Abstract
To conserve scarce energetic resources during winter, seasonal breeders inhibit reproduction and other nonessential behavioral and physiological processes. Reproductive cessation is initiated in response to declining day lengths, a stimulus represented centrally as a long-duration melatonin signal. The melatonin signal is not decoded by the reproductive axis directly, but by an unidentified neurochemical system upstream of gonadotropin-releasing hormone (GnRH). The dorsomedial nucleus of the hypothalamus (DMH) has been implicated in seasonal changes in reproductive function in Syrian hamsters (Mesocricetus auratus), although the specific-cell phenotype decoding photoperiodic information remains unknown. RFamide-related peptide (RFRP; the mammalian homolog of the gonadotropin-inhibitory hormone (GnIH) gene identified in birds) has emerged as a potent inhibitory regulator of the reproductive axis and, significantly, its expression is localized to cell bodies of the DMH in rodents. In the present study, the authors explored the relationship between RFRP expression, photoperiod exposure, and reproductive condition/hormonal status. In male hamsters that respond to short days with reproductive inhibition, RFRP-ir and mRNA expression are markedly reduced relative to long-day animals. Replacement of testosterone in short-day animals did not affect this response, suggesting that alterations in RFRP expression are not a result of changing sex steroid concentrations. A subset of the hamster population that ignores day length cues and remains reproductively competent in short days (nonresponders) exhibits RFRP-ir expression comparable to long-day hamsters. Analysis of cell body and fiber density suggests a potential interplay between peptide production and release rate in differentially regulating the reproductive axis during early and late stages of reproductive regression. Together, the present findings indicate that photoperiod-induced suppression of reproduction is associated with changes in RFRP and mRNA expression, providing opportunity for further exploration on the role that RFRP plays in this process.
Keywords: reproduction, gonad, melatonin, seasonal, photoperiod, DMH
Environmental cycles, such as the annual progression of the seasons, have exerted potent selective pressures resulting in seasonally recurring changes in behavior and physiology across taxa. Survival and reproductive fitness are contingent on an individual’s success at negotiating the challenges imposed by a fluctuating and often severe physical environment. Animals that inhabit temperate and boreal latitudes can gain a significant selective advantage by anticipating extreme seasonal shifts and inhibiting energetically expensive processes prior to winter months when food is scarce and weather inclement (Bronson, 1989). Photoperiod, or day length, serves as the most salient proximate factor enabling the timing of seasonal adaptations (Goldman, 2001). Reproduction and associated behaviors are precisely timed to coincide with abundant local food resources and other environmental conditions that are favorable for rearing offspring (Nelson et al., 1990). By inhibiting breeding during winter, animals with short gestation periods conserve significant energetic resources during times when a mistimed reproductive effort could be fatal for both mother and offspring. Despite the intense selective pressure to inhibit reproduction during winter, an alternative strategy of photoperiodic nonresponsiveness occurs at a much lower frequency (Prendergast et al., 2001). Nonresponsive individuals maintain an active reproductive system in the winter, a strategy with a presumed fitness advantage during mild winters, resulting in an evolutionarily stable coexistence of 2 seasonal reproductive phenotypes.
In mammals, day length information is communicated to the brain via nocturnal secretion of the pineal hormone melatonin (Goldman, 2001; Malpaux et al., 2001). The duration of the melatonin signal is directly proportional to the length of night and allows animals to determine time of year precisely (Bartness et al., 1993; Carter and Goldman, 1983). When held in day lengths of sufficiently short duration (or given melatonin of a significantly long duration), Syrian hamsters undergo testicular involution within 8 weeks; when held in day lengths typical of summer conditions, full reproductive condition is maintained (Paul et al., 2008). Seasonal changes in reproductive state are governed by the interaction of 2 systems: a timing mechanism whose output is communicated by melatonin and the neuroendocrine substrate regulating reproductive function, the hypothalamic-pituitary-gonadal (HPG) axis.
The potency of the negative feedback actions of sex steroids is one of the principal mechanisms driving seasonal changes in reproductive function (Ellis and Turek, 1979, 1980; Karsch, 1987; Karsch et al., 1993; Meyer and Goodman, 1986; Sisk and Turek, 1982; Tamarkin et al., 1976). During the breeding season, testosterone exerts a relatively weak negative feedback influence on gonadotropin secretion. Following exposure to inhibitory day lengths, gonadal hormones become markedly more effective at inhibiting gonadotropin secretion (Ellis and Turek, 1979; Sisk and Turek, 1982; Tamarkin et al., 1976; Turek, 1977). Whereas it is well established that day length is encoded in the duration of the melatonin signal (Bartness et al., 1993), the neural locus(i) where this signal is decoded by the reproductive system to affect negative feedback processing remains undetermined. Given the absence of androgen receptors in gonadotropin-releasing hormone (GnRH) neurons (Huang and Harlan, 1993; Tilbrook and Clarke, 2001), negative feedback presumably occurs upstream of the GnRH system. The dorso-medial nucleus of the hypothalamus (DMH) has emerged as a key neural locus regulating the gonadotropic response to photoperiod in both male and female Syrian hamsters (Mesocricetus auratus; Lewis et al., 2002; Maywood et al., 1996). High affinity melatonin receptors overlap with sex steroid receptors in the DMH, suggesting a possible site for melatonin-driven alterations in negative feedback (Maywood et al., 1996). Lesions of the DMH abolish reproductive regression in response to inhibitory photoperiods or long duration infusions of melatonin (Lewis et al., 2002; Maywood et al., 1996). These lesions destroyed the DMH and fibers of passage, making it impossible to determine whether the DMH is the locus for melatonin’s actions, part of the circuitry mediating its effects, or whether fibers from one or more key nuclei decoding the melatonin signal pass through this brain region. However, the fact that the DMH exhibits a marked overlap in melatonin and androgen receptors strongly suggests participation in seasonal breeding. Despite suggestive evidence for the necessity of the DMH in mediating seasonal breeding in this species, the precise cellular identity of melatonin/androgen-sensitive neurons remains unknown.
