Abstract
The hypothalamic-pituitary-adrenal (HPA) axis is a primary mechanism in the allostatic process through which early life stress (ELS) contributes to disease. Studies of the influence of ELS on children’s HPA axis functioning have yielded inconsistent findings. To address this issue, the present study considers multiple types of ELS (maternal depression, paternal depression, and family expressed anger), mental health symptoms, and two components of HPA functioning (trait-like and epoch-specific activity) in a long-term prospective community study of 357 children. ELS was assessed during the infancy and preschool periods; mental health symptoms and cortisol were assessed at child ages 9, 11, 13, and 15 years. A 3-level hierarchical linear model addressed questions regarding the influences of ELS on HPA functioning and its co-variation with mental health symptoms. ELS influenced trait-like cortisol level and slope, with both hyper- and hypo-arousal evident depending on type of ELS. Further, type(s) of ELS influenced co-variation of epoch-specific HPA functioning and mental health symptoms, with a tighter coupling of HPA alterations with symptom severity among children exposed previously to ELS. Results highlight the importance of examining multiple types of ELS and dynamic HPA functioning in order to capture the allostatic process unfolding across the transition into adolescence.
The regulatory changes of the hypothalamic-pituitary-adrenal (HPA) axis, and alterations in cortisol levels in particular, have long been considered a primary mechanism through which early life stress (ELS) is translated into disease – both physical and psychiatric – across the life span (McEwen, 1998). Previous findings suggest that this broad association of ELS, HPA axis functioning, and disease states may reflect an allostatic process whereby, for some individuals, ELS results in sustained and/or repeated HPA axis dysregulation and the “wear and tear” on the body reflective of allostatic load (Juster, McEwen, & Lupien, 2010; Lupien et al., 2006). There is solid evidence, supported by the findings of adult and preclinical studies (see Gunnar & Quevedo, 2007; Meaney & Szyf, 2005; Sanchez, 2006), that children exposed to ELS exhibit alterations in HPA functioning and increased mental health problems (reviewed in Gunnar & Vasquez, 2006; Trickett, Negriff, Ji, & Peckins, 2011). However, there are many inconsistencies regarding the nature and degree of HPA alterations resulting from different types of ELS, ranging from severe maltreatment and neglect to the less memorable yet prevalent, often co-occurring, day-to-day deficits in the early care-taking environment (reviewed in Gunnar & Quevedo, 2007; McCrory, De Brito, & Viding, 2010). In addition, previous studies of children have not included repeated assessments of HPA functioning and mental health problems over sufficiently long periods of time to investigate the ways in which ELS influences the allostatic process unfolding over time. The present study addresses these issues, using an allostasis framework to investigate the influence of multiple types of ELS on later HPA axis development across a six-year period from childhood into adolescence. In addition to investigating the influence of ELS on children’s basal, or trait-like, HPA activity across this time period, the study explores the ways in which HPA activity might reflect allostatic challenges associated with episodic increases in children’s mental health symptoms.
A substantial body of research has established that children exposed to chronic adversities early in life are more likely than other children to suffer a variety of mental health problems (Essex et al., 2006; Halligan, Herbert, Goodyer, & Murray, 2007; Kim, Cicchetti, Rogosch, & Manly, 2009; Trickett et al., 2011). Numerous individual factors have been proposed to explain this association, including social and academic functioning, cognitive styles and biases, emotional/behavioral dysregulation, and alterations in stress neurobiology (Cicchetti & Rogosch, 2007; Cicchetti & Valentino, 2006; Essex et al., 2006; Gunnar & Quevedo, 2007; Heim, Shugart, Craighead, & Nemeroff, 2010). Of particular relevance to questions regarding the allostatic process is the growing body of research focused on the neurobiology of stress during childhood, including the primary allostatic mediators of the hypothalamic-pituitary-adrenocortical (HPA) axis and sympathetic-adrenomedullary (SAM) system and associated cortico-limbic pathways (reviewed in Gunnar & Quevedo, 2007).
The SAM and the HPA axis are distinct but interrelated systems designed to help the body mobilize resources to deal with psychosocial and physical challenges (Gunnar & Quevedo, 2007; Lupien et al., 2006). The stimulation and subsequent regulation of both systems is integral for survival of any organism (McEwen, 1998); however, the contribution of the HPA axis, and its steroid hormone end-product cortisol, to the process of allostasis is of particular interest in developmental studies. Compared to catecholamines released by the SAM system, cortisol is able to cross the blood-brain barrier, exerting direct effects on the brain. In addition, because the HPA axis continues to develop through childhood, exposure to cortisol during these formative years may alter receptor sensitivity in the brain, resulting in long-lasting effects on HPA basal activity as well as responsivity to episodic challenges (Gunnar & Quevedo, 2007).
Much of the human research on stress neurobiology is derived from preclinical research. Numerous studies of rodents and non-human primates have clearly demonstrated that early adverse environmental conditions, ranging from maternal deprivation to less severe variations in maternal care, can sensitize offspring’s stress response systems, resulting in immediate and long-term alterations in HPA functioning and behavior (reviewed in Meaney & Szyf, 2005; Sanchez, 2006). Further, effects of maternal stress appear to be mediated by disturbances in the quality of the mother-infant interaction (Meaney & Szyf, 2005), and in non-human primates, associated disruptions in caregiver attachment (Sanchez, 2006).
Because of the prolonged period of human development, much of the human research on the long-term effects of ELS has relied on retrospective reports of early maltreatment. While useful, these studies cannot describe the process by which ELS “gets under the skin” to influence HPA development. Substantial support exists for the broad influence of early abuse and severe neglect on HPA functioning, although there are major inconsistencies regarding the nature and direction of effects (Miller, Chen, & Zhou, 2007), especially when comparing children vs. adults (Tarullo & Gunnar, 2006). It has been hypothesized that types of ELS that heighten HPA activity in childhood contribute to the development of blunted HPA activity in adulthood (Gunnar, Morison, Chisholm, & Schuder, 2001). In addition, there is now considerable evidence that children’s HPA functioning varies by type of maltreatment (Cicchetti & Rogosch, 2001; Trickett et al., 2011), and that the association of early maltreatment with altered HPA functioning occurs more commonly among those who are currently experiencing depression or internalizing problems (Harkness, Stewart, & Wynne-Edwards, 2011; Hart, Gunnar, & Cicchetti, 1996; Kaufman, 1991). Importantly, the combination of type of maltreatment and current internalizing symptoms has unique effects on later HPA functioning (Cicchetti, Rogosch, Gunnar, & Toth, 2010). Although genetics undoubtedly play an important role in the association between ELS and HPA functioning in individuals with depression/internalizing problems (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008), the findings of preclinical cross-fostering studies (Weaver et al., 2004) and human interventions (Fisher & Stoolmiller, 2008) demonstrate that the early care-taking environment also exerts a unique influence.
Previous studies of childhood maltreatment provide compelling evidence of the influence of severe ELS on later HPA alterations. However, effects of severe ELS may be different than the effects of less extreme day-to-day deficits in the early care-taking environment. A developmental psychopathology perspective emphasizes the need for investigating the full continuum of experiences from normal to abnormal, and the importance of such mutually informative studies for understanding developmental processes (Cicchetti & Toth, 2009). Among the less extreme types of ELS, maternal depression (and maternal stress more generally) has received considerable attention. Exposure to maternal depression/stress during the infancy and preschool periods is associated with children’s elevated cortisol levels (e.g., Brennan et al., 2008; Halligan et al., 2007; Lupien, King, Meaney, & McEwen, 2000). There is also limited evidence that this association is found only among children with high levels of internalizing problems (Ashman, Dawson, Panagiotides, Yamada, & Wilkinson, 2002). In parallel with animal studies showing sensitization effects (Gunnar & Quevedo, 2007; Sanchez, 2006), we have shown (Essex, Klein, Cho, & Kalin, 2002) that preschool-age children exposed to concurrent maternal stress (especially maternal depression) evidenced increased afternoon cortisol levels only if they had been exposed to similar stress beginning at birth. Extending the findings of preclinical studies (Meaney & Szyf, 2005; Sanchez, 2006), it is postulated that maternal depression/stress impacts child HPA functioning through deficits in mother-child interactions and associated insecure attachments (Gunnar & Vasquez, 2006). Compared to non-depressed mothers, depressed mothers are often less emotionally available and responsive to their children’s needs (Bureau, Easterbrooks, & Lyons-Ruth, 2009; Feng, Shaw, Skuban, & Lane, 2007), and their children are less likely to be securely attached (Milan, Snow, & Belay, 2009; Toth, Rogosch, Sturge-Apple, & Cicchetti, 2009). Unresponsive maternal care and insecure attachment in turn have been associated with children’s heightened HPA reactivity (Bernard & Dozier, 2010; Hastings et al., 2011), flatter diurnal cortisol slopes (Pendry & Adam, 2007), and heightened morning cortisol levels in adolescence (Murray, Halligan, Goodyer, & Herbert, 2010).
Less attention has been paid to effects of paternal care on children. Paternal depression and negative parenting behaviors (Connell & Goodman, 2002; Gross, Shaw, Moilanen, Dishion, & Wilson, 2008; Ramchandani, Stein, Evans, O’Connor, & The ALSPAC Study Team, 2005; Schacht, Cummings, & Davies, 2009; Wilson & Durbin, 2010) are associated with children’s mental health symptoms and other negative developmental outcomes. However, very little is known about the neurobiology underlying these associations. Mills-Koonce and colleagues (Mills-Koonce et al., 2011) found that paternal negative parenting behaviors were associated with heightened cortisol reactivity among infants and elevated cortisol levels in toddlers. In addition, we have shown that the combination of low father involvement in infancy and high cortisol reactivity at age 7 prospectively predicted increased mental health problems at age 9 years (Boyce et al., 2006). Importantly, effects were independent of early maternal depression, suggesting that early paternal care-taking has a unique influence on children’s HPA axis development and mental health.
Negative family dynamics characterized by conflict and anger also may influence children’s HPA axis. A few studies have demonstrated that inter-parental conflict and expressed anger are associated with children’s elevated morning cortisol levels (Davies, Sturge-Apple, Cicchetti, Manning, & Zale, 2009; Pendry & Adam, 2007) and, among kindergartners but not adolescents, elevated bedtime cortisol and flatter diurnal slopes (Pendry & Adam, 2007). A few other studies have shown that the accumulation of several negative aspects of family dynamics (e.g., turmoil, exposure to violence) and physical aspects of the household (e.g., crowding, noise) (Evans, 2003) as well as negative events in the family environment like arguments and punishment (Flinn & England, 1995) are associated with children’s elevated basal cortisol. Although these latter studies did not distinguish the effects of family conflict/anger from other negative aspects of the family environment, Davies and colleagues (Davies, Sturge-Apple, Cicchetti, & Cummings, 2007) found that inter-parental conflict was associated with children’s blunted cortisol reactivity independent of other family processes. This suggests that family conflict/anger has a unique influence on children’s HPA development, although the direction of the effect remains unclear.
