Abstract
Background
Microbubbles (MB) combined with ultrasound (US) have been shown to lyse clots without tissue plasminogen activator (tPA) both in vitro and in vivo. We evaluated sonothrombolysis with three types of MB using a rabbit embolic stroke model.
Methods
New Zealand White rabbits (n=74) received internal carotid angiographic embolization of single 3 day-old cylindrical clots (0.6×4.0-mm). Groups included: 1) control (n=11) embolized without treatment, 2) tPA (n=20), 3) tPA+US (n=10), 4) Perflutren Lipid MB+US (n=16), 5) albumin 3µm MB+US (n=8), and 6) tagged albumin 3µm MB+US (n=9). Treatment began 1 hour post-embolization. Ultrasound was pulsed-wave (1 MHz; 0.8 W/cm2) for 1 hour; rabbits with tPA received intravenous tPA (0.9 mg/kg) over 1 hour. Lipid MB dose was intravenous (0.16 mg/kg) over 30 minutes. Dosage of 3µm MB was 5×109 MB intravenously alone or tagged with eptifibatide and fibrin antibody over 30 minutes. Rabbits were euthanized at 24 hours. Infarct volume was determined using vital stains on brain sections. Hemorrhage was evaluated on H&E sections.
Results
Infarct volume percent was lower for rabbits treated with Lipid MB+US (1.0%±0.6%; P=0.013), 3µm MB+US (0.7%±0.9%; P=0.018), and tagged 3µm MB+US (0.8%±0.8%; P=0.019) compared with controls (3.5%±0.8%). The three MB types collectively had lower infarct volumes (P=0.0043) than controls. Infarct volume averaged 2.2%±0.6% and 1.7%±0.8% for rabbits treated with tPA alone and tPA+US, respectively (P=NS).
Conclusions
Sonothrombolysis without tPA using these MB is effective in decreasing infarct volumes. Study of human application and further MB technique development are justified.
Keywords: ischemia, microbubble, ultrasound, thrombolysis, animal models
Introduction
Ischemic stroke affects over 700,000 Americans annually, is the third most common cause of death (1,2), and delivery of successful treatment remains elusive (3–4). The focus of therapy must be the prompt removal of vascular obstruction to restore blood flow and oxygenation of the brain. Intravenous (IV) tissue plasminogen activator (tPA) therapy is far from optimal. Its use is constrained by numerous patient factors including time since stroke onset, presence of bleeding, hypertension, anticoagulant therapy, and others. In spite of the favorable number needed to treat of 9, the small proportion of cases appropriate for tPA treatment decreases its actual use, and physician fear of the high risk of hemorrhage, sometimes fatal, severely restricts tPA use. Intra-arterial interventional approaches are even harder to utilize, are less widely available, and improve a minority of outcomes while creating additional side effects and complications. Access to treatment is limited, and treatments are successful in a minority of cases (5). To date, neuro-protective methods have not proven practical or effective, and translation to clinical practice is lacking (6). Other and improved stroke treatments are needed desperately.
Thrombolytic treatment with microbubbles and ultrasound may eliminate the need for tPA and its associated severe side effects.
Microbubbles (MB) are well known to augment ultrasound (US) in lysing clot (7–13). Molina (14) and others have demonstrated improved outcomes in acute ischemic strokes with the combination of IV tPA, MB, and US. Studies have also demonstrated successful MB-augmented sonothrombolysis without tPA (15–18) including intracranial thrombolysis in pigs without exogenous tPA using transcutaneous US (19,20). The work in dogs and pigs (16–20) was mechanistically similar to our current stroke model in the rabbit. However, these studies lacked evaluation of the possible therapy side effects on ischemic brain and especially lacked evaluation of hemorrhage associated with reperfusion of ischemic brain. This rabbit model uses infarct volumes as an endpoint, a much more important endpoint than simple large vessel recanalization which neglects distal embolization and potential ‘no reflow’ problems. Apparently, endogenous tPA in animal endothelium is adequate to lyse small clots when coupled with MB+US. Therefore, exogenous tPA is not necessary and associated hemorrhagic complications may also be avoided. Still, sonothrombolysis trials without tPA in humans are lacking.
To evaluate this concept in actual ischemic strokes, we tested MB of various types in an embolic clot stroke model in rabbits. A similar model previously led to successful human stroke therapy with tPA (21). In this study we utilize a single aged clot embolus that is site delivered to a common location of clinical stroke. This refined approach allows direct application of various therapies on infarcts of fairly uniform location and size.
Materials and Methods
Angiographic Procedures
Animal procedures were approved by the Institutional Animal Care and Use Committee. New Zealand White rabbits (n=74; mbw=5.2±0.07 kg) were randomly chosen and randomly placed into the various treatment groups.
