Abstract
mTOR, the mammalian target of rapamycin, has been widely implicated in signals that promote cell cycle progression and survival in cancer cells. Rapamycin, which inhibits mTOR with high specificity, has consequently attracted much attention as an anticancer therapeutic. Rapamycin suppresses phosphorylation of S6 kinase at nanomolar concentrations; however, at higher micro-molar doses, rapamycin induces apoptosis in several human cancer cell lines. While much is known about the effect of low-dose rapamycin treatment, the mechanistic basis for the apoptotic effects of high-dose rapamycin treatment is not understood. We report here that the apoptotic effects of high-dose rapamycin treatment correlate with suppressing phosphorylation of the mTOR complex 1 substrate, eukaryotic initiation factor 4E (eIF4E) binding protein-1 (4E-BP1). Consistent with this observation, ablation of eIF4E also resulted in apoptorsis in MDA-MB 231 breast cancer cells. We also provide evidence that the differential dose effects of rapamycin are correlated with partial and complete dissociation of Raptor from mTORC1 at low and high doses, respectively. In contrast with MDA-MB-231 cells, MCF-7 breast cancer cells survived rapamycin-induced suppression of 4E-BP1 phosphorylation. We show that survival correlated with a hyperphosphorylation of Akt at S473 at high rapamycin doses, the suppression of which conferred rapamycin sensitivity. This study reveals that the apoptotic effect of rapamycin requires doses that completely dissociate Raptor from mTORC1 and suppress that phosphorylation of 4E-BP1 and inhibit eIF4E.
Key words: rapamycin, mTOR, 4E-BP1, eIF4E, Akt, apoptosis
Introduction
The mammalian target of rapamycin (mTOR) is an important integrator of signals that sense nutrients and energy.1 mTOR is also critical for controlling cell cycle progression and survival and is commonly activated by oncogenic alterations in human cancer.2 Consequently, there has been strong interest in targeting mTOR as an anticancer therapeutic strategy.3,4 mTOR is inhibited with high specificity by rapamycin; however, a confounding aspect concerning the effect of rapamycin on mTOR is that the concentrations of rapamycin required to suppress different actions of mTOR can vary dramatically.5–8 mTOR exists in two complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), most commonly distinguished by their association with the companion proteins Raptor and Rictor, but also by a differential sensitivity to rapamycin. mTORC1 is generally sensitive to rapamycin, but mTORC2 is relatively resistant.9 While rapamycin suppresses phosphorylation of the mTORC1 substrate S6 kinase in the low nano-molar range,10 rapamycin induces apoptosis in several human cancer cell lines but at micro-molar concentrations.7,11,12 Virtually nothing is known about what the high-dose rapamycin treatment is doing to cause apoptosis.
Rapamycin retards cell cycle progression,10 which is considered the basis for its immune suppressive and anticancer properties. Thus, rapamycin has been referred to as a “cytostatic” drug.13 The cytostatic effects of rapamycin and rapamycin analogs (rapalogs) are likely due to the suppression of S6 kinase, because these studies have used nano-molar concentrations that suppress S6 kinase phosphorylation. Although mTORC2 is considered to be rapamycin-insensitive, conditions where rapamycin suppresses mTORC2 have been reported in references 6 and 8. Thus, it is possible that the apoptotic effects of high-dose rapamycin treatment are due to an effect on mTORC2, which has also been implicated in cancer cell survival.5,14 Another plausible target is eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1). Although 4E-BP1 is thought to be an mTORC1 substrate,10 4E-BP1 phosphorylation is insensitive to rapamycin at doses up to 500 nM.10,15,16 Sonenberg and colleagues recently reported that cell proliferation is controlled by 4E-BPs.17 Thus, suppression of 4E-BP1 phosphorylation could be responsible for the effects of high-dose rapamycin treatment.
We report here that the apoptotic effect of high-dose rapamycin is due to the suppression of 4E-BP1 phosphorylation and the subsequent sequestration and inhibition of eIF4E. Interestingly, high-dose rapamycin treatment dramatically elevated Akt phosphorylation at S473 in MCF-7 breast cancer cells, which suppresses the drug's apoptotic effects. These data have significant implications for the use of rapamycin, and other compounds that suppress mTOR, as an anticancer therapeutic agents.
Results
Differential effects of low and high-dose rapamycin treatment on G1 cell cycle progression and cell viability.