We have characterized a novel gonadotropin-inhibitory hormonal system, RFamide-related peptide (RFRP; as the mammalian homolog of gonadotropin-inhibitory hormone (GnIH) identified in birds (Tsutsui et al., 2000)) in the brains of rodents (Kriegsfeld et al., 2006). RFRP belongs to the family of neuropeptides containing the C-terminal Arg-Phe-NH2 (RFamide; Tsutsui et al., 2007; Ukena and Tsutsui, 2005), whose prominent role in the regulation of neuroendocrine function has recently become a topic of considerable interest. Importantly, in the present context, RFRP cell bodies in hamsters are restricted to the DMH with projections directly to GnRH cell bodies, consistent with the possibility that this neuropeptide may link the reception of the melatonin signal in the DMH to the reproductive axis. Additionally, RFRP-immunoreactive (ir) cells express sex steroid receptors and have been implicated as a mediator of sex steroid negative feedback (Greives et al., 2008; Kriegsfeld, 2006). In mice, direct administration of RFRP-3 rapidly and repeatedly inhibits firing rate in GnRH neurons (Ducret et al., 2009), pointing to direct actions on this neuronal system. In agreement with this possibility, intracerebroventricular (ICV) administration of RFRP attenuates FOS expression in GnRH neurons (Anderson et al., 2009). A direct hypophysiotropic action for RFRP has recently received support in rats (Murakami et al., 2008) and sheep, whereby RFRP suppresses GnRH stimulated LH release in cultured pituitary cells (Clarke et al., 2008). Together, these findings suggest that RFRP cells are in a position to receive photoperiodic information via melatonin, alter their response to negative feedback through changes in androgen receptors, and communicate this information to the reproductive axis.
Considering the potent inhibitory effect of RFRP on the reproductive axis in conjunction with the overlapping neural distribution of RFRP-producing neurons with a neural locus identified as critical for mediating the effects of photoperiod on the reproductive axis in Syrian hamsters, we hypothesized that RFRP would be more abundantly produced and expressed under inhibitory day lengths relative to long day conditions. Therefore, the goal of the present studies was to examine whether the pattern of RFRP and mRNA expression is associated with changes in photoperiod and/or reproductive condition in the brains of Syrian hamsters. We utilized 2 methods to disentangle the effects of photoperiod from sex steroids. First, we exploited the natural phenomenon of photoperiodic nonresponsiveness, whereby individuals of a breeding population ignore day length cues and maintain a fully functional reproductive system in the winter (Prendergast et al., 2001). This variable response to short days offers a powerful tool to examine the effects of photoperiod exposure independent of changes in reproductive function in a natural context without manipulating sex steroids. To extend these findings and more closely examine the effects of photoperiod and hormonal status on RFRP expression, we measured photoperiodic effects on RFRP mRNA expression while manipulating steroid concentrations via castration and testosterone replacement.
MATERIALS AND METHODS
Animals
Adult (60–80 days of age), male LVG Syrian hamsters (M. auratus; n = 44 total for all studies) were used. All animals were purchased from Charles River (Wilmington, MA) at 4 to 5 weeks of age and allowed several weeks to acclimate to the laboratory conditions prior to the start of photoperiod manipulations. Animals were housed in translucent polypropylene cages (40 × 27 × 20 cm) and provided ad libitum access to food and water for the duration of the study. Animals were maintained in a colony room at 23 ± 1 °C with a 24-h light/dark cycle. All experimental protocols conformed to the Institutional Animal Care and Use Committee guidelines of the University of California, Berkeley.
Experiment 1
Syrian hamsters (M. auratus) were kept in long days (LD 16:8) until the start of the experiment, after which some (n = 5) of the animals remained in long days while others (n = 20) were moved to short-day (LD 8:16) conditions. Brains were collected from short-day hamsters after 3 weeks (n = 5) or 8 weeks (n = 15) of photoperiod exposure. Short-term photo-period exposure (i.e., 3 weeks) was used to separate the effects of photoperiod from hormonal status; these animals are acclimated to short day lengths but have not undergone alterations in the reproductive axis, and have testosterone profiles equivalent to long-day animals (Stirland et al., 1995, 1996). Animals responsive to short days display fully regressed gonads and basal sex steroids by 8 weeks in photoperiod. Animals in short days for 8 weeks that do not respond to photoperiod have a fully functioning reproductive axis (Prendergast et al., 2001). Animals in the 8-week short-day condition were separated into “responders” (SD-R; n = 9) and “nonresponders” (SD-NR; n = 6) based on gonadal size as described below.
