA | |
| Reverse | GCAGCTTTGAATCCTGCTCT | |
| 3β-HSD (NM_001135932) | 200 | |
| Forward | GGAGACATTCTGGATGAGCAG | |
| Reverse | TCTATGGTGCTGGTGTGGA | |
| CYP11A (NM_001093789) | 172 | |
| Forward | CAGGAGGCAGTAGAGGATGC | |
| Reverse | CAACGTCCCTCCAGAACTGT | |
| Id1 (NM_001097568) | 151 | |
| Forward | TCTGGGATCTGGAGTTGGAG | |
| Reverse | ATACGATCGTCCGCTGGAA | |
Statistical analysis
For each cell culture experiment, cells from at least four sheep were pooled and treatments carried out in quadruplicate. The significance of treatment effects was determined by ANOVA, and individual comparisons between treatments were made using Student's t-test. All hormone data were expressed as picograms of hormone per microliter of cell culture media.
Results
In this study, activin receptors (types IA, IB, IIA and IIB), Smad2/3, and the putative inhibin co-receptor betaglycan (transforming growth factor (TGF) βRIII) were identified in ovine theca and granulosa cells during folliculogenesis (data not shown).
Activin suppressed androgen secretion
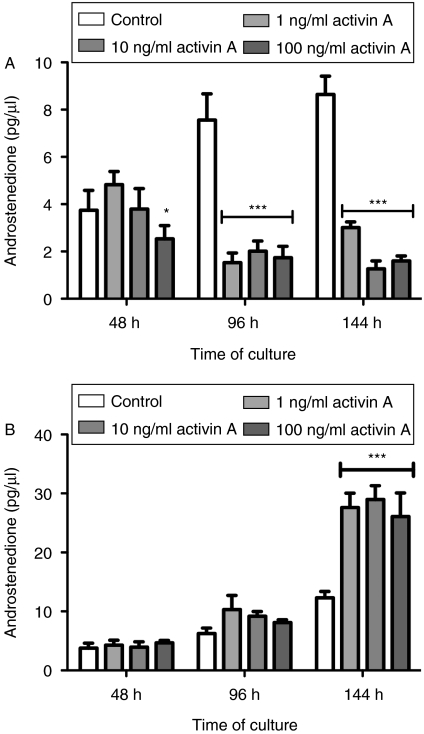
The effects of activin A at 1, 10 and 100 ng/ml on thecal androgen production are shown in Fig. 1A. Activin A was a potent inhibitor of androstenedione production from ovine thecal cells in vitro and was highly effective at the lowest dose tested (1 ng/ml). Viability evaluated by Neutral Red assay on completion of culturing at 144 h was not affected by any dose of activin (data not shown). Analyses also showed that theca cells maintained androgen production during the last 48 h culture period, indicating good health and steroidogenic capabilities. Furthermore, progesterone output measure by RIA (data not shown) remained at initial control levels throughout the culture period, indicating a lack of luteinization in the culture conditions as expected (Campbell et al. 1998).
Figure 1.
Androstenedione production in theca cell cultures (A) in the presence of 0–100 ng/ml activin A and (B) effects of inhibin A on theca androstenedione production. Cell culture media were replaced every 48 h and androstenedione secreted into spent media was evaluated by RIA. Results show average±s.e.m. Data provided are representative of at least four experiments, of which each experiment contained a separate pool of cells (n=4–5 sheep per pool), *P<0·05 and ***P<0·001 compared with the control group.
Inhibin increased androgen production
Inhibin increased (P<0·001) androstenedione production from theca cells cultured in vitro (Fig. 1B). Results show a potent response to inhibin, which was highly effective at the lowest dose evaluated (1 ng/ml). Inhibin added alone is likely to act by blocking the suppressive effects of endogenously produced activin, and possibly also acts on BMPs that may be produced by theca cells in vitro (Wiater & Vale 2003).
Inhibin blocked the suppressive effects of activin on theca steroidogenesis
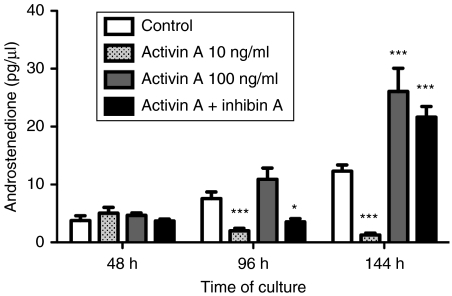
Inhibin blocked the suppressive effects of added activin on theca androstenedione production at 144 h but not at 96 h (Fig. 2). The effects of inhibin became more significant over time, and on completion of the culture, inhibin antagonized (P<0·001) activin suppression.
Figure 2.
Androstenedione production from theca cells in the presence of activin (10 ng/ml), inhibin (100 ng/ml) and both factors added together. Results give data of the average±s.e.m. of four replicate wells of pooled theca cells from four sheep, androstenedione was measured by RIA. *P<0·5 and ***P<0·001 compared with the control group.