Although previous studies generally support an allostasis model of ELS, HPA functioning, and children’s mental health (see reviews in Juster et al., 2010; Lupien et al., 2006), inconsistent findings (Miller et al., 2007) and the lack of long-term developmental studies (Gunnar, 2001) leave it unclear just how the allostatic process works from childhood into adolescence. Inconsistent findings may result, in part, from the focus of most studies on single types of ELS and the relative lack of consideration of children’s current psychiatric state. To our knowledge, there are no studies of children’s HPA functioning and mental health which consider potential differences in the influences of multiple types of ELS representing less severe day-today deficits in the early care-taking environment, including both maternal and paternal factors as well as broader family dynamics. Further, outside of studies of early maltreatment (Cicchetti et al., 2010; Harkness et al., 2011; Hart et al., 1996; Kaufman, 1991), only a few studies of less severe deficits in the early care-taking environment (e.g., Ashman et al., 2002) have considered children’s current mental health problems.
In addition, to our knowledge, there are no long-term prospective developmental studies of ELS and HPA functioning with the repeated assessments of HPA functioning and mental health problems critical for investigating this allostatic process. From the perspective of allostasis, alterations in HPA functioning will occur to help the individual adapt to current challenges – in this case, episodic increases in mental health problems. There is considerable instability of mental health problems throughout childhood (Essex et al., 2009), and recent work shows that the expression of mental health symptoms is capable of dysregulating the HPA axis (Ruttle et al., 2011). Episodic changes in symptom levels may induce an allostatic state to which the child’s regulatory systems must adapt, above and beyond the sustained impact of ELS. An allostasis framework postulates that the HPA component that is responsive to episodic challenges is distinct from the longer-term stable component that contributes to an individual’s homeostatic set-point. Thus, the investigation of the influences of ELS on the development of children’s HPA functioning and mental health problems requires a study capable of distinguishing these two HPA components. Recent methodological advances based on cross-sectional or short-term longitudinal studies have enabled researchers to distinguish a short-term stable component of cortisol within an assessment period (i.e., epoch-specific) from moment-to-moment cortisol fluctuations (Hruschka, Kohrt, & Worthman, 2005; Shirtcliff & Essex, 2008; Shirtcliff, Granger, Booth, & Johnson, 2005; Wismer Fries, Shirtcliff, & Pollak, 2008). However, to our knowledge there have been no longer-term longitudinal studies with repeated assessments of HPA functioning and mental health symptoms capable of distinguishing the influences of ELS on children’s trait-like HPA activity from the influences on the ways in which HPA activity covaries with mental health symptoms. This would provide a strong test of the allostatic process, including ELS effects on basal HPA functioning and the ways in which alterations in HPA functioning unfold at points in children’s development when they are challenged by increased mental health problems.
Present Study
The overall aim of the present study is to investigate the influences of three types of ELS reflecting day-to-day deficits in the early care-taking environment on HPA functioning and its co-variation with mental health symptoms across childhood and into adolescence. The three types of ELS include maternal and paternal depressive symptoms, representing the influences of both types of care-takers, and family expressed anger, representing the influence of broader family dynamics. Mental health symptoms and cortisol were assessed when children were ages 9, 11, 13, and 15 years; and the trait-like component of cortisol that is stable across the entire six-year period was distinguished from the epoch-specific component that varies with the episodic challenge of increased mental health symptoms.
The first major research question is whether ELS influences trait-like HPA functioning and its development. We hypothesize that all three types of ELS will exert long-term effects on trait-like cortisol and its development from childhood into adolescence. Because different types of ELS tend to aggregate in families and frequently co-occur (Repetti, Taylor, & Seeman, 2002), we consider the joint influences, including their interactions, of the three types of ELS. Due to insufficient prior evidence regarding differential influences by type of ELS, we take an exploratory approach to these questions. To investigate the developmental processes from childhood to adolescence, we consider child age rather than pubertal status. Although our larger body of work emphasizes pubertal maturation and tempo (Ellis, Shirtcliff, Boyce, Deardorff, & Essex, 2011), we have found in previous analyses of this same data set that age is a more robust indicator of HPA development (Shirtcliff et al., 2011). Further, a measure of puberty (e.g., pubertal timing) that is uncorrelated with age would be appropriate statistically, but pubertal timing is not a changing developmental phenomenon (Marceau, Ram, Houts, Grimm, & Susman, 2011), which is of central interest here.
The second major research question is whether children’s HPA functioning co-varies with current mental health symptoms at each assessment in childhood and adolescence, and whether the patterns of co-variation are dependent on prior ELS exposure. Because of the high co-occurrence of internalizing and externalizing symptoms at each assessment (range of rs = .46 to .54), we created two scores which distinguished the severity of children’s symptoms (i.e., average of the two standardized scores, reflecting score commonality) from the directionality of symptoms (i.e., half difference of the standardized scores, reflecting what differentiates the scores). Because severity and directionality scores are statistically independent, they can be analyzed together without additional Type I error (Essex, Klein, Cho, & Kraemer, 2003). We hypothesize that children’s HPA functioning will change from one assessment period to the next in correspondence with levels of mental health symptom severity, and that this coupling of HPA functioning with mental health symptoms will differ by ELS exposure. Because of insufficient prior evidence regarding differential influences by type of ELS, we take an exploratory approach to these questions.
Method
Participants
A total of 570 women and their husbands/partners were initially contacted during the second trimester of pregnancy through obstetric/gynecology and low income clinics in the Milwaukee (80%) and Madison (20%), Wisconsin geographical areas, for participation in the Wisconsin Maternity Leave and Health Project (now called the Wisconsin Study of Families and Work, WSFW). Because the project originally focused on parental employment leave, family stress, and women’s health outcomes during the first postnatal year, female participants met the following inclusion criteria: (a) over age 18; (b) living with the biological father; (c) at least one member of the couple working for pay; (d) not a student; and (e) not unemployed (see Hyde, Klein, Essex, & Clark, 1995, for details). Of the original sample, 560 (98.3%) had eligible live births. At the time of the cortisol assessments (beginning at age 9), only those families living within geographic proximity to the project offices were asked to participate in saliva collection; thus, analyses include the 357 families (64% of the original sample) who lived in geographic proximity and agreed to participate in saliva collection. At the time of recruitment, there were no significant differences between the 357 participants and the remaining families from the original sample in terms of parental education, marital or ethnic status, or annual family income; parents in the 357 families were slightly older: mothers M = 29.6 (SD = 4.2) versus 28.9 (SD = 4.6), t(568) = −1.97, p < .05; fathers M = 31.6 (SD = 5.2) versus 30.7 (SD = 4.8), t(548) = −2.17, p < .05. At the time of recruitment, 44% of the 357 mothers and 52% of the fathers had a high school or technical degree or less, while the remainder had at least a college degree; 40% were first-time mothers. Most couples were married (95%); median annual family income was $48,000 (range = <$10,000 to >$180,000). Eleven percent of participating children represented ethnic and/or racial minorities (89% Caucasian, 4% African American, 3% Native American/Alaskan Native, 2% Asian/Pacific Islander, 1% Hispanic, 1% Other). Parents gave informed consent at each time point; child assent was obtained beginning at age 13 in accordance with the policies of the University of Wisconsin Institutional Review Board.
Measures
Data on ELS were collected during the infancy (child age 1, 4, and 12 months) and preschool (child age 3.5 and 4.5 years) periods. Data on HPA functioning and child mental health symptoms were collected at age 9 (M age=9.27, range=8.6–10.3), 11 (M age=11.19, range=10.5–12.3), 13 (M age=13.22, range=11.6–14.2), and 15 (M age=15.50, range=14.8–16.5) years. At age 9, saliva collection was limited to a subset of the children (N = 165 participants with complete data); at ages 11, 13, and 15 years, all participating children were asked to collect saliva (N = 297, N = 306 and N = 273 participants, respectively).
Child age
In data analyses, age was coded as years-since-school-entry to (a) center maturation on a meaningful event (rather than extrapolate downward to birth), and (b) capture important experience-based social transitions, thereby taking both chronological age and life experience into account (Shirtcliff & Essex, 2008).
Child salivary cortisol
Within each of the four assessments, children were asked to collect saliva for three consecutive days across three target collection times: (1) shortly after waking (before brushing teeth or eating breakfast); (2) between 3:00 PM and 7:00 PM (prior to dinner); and (3) just before going to bed. Cortisol was assessed in duplicate using well-established salivary enzymeimmunoassay kits (Salimetrics, State College, PA). Mean intra-assay and inter-assay coefficients of variation (CVs) were 3.8% and 7.4%, respectively. Samples were reanalyzed if the CV for the duplicate measurements were ≤20% for samples with values >.02 μg/dL and ≤30% for samples with values ≤.02 μg/dL. To normalize distributions, raw cortisol was log-transformed and extreme values were winsorized. We tested whether BMI or medication usage impacted salivary cortisol levels and did not find systematic associations (Shirtcliff et al., 2011). Nevertheless, given the likely effect of oral steroids on cortisol levels (Masharani et al., 2005) the one child taking prednisone was omitted from the analyses for that assessment.
Maternal and paternal depression
Parental depression symptoms were assessed with the Center for Epidemiologic Studies Depression scale (CES-D; Radloff, 1977), a 20-item self-report measure of depressive symptoms (e.g., “I felt that I could not shake off the blues even with help from my family or friends”). Parents rated items using a 4-point Likert-type scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time). For each parent separately, scores were averaged within the infancy (child age 1, 4, and 12 months) and preschool periods (child age 3.5 and 4.5 years), and then averaged across the two periods. All αs exceeded .85.
Family expressed anger
In the infancy period (child age 1, 4, and 12 months), family expressed anger was assessed with the average of three items from the Partner Role Quality scale tapping overt marital conflict (e.g., concerned about “arguing or fighting”; Barnett & Marshall, 1989). Participants rated items on a 4-point scale ranging from 1 (not at all) to 4 (extremely). In the preschool period (child age 3.5 and 4.5 years), parents completed the marital conflict measure, the Anger Expression Inventory (STAXI; Spielberger, Krasner, Solomon, & Janisse, 1988; e.g., “When angry or furious, I argue with others”) and the Negative subscale of the Family Expressiveness Questionnaire (FEQ; Halberstadt, 1986; e.g., “We blame one another for family troubles”). Preschool scores were standardized before averaging the three measures for mothers and fathers separately. For each parent, the infancy score was standardized and averaged with the preschool score, and then mother and father scores were averaged together for a single measure of family expressed anger. All αs exceeded .70.
Child mental health symptoms
Mental health symptoms were assessed using mother, teacher, and child reports from the MacArthur Health and Behavior Questionnaire (HBQ; Boyce et al., 2002; Essex, Boyce, et al., 2002). Mothers completed the HBQ as a telephone interview and teachers as a written questionnaire; children completed a parallel questionnaire, the HBQ-Child (HBQ-C). For internalizing symptoms, mothers and teachers rated items for Depression (e.g., “Cries a lot”) and Generalized Anxiety (e.g., “Worries about things in the future”). For externalizing symptoms, mothers and teachers rated items for Oppositional Defiant Disorder (e.g., “Argues a lot with adults”), Conduct Disorder (e.g., “Lies or cheats”), Inattention (e.g., “Does not seem to listen”), Impulsivity (e.g., “Interrupts or butts in on others”), Overt Aggression (e.g., “Gets in many fights”), and Relational Aggression (e.g., “Tries to get others to dislike a peer”). Items were rated on a 3-point scale ranging from 0 (never or not true) to 2 (often or very true). For the HBQ-C, children completed items tapping internalizing and externalizing symptoms parallel to those asked of mothers and teachers (e.g., “I cry a lot/I don’t cry a lot”; “I do what adults ask me to do/I don’t do what adults ask me to do”). For each item, children chose the one statement from each pair that was most like them and marked how much that statement was like them. Specifically, responses were coded on a 6-point scale based on which statement was selected (positive or negative) and whether the response option marked was really like me (6 if positive, 1 if negative), mostly like me (5 if positive, 2 if negative), or sort of like me (4 if positive, 3 if negative). Coefficient αs ranged from .70 to .96 across reporters and assessments. PCA was used to combine mother, teacher, and child reports into multi-informant scores for each construct at each assessment (Kraemer et al., 2003). The resulting scores accounted for over 70% of the total variance. Finally, as described earlier, two independent scales were constructed: Symptom Severity (average of the two standardized scores, reflecting score commonality) and Symptom Directionality (half difference of the standardized scores, reflecting what differentiates the scores; positive scores indicate a preponderance of externalizing symptoms).