Surgical and angiographic procedures were described previously (22). Briefly, rabbits were sedated with an intramuscular injection of ketamine, 35 mg/kg and xylazine, 5 mg/kg, and anesthetized with isoflurane. A femoral artery was exposed, and a 3F vascular sheath was used to place a modified 65-cm angled-tip 3F catheter into the artery. Using standard angiographic techniques, the 3F catheter was advanced to the internal carotid artery (ICA).
Sub-selective ICA magnification angiography (Figure 1A) preceded embolization, accomplished by injecting a 4.0×0.6-mm cylindrical clot with 0.7 to 2.0-mL of saline. Repeat angiography one minute later documented occlusion (Figure 1B).
Figure 1. Rabbit Angiography.
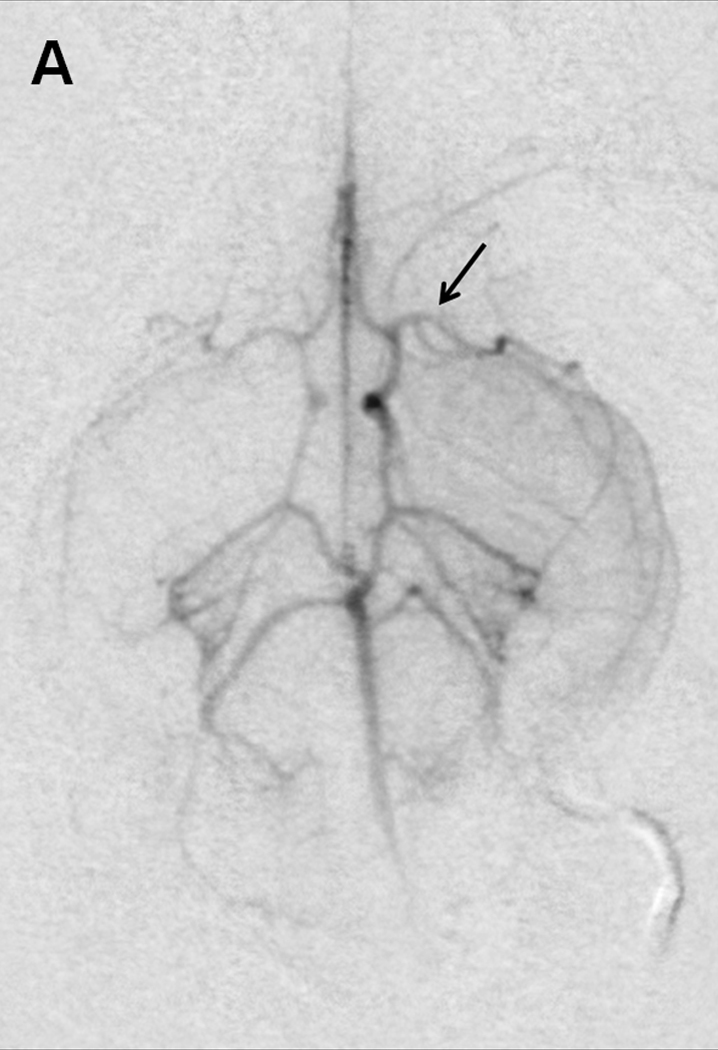
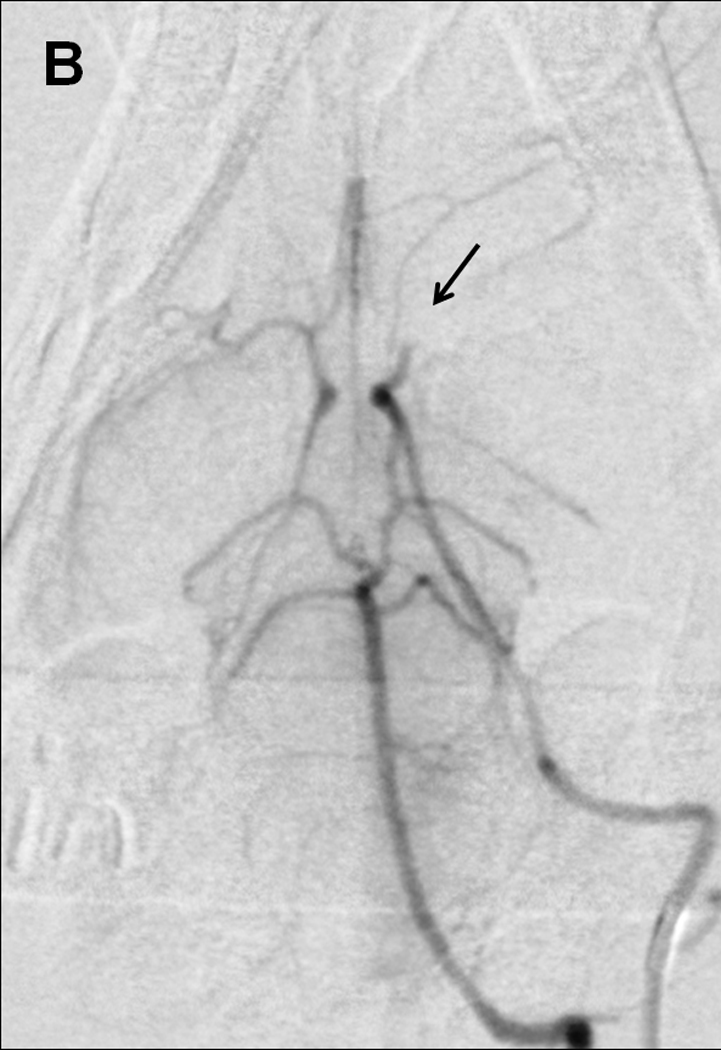
Selective internal carotid angiogram (A) shows the Circle of Willis and its major branches including the middle cerebral artery (arrow). Angiogram one minute after embolization demonstrates (B) occlusion of the anterior cerebral and middle cerebral arteries (arrow).
