Abstract
In mammals, circadian rhythms are generated by the suprachiasmatic nuclei (SCN) of the hypothalamus. SCN neurons are heterogeneous and can be classified according to their function, anatomical connections, morphology and/or peptidergic identity. We focus here on gastrin-releasing peptide- (GRP) and on GRP receptor- (GRPr) expressing cells of the SCN. Pharmacological application of GRP in vivo or in vitro can shift the phase of circadian rhythms, and GRPr-deficient mice show blunted photic phase shifting. Given the in vivo and in vitro effects of GRP on circadian behavior and on SCN neuronal activity, we investigated whether the GRPr might be under circadian and/or diurnal control. Using in situ hybridization and autoradiographic receptor binding, we localized the GRPr in the mouse SCN and determined that GRP binding varies with time of day in animals housed in a light–dark cycle but not in conditions of constant darkness. The latter results were confirmed with Western blots of SCN tissue. Together, the present findings reveal that changes in GRPr are light driven and not endogenously organized. Diurnal variation in GRPr activity probably underlies intra-SCN signaling important for entrainment and phase shifting.
Keywords: circadian, gastrin-releasing peptide, neuropeptide, receptor binding
Introduction
It is well established that the suprachiasmatic nucleus (SCN) of the hypothalamus serves as a brain clock regulating circadian rhythms in physiology and behavior (Klein et al., 1991). A coherent rhythm in metabolic activity, electrical activity and gene expression occurs in the SCN at the tissue level, and results in circadian rhythmicity in the organism as a whole (reviewed by Herzog & Schwartz, 2002; Antle & Silver, 2005). Nevertheless, it is known that individual SCN cells are heterogeneous in peptidergic phenotype, gene expression and function (Moore & Silver, 1998; Silver et al., 1999; Karatsoreos et al., 2004).
Circadian oscillation is a property of individual cells (Welsh et al., 1995; Aton et al., 2005), although some SCN cells lack detectable circadian rhythms in gene expression (Hamada et al., 2001) and electrical activity (Jobst & Allen, 2002). In mouse, gastrin-releasing peptide- (GRP) containing cells lack detectable rhythmic expression of Period genes or proteins, but do respond to photic stimulation, as measured by Per1 mRNA and FOS induction (Dardente et al., 2002b; Karatsoreos et al., 2004). In rat, GRP cells receive direct retinal contacts (Tanaka et al., 1997), and in mouse, GRP-containing cells are located in the retinorecipient region of the SCN (Abrahamson & Moore, 2001; Karatsoreos et al., 2004).
Pharmacological studies suggest that the GRP receptor (GRPr) plays a central role in the photic resetting of the circadian clock. GRP injections in the SCN in vivo produce behavioral phase shifts that are similar to those induced by light (Albers et al., 1995; Piggins et al., 1995; Antle et al., 2005), and GRPr-deficient mice display blunted phase shifts to both light and GRP but have normal circadian rhythms (Aida et al., 2002). Additionally, application of GRP to SCN slices in vitro leads to phase shifts in electrical firing that can be blocked by prior application of GRPr antagonists (McArthur et al., 2000). To assess the contribution of circadian and/or diurnal gating of the GRPr, the present study explored the distribution of GRPr within the SCN using immunohistochemistry and in situ hybridization, and the circadian and diurnal patterns of GRPr binding through receptor autoradiography and Western blotting.
Methods
Animals and housing
Experimental mice (male, C57BL/6J, aged 50–60 days, Charles River, MA, USA) for receptor binding (n = 48) and Western blotting studies (n = 36) were housed in translucent propylene cages (29 × 19 × 12.5 cm), in a light–dark cycle (LD 12 : 12 h) or were transferred to constant darkness (DD) for at least 2 days prior to experimentation. For immunocytochemistry (ICC) and in situ hybridization analysis of GRPr, another group of animals (n = 4) was used. All animals were housed as above in a light–dark cycle (LD 12 : 12 h).
The animal rooms were maintained at 21 ± 1 °C. A white-noise generator (91 dB spl) masked environmental noise and a dim red light (0.5–1 lux) allowed for animal maintenance. All animals were provided with food and water ad libitum, and the experimental procedures were in accordance with the Columbia University Institutional Animal Care and Use Committee and Animal Welfare regulations.
Perfusion, ICC and in situ hybridization
For ICC and GRPr mRNA analysis, animals were deeply anesthetized (pentobarbital: 200 mg/kg i.p.). Animals killed in the dark were anesthetized under the dim red light, with their heads covered with a light-proof hood until perfused. Mice were perfused intracardially with 50 mL of saline followed by 100 mL of 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.3. Brains were post-fixed for 18–24 h at 4 °C, and cryoprotected in 20% sucrose in 0.1 m PB overnight.
Brains were sectioned at 35 µm on a cryostat. Alternate sections were used for ICC and in situ hybridization. For ICC free-floating sections were first incubated in normal donkey serum for 1 h, and then simultaneously in anti-vasopressin made in guinea-pig (Peninsula Laboratories/Bachem, Belmont, CA, USA; 1 : 5000), and anti-GRP made in rabbit (ImmunoStar Inc., Hudson, WI, USA; 1 : 10000) for 48 h. Following the primary incubation, sections were placed in the appropriate donkey secondary antibody conjugated to a CY2 or CY3 fluorescent chromogen (1 : 200, Jackson ImmunoResearch, West Grove, PA, USA) for 1 h. Staining was not observed upon omission of the primary antibody. Sections were mounted onto gelatin-coated slides, and coverslips were applied with Krystalon (EM Science, Gibbstown, NJ, USA).