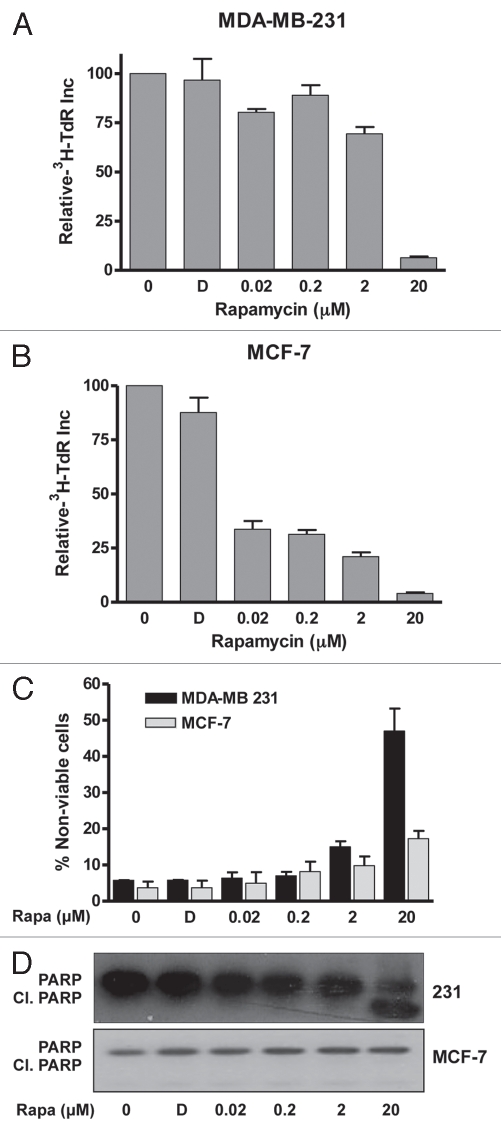
We reported previously that while high-dose rapamycin treatment induced apoptosis in MDA-MB-231 cells in the absence of serum, in the presence of serum, high-dose rapamycin treatment induced G1 cell cycle arrest.11 Cell cycle arrest has been reported with low-dose nano-molar concentrations of rapamycin;18–21 however, it has been noted that rapamycin may only slow cell cycle progression through G1 at the nano-molar concentrations used.10,16 We therefore examined the rapamycin doses required to block G1 cell cycle progression of MDA-MB-231 cells and the less malignant MCF-7 breast cancer cell line. Since MDA-MB-231 cells do not arrest in G1/G0 in response to serum withdrawal, we synchronized the MDA-MB-231 and MCF-7 cells with nocodazole, which reversibly arrests cells at mitosis.22 Mitotic cells were collected and then monitored for progression into S phase by uptake of [3H]-thymidine. Concentrations of rapamycin up to 200 nM slightly reduced the incorporation of [3H]-thymidine in the MDA-MB-231 cells, but 20 µM rapamycin dramatically suppressed uptake of [3H]-thymidine (Fig. 1A). Progression of the MCF-7 cells into S phase was more sensitive to nano-molar levels of rapamycin, but, like the MDA-MB-231 cells, complete G1 arrest required 20 µM rapamycin (Fig. 1B). Thus, while low nano-molar rapamycin concentrations retard G1 cell cycle progression, micro-molar concentrations caused a complete G1 arrest. These data reveal a specific effect of rapamycin that occurs between 2 and 20 µM that results in complete G1 arrest in the presence of serum.
Figure 1.
Differential suppression of G1 cell cycle progression and cell viability by low- and high-dose rapamycin treatment. MDA-MB-231 (A) and MCF-7 (B) cells were plated at 50% confluence in a 10 cm dish in complete medium containing 10% serum. After 24 h, the cells were treated with nocodazole at 200 ng/ml for 16 h to block cells at mitosis. At this point, mitotic cells were collected and plated in complete media containing 10% serum. Four hr later, after the cells reattached, the indicated concentrations of rapamycin or DMSO vehicle (D) were added along with [3H]-thymidine (TdR) (1 µCi/ml). after 24 h, lysates were collected, and the incorporated label was determined as described in Material and Methods. Error bars represent the standard deviation from three independent experiments. (C) MDA-MB-231 and MCF-7 cells were plated in regular (10% serum) medium. Twenty-four hours later, at 90% confluence, cells were exposed to 0% serum and treated with DMSO or rapamycin (Rapa) at indicated doses and harvested after 24 h. Cell viability was determined as described in Materials and Methods. (D) MDA-MB-231 cells were plated as in (A) and treated with DMSO (D) or rapamycin at indicated doses and harvested after 4 h (the 4-h time point established from a time course at 20 µM rapamycin at which strong induction of cleaved PARP was observed). Cell lysates were collected and immunoblotted with PARP antibody. Data shown are representative of experiments repeated at least two times.
