B-1 B cells are a subpopulation of B lymphocytes that are generally considered part of the innate immune system. These cells spontaneously secrete IgM antibodies and provide protection against pathogens that include encapsulated bacteria.1 The developmental origin of B-1 lymphocytes has been a topic of considerable controversy. One view is that they are a separate hematopoietic lineage derived from distinct progenitors, while another is that the development of B-1 cells is linked to that of B-2 B cells. B-2 B cells are produced in the bone marrow from hematopoietic stem cells (HSCs) and predominate in peripheral lymphoid organs, where they take part in adaptive immune responses.2 The nuances of these two, non-mutually exclusive models have been discussed.3
The lineage model evolved from transplantation experiments that showed that progenitors in fetal and neonatal tissues efficiently generated B-1 cells, while the capacity of adult bone marrow to do so was limited.4,5 These observations suggested the existence of a distinct B-1 progenitor population that was produced primarily in the fetus, and this was confirmed in studies from our laboratory that identified a B-1 B cell-specified progenitor. These cells were produced most efficiently in fetal tissues; their numbers waned after birth, and their developmental potential was limited to the production of B-1 B cells.6
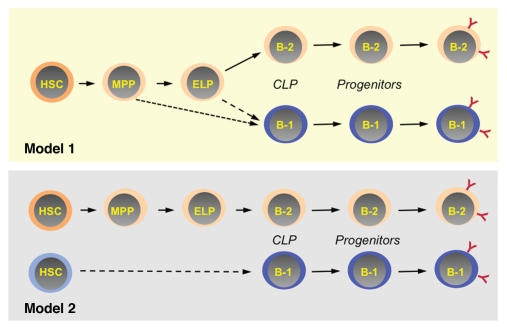
The development of B-2 B cells is increasingly well-defined, and lineage diagrams with cellular intermediates between hematopoietic stem cells (HSCs) and newly produced sIgM+ B cells have been formulated (Fig. 1).2 Where to place B-1 progenitors in this scheme has been an issue. One recent study demonstrated that adult bone marrow HSCs, early lymphoid progenitors (ELPs) and common lymphoid progenitors (CLPs) could generate B-1 progenitors, but pro-B cells could not do so.7 Because HSCs, ELPs and CLPs can also generate B-2 cells, these observations suggested that the B-1 and B-2 developmental programs are linked through the CLP stage of development.
Figure 1.
Schemes of B-cell development indicating possible points of divergence between the B-1 and B-2 pathways. Two models of B-1 development are proposed based on the clonal analyses discussed herein. In Model 1, B-1 and B-2 progenitors are derived from HSCs, but the decision for cells to enter one or the other pathway is made at the MPP or ELP stage of development. Alternatively, as shown in Model 2, distinct B-1- and B-2-specified HSCs may exist. The abbreviated designations for the various stages of hematopoietic development are defined in the text.
A recent observation from our laboratory adds a new twist to the story.8 We questioned whether CLPs were bi-potential and could generate both B-1 and B-2 progeny or if this compartment was developmentally heterogeneous and consisted of distinct B-1- and B-2-specified CLPs. Distinguishing between these alternatives has important implications. The existence of bi-potential CLPs would indicate a close developmental relationship between the two B lymphocyte populations. However, if distinct B-1- and B-2-specified CLPs exist, it would indicate an early separation between the B-1 and B-2 lineages and lend support to the lineage model. In order to address these issues, we developed a clonal assay in which single CLPs were seeded under conditions that allowed the development of either B-1 or B-2 progenitors.9 To our surprise, individual CLPs produced either B-1 or B-2 progenitors, indicating that B-1 vs. B-2 fate decisions are made before the CLP stage of development. We also observed that the number of B-1-specified CLPs was reduced in adult bone marrow, consistent with the observations that B-1 potential is most robust in the fetus and neonate.
The question raised by these results is how early do the B-1 and B-2 lineages diverge? One way to address this would be to apply the clonal approach used to dissect the B-cell potential of CLP to their upstream precursors. Our preliminary studies of single ELPs indicate that they can also generate B-1 and B-2 progenitors. However, analysis of over 1,000 individual ELPs revealed that they preferentially generated B-2 progenitors, regardless of whether they were derived from neonates or adults. This result suggests that ELPs may not be the main intermediate from which the majority of B-1 lineage cells derive, and that most B-1 progenitors are the progeny of more primitive precursors such as multi-potential progenitors (MPPs) (Fig. 1, Model 1). Alternatively, it is intriguing to consider the possibility that B-1- vs. B-2-biased HSCs might exist (Ghosn E and Herzenberg L, personal communication; Fig. 1, Model 2). If so, it will be important to investigate when during ontogeny they are produced and to identify the genetic programs that under-lie their formation and maintenance.
An understanding of the developmental relationships between B-1 and B-2 B cells and defining the stem and progenitor cells from which they arise has practical considerations. For example, full reconstitution of humoral immunity in patients may be dependent on ensuring that stem/progenitors with B-1 and B-2 potential are transplanted. A recent report that identified a candidate human B-1 cell indicates the relevance of this issue.10
Comment on: Barber CL, et al. Proc Natl Acad Sci USA. 2011;108:13700–13704.
References
- 1.Baumgarth N. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 2.Monroe JG, et al. Adv Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 3.Herzenberg LA, et al. Nat Immunol. 2006;7:225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa K, et al. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantor AB, et al. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 6.Montecino-Rodriguez E, et al. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 7.Esplin BL, et al. Proc Natl Acad Sci USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber CL, et al. Proc Natl Acad Sci USA. 2011;108:13700–13704. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montecino-Rodriguez E, et al. Nat Protoc. 2006;1:1140–1144. doi: 10.1038/nprot.2006.163. [DOI] [PubMed] [Google Scholar]
- 10.Griffin DO, et al. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]