Treatment began 1 hour later. An ear vein catheter was used for tPA and MB administration. The angiographic catheter was removed and the incision sutured. Rabbits recovered and were monitored for adverse effects.
Preparation of Embolus
Donor rabbit arterial blood was immediately transferred into 1.5-mm inner diameter glass tubes (Natelson Blood Collecting Tube, Fisher Scientific, Waltham, MA), clotted at 37°C for 6 hours, and incubated at 4°C for 66 hours. The clot was expelled from the tubing and cut to size (4.0×0.6-mm cylinder). A single clot was drawn into a 3.0-mL syringe containing physiological saline for injection into the ICA.
Ultrasound
Rabbits with US received transcutaneous pulsed-wave (20% duty cycle) US at 1 MHz, 0.8 W/cm2, calculated peak negative pressure 0.1575 MPa, (Sonicator 716; Mettler Electronics, Anaheim, CA) for 1 hour. The side of the head was clipped and depilatory cream applied. A 10-cm2 therapeutic transducer was placed in front of the ear and behind the eye and was coupled to the skin with US gel. Positioning was confirmed fluoroscopically.
Treatments
Rabbits were randomly assigned to groups: 1) control (n=11), 2) tPA only (n=20), 3) tPA+US (n=10), 4) lipid MB+US (n=16), 5) 3µm MB+US (n=8) and 6) tagged 3µm MB+US (n=9). Control rabbits were embolized but received no therapy. Rabbits administered tPA received intravenous tPA in a standard dose (0.9 mg/kg) with an initial 10% bolus and the remainder administered over 1 hour. This corresponds to the standard human dosage and a classic previous rabbit study (21) that has been efficacious in our preliminary studies. Rabbits administered lipid MB (LMB) received intravenous Perflutren Lipid Microspheres (Definity; Lantheus Medical Imaging; North Billerica, MA) at a dose rate of 0.16 mg/kg, a 10-fold increase over standard image contrast dose, over 30 minutes.
Lipid MB were activated by vigorous mechanical shaking per manufacturer’s instructions. The required dose was diluted to 6.0-mL with physiological saline and administered in 1-mL boluses every 5 minutes.
Custom 3µm MB were prepared by sonicating a decafluorobutane gas-saturated solution of 5% w/V human serum albumin (Plasbumin-25, Talecris Biotherapeutics, Inc., Research Triangle Park, NC) and 10% w/V dextrose (Sigma-Aldrich Co., St. Louis, MO) using a Fisher 500 Sonic Dismembrator (Fisher Scientific, Waltham, MA). Sonication was in two steps: 30 sec at 250 W and 20 sec at 450 W. The 3µm MB were isolated on the basis of differential buoyancy. Some 3µm MB were then dual tagged using a 3b2a inhibitor, eptifibatide, at a very low dose (20) and a monoclonal antibody to the human D-dimer fibrin degradation product (American Diagnostics, Inc., Stamford, CT). Antibody was chemically cross-linked to the MB with a chemical cross-linker using 100 µg of antibody per 1010 MB. Our testing confirmed the reaction and binding to clotted rabbit blood, in agreement with another study (23). The 3µm MB were diluted to provide 5×109 MB for injection as above.
Functional Testing
Before sacrifice each rabbit received a neurological assessment score (NAS) using the wryneck test procedure described previously (24). The NAS tests motor, sensory, balance, and reflex measures and ranges from 0 to 10, with higher scores indicating greater neurological injury.