Perfusions and Tissue Collection
At the conclusion of the experiment, hamsters were weighed to the nearest 0.1 g, anesthetized deeply with sodium pentobarbital (200 mg/kg), and perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.3). All hamsters were killed between the hours of 1200 h and 1500 h PST. A previous report (Revel et al., 2008) noted that there was no circadian fluctuation in levels of RFRP expression in the brains of hamsters. Brains were postfixed for 3 h at room temperature in 4% paraformaldehyde and cryoprotected in 20% sucrose in 0.1 M PBS and stored at 4 °C until processed. Coronal sections (40 μm) were cut on a cryostat and processed as free-floating sections beginning rostrally at the medial septum/diagonal band of Broca and extending caudally to the brain stem. Necropsies were performed and reproductive organs (paired testes, epididymides, and seminal vesicles) were collected, cleaned of fat and connective tissue, and weighed to confirm the effects of photoperiodic treatments on reproductive condition. After 8 weeks in short days, animals that had paired testes weighing more than 0.15 g were classified as nonresponders; animals with paired testes weighing <0.15 g were classified as responders. The threshold value of 0.15 g constitutes >2 standard deviations above the mean gonadal mass observed for a typical responder.
Single-Label Immunofluorescence
For visualization of RFRP, sections were washed in PBS, incubated in 0.5% H2O2, and incubated in normal goat serum in 0.1% Triton X-100 (PBT) for l h. Sections were then incubated for 48 h at 4 °C in antiserum generated against white-crowned sparrow GnIH (PAC 123a) diluted at 1:100,000 with 0.1% PBT as previously validated in Syrian hamsters with this antibody (Gibson et al., 2008; Kriegsfeld et al., 2006). After incubation in anti-GnIH, brains were incubated for 1 h in biotinylated goat anti-rabbit (1:300; Vector Laboratories, Burlingame, CA), followed by incubation in avidin-biotin-horseradish peroxidase complex (ABC Elite kit, Vector Laboratories). Brains were then incubated in a biotinylated tyramide solution (0.6%) for 30 min. Cells were then labeled by using Cy-2 conjugated to streptavidin (The Jackson Laboratory, Sacramento, CA) as the fluorophore. Control staining was accomplished by preadsorbing the primary RFRP antibody with rat or hamster RFRP peptides (each of which eliminated staining).
Measures
RFRP cell numbers, size, and optical density were measured. To gain a more detailed understanding of peptide expression, fiber density was evaluated at major RFRP terminal zones. In brief, sections were investigated using a Zeiss Z1 microscope, using the standard wavelength for Cy-2 (488 nm). Every 4th section from the medial septum to the brainstem was assessed. RFRP-ir cell bodies were located by visually scanning the brains under 200× magnification and were found to be restricted to the DMH, as previously reported. All RFRP-ir cells were counted through the rostrocaudal extent of the DMH. Immunoreactive cells were photographed in gray-scale with a Zeiss Axiocam Cooled CCD camera at 400× magnification for cell size and density analyses. Cell bodies were outlined and the 2-dimensional area was calculated using NIH Image 1.61. Each pixel in the grayscale image capture, which is easily produced by inverting (i.e., negative image) a single-label immunofluorescent image, has a measurable specific intensity, with values ranging from 0 (white) to 256 (black). The average value for all pixels in an outlined area is taken as the mean intensity of staining for a given region of the image. OD measures were normalized to minimize differences between replications of immunohistochemistry. First, a background measurement was taken by placing a square outline, 4 times, on nonoverlapping, unstained areas of each section. The mean of these 4 measures provided the background OD for each section. The OD for each cell body was assessed by outlining the cell body, obtaining a density measure using NIH Image, and subtracting the background OD from the OD of each cell. To account for potential overcounting, an Abercrombie correction was applied to cell count data prior to analysis.
Experiment 2
To further investigate the role of photoperiod and hormonal status on RFRP mRNA expression, animals were castrated and either given a blank, control Silastic (Dow Corning, Inc., Midland, MI) capsule, or a capsule filled with testosterone (15-mm length, 1.45-mm ID, 1.93-mm OD). This capsule length has been shown to restore circulating T levels to average basal levels of an intact LD Syrian hamster (Bittman et al., 1999; Faruzzi et al., 2005). Animals (n = 20) were held in long days (LD 16:8) prior to surgery. More specifically, all hamsters (>60 days of age) were gonadectomized under isoflurane anesthetic, after which half (n = 10) were implanted with testosterone and the other half (n = 10) received an empty capsule. Upon recovery from surgery, half of the animals from each hormone condition (n = 5/group) remained in long days (LD 16:8) whereas the other half were transferred to short days (LD 8:16) for 8 weeks prior to brain collection for in situ hybridization.