Major Analytic Strategy
Growth curve techniques were chosen as particularly appropriate for investigating questions of allostasis. Specifically, hierarchical linear modeling (HLM) permits simultaneous modeling of cortisol levels and diurnal rhythms so that short-term (i.e., epoch-specific) stability, developmental change over time, and long-term (i.e., trait-like) stability in an allostatic system can be considered at the same time. Further, HLM allows growth trajectories to be defined at the intra-individual difference level which, in addition to charting how an individual’s HPA axis develops over time, provides the opportunity for investigating how individual HPA functioning co-varies with other changing processes (here, mental health symptoms at each assessment period). ‘Fixed’ factors like ELS can be modeled in HLM as person-specific predictors of HPA functioning and its co-variation with other changing processes. Since missing data is common in longitudinal investigations, HLM is also advantageous because it allows for inclusion of subjects with incomplete data without violating missing data assumptions (Schafer & Graham, 2002).
As described in detail below and shown in Table 1, the present study uses a three-level HLM. First, the total variance in HPA activity (cortisol levels and diurnal rhythms) is parsed into the percentages uniquely (i.e., independently) attributable to situation-specific fluctuations (due to unmeasured contextual forces or momentary experience within any given day) (Level 1), which is then distinguished from the two components of primary interest: epoch-specific, and developmental change over assessment periods (Level 2), and trait-like (Level 3). Next, other “dynamic”, or epoch-specific, predictors (age, quadratic age, and children’s mental health symptom severity and directionality at each assessment period) are included in Level 2; and other “fixed”, or trait-like, predictors (child sex, the three types of ELS, and all two-way multiplicative ELS interactions) are included in Level 3. Finally, cross-level interactions of ELS (Level 3) with mental health symptoms (Level 2) are included.
Table 1.
Illustration of the Three Levels of the HLM
| Level 1: [momentary experience] | Cort = π0 − π1TSW + π2TSWsq − π3TSWcubic − π4clocktime + e |
|
|
|
| Level 2: [epoch-specific functioning and developmental change] | π0 = β00 + β01severity + β02directionality + β03age + β04agesq +R0
|
| π1TSW = β10 + β11severity + β12directionality + β13age + R1
| |
| π2TSWsq = β20 + β21severity + β22directionality
| |
| π3TSWcubic = β30 | |
| π4clocktime = β40 | |
| Level 3: | |
|
| |
| [set-point in cortisol level] | β00 = γ000 + γ001sex + γ002mdep − γ003pdep − γ004anger + γ005mdep*anger + U00 |
| [cross-level interactions with mental health symptoms on cortisol level] | β01severity = −γ010 + γ011mdep − γ012pdep + γ013anger − γ014mdep*anger + γ015pdep*anger |
| β02directionality = γ020 − γ021sex + γ022mdep − γ023pdep + γ024anger | |
| [cross-level interactions with age on cortisol level] | β03age = γ030 − γ031sex − γ032mdep + γ033pdep + γ034anger − γ035mdep*pdep − γ036mdep*anger |
| β04agesq = −γ040 + γ041sex + γ042mdep − γ043pdep − γ044anger | |
|
| |
| [set-point in linear slope] | β10 = −γ100 − γ101sex − γ102mdep − γ103pdep + γ104anger + γ105mdep*pdep − γ106mdep*anger + U10 |
| [cross-level interaction with mental health symptoms on linear slope] | β11severity = γ110 − γ111mdep + γ112pdep + γ113anger + γ114mdep*anger + γ115pdep*anger |
| β12directionality = −γ120 + γ121sex − γ122mdep + γ123pdep + γ124anger − γ125mdep*pdep | |
| [cross-level interaction with age on linear slope] | β13age = γ130 − γ131mdep + γ132pdep − γ133anger + γ134mdep*anger + U13 |
|
| |
| [set-point in quadratic slope] | β20 = γ200 + γ201sex + γ202mdep + γ203pdep − γ204anger − γ205mdep*pdep + γ206pdep*anger |
| [cross-level interaction with mental health symptoms on quadratic slope] | β21severity = −γ210 + γ211mdep − γ212pdep − γ213anger − γ214mdep*anger |
| β22directionality = γ220 | |
|
| |
| [fixed effect of cubic slope] | β30TSWcubic = γ300 |
| [fixed effect of clock-time] | β40clocktime = γ400 |
Note. Significant coefficients in the final model are denoted in bold.
Level 1: Distinguishing HPA axis diurnal rhythm and separating situation-specific HPA axis fluctuations
Level 1 examines each single cortisol measure (N = 9144; maximum of 9 samples/assessment and 4 assessment periods; on average participants provided 25 samples each). Beyond situation-specific fluctuations within a day, the diurnal rhythm is the largest influence on a single cortisol sample at Level 1. To distinguish situation-specific variability and the diurnal rhythm, Level 1 includes Time (in hours) Since Waking (TSW) as a random predictor of cortisol levels. The diurnal slope is steeper in the morning than in the afternoon. Therefore, curvature of the slope is modeled with (fixed) quadratic and cubic terms although they are not outcomes-of-interest. TSW holds the advantage of centering the slope on the individual’s biorhythm, but there are also extrinsic influences on the circadian rhythm (Shirtcliff & Essex, 2008). To model that samples taken later in the day may be lower, we include a fixed predictor of “clock time” (i.e., Time Since Noon, residualized from TSW to prevent multicollinearity).
Level 2: Distinguishing epoch-specific HPA axis functioning, development across assessments, and co-variation with mental health symptoms
The growth curve model simultaneously includes a Level 2 hierarchy that captures cortisol measures within each assessment period (N = 1041; maximum of 4 assessment periods per person). Both cortisol levels and rhythms (distinguished in the Level 1 model) are outcomes of interest at each assessment period using a slopes-as-outcomes approach. To examine the developmental trajectory of HPA axis maturation across assessment periods, age-since-school-entry is a fixed predictor of cortisol level and its rhythm. A fixed quadratic term of age is included to test if growth across the six-year period of childhood into adolescence is nonlinear (Schreiber et al., 2006).
Most importantly for the present analyses, at each of the four assessments, concurrent mental health symptom severity and directionality are included as predictors of children’s morning cortisol level and diurnal slope, thereby capturing whether HPA functioning and mental health symptoms co-vary concurrently (i.e., a co-varied rate of change model; Coe, Lubach, & Shirtcliff, 2007). Because age and quadratic-age are simultaneously modeled, significant associations between HPA functioning and mental health symptoms indicate co-variation beyond developmental effects. This component is notable because mental health symptoms are allowed to be dynamic and time-sensitive, co-varying with epoch-specific HPA functioning over time. This fits with the allostatic process of “maintaining stability through change” (McEwen, 1998). Like other stress- or health-related outcomes (Koob & Le Moal, 2008a, 2008b; Weems & Carrion, 2009), the episodic changes in mental health symptoms may place the individual in an allostatic state that requires counter-regulatory opponent HPA axis processes to help restore functioning at the individual’s established set-point.
Level 3: Distinguishing trait-like HPA axis functioning and the influence of early life stress
The third level of the hierarchy distinguishes a single estimate of morning cortisol level and diurnal rhythm for each individual that is trait-like across all four assessment periods (N = 357). In addition to child sex, the three types of ELS are included as predictors of children’s trait-like morning cortisol level and diurnal slope. The three two-way interactions between the ELS measures (maternal depression * paternal depression; maternal depression * family expressed anger; paternal depression * family expressed anger) are also tested as predictors of trait-like HPA functioning, and if significant, remain in the model. Results from the Level 3 analyses address the first research question, i.e., whether ELS has long-term effects on trait-like cortisol levels and diurnal rhythms across childhood and into adolescence. This fits within an allostasis framework by considering the long-term effects of ELS on the development of individual’s trait-like cortisol, and the “wear and tear” on the body associated with sustained hyper- or hypo-cortisolism (Gunnar & Quevedo, 2007; McEwen, 1998).
Cross-level interactions
The second major research question is addressed with cross-level interactions, which consider whether the co-variation of epoch-specific HPA functioning and concurrent mental health symptoms (“dynamic” factors at Level 2) are influenced by ELS (a “fixed” factor at Level 3), i.e., interactions of ELS and mental health symptoms predicting HPA functioning. Other cross-level interactions captured whether ELS influences the development of the HPA axis across the four assessments (interaction of ELS * age). Overall, these cross-level interactions were designed to capture how the influence of ELS on HPA functioning can dynamically unfold years later. This fits within an allostasis framework by (a) allowing for the process of maintaining stability through change – in this case, epoch-specific HPA alterations occurring over time in tandem with mental health symptoms – to be different depending on ELS; and (b) considering effects of ELS on individuals’ patterns of HPA axis–mental health symptom co-variation, and the “wear and tear” on the body associated with repeated HPA alterations to episodic challenges (McEwen, 1998).
Results
Descriptive Statistics
Average depression symptom scores for both mothers and fathers across the infancy and preschool periods (5 assessments) ranged from 0 to 30 (maternal depression: M = 6.73, SD = 5.21; paternal depression: M = 8.34, SD = 6.01). For mothers, 5% had average scores of 16 or higher, indicating clinically significant levels of symptoms on average across the infancy and preschool periods, and another 9% had average scores above 12, indicating sub-threshold levels of depression symptoms; for fathers, 11% had average scores of 16 or higher, and another 12% had average scores above 12. Because family expressed anger is a standardized composite measure of three components, descriptive statistics are limited to each separate component. Average marital conflict ranged from 1 to 4 for mothers (M = 1.40, SD = 0.48) and fathers (M = 1.51, SD = 0.52); preschool anger expression ranged from 3 to 45 for mothers (Mdn = 21, SD = 7.6) and 2 to 43 for fathers (Mdn = 21, SD = 7.7); and preschool negative family expressiveness ranged from 20.5 to 68.2 for mothers (M = 41.9, SD = 9.1) and 19 to 64.2 for fathers (M = 39.6, SD = 8.7). On average, 10% of mothers and 18% of fathers reported marital conflict across the infancy and preschool periods; 10% of mothers and 8% of fathers reported difficulties controlling anger; and 20% and 12%, respectively, reported negative family expressiveness.
The HLM analyses provide descriptive statistics for cortisol’s morning levels, diurnal rhythms, and developmental changes. Level 1 results showed that morning cortisol levels were lower as the day progressed (β = −.02, p = .0001 for linear); significant quadratic and cubic terms indicated this diurnal decline was steeper in the morning than afternoon (β = .001, p = .0001 for quadratic; β = −.00003, p = .0001 for cubic). Samples collected later in the day were lower than samples collected earlier in the day (β = −.005, p = .0001 for “clock time”). Level 2 results showed that morning cortisol and the diurnal rhythm showed substantial developmental changes across the four assessments from age 9 to 15 years. Cortisol level declined as children grew older (β = −.006, p = .025) but this was modified by quadratic age (β = .0003, p = .038) such that cortisol levels were lowest when children were age 13 and increased again by age 15. In parallel, cortisol’s diurnal rhythm flattened as children matured (β = .0004, p = .0001). There was no effect of quadratic age on cortisol’s slope, so it was removed from further analyses of slope.