Measurement of Infarct Volume
At 24 hours rabbits were euthanized by intravenous administration of 1.5-mL of pentobarbital. The brain was harvested, chilled in saline for 1 hour, and sliced at 0.4-cm intervals using a chilled brain mold (RBM-7000C; ASI Instruments Inc.; Warren, MI). Coronal brain sections (n=8 in each rabbit) were placed in 1% 2,3,5-triphenyltetrazolium chloride (TTC) for 45 minutes at 37°C, fixed in 10% formalin, and digitally photographed (Figure 2). Areas of infarction were measured using digital analysis (NIH ImageJ). Each brain section volume and stroke volume was calculated by multiplying the section area by the slice thickness (0.4-cm). Images were measured by technicians blinded to treatment group. Percent infarct volume was calculated.
Figure 2. Triphenyltetrazolium chloride (TTC) vital stain of brain.
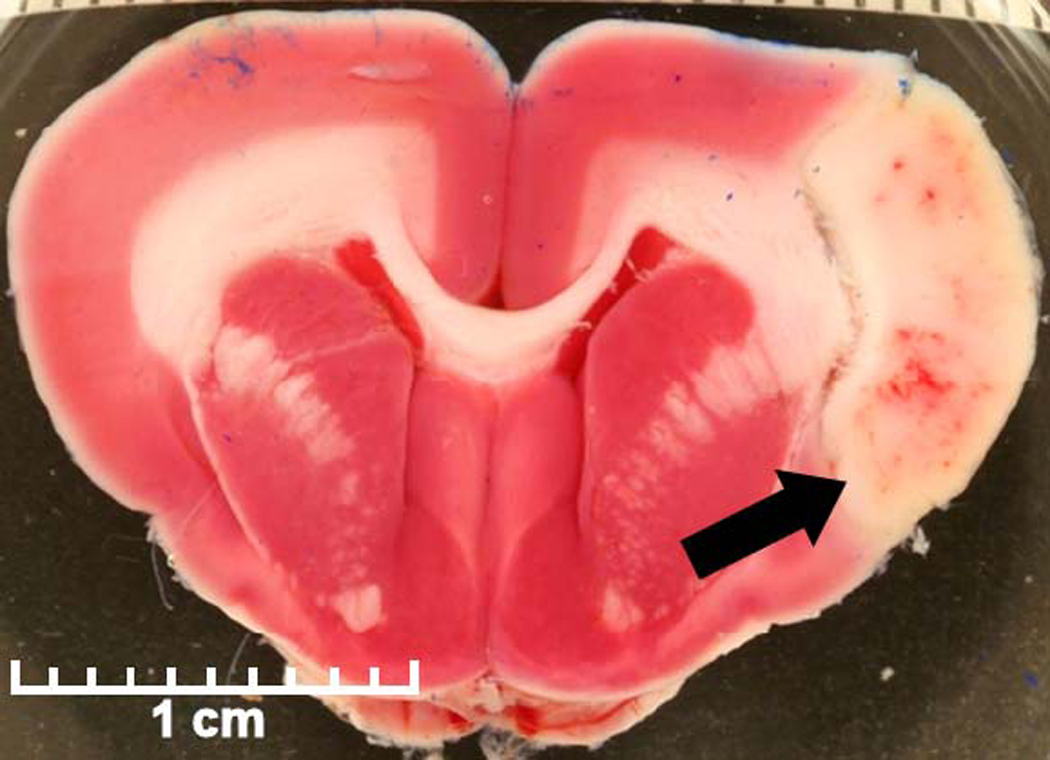
A stained fresh brain section shows typical pale infarct area (arrow) in the middle cerebral artery distribution with associated dark spots of hemorrhage.
Hemorrhage Determination
Fixed brain sections were embedded in paraffin, sectioned at 4-µm, stained with hematoxylin and eosin (H&E), and evaluated by a veterinary pathologist. Intracranial hemorrhage was defined as extravasation of erythrocytes and fluid into the extravascular space. The presence of ICH and its location were recorded (Figure 2). All hemorrhage analyses were performed by a veterinary pathologist blinded to treatment group.
Blood Tests
Blood samples were collected from an auricular artery before embolization (baseline) and at 3 and 24 hours post-embolization. The serum was stored at −80°C until analysis. S-100B serum concentrations were measured by enzyme-linked immunosorbent assay.
Statistical Analysis
Percent infarct volume was compared among experimental groups using one-way ANOVA. Percent infarct volume for each group is reported as least square mean±standard error as generated by PROC GLM in the SAS® software (SAS Inst. Inc.; Cary, NC). Median NAS scores are reported for each group, and the distribution of scores among the treatment groups compared with the Kruskal-Wallis test. The incidence of hemorrhage within or outside the stroke was compared using the chi-square test. Three hour and 24-hour S-100B values for each rabbit were divided by that rabbit’s baseline measurement to create 3- and 24-hour fold change values. The effects of treatment and time on these S-100B fold changes were tested using an unstructured covariance matrix in PROC MIXED in SAS®, a repeated-measures ANOVA procedure, followed by individual group comparisons using least squares means. Fold changes are reported as least squares means±standard errors. Pearson correlation was used to evaluate the association between percent infarct volume and 24-hour S-100B values.