To detect GRPr mRNA, in situ hybridization histochemistry was performed as described previously (Karatsoreos et al., 2004). In brief, sections were processed with proteinase K (1 mg/mL, 0.1 m Tris buffer, pH 8.0; 50 mm EDTA; 10 min) at 37 °C and 0.25% acetic anhydride in 0.1 m triethanolamine for 10 min. Sections were incubated in hybridization buffer [60% formide, 10% dextran sulphate, 10 mm Tris/HCl (pH 8.0), 1 mm EDTA (pH 8.0), 0.6 m NaCl, 500 mg/mL of 0.2% n-laurylsarcosine, 200 mg/mL tRNA, 1× Denhardt’s solution, 0.25% SDS and 10 mm dithiothreitol (DTT)] containing digoxigenin-labeled GRPr antisense cRNA probes (plasmid, generous gift of Dr L. Hampton) for 16 h at 60 °C. A 1.4-kb sequence raised against the entire coding region of mouse GRPr mRNA was used (Shumyatsky et al., 2002). After a high-stringency post-hybridization wash, sections were treated with RNase A, and were then further processed for immunodetection with a nucleic acid detection kit (Boehringer Mannheim, Germany). Sections were incubated in 1.0% blocking reagent in buffer 1 (100 mm Tris/HCl buffer, 150 mm NaCl, pH 7.5) for 1 h at room temperature, then incubated at 4 °C in an alkaline phosphatase-conjugated digoxigenin antibody diluted 1 : 5000 in buffer 1 for 3 days. On the following day, sections were washed in buffer 1 twice (5 min each), and incubated in buffer 3 (100 mm Tris/HCl buffer, pH 9.5, containing 100 mm NaCl and 50 mm MgCl2) for 5 min. They were then incubated in a solution containing nitroblue tetrazolium salt (0.34 mg/mL) and 5-bromo-4-chrolo-3-indolyl phosphate toluidinium salt (0.18 mg/mL) (Roche Applied Science, Indianapolis, IN, USA) for 8 h. The colorimetric reaction was halted by immersing the sections in buffer 4 (10 mm Tris/HCl containing 1 mm EDTA, pH 8.0). Tissue hybridized with a sense probe resulted in no staining. Sections were mounted onto gelatin-coated slides, and coverslips were applied with Permount (Fisher Scientific, Houston, TX, USA).
Microscopy
Sections were examined on a Nikon Eclipse E800 microscope. Images were captured with a cooled CCD camera (SPOT; Morrell, Melville, NY, USA) and stored on a PC for subsequent analysis. Immunostained sections were excited using filters for CY2 (480 ± 20 nm) and CY3 (560 ± 40 nm), with each channel acquired independently, and then combined digitally within the SPOT software. For in situ processed tissue, images were captured in 8-bit grayscale. Images were then superimposed, and processed using Photoshop 7 (Adobe Systems, Mountain View, CA, USA).
Receptor autoradiography
For the receptor binding experiments, groups of animals were killed at circadian time (CT) 0, 4, 8, 12, 16 or 20 (n = 4 per group), or while in LD at Zeitgeber time (ZT) 0, 4, 8, 12, 16 or 20 (n = 4 per group). Animals were quickly anesthetized using CO2 and decapitated. Brains were rapidly removed and snap frozen on powdered dry ice. Brains were then stored at −80 °C until sectioning. Coronal sections (20 µm) were cut on a cryostat and thaw mounted on Fisher Brand Plus slides, and stored at −80 °C until processed.
For receptor autoradiography, slides were thawed, dried completely and pre-incubated in 50 mm Tris-acetate buffer (TAB, pH 7.4) for 45 min at room temperature to remove any endogenous ligand. The slides were then fan-dried for 20 min. One slide (eight coronal sections) per brain was incubated in 0.02 nm [125I]GRP (2200 Ci/mmol; Perkin-Elmer, Boston, MA, USA) in TAB, and one alternate slide was incubated in 0.02 nm [125I]GRP plus 0.2 mm GRP (Sigma, St. Louis, MO, USA). After incubation, sections were rinsed five times for 5 s each in ice-cold TAB and dried rapidly. Sections were placed in contact with Kodak MS film for 24 h with 125I-labeled plastic standards (Amersham Pharmacia Biotech, Piscataway, NJ, USA), which contained known quantities of radioactivity. Films were developed for 4 min in Kodak D-19 developer and fixed for 5 min in Kodak rapid fix.
Quantification of binding
In order to visualize and quantify GRP binding, films were placed upon a light table and analysed using computerized image analysis software (MCID-M4, Imaging Research, Inc., St. Catharines, ON, Canada) and the relative optical density (ROD) of the standardized strips (Amersham Pharmacia Biotech) was measured. Once a standard curve was generated, the ROD of both sides of the SCN in each section was taken.