In Situ Hybridization
Fresh frozen brain tissue was used for this experiment. Coronal sections (20 μm) through the medial basal hypothalamus were cut on a cryostat and thaw mounted onto silane-coated slides (Electron Microscopy Sciences, Hatfield, PA). In brief, fixed slices were hybridized with DIG-labeled sense or antisense RNA probes (200 ng/mL) at 50 °C overnight followed by an additional 30-min RNase digestion step to further reduce the background caused by free DIG-labeled single strand RNA probe. The DIG-labeled probes were produced by a dual promoter (SP6 and T7) plasmid (Topo-II vector from Invitrogen) with hamster RFRP partial sequence inserted (GenBank accession number: DQ371799). After digestion and washing, the sections were treated with 1.5% blocking reagent (Roche Diagnostics, Indianapolis, IN) in PBS and incubated with alkaline phosphatase-labeled sheep anti-digoxigenin antibody (1:1000 dilution in the blocking solution; Roche Diagnostics, Indianapolis, IN) for 1 h. Immunoreactive products were detected by immersing the sections for 12 h in NBT/BCIP substrate solution (Roche Diagnostics), and the expression of RFRP mRNA was observed with a Zeiss M1 microscope.
Statistics
All statistical analyses were conducted using the SigmaStat analysis package (Systat Software, Inc., Chicago, IL). Data from experiment 1 were analyzed as a series of 1-way analyses of variance (ANOVAs). Experiment 2 was analyzed with a 2-way (photoperiod × hormone treatment) ANOVA. All values are reported as means (± SEM) and all tests were considered significant if p < 0.05. Group differences were assessed using post hoc Tukey tests.
RESULTS
Experiment 1
Reproductive organ masses are influenced by photoperiod
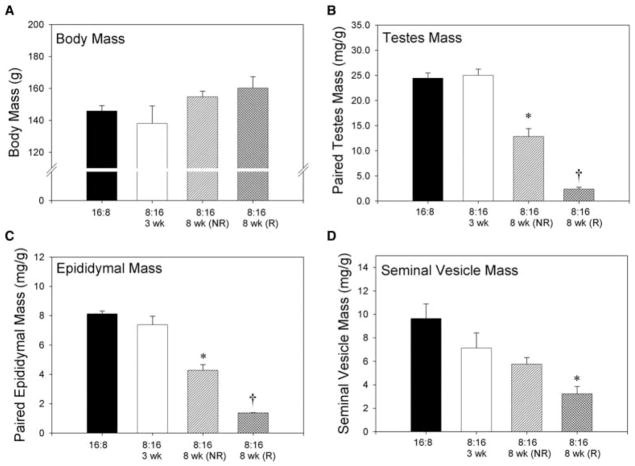
Short-day exposure for 8 weeks significantly reduced reproductive organ (i.e., testes, seminal vesicles, and epididymides) masses in reproductively responsive animals relative to long-day controls, short-day 3-week animals, and short-day 8-week nonresponders (p < 0.05 in each case; Fig. 1). Seminal vesicle mass did not differ among nonresponsive animals exposed to short days for 8 weeks, long-day animals and short-day 3-week hamsters (p > 0.05 in each case). In contrast, epididymal and paired testes masses of short-day 8-week nonresponders were lower than long-day hamsters and short-day 3-week animals (p < 0.05 in each case) and greater than those of short-day 8-week responders (p < 0.05). There was no effect of photoperiod on body mass (p > 0.05).
Figure 1.
Body and reproductive organ masses from animals held in long (LD 16:8) or short (LD 8:16) day lengths. Animals held in short days for 8 weeks were separated into “responders” (R) or “nonresponders” (NR) based on gonadal size. Body size (A) was unaffected by photoperiodic treatment. Testes mass (B) and epididymal mass (C) were significantly diminished in R hamsters held in short days for 8 weeks. NR animals held in short days for 8 weeks had significantly decreased testes and epididymal masses than either long-day or short-day 3-week animals. Seminal vesicle mass (D) was significantly less in short-day 8-week animals than all other groups.