Results
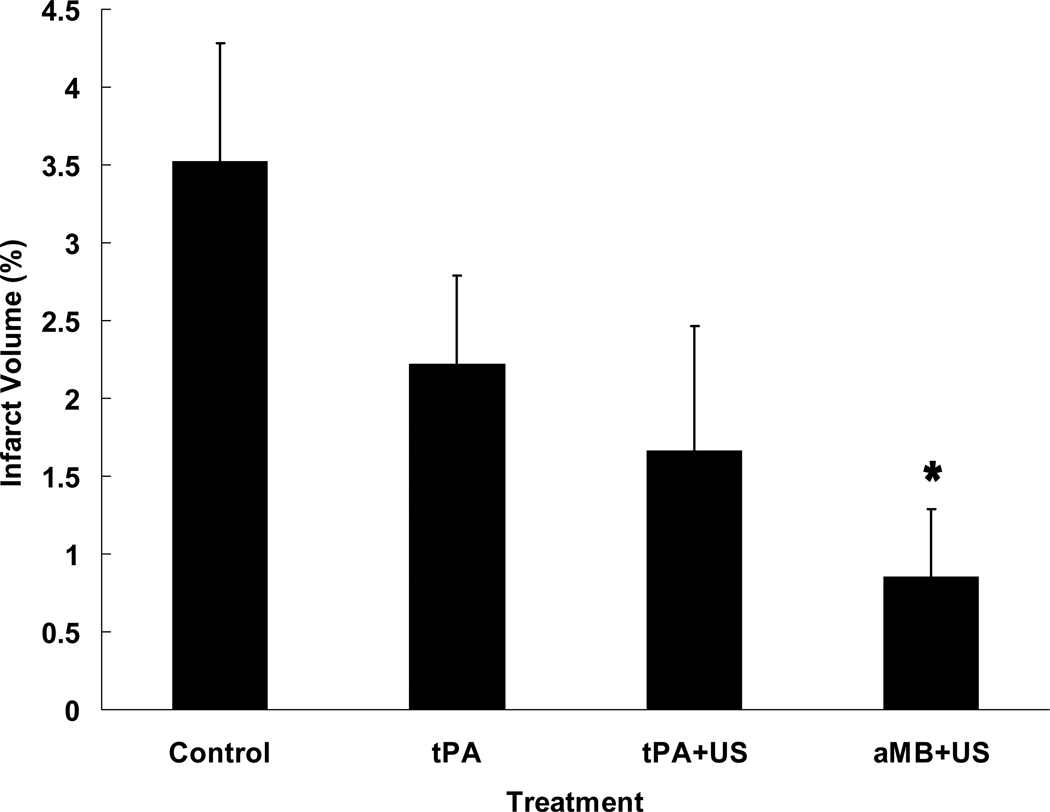
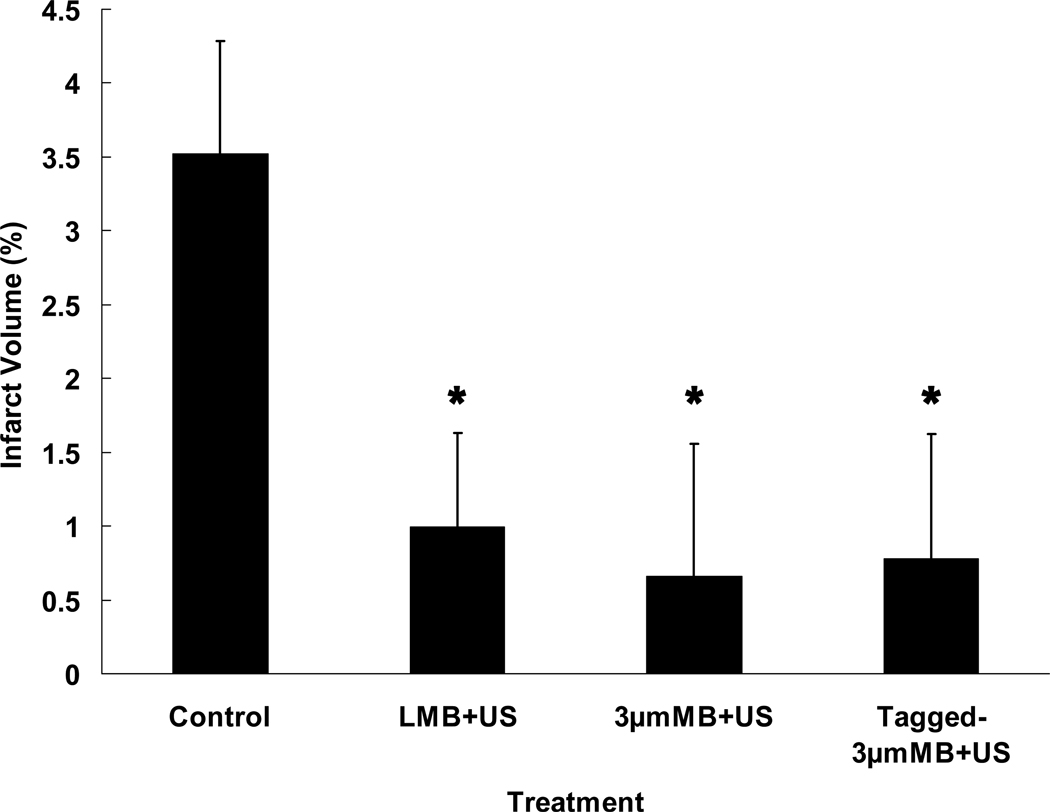
Seventy-four rabbits completed the protocol and were evaluated (Figure 3). Mean infarct volume percent was lower for rabbits treated with LMB+US (1.0%±0.6%; P=0.013), 3µm MB+US (0.7%±0.9%; P=0.018), and tagged 3µm MB+US (0.8%±0.8%; P=0.019) compared with control rabbits (3.5%±0.8%). Infarct volume averaged 2.2%±0.6% and 1.7%±0.8% for rabbits treated with tPA alone and tPA+US, respectively, and did not differ from controls, P=0.18 and P=0.10, respectively. The three MB types collectively differed (P=0.0043) from control, showed a trend vs. tPA, P=0.058, and were not different from tPA+US, P=0.36.
Figure 3. Infarct Volume vs. Treatment.
Percent infarct volumes of rabbits following embolization and various treatments show all microbubble groups (aMB+US) had significantly less infarct than controls (A), P=0.004. MB types were not significantly