Western blotting
For Western blotting, animals were deeply anesthetized by exposure to CO2, decapitated and brains placed into ice-cold saline solution. Animals were killed every 4 h, starting at ZT0 and CT0 (for the LD and DD groups, respectively; n = 3 per time point). The caudal aspect of the brain was blocked, and sectioned using a Vibratome (Ted Pella, Inc., Redding, CA, USA). Ten 10-µm serial sections were cut until the rostral aspect of the SCN was visible. At this point, 400-µm coronal sections of hypothalamus were collected in ice-cold saline. The SCN, visible as an opaque area under the dissecting scope, was separated from the optic tract and adjacent hypothalamus using a no. 7 dissecting blade. To prepare SCN lysates, the tissues were homogenized by sonication in lysis buffer [1% SDS in dH2O with Roche complete, Mini, EDTA-free protease inhibitor cocktail (no. 1836170, Roche Diagnostics, Basel, Switzerland)] and then incubated in a boiling water bath for 10 min. Lysates were spun in a microfuge (17 383 g for 30 s) to remove insoluble material. The protein concentration of the cleared supernatant was determined by the BCA method (Pierce, Rockford, IL, USA). Lysates (5 µg per lane) were subjected to SDS gel electrophoresis, blotted to a nitrocellulose membrane and probed with the GRPr antibody (1 : 500; gift from Dr R. V. Benya, University of Illinois, Chicago). In all cases, an actin loading control was run to ensure that no errors were made in the process of loading the gels. A single band was observed at ~37–50 kDa, and deletion of primary antibody eliminated this band. Gels were run in triplicate, and all runs were used in the analysis.
Quantification of Western blotting
Proteins were visualized by chemiluminescence according to the manufacturer’s instructions (Lumiglo; Upstate Cell Signaling, Charlottesville, VA, USA). ROD of the Western blots was measured using the image analysis software (MCID) by comparing intensity of the target band against the background intensity (target intensity/background intensity).
Statistical analyses
For receptor binding and Western blots, RODs were analysed by using a one-way anova (Sigma Stat, SPSS, Chicago, IL, USA). Significant main effects were further analysed using the Tukey HSD test. Differences were considered significant if P < 0.05. All data are presented as mean (± SEM).
Results
Anatomical distribution of GRP protein and GRPr mRNA within the SCN
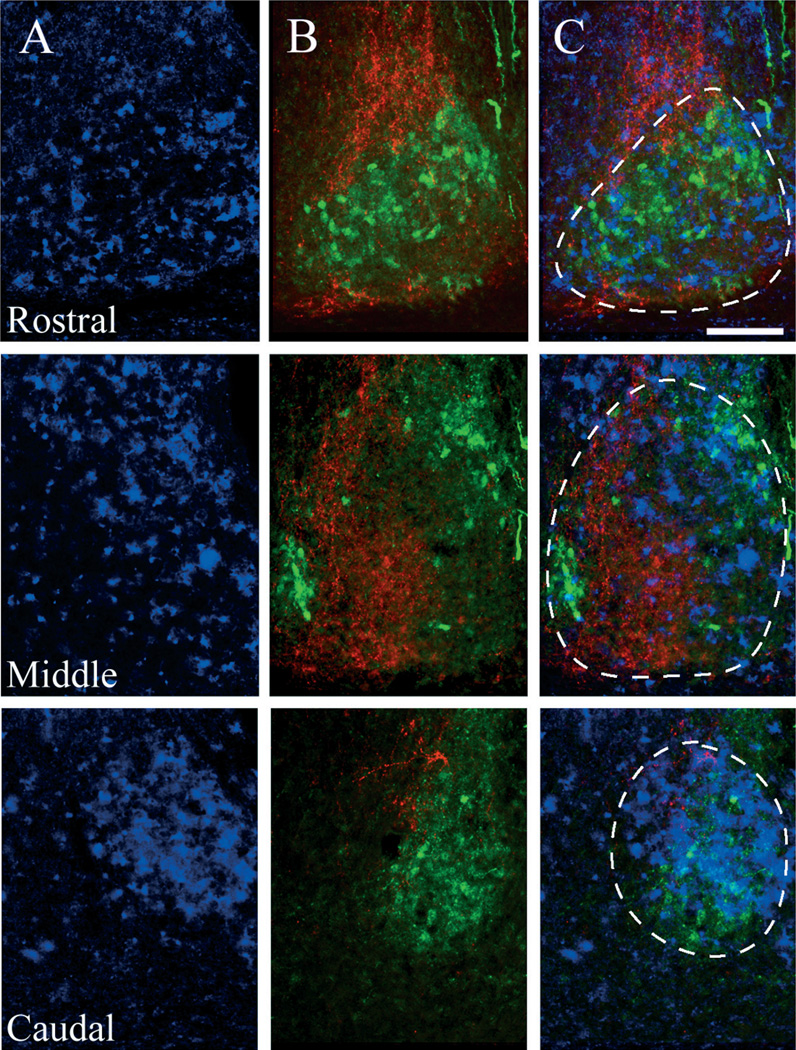
We examined the localization of GRP protein and GRPr mRNA in the mouse SCN using both ICC and digoxigenin in situ hybridization. GRPr mRNA expression is detected in the dorsal and medial aspects of the caudal SCN (Fig. 1A). We used immunostaining for well-characterized SCN peptides to delineate distinct SCN regions. Arginine vasopressin (AVP) immunoreactive (-ir) cells lie mostly in the dorsal and medial SCN, with a small cluster located on the extreme ventro-lateral portion of the SCN (Fig. 1B, green). GRP cell bodies are localized in the lateral and ventral aspects of the caudal portion of the SCN (Fig. 1B, red). The overlay of the mRNA in situ hybridization images with the corresponding protein immunofluorescence signal from adjacent sections demonstrates the compartmentalization of GRPr- (blue), AVP- (green) and GRP- (red) containing cell bodies (Fig. 1C). From rostral to caudal, GRPr mRNA is observed throughout the SCN. Highest expression appears to occur in the dorsal and medial regions, and lowest in the areas of GRP expression (Fig. 1C).
Fig. 1.