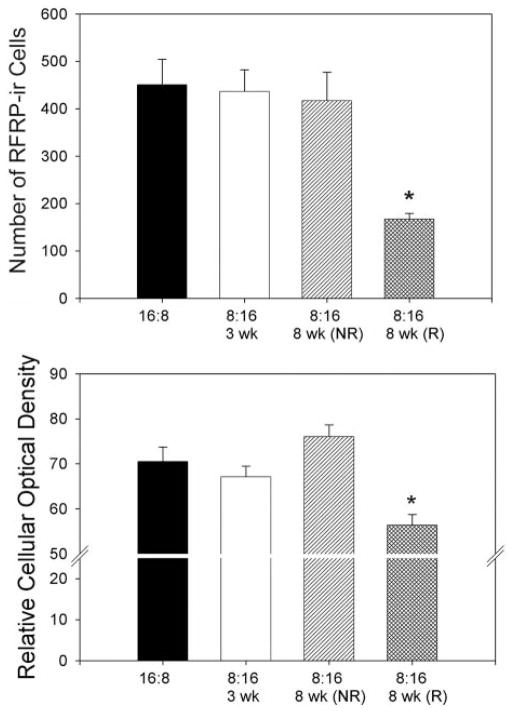
RFamide-related peptide immunoreactive (RFRP-ir) cell numbers and optical density are associated with reproductive condition, independent of photoperiod
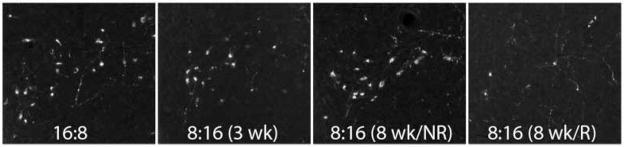
To determine the effects of photoperiod and reproductive condition on RFRP expression, Syrian hamster brains were processed immunohistochemically to examine RFRP-ir (Fig. 2). As we have previously reported, RFRP-ir cells were restricted to the DMH, with extensive projections to midline brain regions. Reproductive state significantly affected the number and optical density (p < 0.05, in both cases) of RFRP-ir neurons in the DMH. Animals that responded with reproductive inhibition to 8 weeks’ exposure in short-day (LD 8:16) photoperiod displayed significantly fewer (p < 0.05) and less dense (p < 0.05) RFRP-ir neurons than all other groups; all other groups displayed a similar number and size of RFRP-ir neurons (p > 0.05; Fig. 3). Photoperiod alone did not significantly affect RFRP-ir cell number or size, as hamsters non-responsive to 8 weeks of short-day photoperiod, as well as ones held in short days for only 3 weeks, resembled their long-day (LD 16:8) counterparts in measures of cell number and size (p > 0.05).
Figure 2.
RFRP immunohistochemical labeling. Representative photomicrographs of GnIH-ir staining in the DMH of hamsters held in long-day (LD 16:8) photoperiod (A) for 8 weeks, short-day (LD 8:16) photoperiod (B) for 3 weeks, short-day nonresponders (NR; C) held for 8 weeks, and short-day responders (D) held for 8 weeks. High power (200×) photomicrographs are shown for each condition.
Figure 3.
RFRP-ir cell numbers and optical density are associated with reproductive condition, independent of photoperiod. Mean (± SEM) number of RFRP-ir cells (top) and cellular optical density (bottom) of animals held in long days for 8 weeks (LD 16:8) or short days (LD 8:16) for 3 weeks or 8 weeks (responders (R) and nonresponders (NR)). Short-day R hamsters held for 8 weeks in short days display significantly fewer number of RFRP-ir cells. Cellular optical density was unaffected by photo-period. *p < 0.05.
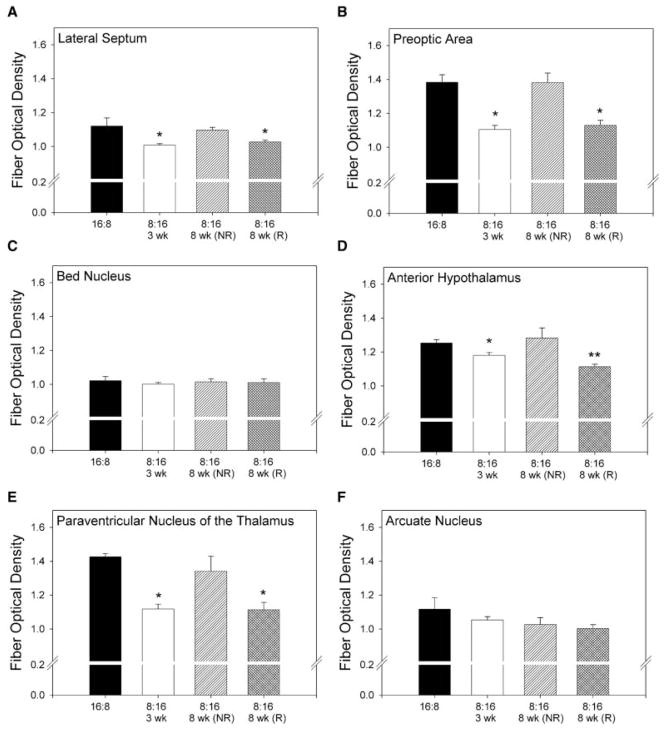
Fiber density is associated with changes in photoperiod and reproductive condition
In an effort to characterize further the changes in RFRP-ir expression in different photoperiods, we examined the density of RFRP-ir fiber staining in many of the key targets of RFRP terminals (lateral septum (LS), preoptic area (POA), bed nucleus of the stria terminalis (BNST), anterior hypothalamus (AH), paraventricular nucleus of the thalamus (PVT), and the arcuate nucleus (Arc)). Hamsters with regressed gonads held for 8 weeks in short-day (LD 8:16) photoperiods had significantly decreased (p < 0.05) fiber optical density in the LS, POA, AH, and the PVT compared to either long-day (LD 16:8) or short-day nonresponder groups. Hamsters held for 3 weeks in short days (LD 8:16) had significantly (p < 0.05) decreased fiber optical density in all of the same target nuclei (Fig. 4).