different among themselves, but each was different from control (B). The tPA and tPA+US groups showed a trend towards improvement. (*P<0.02 vs. Control.)
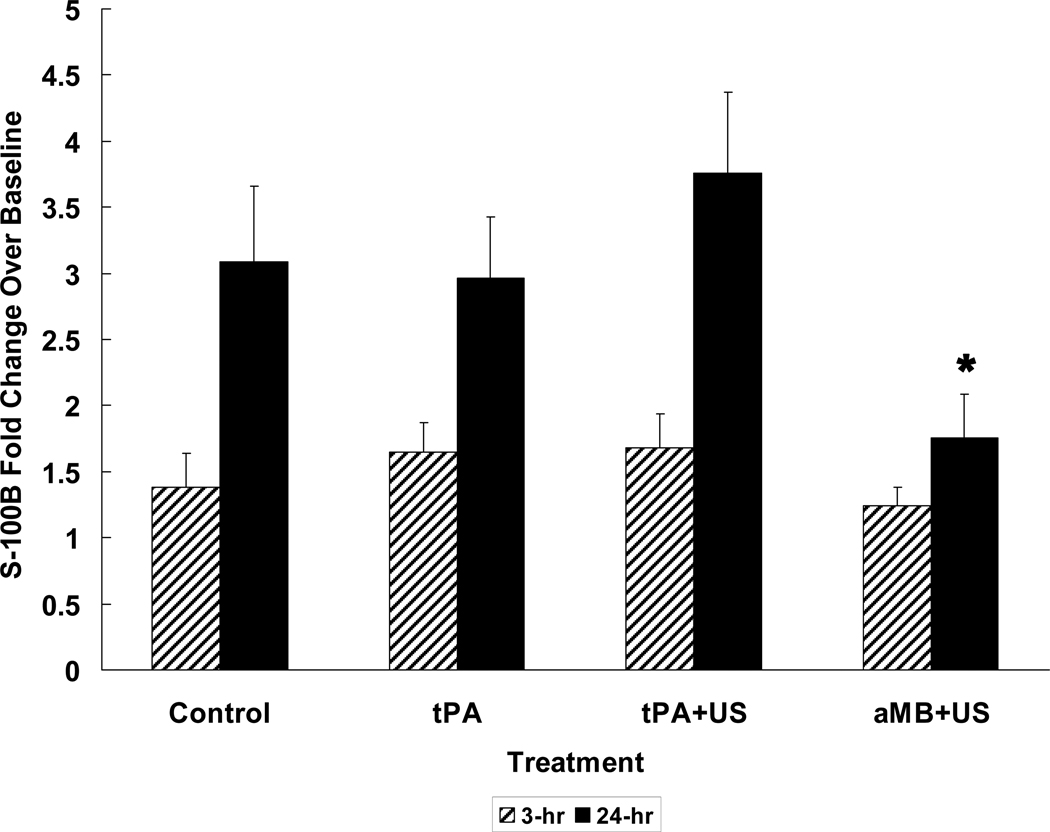
Mean fold increases over pre-embolization levels at 3 and 24 hours post-embolization in S-100B values (n=58) reveal significant increases at 3 hours for the tPA (1.6-fold) and tPA+US (1.7-fold) groups (p=0.01 each) and at 24 hours for control (3.1-fold), tPA (3.0-fold), and tPA+US (3.7-fold) groups (p≤0.001 each) (Figure 4). LMB+US, 3µm MB+US, and tagged 3µm MB+US did not increase S-100B levels significantly at 24 hours (1.7-fold, 1.6-fold, and 2.2-fold, respectively, P=0.21, 0.46, and 0.19, respectively). Twenty-four hour S-100B increases of the three MB types collectively were lower than those of control, tPA, and tPA+US (P<0.05 for each). S-100B values at 24 hours were positively correlated with infarct volume (r=0.45, P<0.001).