Distribution of GRPr mRNA with respect to arginine vasopressin (AVP) and GRP cells within the mouse SCN. (A) In situ hybridization for GRPr mRNA in the mouse SCN, representing the first ~40–80 µm (rostral), middle ~160–200 µm (middle) and caudal ~320–360 µm (caudal) aspects of the SCN. (B) Double labeled immunoflourescence of AVP- (green) and GRP- (red) containing cells in the mouse SCN, rostral to caudal (top to bottom), from sections directly adjacent to the sections used in A. (C) Overlay of the adjacent GRPr mRNA (blue) signal and AVP/GRP immunofluorescence. GRPr mRNA is detected throughout the SCN, but is highest in regions of AVP expression, and lowest in the GRP-containing region. Dotted lines delineate the SCN. Scale bar, 50 µm.
125I-GRP binding in the SCN
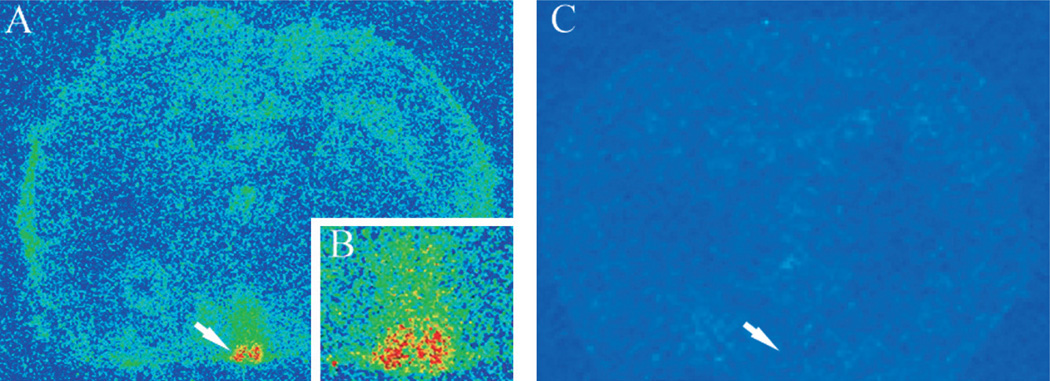
Radiolabeled GRP (125I-GRP) was used to examine GRP binding in the SCN. The highest levels of GRP binding were detected in the SCN (shown in yellow and red, Fig. 2A and B). In addition to that in the SCN, high levels of binding were detected in the amygdala, periventricular hypothalamic nucleus and ventral portion of the paraventricular hypothalamic nucleus, and moderate levels of GRP binding throughout the hypothalamus (data not shown). All GRP binding was eliminated when brain slices were co-incubated with 125I-GRP and excess unlabeled GRP (Fig. 2C).
Fig. 2.
Autoradiographic signal of 125I-GRP binding in the mouse brain. Low-magnification 20-µm coronal section, pseudo-colored to show distribution of GRP binding within the mouse brain. Yellow and red signals indicate regions of high binding, blue and green signals indicate regions of low binding. Arrows indicate the location of the SCN, just above the optic chiasm. (A) Brain section treated with 125I-GRP, with a higher magnification of the SCN in the inset (B), and (C) adjacent brain section treated with 125I-GRP, and with 1000-fold excess unlabeled GRP. The SCN shows very high levels of 125I-GRP binding. Moderate levels of binding are also observed in the lateral hypothalamus and the amygdalar region.
GRPr binding in the SCN is modulated by environmental lighting conditions
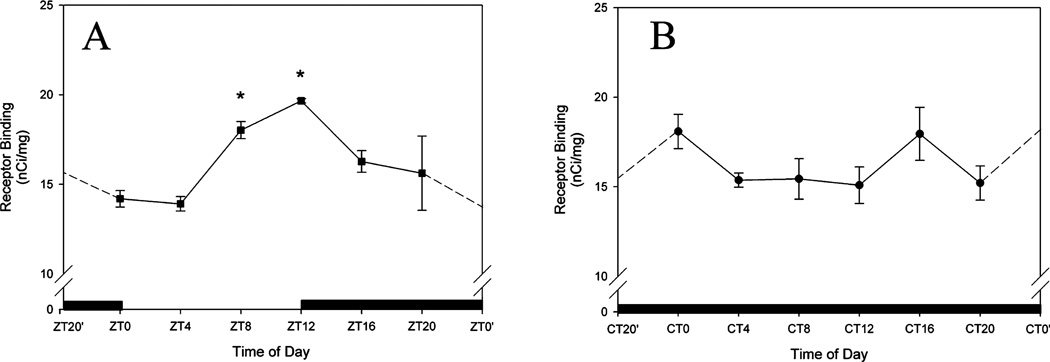
To measure variation in GRPr binding as a function of time of day we used receptor autoradiography in animals housed in both LD and DD, and killed at 4-h intervals. In animals that were maintained on an LD cycle, SCN GRP binding density was rhythmic with a peak at ZT12 (P < 0.05; Fig. 3A). Rhythmicity was not observed in animals maintained in DD (P > 0.05; Fig. 3B).
Fig. 3.
Quantification of 125I-GRP binding in a light–dark (LD) cycle (A) or constant darkness (DD, B). Data were measured against known levels of radioactivity, and plotted as nCi/mg. Time of day (ZT for LD, and CT for DD) is plotted along the x-axis. CT/ZT0′ and CT/ZT22′ indicate re-plotting of time point to allow for ease of reference. Asterisks indicate a significant difference from all other time points examined (one-way anova, P < 0.05). A peak in GRP binding was observed in LD animals at ZT12. No significant differences were detected in DD housed animals.