In addition to these developmental effects, we also found that child sex influenced nearly every component of HPA functioning. Compared to boys, girls had higher morning cortisol levels (β = .038, p = .019), steeper slopes (β = −.002, p < .0001), and more curvature to their slopes (β = .0001, p < .004). Girls also showed stronger developmental changes in HPA functioning (level*age, β = −.009, p = .042, level*quadratic age, β = .0008, p = .016). Thus, gender is included as a control variable at Level 3 in all subsequent models.
Bivariate Associations
Table 2 shows that there were small to moderate significant associations among the three types of ELS, and between each type of ELS and children’s later mental health symptom severity; there were no associations of ELS with mental health symptom directionality. In addition, there were no significant associations of ELS with children’s HPA functioning. However, there were numerous, but small, significant associations of children’s mental health symptom severity, and especially directionality, with children’s HPA functioning.
Table 2.
Pearson Correlations Among Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Life Stress | ||||||||||||||||||||
| 1. Maternal Depression | ||||||||||||||||||||
| 2. Paternal Depression | .38*** | |||||||||||||||||||
| 3. Family Exp. Anger | .42*** | .41*** | ||||||||||||||||||
| Mental Health Symptoms | ||||||||||||||||||||
| 4. Severity, Age 9 | .36*** | .24*** | .23*** | |||||||||||||||||
| 5. Severity, Age 11 | .36*** | .18** | .26*** | .72*** | ||||||||||||||||
| 6. Severity, Age 13 | .31*** | .21*** | .29*** | .61*** | .70*** | |||||||||||||||
| 7. Severity, Age 15 | .33*** | .19** | .25*** | .55*** | .60*** | .70*** | ||||||||||||||
| 8. Directionality, Age 9 | −.10 | −.06 | .04 | .00 | .14** | .15** | .11* | |||||||||||||
| 9. Directionality, Age 11 | .02 | −.05 | .04 | .12* | .00 | .11* | .05 | .53*** | ||||||||||||
| 10. Directionality, Age 13 | .00 | −.04 | .03 | .09 | .09 | .00 | .05 | .54*** | .63*** | |||||||||||
| 11. Directionality, Age 15 | −.05 | −.06 | .01 | .10 | .14* | .11 | .00 | .51*** | .53*** | .66*** | ||||||||||
| Cortisol | ||||||||||||||||||||
| 12. Level, Age 9 | .11 | −.04 | .00 | .02 | −.02 | .04 | −.02 | −.15 | −.12 | −.12 | −.07 | |||||||||
| 13. Level, Age 11 | −.04 | −.01 | .03 | −.13* | −.08 | −.02 | −.10 | −.07 | −.11 | −.05 | −.12 | .34*** | ||||||||
| 14. Level, Age 13 | −.03 | −.03 | .01 | −.09 | .01 | .00 | −.03 | −.01 | −.11 | .00 | −.02 | .37*** | .34*** | |||||||
| 15. Level, Age 15 | −.01 | −.04 | −.01 | −.08 | −.05 | −.04 | .02 | −.12 | −.18** | −.07 | −.09 | .30*** | .37*** | .41*** | ||||||
| 16. Slope, Age 9 | −.11 | −.01 | −.04 | .05 | .08 | .02 | .05 | .23** | .14 | .18* | .16 | −.88*** | −.42*** | −.34*** | −.39*** | |||||
| 17. Slope, Age 11 | .07 | .03 | .02 | .15** | .11 | .08 | .12 | .08 | .14* | .10 | .16* | −.44*** | −.90*** | −.36*** | −.44*** | .59*** | ||||
| 18. Slope, Age 13 | .06 | .06 | .02 | .11 | .02 | .02 | .05 | .04 | .15* | .03 | .05 | −.37*** | −.36*** | −.95*** | −.43*** | .38*** | .41*** | |||
| 19. Slope, Age 15 | .04 | .05 | .01 | .08 | .04 | .04 | −.02 | .07 | .18** | .06 | .06 | −.23** | −.27*** | −.37*** | −.82*** | .25** | .32*** | .44*** | ||
| 20. Trait-Like Level | −.02 | −.04 | .00 | −.13* | −.06 | −.03 | −.07 | −.11* | −.16** | −.08 | −.12* | .74*** | .70*** | .74*** | .75*** | −.82*** | −.80*** | −.75*** | −.59*** | |
| 21. Trait-Like Slope | .02 | .02 | .01 | .13* | .08 | .07 | .08 | .12* | .12* | .09 | .14* | −.54*** | −.54*** | −.51*** | −.64*** | .84*** | .74*** | .52*** | .28*** | −.86*** |
p < .05.
p < .01.
p < .001.
Influence of ELS on Trait-like HPA Axis Functioning and Its Development
Table 3 presents results of the Level 3 and cross-level analyses addressing the first major research question regarding the long-term influence of ELS on children’s trait-like HPA functioning and developmental changes from ages 9 to 15 years. There were no significant main effects of ELS – maternal depression, paternal depression, or family expressed anger – on trait-like morning cortisol level, diurnal rhythm, or developmental changes in cortisol level or slope. There were, however, significant interaction effects, and especially, broad effects of the interaction of early maternal depression * family expressed anger on trait-like morning cortisol level and slope as well as their developmental changes.
Table 3.
Hierarchical Linear Model Predicting Trait-like Cortisol Levels and Diurnal Rhythm, and Developmental Change in Cortisol Levels and Diurnal Rhythm From Early Life Stress Exposures
| Cortisol levels β (SE) | Diurnal rhythm β (SE) | Developmental change in cortisol levels β (SE) | Developmental change in diurnal rhythm β (SE) | |
|---|---|---|---|---|
| Maternal depression | .0016 (.0018) | <.0001 (.0002) | −.0006 (.0005) | <.0001 (<.0001) |
| Paternal depression | −.0020 (.0014) | <.0001 (.0001) | .0006 (.0003) | <.0001 (<.0001) |
| Family expressed anger | −.0448 (.0232) | .0013 (.0013) | .0121 (.0063) | <.0001 (.0001) |
| Maternal depression x family exp. anger | .0115 (.0041)** | −.0009 (.0004)* | −.0013 (.0005)* | .0001 (<.0001)* |
| Maternal depression x paternal depression | .0003 (.0001)* | |||
| Paternal depression x family exp. anger |
Note. All models controlled for child sex, as well as quadratic age on cortisol levels. Non-significant interaction terms were dropped from final models.
p < .05.
p < .01.
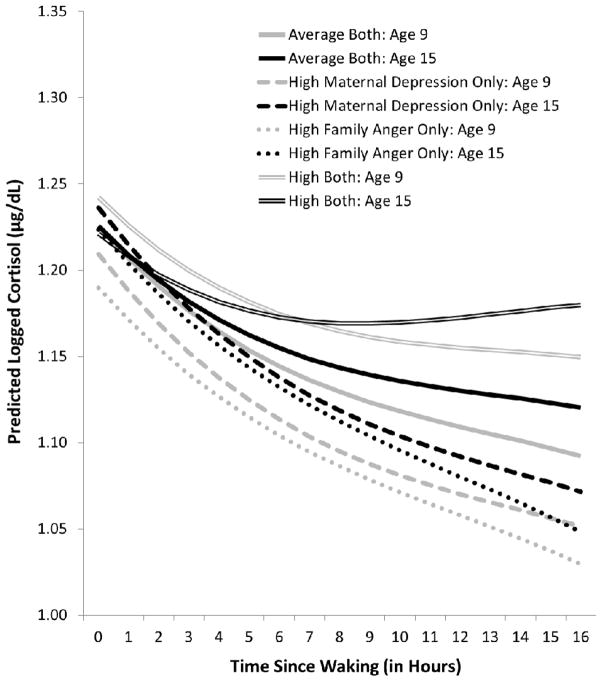
Figure 1 illustrates these maternal depression * family expressed anger interaction effects by comparing the effects of exposure to average levels of both types of ELS (solid lines) with the effects of exposure to high levels of early maternal depression only (dashed lines), family expressed anger only (dotted lines), or both types of ELS (double lines); to simplify the presentation, only the age 9 (grey lines) and age 15 (black lines) effects are shown since the developmental change was linear. At age 9, children exposed to average levels of both types of ELS showed moderately high trait-like morning cortisol levels which did not change substantially as they grew older; their diurnal slopes were moderately steep and became flatter with age. In comparison, at age 9, children who had been exposed only to high levels of maternal depression or family expressed anger showed lower trait-like morning cortisol levels but increases in levels as they grew older such that by age 15, their cortisol levels were comparable to those of the children exposed to average levels of ELS; their diurnal slopes were steeper and did not change substantially as they grew older. In contrast, at age 9, children exposed to high levels of both types of ELS showed the highest trait-like morning cortisol levels, which decreased as they grew older such that by age 15, their cortisol levels also were comparable to those of the other children; their diurnal slopes were relatively flat and continued to flatten with age such that by age 15, they exhibited a very flat daytime rhythm.
Figure 1.
Overall group comparison of and developmental changes in trait-like cortisol level and diurnal rhythm by early life exposure to maternal depression and/or family expressed anger. Because the developmental change was linear, the age 11 and age 13 effects are omitted and only age 9 (grey) and age 15 (black) are shown.
In addition to broad effects of the interaction of maternal depression * family expressed anger on trait-like cortisol, results revealed a single modest (p = .04) effect of maternal depression * paternal depression on children’s trait-like diurnal rhythm (not illustrated in a figure). Children exposed only to early maternal depression evidenced flatter slopes, children exposed only to early paternal depression evidenced steeper slopes, and children exposed to both types of ELS evidenced slopes comparable to the children with average stress exposure.
Influence of ELS on the Co-variation of Epoch-specific HPA Axis Functioning and Concurrent Mental Health Symptoms
Table 4 presents results of the Level 2 and cross-level analyses addressing the second major research question regarding the influence of ELS on the co-variation of epoch-specific HPA functioning with children’s concurrent mental health symptoms. Results revealed numerous significant main and interactive effects of ELS on the co-variation of HPA functioning and symptom severity, with a single modest effect involving symptom directionality.
Table 4.
Hierarchical Linear Model Predicting the Interaction of Epoch-specific Cortisol Levels/Diurnal Rhythm and Mental Health Symptom Severity/Directionality from Early Life Stress Exposures
| Cortisol level x severity β (SE) | Diurnal rhythm x severity β (SE) | Cortisol level x directionality β (SE) | Diurnal rhythm x directionality β (SE) | |
|---|---|---|---|---|
| Maternal depression | .0014 (.0005)** | −.0002 (.0001)* | .0001 (.0005) | <.0001 (<.0001) |
| Paternal depression | −.0004 (.0004) | .0001 (.0001) | −.0008 (.0006) | <.0001 (.0001) |
| Family expressed anger | −.0126 (.0043)** | .0017 (.0009) | −.0053 (.0048) | .0003 (.0004) |
| Maternal depression x family exp. anger | −.0040 (.0017)* | .0008 (.0003)* | ||
| Maternal depression x paternal depression | −.0002 (.0001)* | |||
| Paternal depression x family exp. anger | .0032 (.0013)* | .0004 (.0001)** |
Note. All models controlled for child sex, as well as quadratic age on cortisol level. Non-significant interaction terms were dropped from final models.
p < .05.
p < .01.