Figure 4. S-100B Change with Treatment.
Using initial pre-stroke values as the base, normalized fold S-100B values increase significantly with time following stroke in controls and tPA and tPA+US groups. All MB groups (aMB+US) show much less increase, reflecting less damage to the brain. (*P<0.05 vs. all other groups.)
Microscopic hemorrhage rates were similar in all groups, both in areas of stroke, P=0.47, and areas outside stroke, P=0.85. Hemorrhage within stroke was seen in controls 73%; tPA 50%; tPA+US 50%; LMB+US 31%; 3µm MB+US 50%; and tagged 3µm MB 56%. Hemorrhage outside of stroke was 36%, 45%, 50%, 50%, 25%, and 33% respectively.
Median NAS values were also similar in all groups: control 3.0, tPA 1.0, tPA+US 1.0, LMB+US 2.5, 3µm MB+US 0.0, tagged 3µm MB+US 2.0. P=0.27.
Angiography (n=74) showed occlusions of the middle cerebral artery in 88%, anterior cerebral artery in 47% and posterior cerebral artery in 4%. No visible embolus was seen in 5%, but 3 of these 4 had measurable areas of appropriate stroke on TTC stains. The fourth had no visible stroke but was in the control group, lowering that mean value. Eleven other animals did not complete the protocol: 5 with posterior cerebral artery occlusions and symptoms requiring early euthanasia (1 control, 2 tPA+US, 1 MB+US, 1 tagged 3µm MB+US), 4 with superior cerebellar artery occlusion and early death (1 control, 1 tPA, 1 MB+US, 1 3µm MB+US), 1 with occlusion of 4 vessels and outlier severe stroke volume (1 control), and 1 died overnight and volumes could not be measured (1 tPA). These were distributed randomly in the groups and not included in statistical evaluations.
Discussion
Improved stroke therapy with better efficacy and without the severe hemorrhagic complications associated with tPA is needed urgently. While microbubble augmentation of sonothrombolysis has been previously established, demonstration in an ischemic stroke model is required before human trials without IV tPA, the standard of care, can be seriously considered.
This series of rabbits clearly shows good efficacy of MB+US without any exogenous tPA when using any of three MB techniques (Figure 3). Significantly smaller stroke volumes and attenuated S-100B increases confirm improved end organ (brain) status with these techniques. Treatment with standard tPA or tPA+US trended toward improvement in stroke volume but did not reach statistical significance with the power available in this study. Potential mechanisms for reduced infarct volume may include: arterial thrombolysis and prompt reperfusion, ultrasound dilation of vessels and improved blood flow (25), and collateral augmentation (26). Unknown factors dealing with endogenous immune mechanisms are suspect but not yet proven (27). This suggests that MB+US therapy is even more efficacious than tPA alone, but this study did not have sufficient power to differentiate between tPA and MB techniques.
These results are based primarily on direct measurement of stroke volumes, a 24-hour TTC measurement reflective of basic brain infarction and confirmed with histological evidence. The improved brain outcomes are apparent, whether they are due to large vessel clearing, small vessel dilatation, collateral recruitment, or a combination of factors (11). Overall survival of more brain, the object of therapy, was clearly demonstrated.
As a peripheral blood biochemical marker of brain injury, S-100B elevation has been correlated with infarct volume, hemorrhagic transformation, and functional outcomes. Serum levels of S-100B, an astroglial protein, have been used to support conclusions drawn about therapeutic agents in ischemic stroke (28–30). The S-100B scores support the stroke volume data with detection of less damage in the MB groups than in controls (Figure 4).