Light modulates GRPr protein level in the SCN
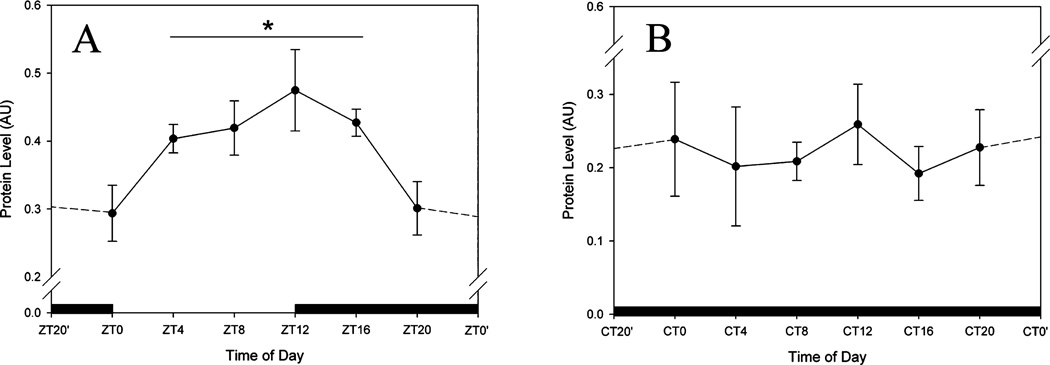
To measure levels of GRPr protein we performed Western blots on SCN dissections from animals housed in both LD and DD, taken at 4-h intervals. Our data reveal that GRPr protein levels are also modulated by photic conditions. In LD housed animals, the highest levels of GRPr protein expression are observed at ZT12 (P < 0.05; Fig. 4A). No rhythm is detected in DD housed animals (P > 0.05; Fig. 4B). It is interesting to note that in LD the levels of GRPr protein and GRPr binding both peak at ZT12.
Fig. 4.
Quantification of Western blots from SCN lysates treated with a GRP receptor (GRPr) antibody. Time of day (ZT for LD, and CT for DD) is plotted along the x-axis. (A) GRPr levels in the SCN of animals held in LD. (B) GRPr levels in the SCN of animals housed in DD. CT/ZT0′ and CT/ZT22′ indicate re-plotting of time point to allow for ease of reference. In LD, GRPr protein levels were significantly higher during the middle of the day, and peaked at ZT12 (P < 0.05), reaching a nadir between ZT20 and ZT0. No rhythm was detected in animals held in DD. *P < 0.05, compared with all other time points examined (one-way ANOVA).
Discussion
The present results indicate that in mouse SCN, GRPr binding and GRPr protein expression are rhythmic in LD cycles, peaking at the beginning of the night and reaching a trough at the end of the night. In DD, there is no detectable rhythm in either receptor binding or receptor protein levels. A parallel pattern of change in GRP peptide, with a peak in the day and a trough in the night, is seen using immunohistochemistry, enzyme immunoassay and in situ hybridization (Zoeller et al., 1992; Shinohara et al., 1993; Inouye & Shibata, 1994; Okamura & Ibata, 1994; Dardente et al., 2004). Together, these results suggest that the activity of the GRP signaling system within the SCN is modulated by the external LD cycle, and is not under control of the endogenous, circadian clock. The occurrence of a rhythm in GRPr mRNA has not been detected previously (McArthur et al., 2000).
Substantial evidence indicates that the SCN has at least two functionally distinct compartments, such that localization of light-induced gene and protein expression occurs in the ventromedial SCN ‘core’ in rat (Yan et al., 1999; Dardente et al., 2002a), hamster (Hamada et al., 2001) and mouse SCN (Karatsoreos et al., 2004). The precise peptidergic identity of directly retinorecipient cells depends upon the species examined. In rat, vasoactive intestinal polypeptide (VIP) and GRP cells of the ventrolateral SCN receive retinal input (Tanaka et al., 1993, 1997), and respond to photic stimulation with changes in both gene and protein expression (Dardente et al., 2002b; Yan & Okamura, 2002). In hamster, cells containing the calcium binding protein calbindin-D28k [of which 40–50% co-express GRP (LeSauter et al., 2002)] are among those receiving direct retinal input (Bryant et al., 2000), and these cells express the immediate early gene c-fos following a phase-shifting light pulse (Silver et al., 1996). In addition to calbindin-D28k cells, both GRP and VIP cells of the hamster SCN receive retinal input, with most GRP-positive perikarya expressing FOS following a light pulse (Aioun et al., 1998). In mouse, GRP cells in the core SCN are retinorecipient and express FOS following a photic phase-resetting stimulus (Karatsoreos et al., 2004). The present results suggest that GRPr mRNA is expressed strongly in the dorsal and medial aspects of the SCN.
Considerable work shows that GRP is key in the mechanism whereby retinorecipient SCN neurons communicate phase setting information to rhythmic SCN cells. GRP-containing cells are localized primarily to the non-rhythmic light-responsive core region (Kawamoto et al., 2003; Karatsoreos et al., 2004), whereas GRPr is expressed predominantly in the rhythmic dorsal shell region (present results, and Aida et al., 2002). This arrangement of GRP cells and GRPr cells provides a basis for intra-SCN communication between functionally distinct SCN regions.