Activin receptor inhibitor stimulated steroidogenesis
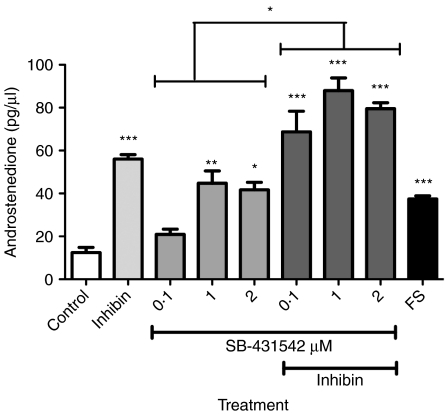
The small molecule inhibitor SB-431542 is a potent and specific inhibitor of activin receptors type IB (ALK4), TGFβRI (ALK5) and activin receptor type IC (ALK7) (Inman et al. 2002). In this experiment, the effects of this chemical activin receptor inhibitor were compared with those of inhibin. The manufacturer's instructions indicate that 10 μM SB-431542 is required for complete ablation of Smad2 phosphorylation by activin receptors IB, IC and TGFβRI. A dose response was therefore established from 0·1 to 10 μM, and the total androstenedione produced over time in culture is shown in Fig. 3. Results showed that SB-431542 increased androstenedione secretion at 0·1–2 μM, but had no effect from 5 to 10 μM (data not shown). Cell viability assessed on completion of the 144 h culture period showed that at concentrations of ≥5 μM SB-431542, cell viability decreased indicating that SB-431542 had a detrimental effect on these cells (data not shown).
Figure 3.
Androstenedione secretion from theca cells in the presence of inhibin (10 ng/ml), SB-431542 (0·1–2 μM), or both SB-431542 and inhibin (10 ng/ml), and follistatin (FS, 500 ng/ml) over an accumulative period of 144 h. Data presented are the average±s.e.m. of four replicate wells, all from the same pool of cells (n=4 sheep pooled). *P<0·05, **P<0·01 and ***P<0·001 compared with the control group. The statistical significance of SB-431542 alone and together with inhibin is also shown above each treatment group as *P<0·05.
SB-431542 was added at doses ranging from 0·1 to 2 μM to maintain cell viability. Inhibin was used in combination with SB-431542 to evaluate whether the level of androgen secreted from cells was equal in cells treated with either inhibitor. If inhibin was merely causing androgen production by blocking the suppressive effects of activin, then the expected result would be equivalent to that observed when the chemical activin receptor inhibitor was present. Interestingly, in this experiment, the level of androstenedione secretion was greater in cell cultures treated with both inhibin and SB-431542 (Fig. 3).
Follistatin inhibited activin suppression
Follistatin is a known antagonist of TGFβ superfamily members, including activin and BMPs, and was originally identified from the follicular fluid of ovarian follicles (Ueno et al. 1987). Follistatin (500 ng/ml) was added to cultures and androstenedione production measured (Fig. 3). The results show that the addition of follistatin increased androgen accumulation over 144 h in culture (P<0·001).
Inhibitory Smads 6 and 7 increased androgen production
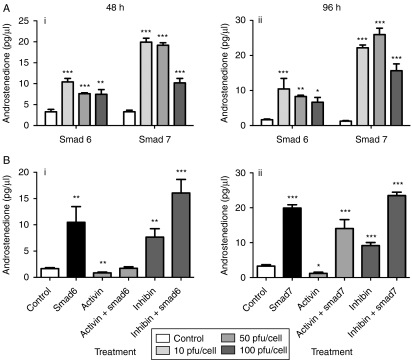
Smads 6 and 7 are intracellular signaling molecules that inhibit the signal transduction of BMP (Smad1/5/8), or BMP and activin (Smad2/3) signaling respectively. These inhibitory factors were transfected into theca cultures using adenoviruses to manipulate Smad signal transduction specifically. To establish the rate of infection required, the adenovirus construct was titred across concentrations from 10 to 100 pfu/cell seeded (Fig. 4A). Results indicated that 10 pfu/cell was sufficient to infect cells, and that at higher concentrations theca cell viability decreased. Overexpression of Smad6 or 7 by infecting cells at 10, 50 and 100 pfu/cell resulted in increased androstenedione production from theca cells in culture over two consecutive 48 h periods postinfection (Fig. 4A). It is not unexpected that the effects of Smad7 are more pronounced than those of Smad6 given that Smad7 inhibits both Smad1/5/8 and Smad2/3 (rather than just Smad1/5/8 for Smad6). BMPs have been previously shown to suppress androstenedione production in bovine and ovine theca cultures (Glister et al. 2005, Campbell et al. 2006), so blocking BMP signaling with Smad6 would remove this suppression which is presumably due to BMPs being produced endogenously by the thecal cells themselves. Adenovirus transfections were carried out at 10 pfu/cell thereafter.
Figure 4.
(A) Androstenedione secretion from theca cells infected with either Smad6 or 7 for a 48 h period of culture. Cells were infected with 10, 50 and 100 pfu/cell overnight before addition of new media and the beginning of the first 48 h period. (B) Androstenedione production from theca cells infected with Smad6 or 7 in combination with activin (10 ng/ml) and inhibin (10 ng/ml). Cells were infected with 10 pfu adenovirus/cell overnight before addition of appropriate ligands. Data presented are the average±s.e.m. of four replicate wells, all from the same pool of cells (n=4 sheep pooled). *P<0·05, **P<0·01 and ***P<0·001 compared with the control group.
As expected, when activin was added to cells overexpressing Smad6, the suppressive effects of activin were not markedly altered. However, when Smad7 was overexpressed, activin was unable to suppress androgen production (P<0·001; Fig. 4B). Inhibin effects were not affected by overexpression of either Smad6 or 7.