In contrast to the rapamycin-induced G1 cell cycle arrest observed in the presence of serum, we have previously noted that in the absence of serum, high-dose rapamycin treatment causes apoptosis.11,12 To establish the precise concentrations needed to induce apoptosis, dose curves were performed for the effect of rapamycin on the viability of MDA-MB-231 and MCF-7 cells in the absence of serum. As shown in Figure 1C, loss of cell viability in the MDA-MB-231 cells occurred between 2 and 20 µM, notably, the same concentration range that induced complete G1 cell cycle arrest in the presence of serum. A corresponding increase in cleavage of the caspase 3 substrate poly-ADP-ribose polymerase (PARP) was observed at 20 µM (Fig. 1D, indicating apoptotic cell death. Surprisingly, the MCF-7 cells survived the rapamycin treatment (Fig. 1C) and no PARP cleavage was observed (Fig. 1D). Thus, compared with the MCF-7 cells, MDA-MB-231 cells are less sensitive to low-dose rapamycin treatment with regard to G1 cell cycle progression but more sensitive to the apoptotic effect of high-dose rapamycin treatment. These data further establish a critical effect of rapamycin between 2 and 20 µM.
Differential rapamycin sensitivity of mTOR substrates in MDA-MB-231 cells.
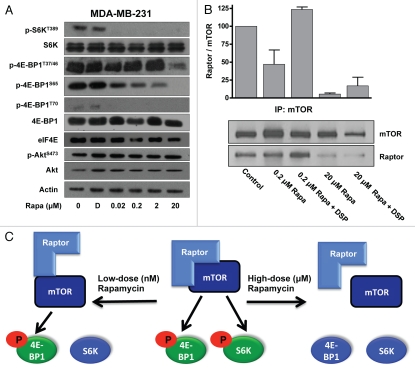
We next examined the sensitivity of mTOR substrates to rapamycin in MDA-MB-231 cells. As shown in Figure 2A, S6 kinase phosphorylation at the mTORC1 site at T389 was sensitive to 20 nM rapamycin, significantly lower than the concentration needed to induce apoptosis. Rapamycin at 20 µM had no effect on the phosphorylation of Akt at the mTORC2 site at S473, indicating that the apoptotic effect of rapamycin was not likely due to suppression of mTORC2. There are four sites on 4E-BP1 that are phosphorylated in response to the activation of mTORC1: T37/T46, S65 and T70.23 Phosphorylation at all of these sites was sensitive to rapamycin; however, there were profound differences in the doses needed to suppress the different sites. Phosphorylation at T70 was sensitive to doses of rapamycin that suppressed S6 kinase phosphorylation; however, phosphorylation of T37/46 and S65 were sensitive to the doses that induced apoptosis. Thus, rapamycin induces apoptosis at the same concentration that suppress 4E-BP1 phosphorylation, most significantly at T37/46 and S65.
Figure 2.
Differential rapamycin sensitivity of mTOR substrates in MDA-MB-231 cells. (A) MDA-MB-231 cells were plated in regular medium. Twenty-four hours later, at 90% confluence, cells were exposed to 0% serum and treated with DMSO (D) or rapamycin (Rapa) at the indicated concentrations for 4 h. Cells were harvested and lysates were immunoblotted with the indicated antibodies. (B) Cells were plated as in (A) exposed to 0% serum and treated with indicated rapamycin concentrations for 4 h. Cells were harvested and then lysed in the absence or presence of DSP cross-linking reagent and immunoprecipitated with mTOR. The immunoprecipitates were then subjected to protein gel blot analysis using either mTOR or Raptor antibody. The data shown are representative of experiments repeated at least two times. (C) A model for the differential sensitivity of mTORC1 substrate phosphorylation by rapamycin.
It has been reported that nano-molar rapamycin concentrations disrupt the association of mTOR with Raptor, a critical component of the mTORC1 complex.24 However, co-treatment with a cross-linking reagent in lysis buffer maintained the mTOR-Raptor association, suggesting that low-dose rapamycin treatment weakens but does not abolish the association between mTOR and Raptor. This finding suggests that low-dose rapamycin treatment disrupts the structure of mTORC1 sufficiently to inhibit phosphorylation of S6 kinase, but not 4E-BP1. We therefore examined the association of mTOR with Raptor in the presence of low (200 nM) and high (20 µM) rapamycin doses with and without cross-linking. As shown in Figure 2B, low-dose rapamycin treatment partially dissociated mTOR from Raptor, and as reported previously in references 6 and 24, the cross-linking reagent reversed this effect and rescued the association between mTOR and Raptor. In contrast, high-dose rapamycin proved to irreversibly dissociate mTOR from Raptor, as cross-linking failed to rescue the complex association. These data suggest that the differential effects of high- and low-dose rapamycin treatment on mTORC1 is due to differential disruption of the mTORC1 complex where low doses loosely disrupt the structure of mTORC1, such that S6 kinase is no longer recognized as a substrate, and high doses completely dissociate the complex such that 4E-BP1 is no longer recognized. This is shown schematically in Figure 2C.
Hyperphosphorylation of akt at S473 suppresses rapamycin-induced apoptosis.
