Abstract
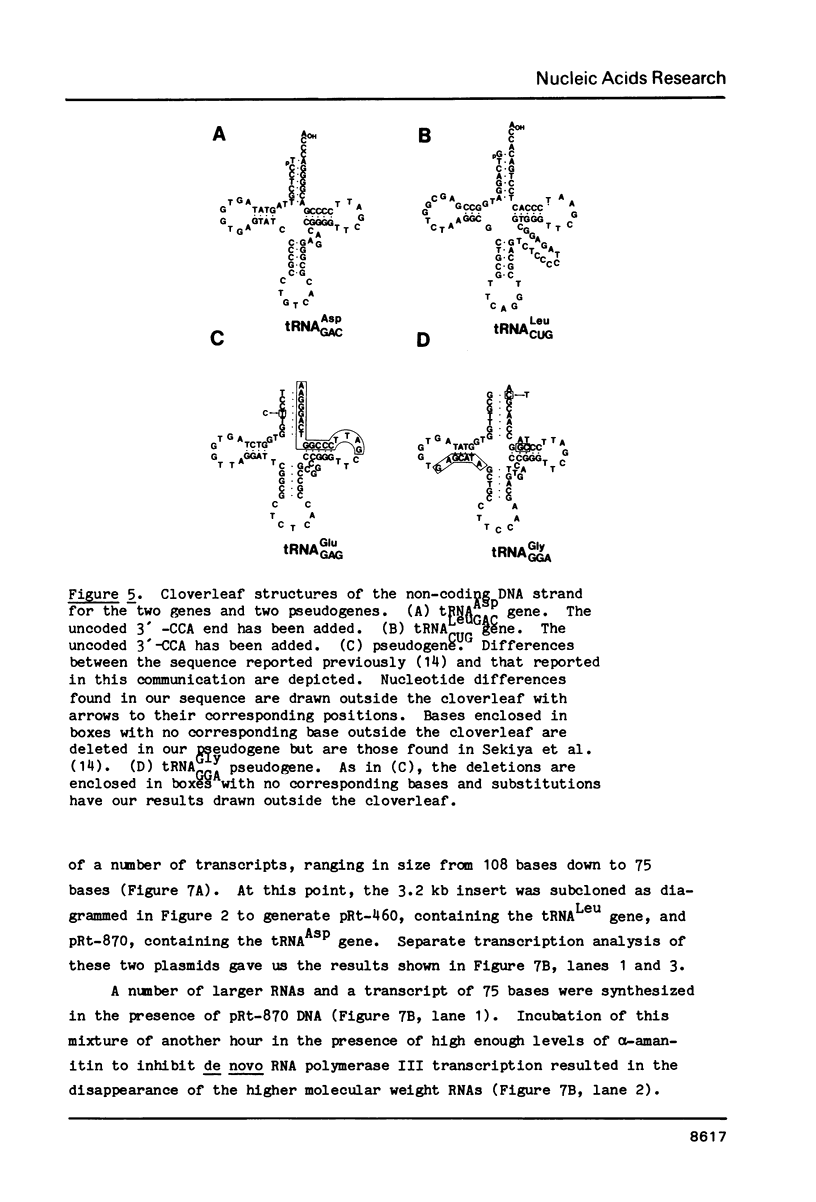
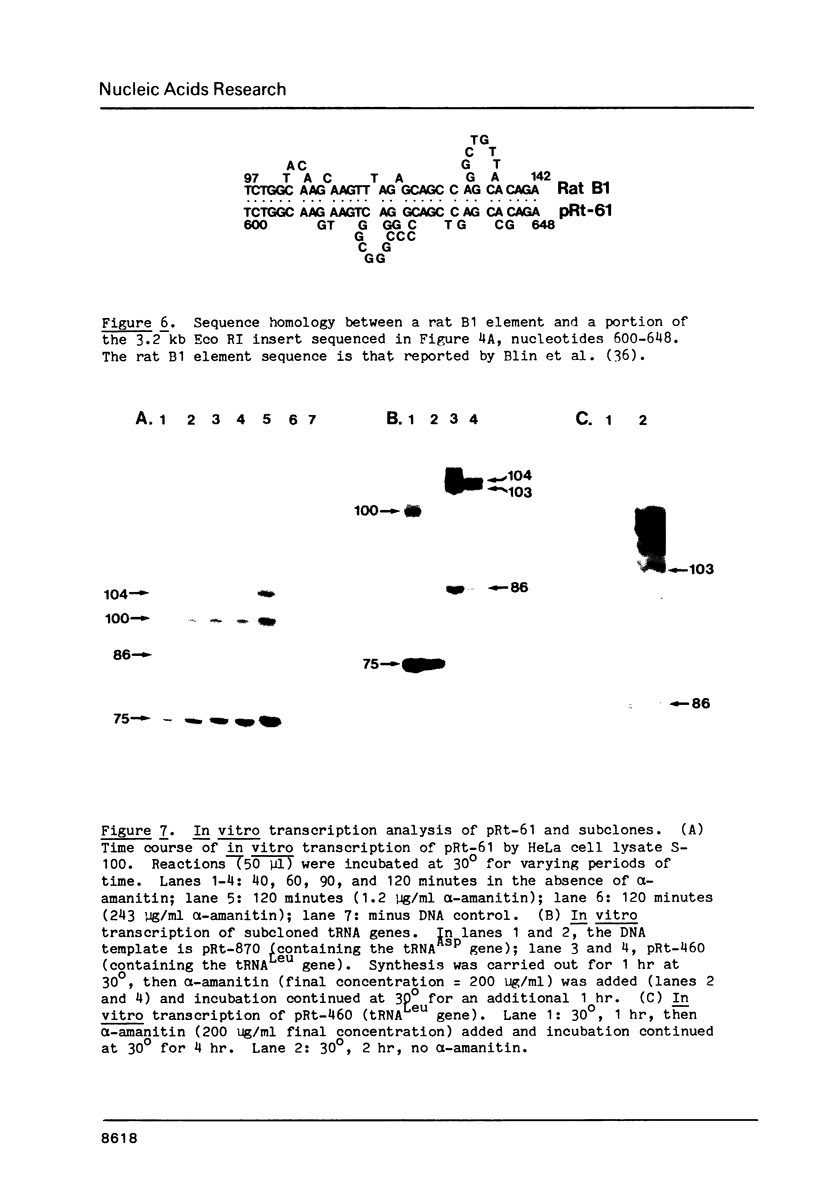
A rat genomic DNA fragment containing a tRNA gene cluster was isolated from a lambda phage library. Hybridization and nucleotide sequence analysis revealed the presence of a 83 bp tRNALeuCUG gene and a 72 bp tRNAAspGUG gene. Both genes possessed intact coding regions and putative transcription termination signals at their respective 3' ends. In vitro transcription analysis of the two subcloned genes in a HeLa cell S-100 system demonstrated the specific synthesis of a number of RNAs by RNA polymerase III. Studies carried out in the presence of alpha-amanitin showed that the larger RNAs are precursors for the final processed transcripts of the tRNALeu and tRNAAsp genes, respectively. Further nucleotide sequence analysis of the cluster revealed the presence of tRNAGly and a tRNAGlu pseudogenes with missing areas within their coding regions which are essential for transcription by RNA polymerase III. Within the region of DNA between the tRNALeu and tRNAAsp genes is a sequence which is 65% homologous to a region of the rat B1 element. The significance of this latter structure within the gene cluster is unknown.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Gait M. J., Mayol L., Young I. G. A short primer for sequencing DNA cloned in the single-stranded phage vector M13mp2. Nucleic Acids Res. 1980 Apr 25;8(8):1731–1743. doi: 10.1093/nar/8.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M., Bevan M. Kilo-sequencing: an ordered strategy for rapid DNA sequence data acquisition. Nucleic Acids Res. 1983 Jan 25;11(2):349–368. doi: 10.1093/nar/11.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann J. S., Johnson P. F., Abelson J. Cloning of yeast transfer RNA genes in Escherichia coli. Science. 1977 Apr 8;196(4286):205–208. doi: 10.1126/science.322282. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Brown D. D., Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978 Nov;15(3):1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Blin N., Weber T., Alonso A. Cross-reaction of snRNA and an Alu I-like sequence from rat with DNAs from different eucaryotic species. Nucleic Acids Res. 1983 Mar 11;11(5):1375–1388. doi: 10.1093/nar/11.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto G., Castagnoli L., Melton D. A., Cortese R. Promoter of a eukaryotic tRNAPro gene is composed of three noncontiguous regions. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1195–1199. doi: 10.1073/pnas.79.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H. Arangement of transfer-RNA -genes in yeast. Nucleic Acids Res. 1976 Sep;3(9):2379–2386. doi: 10.1093/nar/3.9.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. D., Clarkson S. G., Tocchini-Valentini G. Transcription initiation of eucaryotic transfer RNA genes. Cell. 1982 May;29(1):3–5. doi: 10.1016/0092-8674(82)90083-6. [DOI] [PubMed] [Google Scholar]
- Han J. H., Harding J. D. Using iodinated single-stranded M13 probes to facilitate rapid DNA sequence analysis--nucleotide sequence of a mouse lysine tRNA gene. Nucleic Acids Res. 1983 Apr 11;11(7):2053–2064. doi: 10.1093/nar/11.7.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., RajBhandary U. L. Organization of tRNA and rRNA genes in N. crassa mitochondria: intervening sequence in the large rRNA gene and strand distribution of the RNA genes. Cell. 1979 Jul;17(3):583–595. doi: 10.1016/0092-8674(79)90266-6. [DOI] [PubMed] [Google Scholar]
- Hipskind R. A., Mazabraud A., Corlet J., Clarkson S. G. DNA conformation and transcription initiation of eukaryotic tRNA genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):873–878. doi: 10.1101/sqb.1983.047.01.100. [DOI] [PubMed] [Google Scholar]
- Lasser-Weiss M., Bawnik N., Rosen A., Sarid S., Daniel V. Isolation and characterization of cloned rat DNA fragment carrying tRNA genes. Nucleic Acids Res. 1981 Nov 25;9(22):5965–5978. doi: 10.1093/nar/9.22.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Gefter M. L. Transcription of mammalian genes in vitro. Gene Amplif Anal. 1981;2:369–382. [PubMed] [Google Scholar]
- Martin S. L., Zimmer E. A., Davidson W. S., Wilson A. C., Kan Y. W. The untranslated regions of beta-globin mRNA evolve at a functional rate in higher primates. Cell. 1981 Sep;25(3):737–741. doi: 10.1016/0092-8674(81)90181-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. R., Kramer F. R. Structure-independent nucleotide sequence analysis. Proc Natl Acad Sci U S A. 1979 May;76(5):2232–2235. doi: 10.1073/pnas.76.5.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. V., Hall B. D., Cameron J. R., Davis R. W. Cloning of the yeast tyrosine transfer RNA genes in bacteriophage lambda. J Mol Biol. 1979 Jan 25;127(3):285–295. doi: 10.1016/0022-2836(79)90330-9. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Cooke H., Buckland R. Nucleotide sequence of a segment of human DNA containing the three tRNA genes. Nucleic Acids Res. 1982 Nov 25;10(22):7313–7322. doi: 10.1093/nar/10.22.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Kuchino Y., Nishimura S. Mammalian tRNA genes: nucleotide sequence of rat genes for tRNAAsp, tRNAGly and tRNAGlu. Nucleic Acids Res. 1981 May 25;9(10):2239–2250. doi: 10.1093/nar/9.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., DeFranco D., Silberklang M., Hosbach H. A., Schmidt T., Kubli E., Gergen J. P., Wensink P. C., Söll D. The initiator tRNA genes of Drosophila melanogaster: evidence for a tRNA pseudogene. Nucleic Acids Res. 1981 Nov 25;9(22):5867–5882. doi: 10.1093/nar/9.22.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K., Noguchi S., Nishimura S., Sekiya T. Characterization of a rat tRNA gene cluster containing the genes for tRNAAsp, tRNAGly and tRNAGlu, and pseudogenes. Nucleic Acids Res. 1982 Jul 24;10(14):4441–4448. doi: 10.1093/nar/10.14.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Venegas A., Rutter W. J. Yeast tRNA3Leu gene transcribed and spliced in a HeLa cell extract. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5963–5967. doi: 10.1073/pnas.78.10.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterman R. D., Lynch K. R., Nakhasi H. L., Dolan K. P., Hamilton J. W., Cohn D. V., Feigelson P. Cloning and sequence of several alpha 2u-globulin cDNAs. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3478–3482. doi: 10.1073/pnas.78.6.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Weber L., Berger E. Base sequence complexity of the stable RNA species of Drosophila melanogaster. Biochemistry. 1976 Dec 14;15(25):5511–5519. doi: 10.1021/bi00670a015. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Segall J., Harris B., Ng S. Y., Roeder R. G. Faithful transcription of eukaryotic genes by RNA polymerase III in systems reconstituted with purified DNA templates. J Biol Chem. 1979 Jul 10;254(13):6163–6173. [PubMed] [Google Scholar]
- Yen P. H., Davidson N. The gross anatomy of a tRNA gene cluster at region 42A of the D. melanogaster chromosome. Cell. 1980 Nov;22(1 Pt 1):137–148. doi: 10.1016/0092-8674(80)90162-2. [DOI] [PubMed] [Google Scholar]