Figure 4.
RFRP-ir fiber density is associated with changes in photoperiod and reproductive condition in some brain targets. Fiber optical density in the (A) lateral septum, (B) preoptic area, (D) anterior hypothalamus, and (E) paraventricular nucleus of the thalamus was significantly affected by photoperiod in short-day responsive (R) animals held for 8 weeks in short days. In these same nuclei, fiber density was significantly decreased in animals held in short days for 3 weeks. Fiber density was unaffected by photoperiod in the (C) bed nucleus of the stria terminalis and the (F) arcuate nucleus. *Significantly less than all groups, excluding those bearing a single asterisk (p < 0.05). **Significantly less than all groups (p < 0.05).
Experiment 2
RFamide-related peptide mRNA expression is affected by photoperiod treatment, independent of hormone levels
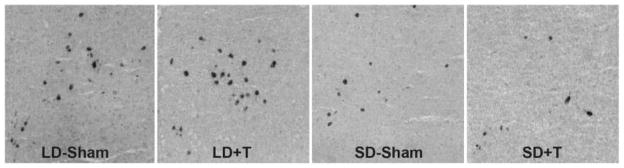
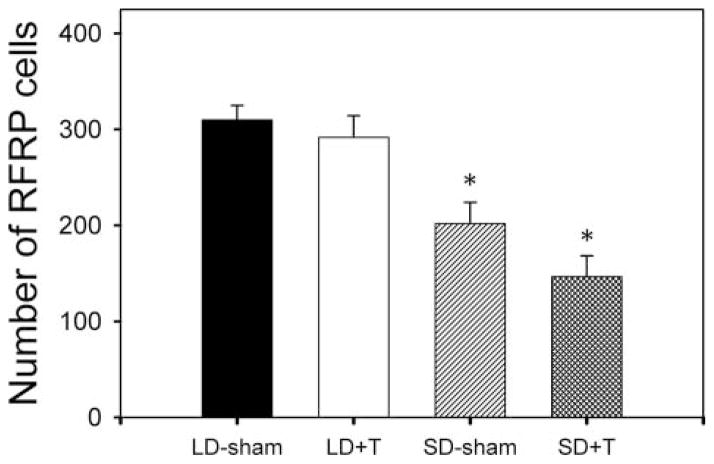
To determine whether differences in peptide expression in experiment 1 were the result of changes in RFRP transcription, we examined RFRP mRNA expression using in situ hybridization in animals whose testosterone concentrations were manipulated in each photoperiodic condition. Hamsters held in short days (LD 8:16) for 8 weeks exhibited significantly fewer (p < 0.05) RFRP mRNA-expressing cells than groups held in long days, regardless of testosterone treatment. There was no significant effect of testosterone in either photoperiod (p > 0.05), suggesting that RFRP expression is modulated by day length, and not a secondary result of changes in sex steroid concentrations (Figs. 5 and 6).
Figure 5.
RFRP mRNA labeling. Representative photomicrographs depicting RFRP mRNA expression across long-day (LD) and short-day (SD) photoperiods in gonadectomized animals receiving either testosterone replacement (+T) or an empty capsule (sham).
Figure 6.
RFRP mRNA expression is affected by photoperiod, independent of testosterone levels. Mean (± SEM) number of RFRP mRNA-expressing cells of gonadectomized animals held in long-day (LD 16:8) and short-day (LD 8:16) photoperiods and receiving either testosterone replacement (+T) or empty capsules (sham). Hamsters held in short days (LD 8:16) exhibited significantly fewer (p < 0.05) RFRP mRNA-expressing cells than groups held in long days, regardless of testosterone treatment. There was no significant effect of testosterone in either photoperiod. *p < 0.05.
DISCUSSION
The present findings uncover an unanticipated pattern of RFRP expression associated with seasonal changes in reproductive function in Syrian hamsters. Males exposed to short, winter-like photoperiods with a regressed reproductive apparatus exhibit a marked reduction in RFRP-ir and mRNA expression relative to their long-day counterparts. Importantly, these differences were not secondary consequences of diminished sex steroid concentrations, as testosterone replacement did not elevate RFRP mRNA expression to long-day values in short-day hamsters. Likewise, RFRP-ir cells were unaffected in short-day animals with a reproductive system that did not undergo regression, suggesting an association between changes in RFRP and reproductive competency.