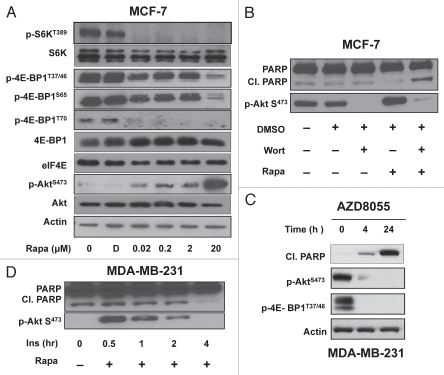
The sensitivity of mTOR substrate phosphorylation to rapamycin was also examined in the MCF-7 cells, which did not undergo apoptosis in response to high-dose rapamycin but did arrest in G1. As shown in Figure 3A, rapamycin suppressed the phosphorylation of S6 kinase at nanomolar doses and 4E-BP1 at micro-molar doses in MCF-7 cells as was observed in the MDA-MB-231 cells. Thus, while high-dose rapamycin treatment also suppressed the 4E-BP1 phosphorylation in the MCF-7 cells, this treatment did not induce apoptosis. Interestingly, there was a substantial increase in the phosphorylation of Akt at S473 observed between 2 and 20 µM rapamycin (Fig. 3A). A modest increase in Akt phosphorylation was observed with nano-molar doses of rapamycin as has been reported previously in references 25–27. However, at 20 µM rapamycin, there was dramatic hyperphosphorylation of Akt at S473 in the MCF-7 cells. Since Akt phosphorylation at S473 has been implicated in survival signaling,28 we examined whether suppression of Akt phosphorylation sensitized the MCF-7 cells to high-dose rapamycin. Phosphorylation of Akt at S473 is dependent on phosphatidylinositol-3-kinase.28 Treatment with the phosphatidylinositol-3-kinase inhibitor wortmannin suppressed Akt phosphorylation at S473 and sensitized the MCF-7 cells to high-dose rapamycin, which now induced PARP cleavage (Fig. 3B). These data indicate that the hyperphosphorylation of Akt in MCF-7 cells protects against the apoptotic effects of high-dose rapamycin.
Figure 3.
Phosphorylation of Akt at suppresses rapamycin-induced apoptosis. (A) MCF-7 cells were plated and treated as in Figure 2A. Cells were harvested, and lysates were immunoblotted with the indicated antibodies. (B) MCF-7 cells plated as in (A) and treated in 0% serum with DMSO (D), wortmannin (Wort) (1 µM) or rapamycin (Rapa) (20 µM) for 4 h. Cell lysates were then immunoblotted with the indicated antibodies. (C) MCF-7 were prepared as in (A) and then treated with 500 nM µM AZD8055 for the indicated times. The cells were harvested at the indicated times, lysates were then prepared and immunoblotted with the indicated antibodies. (D) MDA-MB 231 were plated as in (A) and treated in 0% serum with insulin or rapamcyin (20 µM) for indicated times. Cell lysates were immunoblotted with the indicated antibodies. All data are representative of at least two independent experiments.
If MCF-7 cells are surviving the high-dose rapamycin by increasing Akt phosphorylation at S473, then catalytic mTOR inhibitors,4,29 which inhibit both mTORC1 and mTORC2, the kinase that phosphorylates Akt at S473, should induce apoptosis in the MCF-7 cells. As shown in Figure 3C, the catalytic mTOR inhibitor AZD8055 induced PARP cleavage in the MCF-7 cells,30 providing further evidence that the hyperphosphorylation of Akt at S473 was responsible for the resistance to the high-dose rapamycin treatment.
Next, we examined whether inducing Akt phosphorylation could desensitize MDA-MB-231 cells to high-dose rapamycin treatment. We previously reported that insulin stimulates Akt phosphorylation at S473 in these cells.6 As shown in Figure 3D, insulin treatment caused transient stimulation of Akt phosphorylation, which completely abolished PARP cleavage induced by high-dose rapamycin treatment. Collectively, these data reveal that stimulating Akt phosphorylation at S473 prevents rapaymcin-induced apoptosis.
Suppression of eIF4E expression leads to apoptosis.
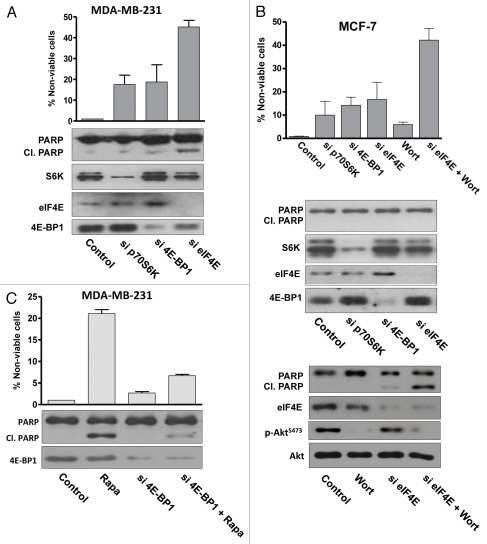
Upon phosphorylation of 4E-BP1 by mTORC1, eIF4E dissociates and facilitates translation of RNAs encoding proteins involved in cell cycle progression.31,32 Therefore, if suppressed 4E-BP1 phosphorylation induces apoptosis, then ablation of eIF4E expression should mimic the effect of high-dose rapamycin treatment. siRNAs targeted against S6 kinase, 4E-BP1 and eIF4E were introduced into the MDA-MB-231 cells, which were then examined for PARP cleavage. As shown in Figure 4A, a substantial increase in PARP cleavage and loss of cell viability was observed with eIF4E ablation but not with S6 kinase or 4E-BP1 ablation. These data further support a model whereby the apoptotic effect of high-dose rapamycin is due to suppression 4E-BP1 phosphorylation and subsequent inhibition of eIF4E.