Activin and inhibin modulate expression of genes required for steroidogenesis
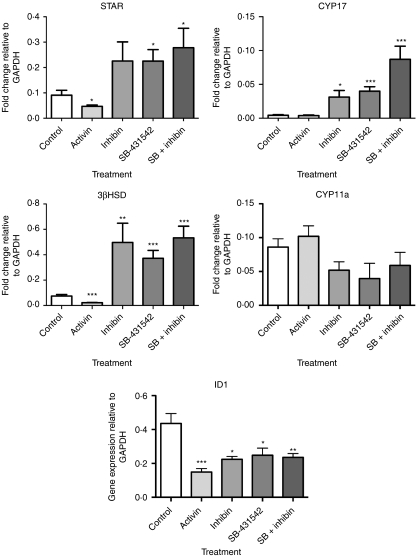
Given that activin and inhibin modulate androstenedione production from ovine theca cells in culture, we assessed the effects of these factors on the expression levels of components of the steroidogenic pathway (Fig. 5). The expression of steroidogenic acute regulatory protein (STAR), which is responsible for transportation of cholesterol esters into the mitochondria for initiation of steroidogenesis, was decreased in the presence of added activin (50 ng/ml), and increased by SB-431542 alone (2 μM) and in combination with inhibin (100 ng/ml; P<0·05). Activin alone did not affect the expression of CYP17 encoding 17α-hydroxylase (17αOH), whereas it was increased by inhibin (P<0·05) and SB-431542 (P<0·001), and when added in combination, the stimulatory effect was further enhanced (P<0·001). The expression level of 3β-hydroxysteroid dehydrogenase (3β-HSD) was significantly decreased (P<0·001) by activin, while inhibin alone (P<0·01) and in combination with SB-431542 (P<0·001) both increased the expression of 3β-HSD. There were no significant effects on the expression of cytochrome P450, family 11, subfamily A and polypeptide 1 (CYP11A), indicating that the effects of activin and inhibin are targeted primarily to the expression of 3β-HSD.
Figure 5.
The expression of steroidogenic acute regulatory protein (STAR), 17α-hydroxylase (CYP17), 3β-hydroxysteroid dehydrogenase (3β-HSD), cholesterol side-chain cleavage cytochrome P450 (CYP11A) and inhibitor of DNA binding 1 (ID1) was assessed in theca cells cultured alone, or in the presence of activin (50 ng/ml), inhibin (100 ng/ml) and/or SB-431542 (2 μM). Cells were cultured for a total of 64 h, whereby fresh ligand was added to each well at 24 and 48 h and then an hour before RNA isolation. Data are the average±s.e.m. for four replicate experiments (each experiment from a pool of cells harvested from four to five sheep) containing four replicate wells per treatment. *P<0·05, **P<0·01 and ***P<0·001 were assessed by Student's t-tests compared with the control group.
Expression of inhibitor of DNA-binding (ID) genes
ID1 expression levels were significantly reduced in the presence of activin, inhibin and SB-431542 (P<0·05), but the levels of ID2 did not change significantly for any treatment group. ID3 and ID4 mRNA levels were below detection levels (data not shown).
Discussion
There is contradictory evidence for a role of activin in follicle development, where some studies have shown stimulatory effects (Li et al. 1995, Smitz et al. 1998, Liu et al. 1999, Zhao et al. 2001, Thomas et al. 2003, McLaughlin et al. 2010), while others suggest activin inhibits development (Mizunuma et al. 1999, Ding et al. 2010), prevents luteinization (Myers et al. 2008), or has no effect at all (Fortune et al. 2000). Moreover, the concept of activin acting alone is far removed from the in vivo situation where many other factors are present that can antagonize and modulate the efficiency of activin signaling.
Studies on ovarian somatic cells have been conducted using media containing serum, which have been shown to induce luteinization of cells, thus changing biological functionality to principally the secretion of progesterone rather than androgens (Hillier et al. 1991b, Shukovski et al. 1993, Wrathall & Knight 1995). In this study, serum-free conditions were employed, and theca cells were cultured for a maximum of 144 h in the presence of low-dose LH to maintain androgen steroidogenic capacity. This study extends on previous findings in luteinized theca cells that activin A suppresses androgen production from thecal cells cultured in vitro without affecting cell viability (Hsueh et al. 1987, Hillier & Miro 1993, Wrathall & Knight 1995).
In the ovary, a major factor modulating activin bioactivity is inhibin, which is produced by granulosa cells once follicles reach the preantral stage of development (Roberts et al. 1993). It has been unclear whether inhibin acts by simply blocking binding of activin and BMPs to their receptors or it can elicit its biological effects through an unidentified mechanism not involving antagonism of activin/BMP receptors since no signaling pathways have been identified with inhibin bioactivity (Bernard et al. 2001, 2002). Association of inhibin with the membrane-anchored proteoglycan, betaglycan (also known as TGFβRIII), has been shown to increase the affinity of inhibin binding to activin type II receptors (Lewis et al. 2000). However, it is unclear if inhibin can elicit any signaling through betaglycan in a manner similar to TGFβ2 that utilizes the small cytoplasmic domain of this receptor for downstream signal transduction (Blobe et al. 2001). In bovine follicles, the abundance of betaglycan increased approximately fivefold as bovine follicles grew and decreased by 50% when follicles reached the final stages of development, indicating an important role during the stages of development (Glister et al. 2010). Betaglycan is also found in granulosa and thecal cells of sheep follicles (data not shown).
In this study, inhibin had a potent and robust stimulatory effect on androstenedione production when added to cell culture media alone. Furthermore, it blocked the suppressive effects of activin added to culture medium, confirming previous findings (Hillier et al. 1991b, Hillier & Miro 1993, Wrathall & Knight 1995, Campbell & Baird 2001). The observation that inhibin-treated cells produce considerably more androgen than cells with no inhibin added indicates that theca cells might produce activin and/or BMPs that act in an autocrine manner to maintain the level of androgen production at a basal level. The production of activin βA subunit was investigated in these cultures, and it was confirmed that theca cells produce activin βA (data not shown).