GRP injection into the SCN (alone or in a cocktail of other SCN peptides) results in behavioral phase shifts, similar to those induced by light (Piggins & Rusak, 1993; Albers et al., 1995; Piggins et al., 1995; McArthur et al., 2000). GRP injections cause FOS expression in a subset of SCN cells (Piggins et al., 2005), as well as phosphorylation of the extracellular signal-regulated kinase 1/2 (ERK 1/2) (Antle et al., 2005). Pharmacological blockade of this phosphorylation by pre-application of the MAP-erk kinase (MEK) 1/2 inhibitor U0126 mitigates GRP-induced behavioral phase shifts (Antle et al., 2005). As noted above, GRP applied to the SCN in vitro can phase shift circadian firing rhythms and these shifts are blocked by the application of GRP antagonists (McArthur et al., 2000). Additionally, GRPr-deficient mice have intact circadian locomotor rhythms, but impaired light- and GRP-induced phase shifts, particularly in the delay region (Aida et al., 2002). Thus, the activation of shell GRPr-containing cells following application of either GRP or a photic stimulus seems to be necessary for normal phase shifting.
The relationship between VIP and GRP signaling in the SCN is not well understood. Neither GRP nor VIP are rhythmic in constant darkness (Shinohara et al., 1993), whereas in a light–dark cycle, GRP content increases and VIP decreases over the course of the day. In vitro,GRP and VIP application to SCN slices increases neuronal firing rates (Albers et al., 1991; Piggins & Rusak, 1993; Piggins et al., 1994). Additionally, rhythmic electrical activity of SCN neurons can be shifted by both VIP (Reed et al., 2001) and GRP (McArthur et al., 2000). Importantly, intra- SCN injections of both GRP and VIP produce phase-shifts in behavioral rhythms (Albers et al., 1991, 1995; Piggins et al., 1995). Some reports indicate that SCN injections of GRP result in equal phase delays and advances, with VIP treatment leading to much larger phase advances than phase delays (Piggins et al., 1995). By contrast, Albers et al. (1991) demonstrated small behavioral phase-shifting effects of GRP alone, and larger, non-additive, shifts following co-injection of a GRP/VIP/peptide histidine. In vivo, direct SCN GRP and VIP injections induce Per1, Per2, FOS and ERK 1/2 expression in the SCN, all of which are associated with photic phase resetting (Nielsen et al., 2002; Antle et al., 2005; Piggins et al., 2005). Although there seem to be inconsistencies between the behavioral studies, these may be a result of methodological and technical parameters of the individual studies. For instance, differences in cannula gauge, stereotaxic location of injection cannulae and sample sizes may lead to different mean phase-shifting effects following injection of GRP, VIP or the cocktail of GRP/VIP. Regardless, the bulk of the data to date suggest that in all rodent species examined, both GRP and VIP play an important role in photic phase resetting. Together, these studies suggest that the relationship between GRP and VIP in the SCN is complex and dynamic, and though there may be differences between studies, all data show that both GRP and VIP can phase shift the circadian clock in vivo and in vitro.
Knock-out strategies have allowed for the generation of mouse models that can more specifically address the independent roles of GRP and VIP in circadian function. Mice lacking the VIP receptor VPAC2 display disrupted behavioral rhythms, have aberrant entrainment (inability to entrain to an LD cycle) and show a decreased number of rhythmically firing SCN neurons in vitro (Aton et al., 2005). VPAC2-deficient mice also show aberrant gating of photic information to the SCN brain clock in that they express pERK and c-FOS to light pulses during subjective day while wild-type animals do not (Hughes et al., 2004). Based on these data, it has been proposed that VIP and its cognate receptor VPAC2 are fundamental to the generation of circadian rhythms (Aton et al., 2005). In contrast to VPAC2 knock-out animals, mice lacking the gene for GRPr show normal circadian free-running periods, but have aberrant photic phase shifting (Aida et al., 2002). Following photic stimulation, the latter animals show blunted phase shifts, and decreased expression of the circadian clock genes Per1 and Per2 in the SCN, implicating GRP in intra-SCN signaling (Aida et al., 2002). Importantly, electrical rhythmicity can be restored in neurons from behaviorally arrhythmic VPAC2 mice by application of GRP, and blocked by application of a GRPr antagonist (Brown et al., 2005), indicating that VIP and the VPAC2 receptor are not necessary for rhythmicity, and that interactions between VIP and GRP are integral to coherent SCN rhythms. These data suggest that VIP and GRP systems may serve overlapping and partially redundant roles.
In summary, GRP produces a light-like phase-response curve in the subjective night (Albers et al., 1992; Piggins et al., 1995; Antle et al., 2005). We show that GRPr binding and GRPr protein expression are rhythmic in LD, but not in DD, suggesting that the phase-shifting effects of GRP are mediated downstream of the GRPr. These downstream mediators could include signals such as the phosphoinositide pathway (Mason & Biello, 1992; Hamada et al., 1999), the nitric-oxide–cGMP–PKG pathway (reviewed in Golombek et al., 2004) or the mitogen-activated protein kinase pathway (Butcher et al., 2002, 2003, 2005; Dziema et al., 2003; Nakaya et al., 2003; Coogan & Piggins, 2004), all of which can be activated by the GRPr (Moody & Merali, 2004). Taken together, the present results affirm the role of GRP in the photic entrainment/phase-resetting pathway, and point to an SCN circuit modulated by photic signals.
Acknowledgements
This work was supported by NIH grant NS37919 (R.S.) and an NSERC predoctoral fellowship (I.N.K.). We would like to thank Dr Joseph LeSauter for assistance with the tissue dissections, and Jasmine Sasanian and Alice Wang for their expert technical assistance.