Figure 4.
Suppression of eIF4E expression leads to apoptosis. (A) MDA-MB-231 cells were transfected at 50% confluence with negative control siRNA or siRNA targeted for S6 kinase, 4E-BP1 or eIF4. 48 h later, immunoblotting was used to analyze the indicated proteins. The percentage of non-viable cells was determined at 48 h as described in Materials and Methods. (B) MCF-7 cells were transfected with indicated siRNAs as in (A). Cells were evaluated for the indicated proteins and phosphoproteins and cell viability at 48 h. Wortmannin (1 uM) was added where indicated 24 h prior to evaluating cell viability and 4 h prior to evaluating protein levels and PARP cleavage. (C) MDA-MB-231 cells were either transfected at 50% confluence with negative control siRNA or siRNA targeted for 4E-BP1. Cells were treated with rapamycin (Rapa) (20 µM) for 4 h for PARP cleavage and 24 h for cell viability. All results are representative of experiments repeated at least two times.
We also examined the effect of suppressing eIF4E in the MCF-7 cells. In contrast with MDA-MB-231 cells, suppression of eIF4E did not significantly increase PARP cleavage or reduce cell viability (Fig. 4b). However, if MCF-7 cells were treated with wortmannin to suppress phosphorylation of Akt at S473, then a substantial increase in PARP cleavage and loss of cell viability was observed in the eIF4E-ablated cells (Fig. 4B). These data reinforce the observation that the MCF-7 cells are resistant to the suppression of 4E-BP1 phosphorylation by rapamycin and that this resistance is due at least in part to a feedback activation of Akt.25–27
Since, it appeared that the apoptotic effect of high-dose rapamycin treatment is due to sequestration eIF4E by unphosphorylated 4E-BP1, we reasoned that suppressing 4E-BP1 expression would desensitize the cells to high-dose rapamycin because there would be no 4E-BP1 to bind eIF4E. We therefore examined the effect of 20 µM rapamycin on cell viability on MDA-MB-231 cells treated with siRNA targeted for 4E-BP1. As shown in Figure 4C, MDA-MB-231 cells treated with a control siRNA were sensitive to 20 µM rapamycin. However, cells treated with siRNA for 4E-BP1 were resistant to 20 µM rapamycin. These data further support the hypothesis that the effects of high-dose rapamycin are due to the suppression of 4E-BP1 phosphorylation and the suppression of eIF4E. Importantly, this result also establishes that the apoptotic effect of high-dose rapamycin treatment is not a nonspecific effect of high-dose rapamycin on another cellular protein.
Discussion
In this study, we investigated the effect of cytotoxic doses of rapamycin on mTOR substrates. A clear correlation emerged between the induction of apoptosis and the suppression of 4E-BP1 phosphorylation at T37/46 and S65. Suppression of 4E-BP1 phosphorylation results in the sequestration of eIF4E by unphosphorylated 4E-BP1,23 and consistent with these findings, ablation of eIF4E expression resulted in apoptotic cell death. Thus, rapamycin-induced apoptosis appears to be a direct result of suppressing 4E-BP1 phosphorylation by mTORC1 and the indirect result of inactivating eIF4E. The finding that suppression of 4E-BP1 phosphorylation by high-dose rapamycin treatment is responsible for the observed apoptosis is consistent with reports implicating eIF4E in cell proliferation and oncogenic transformation.17,33–38 Significantly, phosphorylated 4E-BP1 is also associated with poor patient survival in melanoma.39 Thus, data provided here are consistent with an emerging paradigm that mTOR survival signals are mediated by the phosphorylation of 4E-BP1 and the subsequent release of eIF4E. These data have important implications for the many ongoing clinical trials involving rapamycin and the targeting of mTOR.