Follistatin is produced by granulosa cells and is abundant in follicles as soon as two layers of granulosa cells have developed, and is maintained at high levels of expression through all stages of follicle development up to ovulation (Shimasaki et al. 1989, Nakatani et al. 1991, Roberts et al. 1993, Braw-Tal et al. 1994, Tisdall et al. 1994, Izadyar et al. 1998, Sidis et al. 1998, Silva & Knight 1998). While follistatin was shown to have only a 10% affinity for BMPs as for activins (Glister et al. 2004), it was shown to reverse the suppressive effects of added activin A, TGFβ and BMP4/6/7 on thecal androgen production (Hsueh et al. 1987, Hillier & Miro 1993, Wrathall & Knight 1995, Cortvrindt et al. 1997, Glister et al. 2005). In our study, when added in excess, follistatin alone increased androgen production from theca cells, supporting the concept that theca cells produce activins/BMPs endogenously in vitro.
To further address this concept, we used the non-signaling chemical activin receptor inhibitor SB-431542 as a comparative measure against inhibin (Bak et al. 2009). Androstenedione production was enhanced from theca cultures to levels similar to those produced by inhibin-treated cells, and an additive effect was observed when inhibin and SB-431542 were added in combination. This may be due to a dynamic mechanism of inhibin action compared with the binding of the chemical compound, or the fact that inhibin also interferes with BMP signaling in addition to blocking activin signaling since SB-431542 does not appear to block BMPs effectively.
Intracellular inhibitory Smads were utilized to investigate signaling mechanisms. The overexpression of inhibitory Smads 6 and 7 alone (Fujii et al. 1999, Nakao et al. 1999, Kaivo-Oja et al. 2003) resulted in increased androstenedione production from theca cultures over time. Moreover, the inhibition of signaling by Smad7 was observed to have a more potent effect than that caused by Smad6. This result is logical given that Smad6 blocks BMP signaling through Smad1/5/8, whereas Smad7 blocks both Smad1/5/8 and Smad2/3 pathways. Of importance, these results show that by blocking activin and BMP signal transduction intracellularly, theca cells produce greater amounts of androstenedione than control cells over time.
As expected, since Smad6 affects the Smad1/5/8 pathway, it had no effect on the ability of activin A to suppress androstenedione production and equally did not block the stimulatory effects of inhibin. Activin signaling was blocked by the overexpression of Smad7 but not by Smad6. Inhibin had an additive effect on androstenedione production when Smad6 or 7 was overexpressed. Taken together, these studies indicate that inhibin acts similar to Smad signaling inhibitors Smad6 or 7 by blocking Smad signal transduction and increasing androgen production.
While activin and inhibin modulate theca cell steroidogenesis, it has not been clear how this is achieved. Gene expression analyses showed that activin suppressed expression levels of STAR and 3β-HSD, whilst inhibin and SB-431542 induced expression of CYP17 and 3β-HSD. A previous study using a CYP17 promoter–luciferase reporter system in the H295R cell line in vitro suggested that Smad3 directly inhibited CYP17 promoter activity (Derebecka-Holysz et al. 2008), but this was not replicated in this study with activin-induced Smad2/3 in primary thecal cells. The most potent and significant effects of activin, inhibin and SB-431542 were found on the expression levels of 3β-HSD, indicating an important role for these factors in modulating 3β-HSD abundance. 3β-HSD is required for synthesis of both progesterone and androgens in theca cells, and 17αOH is required for androgen production. If inhibin stimulates or removes an activin-induced inhibition of 3β-HSD expression, then it would make sense that more progesterone would be produced compared with androgens since the substrate pregnenolone is being used to make progesterone, therefore limiting the substrate available to 17αOH for 17-hydroxypregnenolone synthesis. However, past research has not shown any effect of inhibin on progesterone secretion from theca cells cultured in media containing 5% FCS (Hillier et al. 1991b), which may be due to luteinization. Testosterone is synthesized from androstenedione in theca cells by the enzyme 17β-hydroxysteroid, which has not been assessed in these studies, as this enzyme is not thought to be regulated by activins/inhibins due to the lack of response in testosterone levels in these and other studies (Shukovski et al. 1993). Furthermore, in this study, activin, inhibin, SB-431542, Smad6 or 7 did not have any effect on testosterone production (data not shown).
Expression of the inhibitor of DNA-binding protein genes (ID1–4) was assessed and the results agreed with previous studies on granulosa cells from sheep follicles (Hogg et al. 2010). Levels of mRNA encoding ID1 were suppressed in the presence of activin A, but no changes in ID2 expression were observed while ID3 and ID4 were undetectable. In our previous study (Hogg et al. 2010), activin decreased ID3 expression in granulosa cells; moreover, this study also provided evidence that in atretic follicles, ID3 and ID4 expression was partial or absent. Since primary theca cells were cultured for a short period of time and cells remained healthy and viable, changes in ID2 were not expected. In other experiments, BMPs increased ID1–4 expression in theca cultures (data not shown). BMPs are functional in the sheep follicle as evidenced by the presence of p-Smad1/5/8 in theca and granulosa cells (Hogg et al. 2010) and from previous in vitro studies on bovine thecal cells (Glister et al. 2010) and also our current results using Smads 6 and 7, both of which inhibit BMP signaling. Thus, it may be that in our culture system, any endogenous BMPs that are being produced are insufficient to maintain ID1–4 expression or are counteracted by the endogenous activin being produced by the theca cultures themselves. The failure of inhibin and the activin receptor blocker SB-431542 to maintain or increase ID2 requires further investigation.