Although our data are in agreement with recent findings (Revel et al., 2008), the results are counter to our original prediction that an increase in RFRP-ir and mRNA would be observed in animals with a quiescent reproductive system. Although it appears paradoxical that an inhibitory neuropeptide is decreased during inhibitory day lengths, these findings do not rule out a role for RFRP in seasonal changes in reproductive function. One possibility is that Syrian hamsters require enhanced RFRP activity to suppress GnRH during the initial period of regression, but this level of inhibition is not necessary in hamsters with a fully regressed reproductive apparatus. Further empirical examination of the pattern and kinetics of RFRP expression throughout the development of reproductive quiescence is necessary to fully explore this possibility. The literature in birds is supportive of a transient effect of GnIH on the reproductive axis. For example, hypothalamic GnIH content is increased at the onset of gonadal regression in seasonally breeding song sparrows, but this increase is not seen following gonadal regression (Bentley et al., 2003). Likewise, Calisi et al. observed an increase in hypothalamic GnIH content in response to stress in house sparrows at the start of the breeding season but not at the end (Calisi et al., 2008).
Immunohistochemical findings for RFRP expression in hamsters after 3 weeks of short-day exposure are consistent with the possibility that the release of RFRP is increased during initial stages of regression, before the reproductive axis begins to regress (Stirland et al., 1996). More specifically, although cell counts in long-day animals and hamsters held for 3 weeks in short days were identical, fiber density in RFRP cell targets was decreased in this latter group. The parsimonious explanation for this pattern of expression is that RFRP production is static during early stages of regression, whereas peptide release is increased at hypothalamic targets. However, it is also possible that RFRP production and transport are decreased at this time and this peptide does not participate in short-day-induced gonadal regression. It should be noted that, because 3 weeks is too early to assess the photoperiodic responsive phenotype of Syrian hamsters, it is possible that a small number of animals that ultimately would be classified as nonresponders may have been included in the analysis at the 3-week time point. At 8 weeks, decreased fiber expression, along with reduced RFRP-ir and mRNA expression in SD-R animals, suggests that transcription/translation is reduced at this time point relative to long-day animals, inconsistent with a role for RFRP in maintaining gonadal regression.
Theoretically, the trigger for changes in the RFRP system could be the coincidence of the long-duration melatonin signal with androgen receptor binding in, or upstream of, RFRP neurons. Results examining RFRP mRNA from short-day animals given testosterone suggest that photoperiod acts independent of testosterone negative feedback to suppress RFRP production. Recent studies using an immortalized RFRP cell line generated from rat hypothalamus indicate that these cells express melatonin receptors and respond to melatonin administration with increased expression, suggesting a steroid-independent mechanism of photoperiodic control (Gingerich et al., 2009). Whether or not RFRP cells express melatonin receptors in hamsters requires further investigation. It is also possible that the RFRP system responds secondarily to melatonin’s impact on other target loci. For example, a thyroid hormone-dependent cascade has recently been implicated in modulating the effects of photoperiod on the reproductive axis in a number of species. In sheep, the melatonin signal is decoded in the pars tuberalis through the local secretion of thyroid-stimulating hormone (TSH). In turn, TSH activates type II deiodinase (DIO2) leading to increased tri-iodothyronine production (Hanon et al., 2008). A similar mechanism of control has been identified in Siberian hamsters (Watanabe et al., 2004). The possibility that changes in DIO2 precede or contribute to changes in RFRP, or that these 2 pathways act in parallel to adjust the suite of reproductive traits that change seasonally, represents an interesting avenue for further inquiry.
The present findings uncover novel changes in RFRP-ir and mRNA expression associated with day length and reproductive status. The findings are consistent with the notion that RFRP contributes to the initial suppression of reproductive function, but not to the maintenance of reproductive quiescence following its completion. However, further empirical studies are required to fully understand the role of this neuropeptide in mediating seasonal changes in reproductive function.
Acknowledgments
Funding
National Institutes of Health Grants HD-050470 (L.J.K.) and MH075045 (R.S.) and National Science Foundation Grant IOS-0641188 (G.E.B.)
Footnotes
Declaration of Conflicting Interest
The authors have no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Anderson GM, Relf HL, Rizwan MZ, Evans JJ. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology. 2009;150:1834–40. doi: 10.1210/en.2008-1359. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Tubbiola ML, Foltz G, Hegarty CM. Effects of photoperiod and androgen on proopiomelanocortin gene expression in the arcuate nucleus of golden hamsters. Endocrinology. 1999;140:197–206. doi: 10.1210/endo.140.1.6458. [DOI] [PubMed] [Google Scholar]
- Bronson F. Mammalian Reproductive Biology. Chicago: The University of Chicago Press; 1989. [Google Scholar]
- Calisi RM, Rizzo NO, Bentley GE. Seasonal differences in hypothalamic EGR-1 and GnIH expression following capture-handling stress in house sparrows (Passer domesticus) Gen Comp Endocrinol. 2008;157:283–287. doi: 10.1016/j.ygcen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, et al. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–5821. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- Ducret E, Anderson GM, Herbison AE. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology. 2009;150:2799–2804. doi: 10.1210/en.2008-1623. [DOI] [PubMed] [Google Scholar]