Clinical trials with rapamycin or rapalogs have been largely disappointing.3,40 In general, rapamycin has been considered a cytostatic, rather than cytotoxic compound,13 which may explain the limited response in clinical trials. However, doses of rapamycin that suppress 4E-BP1 phosphorylation are, in fact, cytotoxic and induce apoptosis, at least in the absence of serum.11 In the presence of serum, low-dose rapamycin treatment only retarded cell cycle progression, whereas high-dose rapamycin treatment was required to cause complete G1 cell cycle arrest. The efficacy of rapamycin-based strategies in clinical trials is commonly evaluated by effective suppression of S6 kinase.41,42 The study presented here indicates that 4E-BP1 phosphorylation would be a better indicator of drug efficacy. It is widely believed that phosphorylation of 4E-BP1 is not inhibited by rapamycin; however, as shown here, 4E-BP1 phosphorylation is suppressed by rapamycin, but requires higher doses than are required to suppress phosphorylation of S6 kinase. The question as to whether levels of rapamycin or rapalogs that suppress 4E-BP1 phosphorylation at T37/46 can be achieved with tolerated toxicity has not been evaluated.
The differential effects of low- and high-dose rapamycin treatment were apparently due to the partial vs. complete dissociation of mTOR and Raptor. Low-dose rapamycin treatment reduced association between mTOR and Raptor, but if a cross-linking reagent was added during cell lysis, Raptor remained associated with mTOR. The conclusion was that mTOR was able to phosphorylate 4E-BP1, but not S6 kinase, at the low dosage where Raptor is still weakly associated with mTOR. However, at high dosage, the lack of any Raptor associated with mTOR prevents phosphorylation of 4E-BP1 (Fig. 2C). This model is conceptually similar to one recently proposed based on a structural analysis of mTORC1.43 In this study, prolonged treatment (2 h) with 100 nM rapamycin suppressed the phosphorylation of 4E-BP1 by purified mTORC1 at T37/46 in vitro, whereas S6 kinase phosphorylation was suppressed after a 5 min treatment. Their conclusion was similar to ours, whereby the short-term treatment resulted in partial disruption of the mTORC1 complex and prevented phosphorylation of S6 kinase, and long-term treatment completely disrupted the complex such that 4E-BP1 did not get phosphorylated. Longer-term treatment with intact cells, at least over the 2 h time course used for the in vitro study, is not sufficient to break down the mTOR complex in vivo. This could be due to different effective concentrations of rapamycin when it is administered to intact cells. However, the principle is the same in both studies, that mTORC1 can be partially disrupted by rapamycin such that S6 kinase is no longer a substrate, yet 4E-BP1 still gets phosphorylated.
MCF-7 cells have a more benign phenotype than the MDA-MB-231 cells and retain differentiated epithelial cell characteristics. These cells were not killed by high-dose rapamycin treatment even though 4E-BP1 phosphorylation was suppressed. Significantly, the high-dose rapamycin treatment induced hyperphosphorylation of Akt at S473, substantially higher than that observed at the nano-molar concentrations as reported previously in references 25–27. MCF-7 cells were sensitized to rapamycin treatment upon suppression of Akt phosphorylation. Similarly, stimulation of Akt phosphorylation in MDA-MB-231 cells resulted in resistance to high-dose rapamycin treatment. It is possible that normal and less malignant cells like MCF-7 cells could be protected from high-dose rapamycin treatments by virtue of elevated Akt phosphorylation. A clinical trial for everolimus revealed an increase in the phosphorylation of Akt at S473 observed in 50% of the tumors.44 It was not apparent how this impacted on the effect of everolimus; however, there could be interesting differences in the effect of the rapalogs in cells where Akt phosphorylation is elevated.
Rapamycin-based therapeutic strategies are problematic because of the relative resistance to rapamycin of mTORC2 and the phosphorylation of 4E-BP1 by mTORC1.45 Consequently, there has been strong interest in catalytic inhibitors of mTOR that target both mTORC1 and mTORC2 4,29 and suppress phosphorylation of S6 kinase, 4E-BP1 and Akt at S473 with equal efficiency.15,45 Elevated Akt phosphorylation protected the more benign MCF-7 cells from the apoptotic effect of high-dose rapamycin. As shown here, a catalytic mTOR inhibitor was able to induce apoptosis in the MCF-7 cells. Thus, while the catalytic inhibitors kill cancer cells with elevated Akt phosphorylation, this class of inhibitor may also be more toxic to normal cells. In this regard, rapamycin may be advantageous because of its selective inhibition of mTORC1.
The high doses of rapamycin needed to suppress 4E-BP1 phosphorylation in cultured cells reported here are not likely to be achieved in clinical trials. This is because maximum tolerated doses or rapamycin result in nano-molar levels of the drug in plasma.37 Thus, delivering an effective dosage capable of inducing the apoptotic effects observed here would require a vehicle that targets tumors with high specificity. One possible strategy would be to tag rapamycin with glucose, which is taken up preferentially by most cancer cells due to an altered metabolism known as the Warburg effect.46 This strategy has been used previously to enhance the uptake of a photodynamic therapeutic agent into cancer cells.47 With such a strategy, tolerable levels of rapamycin could result in the delivery of higher toxic levels to tumor. This strategy could also result in additional benefits conferred by the low-dose treatments that have been implicated prolonging lifespan.48–50 Although the low-dose rapamycin treatments are not likely to kill the cancer cells or significantly block cell cycle progression, the low doses have been able to sensitize cancer cells to other treatments.51–53 Thus, the current low-dose strategies may still be of value in combination with other chemotherapeutic agents.