In humans, inhibin B is the predominant form of inhibin present during the follicular phase of the cycle, and inhibin A in the luteal phase (Groome et al. 1996). However, inhibin B is not found in sheep ovaries. So, these studies were carried out using only inhibin A (McNeilly et al. 2002). In patients diagnosed with PCOS, studies have shown that circulating inhibin B levels were higher while activin A was lower in the follicular phase (Anderson et al. 1998, Lockwood et al. 1998, Norman et al. 2001, Shen et al. 2005). Inhibin A levels were reported to be lower in the follicular fluid of PCOS follicles compared with control follicles, and there were no differences in levels of activin A and inhibin B levels (Magoffin & Jakimiuk 1998). Activin has been previously shown to increase theca and granulosa cell proliferation (Miro & Hillier 1996, Duleba et al. 2001). In vitro studies using primary cultures of theca cells from PCOS ovaries produced more androgens than normal controls, had higher levels of steroid intermediates produced during steroidogenesis and had higher levels of STAR, LHR, CYP17 and CYP11A expression than in size-matched control follicles (Gilling-Smith et al. 1994, Nelson et al. 1999, Wickenheisser et al. 2000, Jakimiuk et al. 2001, Nelson et al. 2001). Ovaries from PCOS women have also been shown to have 1·5-fold higher expression of betaglycan than controls that would enhance inhibin binding and ability to block activin action, whereas no difference in activin receptor (types IA, IB, IIA and IIB) expression was detected (Zhu et al. 2010). These alterations in inhibin and activin concentrations coupled with increased thecal layers may allow increased androgen production in PCOS patients, caused not by an active excess secretion, but by a reduction in the local inhibition of androgen production by the reduced exposure to activins.
In conclusion, our present studies have confirmed that the biological effects of activin on theca cell androgen production are inhibitory, whereas inhibin acts in an opposing manner by causing an increase in the production of androgens. Furthermore, these effects of activin are modulated through the Smad signaling pathway that results in decreased levels of STAR and 3β-HSD gene expression. The studies also show that inhibin appears to act almost exclusively by blocking the inhibitory action of activins, a concept supported by the similar responses of increased androgen production when activin action was blocked by the activin receptor blocker SB-431542, the activin-binding protein follistatin and the Smad2/3 signaling pathway by Smad7. All the results also support the novel concept that theca cells themselves produce activins, and potentially BMPs which autoregulate androgen production locally. As follicles develop and acquire aromatase, they require more androgen precursor, and this is induced by increased production of inhibin by the granulosa cells, which then act to negate the suppressive effects of thecal, and granulosa cell activins, and BMPs. Therefore, inhibin does not stimulate androgen production, but acts to remove the effects of the inhibitor, activin, on suppressing CYP17 and 3β-HSD, thus allowing increased androgen production.
Acknowledgements
We acknowledge Kirsten Hogg for her gift of primers for quantitative RT-PCR assays. We also extend thanks to Joan Docherty and staff at the Marshall Building for help with all the animal work.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was funded by an MRC grant to A S M (G00007.01).
References
- Anderiesz C, Trounson AO. The effect of testosterone on the maturation and developmental capacity of murine oocytes in vitro. Human Reproduction. 1995;10:2377–2381. doi: 10.1093/oxfordjournals.humrep.a136302. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Groome NP, Baird DT. Inhibin A and inhibin B in women with polycystic ovarian syndrome during treatment with FSH to induce mono-ovulation. Clinical Endocrinology. 1998;48:577–584. doi: 10.1046/j.1365-2265.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- Bak B, Carpio L, Kipp JL, Lamba P, Wang Y, Ge RS, Hardy MP, Mayo KE, Bernard DJ. Activins regulate 17beta-hydroxysteroid dehydrogenase type I transcription in murine gonadotrope cells. Journal of Endocrinology. 2009;201:89–104. doi: 10.1677/JOE-08-0460. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Chapman SC, Woodruff TK. An emerging role for co-receptors in inhibin signal transduction. Molecular and Cellular Endocrinology. 2001;180:55–62. doi: 10.1016/S0303-7207(01)00500-7. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Chapman SC, Woodruff TK. Inhibin binding protein (InhBP/p120), betaglycan, and the continuing search for the inhibin receptor. Molecular Endocrinology. 2002;16:207–212. doi: 10.1210/me.16.2.207. [DOI] [PubMed] [Google Scholar]