There were significant main effects of maternal depression and family expressed anger on the co-variation of mental health symptom severity with epoch-specific morning cortisol level and, for maternal depression, diurnal rhythm (the effect for family expressed anger was only marginally significant, p = .062). Overall, children exposed to early maternal depression showed increases in morning cortisol levels and steeper slopes at assessments when they were experiencing increased mental health symptom severity, and decreases in morning cortisol levels and flatter slopes at assessments when they were experiencing decreased symptom severity. In contrast, children exposed to early family expressed anger showed the opposite patterns, with decreases in morning cortisol levels and flatter slopes occurring concurrently with increased mental health symptom severity, and increases in morning cortisol levels and steeper slopes occurring concurrently with decreased symptom severity.
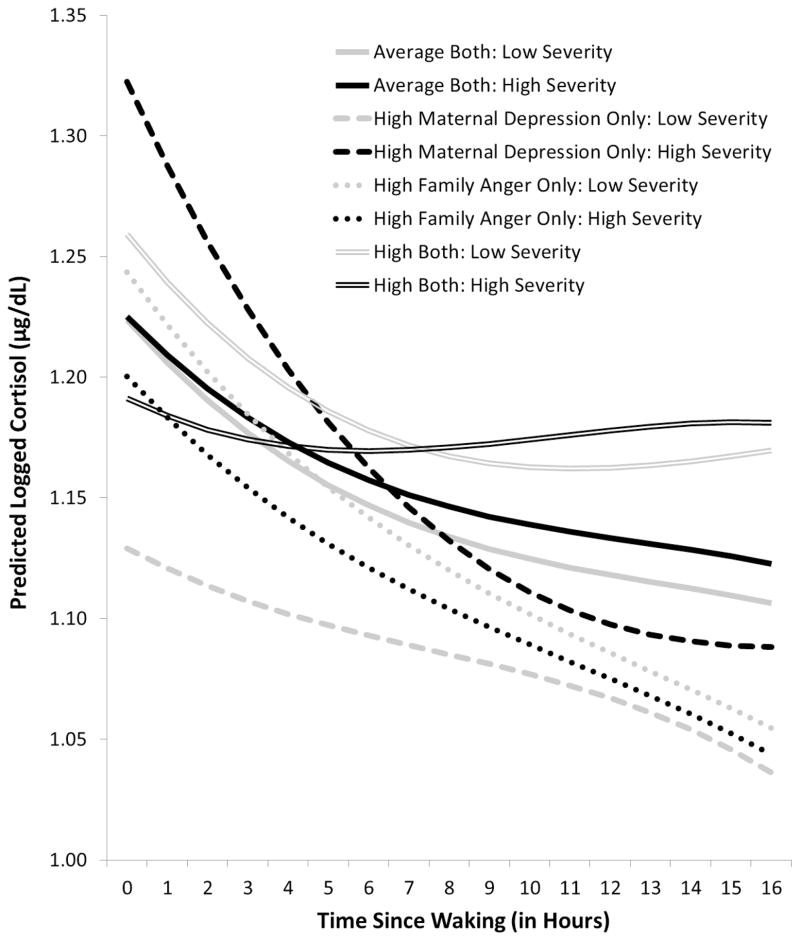
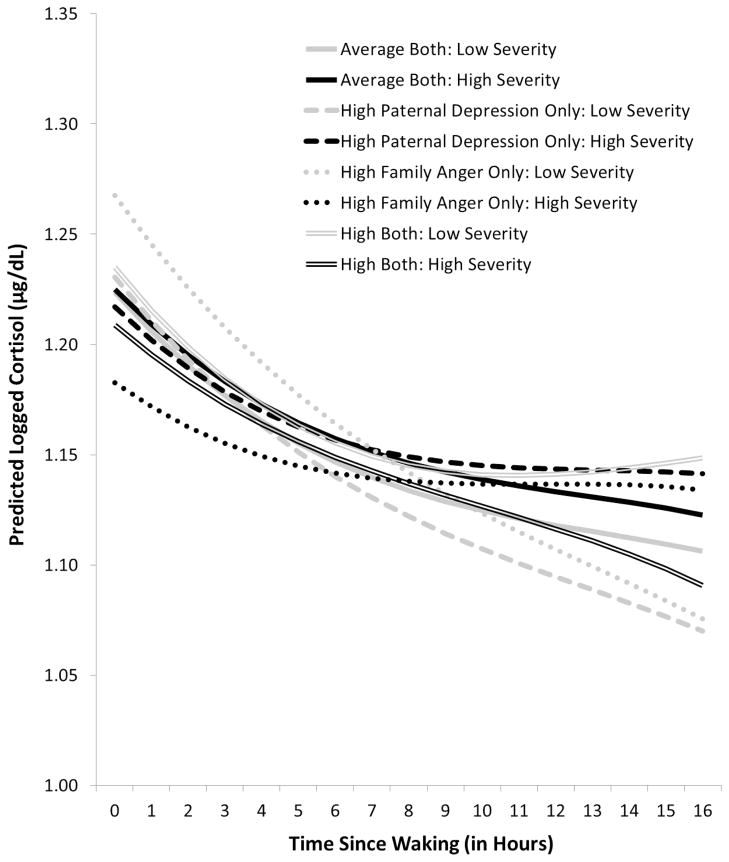
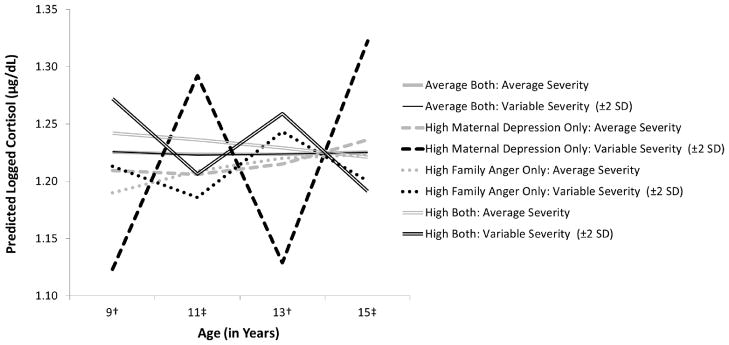
There were also significant interaction effects, especially effects of maternal depression * family expressed anger and paternal depression * family expressed anger on the co-variation of morning cortisol level and diurnal rhythm with mental health symptom severity. Because results are complicated, we simplified their graphical presentation. Figure 2 illustrates the effects of maternal depression * family expressed anger; Figure 3 illustrates the effects of paternal depression * family expressed anger. Each figure compares the effects on cortisol’s morning level and diurnal rhythm of exposure to average levels of both types of ELS (solid lines) with the effects of exposure to high levels of early parental depression only (dashed lines), family expressed anger only (dotted lines), or both types of ELS (double lines). For each type of line, the black version indicates cortisol’s level and slope associated with increased mental health symptom severity (plotted at 2 SD above the mean); the grey version indicates cortisol’s level and slope associated with decreased symptom severity (plotted at 2 SD below the mean). To reduce the complexity of the figures, the average level of symptom severity is not plotted; the average lines, which represent individual’s basal HPA functioning, would simply fall half-way between each pair of black and grey lines. In addition, because there are no differences in the patterns of co-variation by age, we present only the results for age 15. However, such a presentation does not adequately present the alterations, or swings, in HPA activity that occur over time in conjunction with increases or decreases in children’s mental health symptoms. Thus, Figure 4 illustrates this allostatic process for children exposed to early maternal depression and/or family expressed anger, focusing on cortisol’s morning levels and using a hypothetical example in which mental health symptoms alternately increase and decrease from assessment to assessment, beginning with decreased symptoms at age 9.
Figure 2.
Overall group comparison at age 15 of epoch-specific cortisol level and diurnal rhythm by concurrent mental health symptom severity and early life exposure to maternal depression and/or family anger. For clarity of presentation, comparison lines for group effects at average levels of mental health symptom severity are omitted. Results did not differ by age, so age 15 is shown as a representative example.
Figure 3.
Overall group comparison at age 15 of epoch-specific cortisol level and diurnal rhythm by concurrent mental health symptom severity and early life exposure to paternal depression and/or family expressed anger. For clarity of presentation, comparison lines for group effects at average levels of mental health symptom severity are omitted. Results did not differ by age, so age 15 is shown as a representative example.
Figure 4.
Illustration of the co-variation in trait-like morning cortisol levels and mental health symptom severity over time by early life exposure to maternal depression and/or family expressed anger. Cortisol levels at average symptom severity are shown (in grey) for comparison purposes. The illustrative lines (in black) show cortisol levels for the high ELS groups when †symptom severity is set at 2 SD below the mean (ages 9 and 13) or ‡symptom severity is set at 2 SD above the mean (ages 11 and 15).
As illustrated in Figure 2 and Figure 3, children exposed to average levels of ELS (solid lines) showed very small alterations in HPA functioning with increases or decreases (high vs. low severity, respectively) in mental health symptoms. There were no substantial changes in morning cortisol levels; and diurnal rhythms were slightly flatter during times of increased symptom severity (solid black line) and slightly steeper during times of decreased symptom severity (solid grey line). In contrast, children exposed to either or both types of ELS showed wider swings in HPA activity, especially morning levels. Further, there were differences in the direction of the co-variation of HPA functioning with mental health symptom severity depending on the type and combination of ELS.
As shown in Figure 2, children exposed only to maternal depression (dashed lines) showed the widest swings in HPA activity, especially morning cortisol levels (see also Figure 4), in correspondence with increases or decreases in mental health symptom severity. They showed the highest morning cortisol levels and the steepest slopes (dashed black line) at assessments when they were experiencing increased symptom severity, and the lowest morning cortisol level and the flattest slopes (dashed grey line) at assessments when they were experiencing decreased symptom severity. In contrast, children exposed only to family expressed anger (dotted lines) showed the opposite pattern, with narrower swings in HPA activity, especially morning cortisol levels (see also Figure 4). They showed decreases in morning cortisol levels and flatter slopes (dotted black line) at assessments when they were experiencing increased symptom severity, and higher cortisol levels and steeper slopes (dotted grey line) at assessments when they were experiencing decreased symptom severity. Children exposed to both types of ELS (double lines) showed a similar general pattern of co-variation as those exposed only to family expressed anger; in addition, at assessments when they were experiencing increased symptom severity, their morning cortisol levels and slopes decreased to the point that they exhibited a very flat daytime rhythm.
As shown in Figure 3, the interaction of paternal depression*family expressed anger reveals a different pattern. With one exception, children exposed to one or both of these types of ELS showed similar patterns, with the widest swings in HPA activity shown by children exposed only to family expressed anger. Specifically, children exposed to either paternal depression or family expressed anger showed decreases in morning cortisol levels and flatter slopes at assessments when they were experiencing increased mental health symptom severity (black lines) and increases in cortisol levels and steeper slopes at assessments when they were experiencing decreased symptom severity (grey lines). Children exposed to both types of ELS (double lines) also showed this pattern of change in morning cortisol levels; however, unlike the children exposed to only one type of ELS, those exposed both to paternal depression and family expressed anger showed steeper slopes at assessments when they were experiencing increased symptom severity and flatter slopes when they were experiencing decreased symptom severity.