Abbreviations
- AVP
arginine vasopressin
- DD
constant darkness
- GRP
gastrin-releasing peptide
- GRPr
gastrin-releasing peptide receptor
- ICC
immunocytochemistry
- LD
12/12-h light–dark
- ROD
relative optical density
- SCN
suprachiasmatic nucleus
- VIP
vasoactive intestinal polypeptide
References
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol. Pharmacol. 2002;61:26–34. doi: 10.1124/mol.61.1.26. [DOI] [PubMed] [Google Scholar]
- Aioun J, Chambille I, Peytevin J, Martinet L. Neurons containing gastrin-releasing peptide and vasoactive intestinal polypeptide are involved in the reception of the photic signal in the suprachiasmatic nucleus of the Syrian hamster: an immunocytochemical ultrastructural study. Cell Tissue Res. 1998;291:239–253. doi: 10.1007/s004410050994. [DOI] [PubMed] [Google Scholar]
- Albers HE, Gillespie CF, Babagbemi TO, Huhman KL. Analysis of the phase shifting effects of gastrin releasing peptide when microinjected into the suprachiasmatic region. Neurosci. Lett. 1995;191:63–66. doi: 10.1016/0304-3940(95)11559-1. [DOI] [PubMed] [Google Scholar]
- Albers HE, Liou SY, Stopa EG, Zoeller RT. Interaction of colocalized neuropeptides: functional significance in the circadian timing system. J. Neurosci. 1991;11:846–851. doi: 10.1523/JNEUROSCI.11-03-00846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Liou SY, Stopa EG, Zoeller RT. Neurotransmitter colocalization and circadian rhythms. Prog. Brain Res. 1992;92:289–307. doi: 10.1016/s0079-6123(08)61184-x. [DOI] [PubMed] [Google Scholar]
- Antle MC, Kriegsfeld LJ, Silver R. Signaling within the master clock of the brain: localized activation of mitogen-activated protein kinase by gastrin-releasing peptide. J. Neurosci. 2005;25:2447–2454. doi: 10.1523/JNEUROSCI.4696-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J. Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DN, LeSauter J, Silver R, Romero MT. Retinal innervation of calbindin-D28K cells in the hamster suprachiasmatic nucleus: ultrastructural characterization. J. Biol. Rhythms. 2000;15:103–111. doi: 10.1177/074873040001500204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher GQ, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J. Biol. Chem. 2002;277:29519–29525. doi: 10.1074/jbc.M203301200. [DOI] [PubMed] [Google Scholar]
- Butcher GQ, Lee B, Cheng HY, Obrietan K. Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J. Neurosci. 2005;25:5305–5313. doi: 10.1523/JNEUROSCI.4361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher GQ, Lee B, Obrietan K. Temporal regulation of light-induced extracellular signal-regulated kinase activation in the suprachiasmatic nucleus. J. Neurophysiol. 2003;90:3854–3863. doi: 10.1152/jn.00524.2003. [DOI] [PubMed] [Google Scholar]
- Coogan AN, Piggins HD. MAP kinases in the mammalian circadian system – key regulators of clock function. J. Neurochem. 2004;90:769–775. doi: 10.1111/j.1471-4159.2004.02554.x. [DOI] [PubMed] [Google Scholar]
- Dardente H, Klosen P, Caldelas I, Pevet P, Masson-Pevet M. Phenotype of Per1- and Per2-expressing neurons in the suprachiasmatic nucleus of a diurnal rodent (Arvicanthis ansorgei): comparison with a nocturnal species, the rat. Cell Tissue Res. 2002a;310:85–92. doi: 10.1007/s00441-002-0609-9. [DOI] [PubMed] [Google Scholar]
- Dardente H, Menet JS, Challet E, Tournier BB, Pevet P, Masson-Pevet M. Daily and circadian expression of neuropeptides in the suprachiasmatic nuclei of nocturnal and diurnal rodents. Brain Res. Mol. Brain Res. 2004;124:143–151. doi: 10.1016/j.molbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Dardente H, Poirel VJ, Klosen P, Pevet P, Masson-Pevet M. Per and neuropeptide expression in the rat suprachiasmatic nuclei: compartmentalization and differential cellular induction by light. Brain Res. 2002b;958:261–271. doi: 10.1016/s0006-8993(02)03563-1. [DOI] [PubMed] [Google Scholar]
- Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K. The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur. J. Neurosci. 2003;17:1617–1627. doi: 10.1046/j.1460-9568.2003.02592.x. [DOI] [PubMed] [Google Scholar]
- Golombek DA, Agostino PV, Plano SA, Ferreyra GA. Signaling in the mammalian circadian clock: the NO/cGMP pathway. Neurochem. Int. 2004;45:929–936. doi: 10.1016/j.neuint.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Hamada T, LeSauter J, Venuti JM, Silver R. Expression of Period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J. Neurosci. 2001;21:7742–7750. doi: 10.1523/JNEUROSCI.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T, Liou SY, Fukushima T, Maruyama T, Watanabe S, Mikoshiba K, Ishida N. The role of inositol trisphosphate-induced Ca2+ release from IP3-receptor in the rat suprachiasmatic nucleus on circadian entrainment mechanism. Neurosci. Lett. 1999;263:125–128. doi: 10.1016/s0304-3940(99)00111-1. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Schwartz WJ. A neural clockwork for encoding circadian time. J. Appl. Physiol. 2002;92:401–408. doi: 10.1152/japplphysiol.00836.2001. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J. Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye ST, Shibata S. Neurochemical organization of circadian rhythm in the suprachiasmatic nucleus. Neurosci. Res. 1994;20:109–130. doi: 10.1016/0168-0102(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur. J. Neurosci. 2002;16:2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, Silver R. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J. Neurosci. 2004;24:68–75. doi: 10.1523/JNEUROSCI.1666-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Nagano M, Kanda F, Chihara K, Shigeyoshi Y, Okamura H. Two types of VIP neuronal components in rat suprachiasmatic nucleus. J. Neurosci. Res. 2003;74:852–857. doi: 10.1002/jnr.10751. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford University Press; 1991. [Google Scholar]