- Billiar RB, Richardson D, Anderson E, Mahajan D, Little B. The effect of chronic and acyclic elevation of circulating androstenedione or estrone concentrations on ovarian function in the rhesus monkey. Endocrinology. 1985;116:2209–2220. doi: 10.1210/endo-116-6-2209. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Hsueh AJ. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology. 1993;133:2204–2212. doi: 10.1210/en.133.5.2204. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Liu X, Fang SJ, How T, Lodish HF. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. Journal of Biological Chemistry. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- Braw-Tal R, Tisdall DJ, Hudson NL, Smith P, McNatty KP. Follistatin but not alpha or beta A inhibin subunit mRNA is expressed in ovine fetal ovaries in late gestation. Journal of Molecular Endocrinology. 1994;13:1–9. doi: 10.1677/jme.0.0130001. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Baird DT. Inhibin A is a follicle stimulating hormone-responsive marker of granulosa cell differentiation, which has both autocrine and paracrine actions in sheep. Journal of Endocrinology. 2001;169:333–345. doi: 10.1677/joe.0.1690333. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Scaramuzzi RJ, Webb R. Induction and maintenance of oestradiol and immunoreactive inhibin production with FSH by ovine granulosa cells cultured in serum-free media. Journal of Reproduction and Fertility. 1996;106:7–16. doi: 10.1530/jrf.0.1060007. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Baird DT, Webb R. Effects of dose of LH on androgen production and luteinization of ovine theca cells cultured in a serum-free system. Journal of Reproduction and Fertility. 1998;112:69–77. doi: 10.1530/jrf.0.1120069. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Souza CJ, Skinner AJ, Webb R, Baird DT. Enhanced response of granulosa and theca cells from sheep carriers of the FecB mutation in vitro to gonadotropins and bone morphogenic protein-2, -4, and -6. Endocrinology. 2006;147:1608–1620. doi: 10.1210/en.2005-0604. [DOI] [PubMed] [Google Scholar]
- Cortvrindt R, Smitz J, Van Steirteghem AC. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Human Reproduction. 1997;12:759–768. doi: 10.1093/humrep/12.4.759. [DOI] [PubMed] [Google Scholar]
- Daniel SA, Armstrong DT. Androgens in the ovarian microenvironment. Seminars in Reproductive Endocrinology. 1986;4:89–100. doi: 10.1055/s-2007-1022489. [DOI] [Google Scholar]
- Derebecka-Holysz N, Lehmann TP, Holysz M, Trzeciak WH. Smad3 inhibits SF-1-dependent activation of the CYP17 promoter in H295R cells. Molecular and Cellular Biochemistry. 2008;307:65–71. doi: 10.1007/s11010-007-9585-4. [DOI] [PubMed] [Google Scholar]
- Ding CC, Thong KJ, Krishna A, Telfer EE. Activin A inhibits activation of human primordial follicles in vitro. Journal of Assisted Reproduction and Genetics. 2010;27:141–147. doi: 10.1007/s10815-010-9395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duleba AJ, Pehlivan T, Carbone R, Spaczynski RZ. Activin stimulates proliferation of rat ovarian thecal–interstitial cells. Biology of Reproduction. 2001;65:704–709. doi: 10.1095/biolreprod65.3.704. [DOI] [PubMed] [Google Scholar]
- Faiman C, Reyes FI, Dent DW, Fuller GB, Hobson WC, Thliveris JA. Effects of long-term testosterone exposure on ovarian function and morphology in the rhesus monkey. Anatomical Record. 1988;222:245–251. doi: 10.1002/ar.1092220305. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Molecular and Cellular Endocrinology. 2000;163:53–60. doi: 10.1016/S0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, Enomoto S, Kawabata M, Kato M, Ichijo H, Miyazono K. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Molecular Biology of the Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. Journal of Clinical Endocrinology and Metabolism. 1994;79:1158–1165. doi: 10.1210/jc.79.4.1158. [DOI] [PubMed] [Google Scholar]
- Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clinical Endocrinology. 1997;47:93–99. doi: 10.1046/j.1365-2265.1997.2321049.x. [DOI] [PubMed] [Google Scholar]
- Glister C, Kemp CF, Knight PG. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction. 2004;127:239–254. doi: 10.1530/rep.1.00090. [DOI] [PubMed] [Google Scholar]
- Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146:1883–1892. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- Glister C, Satchell L, Knight PG. Changes in expression of bone morphogenetic proteins (BMPs), their receptors and inhibin co-receptor betaglycan during bovine antral follicle development: inhibin can antagonize the suppressive effect of BMPs on thecal androgen production. Reproduction. 2010;140:699–712. doi: 10.1530/REP-10-0216. [DOI] [PubMed] [Google Scholar]
- Groome NP, Illingworth PJ, O'Brien M, Pai R, Rodger FE, Mather JP, McNeilly AS. Measurement of dimeric inhibin B throughout the human menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 1996;81:1401–1405. doi: 10.1210/jc.81.4.1401. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Ross GT. Effects of exogenous testosterone on ovarian weight, follicular morphology and intraovarian progesterone concentration in estrogen-primed hypophysectomized immature female rats. Biology of Reproduction. 1979;20:261–268. doi: 10.1095/biolreprod20.2.261. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Miro F. Inhibin, activin, and follistatin. Potential roles in ovarian physiology. Annals of the New York Academy of Sciences. 1993;687:29–38. doi: 10.1111/j.1749-6632.1993.tb43850.x. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant activin on androgen synthesis in cultured human thecal cells. Journal of Clinical Endocrinology and Metabolism. 1991a;72:1206–1211. doi: 10.1210/jcem-72-6-1206. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Yong EL, Illingworth PJ, Baird DT, Schwall RH, Mason AJ. Effect of recombinant inhibin on androgen synthesis in cultured human thecal cells. Molecular and Cellular Endocrinology. 1991b;75:R1–R6. doi: 10.1016/0303-7207(91)90234-J. [DOI] [PubMed] [Google Scholar]