Finally, there was a modest (p = .04) interaction involving mental health symptom directionality (not illustrated in a figure). The interaction of maternal depression * paternal depression influenced the co-variation of symptom directionality with cortisol’s diurnal rhythm. Children who were exposed to average levels of both types of ELS showed a modest flattening of the slope at assessments when they were experiencing a preponderance of externalizing symptoms, and a modest increase in the slope at assessments when their symptom profile shifted to a preponderance of internalizing symptoms. In comparison, children exposed to either early maternal depression or paternal depression showed similar, but stronger, patterns. In contrast, children who were exposed to both types of ELS showed steeper slopes during times they were experiencing a preponderance of externalizing symptoms and flatter slopes during times when their symptom profile shifted to a preponderance of internalizing symptoms.
Discussion
We investigated the influences of multiple types of ELS representing day-to-day detriments in the early care-taking environment – maternal depression, paternal depression, and family expressed anger – on later HPA functioning and its co-variation with mental health symptoms across a six-year period from childhood into adolescence. Consistent with an allostasis framework, which emphasizes the establishment of stable HPA functioning and the maintenance of that stability through change, we distinguished the trait-like component of HPA functioning from the epoch-specific HPA component which co-varies with episodic challenges – in this case, periodic increases in children’s mental health symptoms. Overall, results supported our hypotheses that ELS influences trait-like cortisol and its development as well as the covariation of epoch-specific cortisol with children’s concurrent mental health symptoms. Consistent with previous findings (Gunnar & Quevedo, 2007; Knutsson et al., 1997), children exposed to typical levels of ELS showed moderately high trait-like morning cortisol with moderately steep slopes across the day, and modest developmental change from age 9 to 15. These children similarly showed very modest swings in HPA activity around their trait-like basal functioning concurrent with episodic increases in mental health symptom severity. In comparison, children exposed to higher levels any of the three types of ELS evidenced alterations (varying in nature according to the type of ELS) in trait-like morning cortisol and diurnal rhythm, and greater developmental alterations from ages 9 to 15 years. Further, they evidenced substantially wider swings in epoch-specific HPA activity around their basal functioning concurrent with increases or decreases in mental health symptom severity across the four assessment periods.
Our investigation extends previous research demonstrating that ELS has profound effects on children’s HPA axis (reviewed in Gunnar & Vasquez, 2006; Trickett et al., 2011) by taking a longer-term prospective approach and distinguishing alterations in the trait-like component of HPA activity associated with basal functioning and its development from alterations related to the component that regulates responses to challenges. As part of the allostatic process (McEwen, 1998), the HPA axis operates at an optimal level with efficient recovery from challenges, as illustrated here by the patterns of HPA functioning shown by children exposed to typical levels of ELS. However, patterns shown by children exposed to higher levels of ELS result in HPA alterations (either heightened or blunted) which are (a) sustained over time as trait-like levels and rhythms, and (b) show wider swings above and below the individual’s trait-like basal level. The additional energy required to regulate and counter-regulate these sustained alterations in trait-like HPA functioning as well as the repeated alterations associated with the episodic challenge of increased mental health symptoms may contribute, both separately and jointly, to the “wear and tear” on the body, eventually culminating as allostatic load (Lupien et al., 2006).
The results of this investigation highlight the cumulative effects of multiple types of ELS on HPA functioning and development across the transition from childhood into adolescence. We found that cumulative exposure to early maternal depression and family expressed anger is particularly influential in defining children’s trait-like basal HPA functioning. Compared to children exposed to typical levels of these two types of ELS, children exposed to high levels of only one – either maternal depression or family expressed anger – evidenced lower trait-like morning cortisol and steeper slopes at age 9, with increases in morning levels over time such that by age 15, they evidenced comparable morning levels with lower levels across the remainder of the day. In contrast, children exposed to both types of ELS evidenced the highest morning cortisol and the flattest slopes at age 9, with decreases in morning levels and a continued flattening of the slope such that by age 15, they showed a pattern of increasing dysregulation of trait-like HPA functioning characterized by a very flat daytime rhythm (i.e., comparable morning levels with a lack of the typical decline across the day). The sustained HPA activation across the day shown by children exposed to multiple types of ELS is consistent with the pattern found in other studies of cumulative stress (Evans, 2003; Flinn & England, 1995). In addition, the longitudinal findings extend this work to suggest that, as part of an allostatic process, the “wear and tear” associated with sustained HPA activation may result over time in a down-regulation of HPA functioning (Miller et al., 2007). Because we investigated this allostatic process only into early adolescence, it is not clear if the trajectory of down-regulation continues into adulthood. However, the findings suggest that the pattern of hypercortisolism often found in studies of maltreated children compared with the pattern of hypocortisolism often found in adults exposed to early maltreatment (Miller et al., 2007) may reflect, in part, a continuation of the developmental process identified in this study.
The present results also contribute to our understanding of the roles of ELS in defining the later co-variation of children’s HPA functioning with mental health symptoms. Across all children, there was little evidence that HPA functioning was coupled with mental health symptoms. However, in addition to the cumulative effects of exposure to early maternal depression and family expressed anger on trait-like HPA functioning, children exposed to both types of ELS showed a further blunting of HPA activity when faced with the episodic challenge of increased mental health symptoms. In addition, we found domain-specific effects of each type of ELS. Children exposed only to early maternal depression showed an up-regulation in HPA activity with concurrent increases in symptom severity and a down-regulation with concurrent decreases in symptom severity. They also showed the widest swings in HPA activity (especially morning cortisol levels) around their basal functioning concurrent with increases and decreases in symptom severity. In contrast, children exposed only to family expressed anger showed a down-regulation in HPA activity with concurrent increases in symptom severity and an up-regulation with concurrent decreases in symptom severity. Other analyses showed that children exposed only to early paternal depression showed the same pattern of down-regulation in HPA activity with increases in symptom severity as that shown by children exposed to family expressed anger. Similar to other stress- or health-related outcomes (Koob & Le Moal, 2008a; Weems & Carrion, 2009), increases in mental health symptoms may create an allostatic state requiring the counter-regulatory opponent process (i.e., swings) of the HPA axis to aid in reestablishing functioning at basal levels. Although speculative, it is possible that the allostatic state may, over time, reset physiological set-points if mental health problems are persistent or recurrent (Koob & Le Moal, 2001, 2008b).
Our findings extend previous research which examines children’s current psychiatric status as a moderator of the association of ELS with alterations in HPA functioning (Ashman et al., 2002; Cicchetti et al., 2010; Tarullo & Gunnar, 2006) in two major ways. First, the findings document that HPA activity and mental health symptoms co-vary over time, reframing the question to consider ELS as the moderator of those patterns of co-variation, or co-regulation. The expression of mental health symptoms may bring an additional stressor to the system (Ruttle et al., 2011). It is also likely that cortisol and mental health symptoms work in a bi-directional manner, with each functioning in relation to the other, and possibly exacerbating the other. Second, different types of ELS have unique effects on the co-variation of HPA functioning with mental health symptoms. It is notable that there are domain-specific effects only on the covariation of HPA functioning with mental health symptoms, and not on trait-like HPA functioning. This suggests that very early in life, prior to the development of children’s cognitive and emotion regulation strategies, it is the accumulation or severity of ELS which most prominently influences the establishment of trait-like HPA functioning. However, as children grow older, they develop cognitive and emotion regulation strategies associated with exposure to each type of ELS (Cummings & Davies, 2002; Goodman & Gotlib, 1999) and, with repeated exposure, may establish preferential patterns of cognitions and emotions which might underlie the differential calibrations of the HPA axis we see when children are faced with the episodic challenge of increased mental health symptoms.
The patterns of differential calibration of the HPA axis to concurrent mental health symptoms found in this investigation are consistent with previous theory and research on the cognitions and emotions, as well as HPA activity, associated with exposure to maternal depression vs. family expressed anger. The wide swings in HPA activity in association with increases in symptom severity that were shown by children exposed to early maternal depression may reflect the potency of maternal care in the early care-taking environment. According to parental investment theory (Bjorklund & Kipp, 1996), it is evolutionarily adaptive for a child to be emotionally, physiologically, and behaviorally attuned to the mother. This bond begins physically during pregnancy and through the infancy period (Uvnäs-Moberg, Arn, & Magnusson, 2005) and continues throughout early development as children calibrate their development to the mother’s emotional and social cues. The up-regulation of HPA activity shown by children of depressed mothers when faced with increased symptom severity is consistent with a substantial literature demonstrating increased HPA responsivity in animals as well as human children exposed to deficits in early maternal care-taking (Gunnar & Quevedo, 2007; Meaney & Szyf, 2005; Sanchez, 2006). These effects are mediated by deficits in mother-child interactions and consequent insecure attachments. When mothers are depressed, they exhibit deficits in communication, sensitivity, and attunement (Hwa-Froelich, Cook, & Flick, 2008; Murray, Fiori-Cowley, Hooper, & Cooper, 1996; Trampolini, Ungerer, & McMahon, 2008) which impair the mother-child attachment relationship and negatively influence the child’s cognitive representations of the self and parents (Toth et al., 2009). Less secure attachments to caregivers and more negative cognitive representations of self and others are associated with the development of less successful emotional- and self-regulatory strategies across childhood (Calkins & Hill, 2007; Crugnola et al., 2011; Kochanska, Philibert, & Barry, 2009) that, in turn, impact the way in which physiological processes are regulated (Fox & Calkins, 2003). In contrast, the down-regulation of HPA activity shown by children exposed to family expressed anger when they confront episodic increases in mental health symptom severity is consistent with studies showing that perceptions of uncontrollability are associated with lower morning cortisol, and perceptions of physical threat are associated with flatter cortisol slopes across the day (Miller et al., 2007). Parental anger and conflict has been associated with children’s cognitive perceptions of threat and self-blame (Grych, Fincham, Jouriles, & McDonald, 2000), perceptions moderated by levels of family cohesiveness (Lindahl & Malik, 2011). Further, children exposed to contrived laboratory adult conflicts typically recognize and experience greater anger, sadness, and fear when faced with escalating conflict or conflicts about children (Koss et al., 2011; Pollak, Messner, Kistler, & Cohn, 2009), situations likely to be perceived as less controllable by the child.
Notably, our findings suggest that exposure to maternal vs. paternal depression represent very different experiences for the child’s psychobiology, possibly because paternal depression is more phenotypically similar to family expressed anger than to maternal depression. While females tend to display the more “classic” symptoms of depression including depressed mood, feelings of worthlessness, and social withdrawal, males with depression typically display higher levels of emotional restriction, aggression and irritability (Brownhill, Wilhelm, Barclay, & Schmied, 2005; Winkler, Pjrek, & Kasper, 2005). Given that children may experience the phenomenon of paternal depression in terms of increased levels of threat, children exposed to paternal depression may evidence patterns of HPA–mental health covariation similar to those displayed by children exposed to family expressed anger.
When interpreted within an allostasis framework, the results highlight the trade-offs of adaptive processes that permit an individual to meet the demands of their environment. Recent models of biological sensitivity to context (BSC; Boyce & Ellis, 2005; Ellis, Essex, & Boyce, 2005) and its extension to adaptive calibration (ACM; Del Giudice, Ellis, & Shirtcliff, 2011) postulate that neurological and neuroendocrine systems reorganize and adapt to contextual and physiological perturbations. However, when individuals must adapt to a stressful environment, the alterations in HPA activity can incur a cost, promoting a degeneration of functioning and a cascade of change leading to differential developmental trajectories as maturation occurs (Cicchetti & Curtis, 2006).