- LeSauter J, Kriegsfeld LJ, Hon J, Silver R. Calbindin-D (28K) cells selectively contact intra-SCN neurons. Neuroscience. 2002;111:575–585. doi: 10.1016/s0306-4522(01)00604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R, Biello SM. A neurophysiological study of a lithium-sensitive phosphoinositide system in the hamster suprachiasmatic (SCN) biological clock in vitro. Neurosci. Lett. 1992;144:135–138. doi: 10.1016/0304-3940(92)90734-o. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Coogan AN, Ajpru S, Sugden D, Biello SM, Piggins HD. Gastrin-releasing peptide phase-shifts suprachiasmatic nuclei neuronal rhythms in vitro. J. Neurosci. 2000;20:5496–5502. doi: 10.1523/JNEUROSCI.20-14-05496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TW, Merali Z. Bombesin-like peptides and associated receptors within the brain: distribution and behavioral implications. Peptides. 2004;25:511–520. doi: 10.1016/j.peptides.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol. Int. 1998;15:475–487. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Sanada K, Fukada Y. Spatial and temporal regulation of mitogen-activated protein kinase phosphorylation in the mouse suprachiasmatic nucleus. Biochem. Biophys. Res. Commun. 2003;305:494–501. doi: 10.1016/s0006-291x(03)00791-5. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Hannibal J, Fahrenkrug J. Vasoactive intestinal polypeptide induces per1 and per2 gene expression in the rat suprachiasmatic nucleus late at night. Eur. J. Neurosci. 2002;15:570–574. doi: 10.1046/j.0953-816x.2001.01882.x. [DOI] [PubMed] [Google Scholar]
- Okamura H, Ibata Y. GRP immunoreactivity shows a day–night difference in the suprachiasmatic nuclear soma and efferent fibers: comparison to VIP immunoreactivity. Neurosci. Lett. 1994;181:165–168. doi: 10.1016/0304-3940(94)90585-1. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J. Neurosci. 1995;15:5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Cutler DJ, Rusak B. Effects of ionophoretically applied bombesin-like peptides on hamster suprachiasmatic nucleus neurons in vitro. Eur. J. Pharmacol. 1994;271:413–419. doi: 10.1016/0014-2999(94)90801-x. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Goguen D, Rusak B. Gastrin-releasing peptide induces c-Fos in the hamster suprachiasmatic nucleus. Neurosci. Lett. 2005;384:205–210. doi: 10.1016/j.neulet.2005.03.072. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Rusak B. Electrophysiological effects of pressureejected bombesin-like peptides on hamster suprachiasmatic nucleus neurons in vitro. J. Neuroendocrinol. 1993;5:575–581. doi: 10.1111/j.1365-2826.1993.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur. J. Neurosci. 2001;13:839–843. doi: 10.1046/j.0953-816x.2000.01437.x. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J. Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/s0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- Silver R, Romero MT, Besmer HR, Leak R, Nunez JM, LeSauter J. Calbindin-D28K cells in the hamster SCN express light-induced Fos. Neuroreport. 1996;7:1224–1228. doi: 10.1097/00001756-199604260-00026. [DOI] [PubMed] [Google Scholar]
- Silver R, Sookhoo AI, LeSauter J, Stevens P, Jansen HT, Lehman MN. Multiple regulatory elements result in regional specificity in circadian rhythms of neuropeptide expression in mouse SCN. Neuroreport. 1999;10:3165–3174. doi: 10.1097/00001756-199910190-00008. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Hayashi S, Tamada Y, Ikeda T, Hisa Y, Takamatsu T, Ibata Y. Direct retinal projections to GRP neurons in the suprachiasmatic nucleus of the rat. Neuroreport. 1997;8:2187–2191. doi: 10.1097/00001756-199707070-00020. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ichitani Y, Okamura H, Tanaka Y, Ibata Y. The direct retinal projection to VIP neuronal elements in the rat SCN. Brain Res. Bull. 1993;31:637–640. doi: 10.1016/0361-9230(93)90134-w. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yan L, Okamura H. Gradients in the circadian expression of Per1 and Per2 genes in the rat suprachiasmatic nucleus. Eur. J. Neurosci. 2002;15:1153–1162. doi: 10.1046/j.1460-9568.2002.01955.x. [DOI] [PubMed] [Google Scholar]
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and Per2 gene expression in the rat suprachiasmatic nucleus: circadian profile and the compartment-specific response to light. Neuroscience. 1999;94:141–150. doi: 10.1016/s0306-4522(99)00223-7. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Broyles B, Early J, Anderson ER, Albers HE. Cellular levels of messenger ribonucleic acids encoding vasoactive intestinal peptide and gastrin-releasing peptide in neurons of the suprachiasmatic nucleus exhibit distinct 24-hour rhythms. J. Neuroendocrinol. 1992;4:119–124. doi: 10.1111/j.1365-2826.1992.tb00354.x. [DOI] [PubMed] [Google Scholar]