- Hogg K, Etherington SL, Young JM, McNeilly AS, Duncan WC. Inhibitor of differentiation (Id) genes are expressed in the steroidogenic cells of the ovine ovary and are differentially regulated by members of the transforming growth factor-beta family. Endocrinology. 2010;151:1247–1256. doi: 10.1210/en.2009-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh AJ, Dahl KD, Vaughan J, Tucker E, Rivier J, Bardin CW, Vale W. Heterodimers and homodimers of inhibin subunits have different paracrine action in the modulation of luteinizing hormone-stimulated androgen biosynthesis. PNAS. 1987;84:5082–5086. doi: 10.1073/pnas.84.14.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Molecular Pharmacology. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Dijkstra G, Van Tol HT, Van den Eijnden-van Raaij AJ, Van den Hurk R, Colenbrander B, Bevers MM. Immunohistochemical localization and mRNA expression of activin, inhibin, follistatin, and activin receptor in bovine cumulus–oocyte complexes during in vitro maturation. Molecular Reproduction and Development. 1998;49:186–195. doi: 10.1002/(SICI)1098-2795(199802)49:2%3C186::AID-MRD9%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. Journal of Clinical Endocrinology and Metabolism. 2001;86:1318–1323. doi: 10.1210/jc.86.3.1318. [DOI] [PubMed] [Google Scholar]
- Kaivo-Oja N, Bondestam J, Kamarainen M, Koskimies J, Vitt U, Cranfield M, Vuojolainen K, Kallio JP, Olkkonen VM, Hayashi M, et al. Growth differentiation factor-9 induces Smad2 activation and inhibin B production in cultured human granulosa–luteal cells. Journal of Clinical Endocrinology and Metabolism. 2003;88:755–762. doi: 10.1210/jc.2002-021317. [DOI] [PubMed] [Google Scholar]
- Laufer N, DeCherney AH, Haseltine FP, Behrman HR. Steroid secretion by the human egg–corona–cumulus complex in culture. Journal of Clinical Endocrinology and Metabolism. 1984;58:1153–1157. doi: 10.1210/jcem-58-6-1153. [DOI] [PubMed] [Google Scholar]
- Lewis KA, Gray PC, Blount AL, MacConell LA, Wiater E, Bilezikjian LM, Vale W. Betaglycan binds inhibin and can mediate functional antagonism of activin signalling. Nature. 2000;404:411–414. doi: 10.1038/35006129. [DOI] [PubMed] [Google Scholar]
- Li R, Phillips DM, Mather JP. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136:849–856. doi: 10.1210/en.136.3.849. [DOI] [PubMed] [Google Scholar]
- Liu X, Andoh K, Abe Y, Kobayashi J, Yamada K, Mizunuma H, Ibuki Y. A comparative study on transforming growth factor-beta and activin A for preantral follicles from adult, immature, and diethylstilbestrol-primed immature mice. Endocrinology. 1999;140:2480–2485. doi: 10.1210/en.140.6.2480. [DOI] [PubMed] [Google Scholar]
- Lockwood GM, Muttukrishna S, Groome NP, Matthews DR, Ledger WL. Mid-follicular phase pulses of inhibin B are absent in polycystic ovarian syndrome and are initiated by successful laparoscopic ovarian diathermy: a possible mechanism regulating emergence of the dominant follicle. Journal of Clinical Endocrinology and Metabolism. 1998;83:1730–1735. doi: 10.1210/jc.83.5.1730. [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Jakimiuk AJ. Inhibin A, inhibin B and activin A concentrations in follicular fluid from women with polycystic ovary syndrome. Human Reproduction. 1998;13:2693–2698. doi: 10.1093/humrep/13.10.2693. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Bromfield JJ, Albertini DF, Telfer EE. Activin promotes follicular integrity and oogenesis in cultured pre-antral bovine follicles. Molecular Human Reproduction. 2010;16:644–653. doi: 10.1093/molehr/gaq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS, Souza CJ, Baird DT, Swanston IA, McVerry J, Crawford J, Cranfield M, Lincoln GA. Production of inhibin A not B in rams: changes in plasma inhibin A during testis growth, and expression of inhibin/activin subunit mRNA and protein in adult testis. Reproduction. 2002;123:827–835. doi: 10.1530/rep.0.1230827. [DOI] [PubMed] [Google Scholar]
- Miro F, Hillier SG. Modulation of granulosa cell deoxyribonucleic acid synthesis and differentiation by activin. Endocrinology. 1996;137:464–468. doi: 10.1210/en.137.2.464. [DOI] [PubMed] [Google Scholar]
- Mizunuma H, Liu X, Andoh K, Abe Y, Kobayashi J, Yamada K, Yokota H, Ibuki Y, Hasegawa Y. Activin from secondary follicles causes small preantral follicles to remain dormant at the resting stage. Endocrinology. 1999;140:37–42. doi: 10.1210/en.140.1.37. [DOI] [PubMed] [Google Scholar]
- Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. Journal of Reproduction and Fertility. 1998;113:27–33. doi: 10.1530/jrf.0.1130027. [DOI] [PubMed] [Google Scholar]
- Myers M, van den Driesche S, McNeilly AS, Duncan WC. Activin A reduces luteinisation of human luteinised granulosa cells and has opposing effects to human chorionic gonadotropin in vitro. Journal of Endocrinology. 2008;199:201–212. doi: 10.1677/JOE-08-0302. [DOI] [PubMed] [Google Scholar]
- Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. Journal of Clinical Investigation. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani A, Shimasaki S, Depaolo LV, Erickson GF, Ling N. Cyclic changes in follistatin messenger ribonucleic acid and its protein in the rat ovary during the estrous cycle. Endocrinology. 1991;129:603–611. doi: 10.1210/endo-129-2-603. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Molecular Endocrinology. 1999;13:946–957. doi: 10.1210/me.13.6.946. [DOI] [PubMed] [Google Scholar]
- Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, Legro RS, Strauss JF, III, McAllister JM. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2001;86:5925–5933. doi: 10.1210/jc.86.12.5925. [DOI] [PubMed] [Google Scholar]