According to the ACM (Del Giudice et al., 2011), there are profiles of children (along a continuum) defined by the level of their biological sensitivity to context. The first profile describes children who are biologically sensitive (high BSC) and thus, have an enhanced ability to encode and attend to salient social information. In a low stress environment, they are largely protected from physiological costs of such sensitivity and incur many benefits of being calibrated, or open, to positive social and emotional cues (e.g., empathy and prosocial behavior; Shirtcliff et al., 2009). Our prior work distinguished large numbers of such sensitive youth within the WSFW (Ellis et al., 2005); and in the present investigation, the HPA functioning of children exposed to typical levels of ELS is suggestive of this profile. Their moderate trait-like basal levels and diurnal rhythms, and moderate developmental trajectory suggest that their HPA axis maintained flexibility to change rhythmically across and day and to develop slowly across adolescence. The maintenance of flexibility, variability, and rhythmicity in HPA functioning are important healthy allostatic components (McEwen & Wingfield, 2003). Further, when the challenge of mental health symptoms arise, sensitive children efficiently return to basal levels likely because they are physiologically open to the protective resources in their environment and use these environmental resources to their benefit to overcome mental health symptoms.
The second profile describes children who are exposed to moderate stress – here, only to maternal depression or family expressed anger – who adapt by developing “buffered” trait-like HPA functioning (low BSC). This permits them to be less attentive or focused on their environment, thereby minimizing costs associated with moderate chronic stress beginning very early in life. Children exposed to a single type of ELS demonstrated somewhat lower trait-like cortisol levels with moderately steep diurnal rhythms; across development, cortisol levels became somewhat higher but remained lower across most of the day. The most striking finding was that the coupling of HPA functioning with mental health symptoms was much tighter for these children, especially those exposed only to early maternal depression, and the direction of co-variation was dependent on the type and combination of ELS. This suggests that while children exposed to moderate ELS may develop a “buffered” pattern of trait-like HPA functioning for dealing with chronic stressors, they remain very responsive at least to the episodic challenge of increased mental health symptom severity. Because previous studies have not distinguished the trait-like component of HPA functioning from the epoch-specific component in a long-term prospective design, the meaning of these findings is unclear. It may be that because mental health symptoms reflect, in part, an individual’s cognitive and emotional biases developed earlier in life, the tight and domain-specific patterns of co-variation of symptoms and HPA function reflects differences in exposure to maternal depression vs. family expressed anger, as discussed above. It is notable, however, that despite the apparent allostatic state of the HPA axis during times of increased mental health symptoms, the HPA axis of youth experiencing a single type of ELS appears to be able to maintain diurnal rhythmicity and flexibility to change across development and manage mental health symptoms. When necessary, buffered youth have the physiological resources to cope with challenges.
The third profile appears physiologically similar to the “sensitive” profile, but the costs of maintaining a high BSC are keenly apparent when these children live in a high stress environment – here, both types of ELS. It is adaptive for children exposed to higher levels of stress to be vigilant and sensitive to contextual cues. High trait-like basal cortisol, which we observed, enhances attention to dangerous or unpredictable stimuli, with some physiological trade-offs. Most striking, however, our results show that children exposed to multiple forms of ELS evidenced flattened diurnal rhythms as early as age 9, and their diurnal rhythm became progressively flatter across development, and flatter still when mental health symptoms increased. These results suggest that the high sensitivity to environmental cues in high ELS children might increase their stress vulnerability primarily by dysregulating the diurnal rhythm. Flexibility and rhythmicity are important tenants of allostatic processes (McEwen, 1998). Cortisol’s diurnal rhythm captures the confluence of intrinsic, biological forces with extrinsic, environmental forces working in concert within an individual on a daily cycle. Individuals exposed to multiple forms of ELS may evidence flattened diurnal rhythms because they are struggling to match their intrinsic physiological set-points with their contextual demands (Skinner, Shirtcliff, Haggerty, Coe, & Catalano, in press). This mismatch of intrinsic and extrinsic forces occurs on a daily cycle, and appears to steadily worsen with time – both over the course of development and during times of increased mental health symptom expression. Thus, prolonged exposure to the cumulative effects of maternal depression and family expressed anger, and the addition of later mental health challenges, likely exerts “wear and tear” on the system, resulting in a down-regulation of HPA functioning over time (Ruttle et al., 2011; Weems & Carrion, 2007; Weems & Carrion, 2009). For high BSC youth from a stressful environment, the cost of vigilance may culminate in a loss of both flexibility and rhythmicity. By exploring the effect of ELS degree and type on HPA functioning and the coupling of HPA functioning with mental health symptoms, we have made strides toward elucidating the unique processes by which typical functioning becomes atypical.
These findings must be considered in light of several potential limitations to inform future research. First, the possibility of a sampling bias within this population, and the non-representativeness of this sample of the larger U.S. population, must be kept in mind, especially regarding the range of ELS. This sample represents largely middle class, white, and, at least at the outset, intact families in a Midwestern state. However, we emphasize that our findings regarding the importance of considering multiple types of ELS and children’s concurrent psychiatric status support recent work with maltreated children (e.g., Cicchetti et al., 2010), and extend it downward to more normative variations in ELS, which is an important tenet of developmental psychopathology. The study of typical and atypical development are mutually informative (Cicchetti, 1990; Cicchetti & Sroufe, 2000). Conceptually integrating this investigation with the ongoing research of children exposed to early maltreatment has the potential to advance our understanding of a wide range of processes in child development.
Second, while this study examined multiple types of ELS, there are many other types of stressors in the lives of young children which should be considered in future studies. Consistent with a multilevel approach (Cicchetti & Curtis, 2007; Cicchetti & Rogosch, 2007), it is particularly important to consider stressors at other levels of analysis. For example, the effects on children’s HPA functioning of the three types of ELS investigated in this study likely operate through deficits in the more intimate qualities of the parent-child relationship and associated attachments (Gunnar & Vasquez, 2006). At another level, children experience stresses associated with the SES of the family into which they were born. Previous studies have demonstrated that, although SES is associated with maternal depression and family expressed anger, each of these levels of stressors exert independent effects on children’s HPA functioning (Davies et al., 2009; Essex, Klein, et al., 2002; Lupien, King, Meaney, & McEwen, 2001). To more fully understand the allostatic process, it is important for future studies to incorporate stressors at multiple levels into longitudinal models of HPA functioning that distinguishes unique and joint effects of ELS on HPA axis development from responsivity to the challenges of life.
Third, although this investigation took a multi-level approach, we considered only the HPA axis as one of the several inter-related primary mediators of the allostatic process (Lupien et al., 2006; McEwen, 1998). Because of the near impossibility of inferring causation within complex developmental systems, it is important to investigate multiple variables and multiple levels of variables simultaneously (Cicchetti & Blender, 2004; Cicchetti & Toth, 2009). Future work should expand the multilevel approach employed in the present investigation to consider the complex interplay of multiple physiological systems (Hastings et al., in press), as well as genetic and epigenetic effects (Gillespie, Phifer, Bradley, & Ressler, 2009; McCrory et al., 2010), aware that specific stressors may not affect all stress-related systems equally or in the same general direction or at the same time.
Our findings suggest several important implications for children exposed to different types of day-to-day detriments in the early caretaking environment, especially when facing the challenges associated with increases in mental health problems. Clearly, when exposure to ELS begins at birth (if not earlier) and is chronic throughout early childhood (and possibly beyond), it has substantial and long-term effects on children’s HPA functioning, suggesting the importance of very early preventive/intervention strategies. In addition, the broad effects of cumulative ELS, especially exposure to early maternal depression and family expressed anger, suggest that multilevel preventive/intervention strategies aimed at reducing maternal depression and promoting more positive family dynamics may be most effective. Given the centrality of early maternal caretaking, the findings also emphasize the importance of supporting mothers during this critical time in children’s development. Perhaps most important, by investigating the association of children’s HPA functioning with mental health symptoms as an allostatic process, the findings highlight that (a) allostatic states often emerge when children are experiencing increases in symptom severity; (b) the direction of the HPA axis alteration varies according to the child’s history of ELS; and (c) children exposed to ELS are most vulnerable to alterations in HPA functioning with increases in mental health symptom severity. Together, these findings make a compelling argument that any strategy aimed at intervening in the allostatic process to reduce the development of allostatic load during childhood and adolescence should consider these issues, and especially the heightened vulnerability of children exposed to ELS when they are experiencing increases in mental health symptoms.
Acknowledgments
This research was supported by NIH grants R01-MH044340, P50-MH052354, P50-MH069315, and P50-MH084051; the HealthEmotions Research Institute; and the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development. Support for EAS was provided by a Career Development Award, K01-MH077687; support for MJS was provided by 1UL1RR025011.