In summary, the effects of rapamycin on cancer cells have been have been difficult to evaluate because of differential effects observed in different cancer cells and the varying concentrations needed to suppress different downstream targets of mTOR. In this study, we have revealed that the cytotoxic effect of high-dose rapamycin treatment is due to the complete dissociation of mTOR from Raptor, which results in the inhibition of 4E-BP1 phosphorylation. We also show that rapamycin-resistance can be achieved by hyperphosphorylation of Akt at the mTORC2 site at S473. These complex responses to rapamycin reveal both problems and opportunities for targeting mTOR, which has been implicated in the signals that promote cell cycle progression and survival in human cancers.
Materials and Methods
Cells, cell culture conditions and cell viability.
The human cancer cell lines MDA-MB-231 and MCF-7 cells were obtained from the American Tissue Type Culture Collection (ATCC) and cultured in Dulbecco's modified Eagle medium (DMEM) (Sigma) supplemented with 10% Fetal Bovine Serum (Sigma). Cell viability was determined by trypan blue exclusion or by counting viable attached cells. For trypan blue exclusion, cells were harvested, washed and treated with trypan blue at a concentration of 0.4% v/v. After 5 min, trypan blue uptake (dead cells) was scored using a hemocytometer. Cell viability was also determined by counting adherent cells 24 h after treatment using a hemacytometer or Coulter counter.
Antibodies and reagents.
The following antibodies were used: PARP, cleaved PARP, p-S6 kinase T389, S6 kinase, p-4E-BP1 T37/46, p-4E-BP1 S65, p-4E-BP1 T70, 4E-BP1, p-Akt S473, Akt (Cell Signaling); α-actin (Sigma); eIF4E (Santa Cruz Biotechnology). Negative control siRNA (Dharmacon), siRNAs targeted against S6 kinase, 4E-BP1 and eIF4E (Santa Cruz Biotechnology) were purchased. Lipofectamine RNAiMax (Invitrogen) was used for transient transfections. Rapamycin and wortmannin were obtained from Calbiochem, and insulin was purchased from Sigma. [3H]-thymidine was from Perkin Elmer and the catalytic mTOR inhibitor AZD8055 was from Axon Medchem.
Protein gel blot analysis.
Extraction of proteins from cultured cells and protein gel blot analysis of extracted proteins was performed using the ECL system (Amersham) as described previously in reference 6.
Transient transfections.
Cells were plated in 6-well plates in medium containing 10% FBS. The next day (50% confluence), transfections with siRNAs (75 nM) in Lipofectamine RNAiMAX were performed. After 6 h, reagents were replaced with fresh 10% FBS, and cells were allowed to incubate for an additional 48 h.
Cross-linking assay and immunoprecipitation.
The cross-linking of mTOR with Raptor dithiobis(succinimidyl) propionate (DSP) (Sigma) and co-immunoprecipitation was performed as described previously in reference 6. Quantitative changes in protein levels were analyzed by densitometry using Image-J software.
Cell cycle synchronization and progression.
Cell cycle progression under different concentrations of rapamycin was studied using thymidine incorporation assay. Cells were plated, synchronized in M phase using nocodazole (Sigma) and then treated with the indicated concentration of rapamycin along with [3H]-thymidine (1 mCi/ml, 20 Ci/mMole) (Perkin Elmer) label as described in the figure legend. Twenty-four hours later, the cells were washed twice with PBS and then precipitated twice with 10% trichloroacetic acid. The precipitates were solubilized in 0.5 ml of 0.5% SDS/0.5 M NaOH solution. The extent of thymidine incorporation was quantified using 75 µl of sample and 3 ml of scintillation fluid.