- Nelson-Degrave VL, Wickenheisser JK, Hendricks KL, Asano T, Fujishiro M, Legro RS, Kimball SR, Strauss JF, III, McAllister JM. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Molecular Endocrinology. 2005;19:379–390. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Milner CR, Groome NP, Robertson DM. Circulating follistatin concentrations are higher and activin concentrations are lower in polycystic ovarian syndrome. Human Reproduction. 2001;16:668–672. doi: 10.1093/humrep/16.4.668. [DOI] [PubMed] [Google Scholar]
- Roberts VJ, Barth S, el-Roeiy A, Yen SS. Expression of inhibin/activin subunits and follistatin messenger ribonucleic acids and proteins in ovarian follicles and the corpus luteum during the human menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 1993;77:1402–1410. doi: 10.1210/jc.77.5.1402. [DOI] [PubMed] [Google Scholar]
- Shen ZJ, Chen XP, Chen YG. Inhibin B, activin A, and follistatin and the pathogenesis of polycystic ovary syndrome. International Journal of Gynaecology and Obstetrics. 2005;88:336–337. doi: 10.1016/j.ijgo.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Koga M, Buscaglia ML, Simmons DM, Bicsak TA, Ling N. Follistatin gene expression in the ovary and extragonadal tissues. Molecular Endocrinology. 1989;3:651–659. doi: 10.1210/mend-3-4-651. [DOI] [PubMed] [Google Scholar]
- Shukovski L, Dyson M, Findlay JK. The effects of follistatin, activin and inhibin on steroidogenesis by bovine thecal cells. Molecular and Cellular Endocrinology. 1993;97:19–27. doi: 10.1016/0303-7207(93)90207-Z. [DOI] [PubMed] [Google Scholar]
- Sidis Y, Fujiwara T, Leykin L, Isaacson K, Toth T, Schneyer AL. Characterization of inhibin/activin subunit, activin receptor, and follistatin messenger ribonucleic acid in human and mouse oocytes: evidence for activin's paracrine signaling from granulosa cells to oocytes. Biology of Reproduction. 1998;59:807–812. doi: 10.1095/biolreprod59.4.807. [DOI] [PubMed] [Google Scholar]
- Silva CC, Knight PG. Modulatory actions of activin-A and follistatin on the developmental competence of in vitro-matured bovine oocytes. Biology of Reproduction. 1998;58:558–565. doi: 10.1095/biolreprod58.2.558. [DOI] [PubMed] [Google Scholar]
- Smitz J, Cortvrindt R, Hu Y, Vanderstichele H. Effects of recombinant activin A on in vitro culture of mouse preantral follicles. Molecular Reproduction and Development. 1998;50:294–304. doi: 10.1002/(SICI)1098-2795(199807)50:3%3C294::AID-MRD5%3E3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Tesarik J, Mendoza C. Nongenomic effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. Journal of Clinical Endocrinology and Metabolism. 1995;80:1438–1443. doi: 10.1210/jc.80.4.1438. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Hillier SG. Differential regulation of aromatase and androgen receptor in granulosa cells. Journal of Steroid Biochemistry and Molecular Biology. 1997;61:233–239. doi: 10.1016/S0960-0760(97)80017-9. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Armstrong DG, Telfer EE. Activin promotes oocyte development in ovine preantral follicles in vitro. Reproductive Biology and Endocrinology. 2003;1:76. doi: 10.1186/1477-7827-1-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall DJ, Hudson N, Smith P, McNatty KP. Localization of ovine follistatin and alpha and beta A inhibin mRNA in the sheep ovary during the oestrous cycle. Journal of Molecular Endocrinology. 1994;12:181–193. doi: 10.1677/jme.0.0120181. [DOI] [PubMed] [Google Scholar]
- Ueno N, Ling N, Ying S-Y, Esch F, Shimasaki S, Guillemin R. Isolation and partial characterization of follistatin: a single-chain Mr 35 000 monomeric protein that inhibits the release of follicle-stimulating hormone. PNAS. 1987;84:8282–8286. doi: 10.1073/pnas.84.23.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. Journal of Clinical Investigation. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendola K, Zhou J, Wang J, Bondy CA. Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Human Reproduction. 1999;14:2328–2332. doi: 10.1093/humrep/14.9.2328. [DOI] [PubMed] [Google Scholar]
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. Journal of Clinical Endocrinology and Metabolism. 1999;84:2951–2956. doi: 10.1210/jc.84.8.2951. [DOI] [PubMed] [Google Scholar]
- Wiater E, Vale W. Inhibin is an antagonist of bone morphogenetic protein signaling. Journal of Biological Chemistry. 2003;278:7934–7941. doi: 10.1074/jbc.M209710200. [DOI] [PubMed] [Google Scholar]
- Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Differential activity of the cytochrome P450 17alpha-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. Journal of Clinical Endocrinology and Metabolism. 2000;85:2304–2311. doi: 10.1210/jc.85.6.2304. [DOI] [PubMed] [Google Scholar]
- Wrathall JH, Knight PG. Effects of inhibin-related peptides and oestradiol on androstenedione and progesterone secretion by bovine theca cells in vitro. Journal of Endocrinology. 1995;145:491–500. doi: 10.1677/joe.0.1450491. [DOI] [PubMed] [Google Scholar]
- Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- Zhao J, Taverne MA, van der Weijden GC, Bevers MM, van den Hurk R. Effect of activin A on in vitro development of rat preantral follicles and localization of activin A and activin receptor II. Biology of Reproduction. 2001;65:967–977. doi: 10.1095/biolreprod65.3.967. [DOI] [PubMed] [Google Scholar]
- Zhu R, Zhou X, Chen Y, Qiu C, Xu W, Shen Z. Aberrantly increased mRNA expression of betaglycan, an inhibin co-receptor in the ovarian tissues in women with polycystic ovary syndrome. Journal of Obstetrics and Gynaecology Research. 2010;36:138–146. doi: 10.1111/j.1447-0756.2009.01103.x. [DOI] [PubMed] [Google Scholar]