References
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Development and Psychopathology. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Barnett RC, Marshall NL. Preliminary manual for the Role-Quality Scales. Wellesley College Center for Research on Women; Wellesley, MA: 1989. [Google Scholar]
- Bernard K, Dozier M. Examining infants’ cortisol responses to laboratory tasks among children varying in attachment disorganization: Stress reactivity or return to baseline? Developmental Psychology. 2010;46:1771–1778. doi: 10.1037/a0020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund DF, Kipp K. Parental investment theory and gender differences in the evolution of inhibition mechanisms. Psychological Bulletin. 1996;120:163–188. doi: 10.1037/0033-2909.120.2.163. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. I: Exploring the “headwaters” of early life morbidities. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:580–587. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe ZN. Maternal depression and infant cortisol: Influences of timing, comorbidity and treatment. Journal of Child Psychology and Psychiatry. 2008;49:1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownhill S, Wilhelm K, Barclay L, Schmied V. ‘Big build’: Hidden depression in men. Australian and New Zealand Journal of Psychiatry. 2005;39:921–931. doi: 10.1080/j.1440-1614.2005.01665.x. [DOI] [PubMed] [Google Scholar]
- Bureau JF, Easterbrooks MA, Lyons-Ruth K. Maternal depressive symptoms in infancy: Unique contribution to children’s depressive symptoms in childhood and adolescence? Development and Psychopathology. 2009;21:519–537. doi: 10.1017/S0954579409000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Hill A. Caregiver influences on emerging emotion regulation: Biological and environmental transactions in early development. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. pp. 229–248. [Google Scholar]
- Cicchetti D. A historical perspective on the discipline of developmental psychopathology. In: Rolf J, Masten AS, Cicchetti D, Nuechterlein KH, Weintraub S, editors. Risk and protective factors in the development of psychopathology. New York: New York: Cambridge University Press; 1990. pp. 2–28. [Google Scholar]
- Cicchetti D, Blender JA. A multiple-levels-of-analysis approach to the study of developmental processes in maltreated children. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17325–17326. doi: 10.1073/pnas.0408033101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Curtis WJ. The developing brain and neural plasticity: Implications for normality, psychopathology, and resilience. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology: Vol 2: Developmental neuroscience. 2. Hoboken, NJ: Wiley; 2006. pp. 1–64. [Google Scholar]
- Cicchetti D, Curtis WJ. Multilevel perspectives on pathways to resilient functioning. Development and Psychopathology. 2007;19:627–629. doi: 10.1017/s0954579407000314. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Personality, adrenal steroid hormones, and resilience in maltreated children: A multilevel perspective. Development and Psychopathology. 2007;19:787–809. doi: 10.1017/S0954579407000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Development. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Sroufe LA. The past as prologue to the future: The times they’ve been a changin’. Development and Psychopathology. 2000;12:255–264. doi: 10.1017/s0954579400003011. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50:16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Valentino K. An ecological-transactional perspective on child maltreatment: Failure of the average expectable environment and its influence on child development. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 3: Risk, disorder, and adaptation. 2. Hoboken, NJ: Wiley; 2006. pp. 129–201. [Google Scholar]
- Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatric Research. 2007;61:520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: A meta-analysis. Psychological Bulletin. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Crugnola CR, Tambelli R, Spinelli M, Gazzotti S, Caprin C, Albizzati A. Attachment patterns and emotion regulation strategies in the second year. Infant Behavior and Development. 2011;34:136–151. doi: 10.1016/j.infbeh.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Davies PT. Effects of marital conflict on children: Recent advances and emerging themes in process-oriented research. Journal of Child Psychology and Psychiatry. 2002;43:31–63. doi: 10.1111/1469-7610.00003. [DOI] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interpersonal conflict and child maladjustment. Developmental Psychology. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Manning LG, Zale E. Children’s patterns of emotional reactivity to conflict as explanatory mechanisms in links between interpartner aggression and child physiological functioning. Journal of Child Psychology and Psychiatry. 2009;50:1384–1391. doi: 10.1111/j.1469-7610.2009.02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neuroscience and Biobehavioral Reviews. 2011 doi: 10.1016/j.neubiorev.2010.11.007. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Shirtcliff EA, Boyce WT, Deardorff J, Essex MJ. Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology. 2011;23:85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kraemer HC. Exposure to maternal depression and marital conflict: Gender differences in children’s later mental health symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:728–737. doi: 10.1097/01.CHI.0000046849.56865.1D. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Kraemer HC, Armstrong JM, Boyce WT, Goldsmith HH, Klein MH, Kupfer DJ. Exploring risk factors for the emergence of children’s mental health problems. Archives of General Psychiatry. 2006;63:1246–1256. doi: 10.1001/archpsyc.63.11.1246. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Kraemer HC, Slattery MJ, Burk LR, Boyce WT, Woodward HR, Kupfer DJ. Screening for childhood mental health problems: Outcomes and early identification. Journal of Child Psychology and Psychiatry. 2009;50:562–570. doi: 10.1111/j.1469-7610.2008.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39:924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Feng X, Shaw DS, Skuban EM, Lane T. Emotional exchange in mother-child dyads: Stability, mutual influence, and associations with maternal depression and child problem behavior. Journal of Family Psychology. 2007;21:714–725. doi: 10.1037/0893-3200.21.4.714. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: Associations with child cortisol levels. Development and Psychopathology. 2008;20:1003–1021. doi: 10.1017/S0954579408000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn MV, England BG. Childhood stress and family environment. Current Anthropology. 1995;36:854–866. [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27:7–26. [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: Genetic and environmental influences on development of the stress response. Depression and Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gross HE, Shaw DS, Moilanen KL, Dishion TJ, Wilson MN. Reciprocal models of child behavior and depressive symptoms in mothers and fathers in a sample of children at risk for early conduct problems. Journal of Family Psychology. 2008;22:742–751. doi: 10.1037/a0013514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grych JH, Fincham FD, Jouriles EN, McDonald R. Interparental conflict and child adjustment: Testing the mediational role of appraisals in the cognitive-contextual framework. Child Development. 2000;71:1648–1661. doi: 10.1111/1467-8624.00255. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vasquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 2: Developmental neuroscience. 2. Vol. 2. Hoboken, NJ: Wiley; 2006. pp. 533–577. [Google Scholar]
- Gunnar MR. The role of glucocorticoids in anxiety disorders: A critical analysis. In: Vasey MW, Dadds MR, editors. Developmental psychopathology of anxiety. New York: Oxford University Press; 2001. pp. 143–159. [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Halberstadt AG. Family socialization of emotional expression and nonverbal communication styles and skills. Journal of Personality and Social Psychology. 1986;51:827–836. [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62:40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Stewart JG, Wynne-Edwards KE. Cortisol reactivity to social stress in adolescents: Role of depression severity and child maltreatment. Psychoneuroendocrinology. 2011;36:173–181. doi: 10.1016/j.psyneuen.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Altered neuroendocrine activity in maltreated children related to symptoms of depression. Development and Psychopathology. 1996;8:201–214. [Google Scholar]
- Hastings PD, Ruttle PL, Serbin LA, Mills RSL, Stack DM, Schwartzman AE. Adrenocortical responses to strangers in preschoolers: Relations with parenting, temperament, and psychopathology. Developmental Psychobiology. 2011 doi: 10.1002/dev.20545. [DOI] [PubMed] [Google Scholar]
- Hastings PD, Shirtcliff EA, Klimes-Dougan B, Allison AL, DeRose L, Usher B, Zahn-Waxler C. Allostasis and the development of internalizing and externalizing problems: Changing relations with physiological systems across adolescence. Development and Psychopathology. doi: 10.1017/S0954579411000538. (in press) [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30:698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hwa-Froelich DA, Cook CAL, Flick LH. Maternal sensitivity and comunication styles: Mothers with depression. Journal of Early Intervention. 2008;31:44–66. [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women’s mental health. Psychology of Women Quarterly. 1995;19:257–285. [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Depressive disorders in maltreated children. Journal of the American Academy of Child & Adolescent Psychiatry. 1991;30:257–265. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- Kim J, Cicchetti D, Rogosch FA, Manly JT. Child maltreatment and trajectories of personality and behavioral functioning: Implications for the development of personality disorder. Development and Psychopathology. 2009;21:889–912. doi: 10.1017/S0954579409000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Bronnegard M, Stierna P, Albertsson-Wikland K. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. Journal of Clinical Endocrinology and Metabolism. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Philibert RA, Barry RA. Interplay of genes and early mother-child relationship in the development of self-regulation from toddler to preschool age. Journal of Child Psychology and Psychiatry. 2009;50:1331–1338. doi: 10.1111/j.1469-7610.2008.02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008a;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review: Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London Series B, Biologial Sciences. 2008b;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss KJ, George MRW, Bergman KN, Cummings EM, Davies PT, Cicchetti D. Understanding children’s emotional processes and behavioral strategies in the context of marital conflict. Journal of Experimental Child Psychology. 2011;109:336–352. doi: 10.1016/j.jecp.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, Kupfer DJ. A new approach to integrating data from multiple informants in psychiatric assessment and research: Mixing and matching contexts and perspectives. American Journal of Psychiatry. 2003;160:1566–1577. doi: 10.1176/appi.ajp.160.9.1566. [DOI] [PubMed] [Google Scholar]
- Lindahl KM, Malik NM. Marital conflict typology and children’s appraisals: The moderating role of family cohesion. Journal of Family Psychology. 2011;25:194–201. doi: 10.1037/a0022888. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Ouellet-Morin I, Hupbach A, Tu MT, Buss C, Walker D, McEwen BS. Beyond the stress concept: Allostatic load – a developmental biological and cognitive perspective. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 2: Developmental neuroscience. 2. Hoboken, NJ: Wiley; 2006. pp. 578–628. [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology. 2011 doi: 10.1037/a0023838. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masharani U, Shiboski S, Eisner MD, Katz PP, Janson SL, Granger DA, Blanc PD. Impact of exogenous glucocorticoid use on salivary cortisol measurements among adults with asthma and rhinitis. Psychoneuroendocrinology. 2005;30:744–752. doi: 10.1016/j.psyneuen.2005.03.003. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. The New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neurosciences. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Milan S, Snow S, Belay S. Depressive symptoms in mothers and children: Preschool attachment as a moderator of risk. Developmental Psychology. 2009;45:1019–1033. doi: 10.1037/a0016164. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mills-Koonce WR, Garrett-Peters P, Barnett M, Granger DA, Blair C, Cox MJ. Father contributions to cortisol responses in infancy and toddlerhood. Developmental Psychology. 2011;47:388–395. doi: 10.1037/a0021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Development. 1996;67:2512–2526. [PubMed] [Google Scholar]
- Murray L, Halligan SL, Goodyer IM, Herbert J. Disturbances in early parenting of depressed mothers and cortisol secretion in offspring: A preliminary study. Journal of Affective Disorders. 2010;122:218–223. doi: 10.1016/j.jad.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. International Journal of Behavioral Development. 2007;31:218–231. [Google Scholar]
- Pollak SD, Messner M, Kistler DJ, Cohn JF. Development of perceptual expertise in emotion recognition. Cognition. 2009;110:242–247. doi: 10.1016/j.cognition.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramchandani P, Stein A, Evans J, O’Connor TG The ALSPAC Study Team. Paternal depression in the postnatal period and child development: A prospective population study. Lancet. 2005;365:2201–2205. doi: 10.1016/S0140-6736(05)66778-5. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: Longitudinal and concurrent associations with cortisol. Hormones and Behavior. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Schacht PM, Cummings EM, Davies PT. Fathering in family context and child adjustment: A longitudinal analysis. Journal of Family Psychology. 2009;23:790–797. doi: 10.1037/a0016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff EA, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, Goldsmith HH. Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31:1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. 2011. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson DR. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: Implications for the development of antisocial behavior. Behavioral Sciences & the Law. 2009;27:137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology. doi: 10.1017/S095457941100054X. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Krasner SS, Solomon EP, Janisse MP. The experience, expression, and control of anger. New York: Springer Verlag Publishers; 1988. [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Toth SL, Rogosch FA, Sturge-Apple M, Cicchetti D. Maternal depression, children’s attachment security, and representational development: An organizational perspective. Child Development. 2009;80:192–208. doi: 10.1111/j.1467-8624.2008.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampolini T, Ungerer JA, McMahon CA. Maternal depression: Relations with maternal caregiving representations and emotional availability during the preschool years. Attachment and Human Development. 2008;10:73–90. doi: 10.1080/14616730801900712. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Negriff S, Ji J, Peckins M. Child maltreatment and adolescent development. Journal of Research on Adolescence. 2011;21:3–20. [Google Scholar]
- Uvnäs-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: The role of the oxytocinergic system. International Journal of Behavioral Medicine. 2005;12:59–65. doi: 10.1207/s15327558ijbm1202_3. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weems CF, Carrion VG. The association between PTSD symptoms and salivary cortisol in youth: The role of time since the trauma. Journal of Traumatic Stress. 2007;20:903–907. doi: 10.1002/jts.20251. [DOI] [PubMed] [Google Scholar]
- Weems CF, Carrion VG. Brief report: Diurnal salivary cortisol in youth – clarifying the nature of posttraumatic stress dysregulation. Journal of Pediatric Psychology. 2009;34:389–395. doi: 10.1093/jpepsy/jsn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Durbin CE. Effects of paternal depression on fathers’ parenting behaviors: A meta-analytic review. Clinical Psychology Review. 2010;30:167–180. doi: 10.1016/j.cpr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Winkler D, Pjrek E, Kasper S. Anger Attacks in Depression: Evidence for a Male Depressive Syndrome. Psychotherapy and Psychosomatics. 2005;74:303–307. doi: 10.1159/000086321. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Shirtcliff EA, Pollak SD. Neuroendocrine dysregulation following early social deprivation in children. Developmental Psychobiology. 2008;50:588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]