Acknowledgments
This work was supported by a grant from the National Cancer Institute (CA46677). Research Centers in Minority Institutions (RCMI) award RR-03037 from the National Center for Research Resources of the National Institutes of Health, which supports infrastructure and instrumentation, is also acknowledged. P.Y. was supported by a Gene Center Fellowship from the RCMI.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Sawyers CL. Will mTOR inhibitors make it as cancer drugs? Cancer Cell. 2003;4:343–348. doi: 10.1016/S1535-6108(03)00275-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YJ, Duan Y, Zheng XF. Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today. 2011;16:325–331. doi: 10.1016/j.drudis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster DA, Toschi A. Targeting mTOR with rapamycin: one dose does not fit all. Cell Cycle. 2009;8:1026–1029. doi: 10.4161/cc.8.7.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22:3937–3942. doi: 10.1038/sj.onc.1206565. [DOI] [PubMed] [Google Scholar]
- 8.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j. molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 10.Choo AY, Blenis J. Not all substrates are treated equally: implications for mTOR, rapamycin-resistance and cancer therapy. Cell Cycle. 2009;8:567–572. doi: 10.4161/cc.8.4.7659. [DOI] [PubMed] [Google Scholar]
- 11.Gadir N, Jackson DN, Lee E, Foster DA. Defective TGFbeta signaling sensitizes human cancer cells to rapamycin. Oncogene. 2008;27:1055–1062. doi: 10.1038/sj.onc.1210721. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Rodrik V, Foster DA. Alternative phospholipase D/mTOR survival signal in human breast cancer cells. Oncogene. 2005;24:672–679. doi: 10.1038/sj.onc.1208099. [DOI] [PubMed] [Google Scholar]
- 13.Easton JB, Houghton PJ. Therapeutic potential of target of rapamycin inhibitors. Expert Opin Ther Targets. 2004;8:551–564. doi: 10.1517/14728222.8.6.551. [DOI] [PubMed] [Google Scholar]
- 14.Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albers MW, Brown EJ, Tanaka A, Williams RT, Hall FL, Schreiber SL. FKBP-rapamycin inhibits a cyclin-dependent kinase activity and a cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J Biol Chem. 1993;268:22825–22829. [PubMed] [Google Scholar]
- 19.Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci USA. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-16.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackman J, O'Connor PM. Methods for synchronizing cells at specific stages of the cell cycle. Curr Protoc Cell Biol. 2001;8:3. doi: 10.1002/0471143030.cb0803s00. [DOI] [PubMed] [Google Scholar]
- 23.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, et al. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 25.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 26.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Thoreen C, Wang J, Sabatini D, Gray NS. mTOR Mediated Anticancer Drug Discovery. Drug Discov Today Ther Strateg. 2009;6:47–55. doi: 10.1016/j.ddstr.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, et al. AZD8055 is a potent, selective and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 31.Bjornsti MA, Houghton PJ. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell. 2004;5:519–523. doi: 10.1016/j.ccr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- 33.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 34.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 35.Petroulakis E, Parsyan A, Dowling RJ, LeBacquer O, Martineau Y, Bidinosti M, et al. p53-dependent translational control of senescence and transformation via 4E-BPs. Cancer Cell. 2009;16:439–446. doi: 10.1016/j.ccr.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 36.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, et al. The translation factor eIF4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17:249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Reilly KE, Warycha M, Davies MA, Rodrik V, Zhou XK, Yee H, et al. Phosphorylated 4E-BP1 is associated with poor survival in melanoma. Clin Cancer Res. 2009;15:2872–2878. doi: 10.1158/1078-0432.CCR-08-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16:1368–1372. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jimeno A, Rudek MA, Kulesza P, Ma WW, Wheelhouse J, Howard A, et al. Pharmacodynamic-guided modified continuous reassessment method-based, dose-finding study of rapamycin in adult patients with solid tumors. J Clin Oncol. 2008;26:4172–4179. doi: 10.1200/JCO.2008.16.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–774. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmaco-dynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 45.Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci USA. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Hui L, Foster DA, Drain CM. Efficient synthesis and photodynamic activity of porphyrinsaccharide conjugates: Targeting and incapacitating cancer cells. Biochemistry. 2004;43:10918–10929. doi: 10.1021/bi049272v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blagosklonny MV. Increasing healthy lifespan by suppressing aging in our lifetime: preliminary proposal. Cell Cycle. 2010;9:4788–4794. doi: 10.4161/cc.9.24.14360. [DOI] [PubMed] [Google Scholar]
- 49.Blagosklonny MV. Validation of anti-aging drugs by treating age-related diseases. Aging. 2009;1:281–288. doi: 10.18632/aging.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster DA. Reduced mortality and moderate alcohol consumption: The phospholipase D-mTOR connection. Cell Cycle. 2010;9:1291–1294. doi: 10.4161/cc.9.7.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephan S, Datta K, Wang E, Li J, Brekken RA, Parangi S, et al. Effect of rapamycin alone and in combination with antiangiogenesis therapy in an orthotopic model of human pancreatic cancer. Clin Cancer Res. 2004;10:6993–7000. doi: 10.1158/1078-0432.CCR-04-0808. [DOI] [PubMed] [Google Scholar]
- 52.Abrams SL, Steelman LS, Shelton JG, Chappell W, Bäsecke J, Stivala F, et al. Enhancing therapeutic efficacy by targeting non-oncogene addicted cells with combinations of signal transduction inhibitors and chemotherapy. Cell Cycle. 2010;9:1839–1846. doi: 10.4161/cc.9.9.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rexer BN, Engelman JA, Arteaga CL. Overcoming resistance to tyrosine kinase inhibitors: lessons learned from cancer cells treated with EGFR antagonists. Cell Cycle. 2009;8:18–22. doi: 10.4161/cc.8.1.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]