- Ellis GB, Turek FW. Time course of the photoperiod-induced change in sensitivity of the hypothalamic-pituitary axis to testosterone feedback in castrated male hamsters. Endocrinology. 1979;104:625–630. doi: 10.1210/endo-104-3-625. [DOI] [PubMed] [Google Scholar]
- Ellis GB, Turek FW. Photoperiodic regulation of serum luteinizing hormone and follicle-stimulating hormone in castrated and castrated-adrenalectomized male hamsters. Endocrinology. 1980;106:1338–1344. doi: 10.1210/endo-106-5-1338. [DOI] [PubMed] [Google Scholar]
- Faruzzi AN, Solomon MB, Demas GE, Huhman KL. Gonadal hormones modulate the display of submissive behavior in socially defeated female Syrian hamsters. Horm Behav. 2005;47:569–575. doi: 10.1016/j.yhbeh.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–4969. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingerich S, Wang X, Lee PK, Dhillon SS, Chalmers JA, Koletar MM, Belsham DD. The generation of an array of clonal, immortalized cell models from the rat hypothalamus: analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neurons. Neuroscience. 2009;162:1134–1140. doi: 10.1016/j.neuroscience.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Kriegsfeld LJ, Bentley GE, Tsutsui K, Demas GE. Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction? Proc Biol Sci. 2008;275:1943–1951. doi: 10.1098/rspb.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- Huang X, Harlan RE. Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res. 1993;624:309–311. doi: 10.1016/0006-8993(93)90094-4. [DOI] [PubMed] [Google Scholar]
- Karsch FJ. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Ann Rev Physiol. 1987;49:365–382. doi: 10.1146/annurev.ph.49.030187.002053. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dahl GE, Evans NP, Manning JM, Mayfield KP, Moenter SM, Foster DL. Seasonal changes in gonadotropin-releasing hormone secretion in the ewe: alteration in response to the negative feedback action of estradiol. Biol Reprod. 1993;49:1377–1383. doi: 10.1095/biolreprod49.6.1377. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ. Driving reproduction: RFamide peptides behind the wheel. Horm Behav. 2006;50:655–666. doi: 10.1016/j.yhbeh.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci U S A. 2006;103:2410–2415. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D, Freeman DA, Dark J, Wynne-Edwards KE, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: mediation by the mediobasal hypothalamus. J Neuroendocrinol. 2002;14:294–299. doi: 10.1046/j.1365-2826.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms. 2001;16:336–347. doi: 10.1177/074873001129002051. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod. 1996;54:470–477. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- Meyer SL, Goodman RL. Separate neural systems mediate the steroid-dependent and steroid-independent suppression of tonic luteinizing hormone secretion in the anestrous ewe. Biol Reprod. 1986;35:562–571. doi: 10.1095/biolreprod35.3.562. [DOI] [PubMed] [Google Scholar]
- Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. J Endocrinol. 2008;199:105–112. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Badura LL, Goldman BD. Mechanisms of seasonal cycles of behavior. Annu Rev Psychol. 1990;41:81–108. doi: 10.1146/annurev.ps.41.020190.000501. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci. 2008;363:341–361. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q Rev Biol. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902–912. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Turek FW. Daily melatonin injections mimic the short day-induced increase in negative feedback effects of testosterone on gonadotropin secretion in hamsters. Biol Reprod. 1982;27:602–608. doi: 10.1095/biolreprod27.3.602. [DOI] [PubMed] [Google Scholar]
- Stirland JA, Grosse J, Loudon AS, Hastings MH, Maywood ES. Gonadal responses of the male tau mutant Syrian hamster to short-day-like programmed infusions of melatonin. Biol Reprod. 1995;53:361–367. doi: 10.1095/biolreprod53.2.361. [DOI] [PubMed] [Google Scholar]
- Stirland JA, Mohammad YN, Loudon AS. A mutation of the circadian timing system (tau gene) in the seasonally breeding Syrian hamster alters the reproductive response to photoperiod change. Proc Biol Sci. 1996;263:345–350. doi: 10.1098/rspb.1996.0053. [DOI] [PubMed] [Google Scholar]
- Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormone in the Syrian hamster. Endocrinology. 1976;99:1528–1533. doi: 10.1210/endo-99-6-1528. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Clarke IJ. Negative feedback regulation of the secretion and actions of gonadotropin-releasing hormone in males. Biol Reprod. 2001;64:735–742. doi: 10.1095/biolreprod64.3.735. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Bentley GE, Ubuka T, Saigoh E, Yin H, Osugi T, Inoue K, Chowdhury VS, Ukena K, Ciccone N, et al. The general and comparative biology of gonadotropin-inhibitory hormone (GnIH) Gen Comp Endocrinol. 2007;153:365–370. doi: 10.1016/j.ygcen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Turek FW. The interaction of the photoperiod and testosterone in regulating serum gonadotropin levels in castrated male hamsters. Endocrinology. 1977;101:1210–1215. doi: 10.1210/endo-101-4-1210. [DOI] [PubMed] [Google Scholar]
- Ukena K, Tsutsui K. A new member of the hypothalamic RF-amide peptide family, LPXRF-amide peptides: structure, localization, and function. Mass Spectrom Rev. 2005;24:469–486. doi: 10.1002/mas.20031. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, Yoshimura T. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology. 2004;145:1546–1549. doi: 10.1210/en.2003-1593. [DOI] [PubMed] [Google Scholar]