The stable levels of hemorrhage are particularly encouraging. Increased bleeding in the TRUMBI Trial of ultrasound augmentation of thrombolysis with tPA was seen with much different equipment and a frequency of 300 kHz (31). Apparently this was not a problem at 2 MHz in Molina’s and Alexandrov’s initial studies (14,32–34). Increased bleeding was encountered in the prematurely terminated TUCSON trial that used 2 MHz US and high levels of MB with normal tPA doses, but this may be due to patient selection differences (33). It was not seen in the current study at 1 MHz using even higher MB levels. While the microscopic hemorrhages seen here have yet to be followed for long-term symptomatic intracranial hemorrhage development, the data herein with unchanged incidence of bleeding is promising. Our earlier work with smaller strokes in rabbits (unpublished data) showed decreased microscopic hemorrhage with MB+US, but this did not continue with these larger strokes. Perhaps larger areas of ischemia elevate hemorrhage risk and the favorable findings in small strokes disappear.
It was hypothesized that tagged 3µm MB would be delivered more effectively to the embolus than other MB and would further improve outcomes. This was not observed, nor was there improvement with the 3µm untagged MB compared with the LMB, although both types of 3µm MB showed superiority to LMB using in vitro sonothrombolysis studies (35). This model did not provide much opportunity for further improvement from the 1.0%±0.6% stroke volume obtained with LMB. A more severe model will be required.
Though lower than controls in all treatment groups, NAS values derived from physiological tests were not significantly improved or different between these groups, reflecting a lack of sensitivity of this scoring system to anterior strokes. Posterior strokes cause dramatic neurological deficits with high scores. However, the targeted anterior circulation in rabbits is more forgiving or silent, even with large infarcts (24).
While this study focused on stroke therapy, the brain is a difficult ultrasound target compared with others. Ultrasound delivery through the skull must contend with high attenuation of acoustic energy in bone. This difficulty has been overcome using narrow beams of focused 2 MHz transcranial Doppler (14,32,34), with larger unfocused 1 MHz beams (19,20,24), and with intravascular delivery using special catheters tipped with 2 MHz transducers (36,37) which travel through the vessel directly to clot. Other anatomical sites including dialysis grafts (17,18) and peripheral blood vessels are easily accessed with either large transcutaneous or intravascular transducers. While deeper structures may require sophisticated approaches, successful transcutaneous sonothrombolysis has been reported in coronary occlusions in pigs and, ultimately, almost all anatomical areas are likely to be served.
Study limitations include the inability to separate in vivo the effects of three different MB which are clearly different in in vitro testing (35). A more severe stroke model is needed and is under development. Another limitation is that correlation of microscopic bleeding at 24 hours in rabbits with symptomatic human bleeding is not yet proven. We are addressing this with long-term survival studies.
In summary, using the rabbit model, MB sonothrombolysis with no exogenous tPA produces significant improvement in strokes without apparent side effect. A long series of animal studies has now shown that human trials without tPA or in patients where tPA is contraindicated are warranted.
Acknowledgments
Acknowledgements and Funding
Sources of Funding
R01 HL082481
R01 CA99178
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Towfighi A, Ovbiagele B, Saver JL. Therapeutic Milestone: Stroke declines from the second to the third leading organ- and disease-specific cause of death in the United States. Stroke. 2010;41:499–503. doi: 10.1161/STROKEAHA.109.571828. [DOI] [PubMed] [Google Scholar]
- 2.Heart Diseases and Stroke Statistics, 2010 Update. American Heart Association; [Google Scholar]
- 3.Marler J. Tissue plasminogen activator for acute ischemic stroke; the National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. for the ECASS Investigators. Thrombolysis with Alteplase 3 to 4.5 Hours after Acute Ischemic Stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 5.Kleindorfer D, Xu Y, Moomaw CJ, Khatri P, Adeoye O. US Geographic Distribution of rt-PA Utilization by Hospital for Acute Ischemic Stroke. Stroke. 2009;40:3580–3584. doi: 10.1161/STROKEAHA.109.554626. [DOI] [PubMed] [Google Scholar]
- 6.Donnan GA. The 2007 Feinberg lecture; a new road map for neuroprotection. Stroke. 2008;39:242–248. doi: 10.1161/STROKEAHA.107.493296. [DOI] [PubMed] [Google Scholar]
- 7.Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148–1150. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- 8.Luo H, Toshihiko N, Fishbein MC, Cercek B, Forrester JS, Kim CJ, et al. Transcutaneous ultrasound augments lysis of arterial thrombi in vivo. Circulation. 1996;94:775–778. doi: 10.1161/01.cir.94.4.775. [DOI] [PubMed] [Google Scholar]
- 9.Rosenschein U, Furman V, Kerner E, Fabian I, Bernheim J, Eschel Y. Ultrasound imaging-guided noninvasive ultrasound thrombolysis: Preclinical results. Circulation. 2000;102:238–245. doi: 10.1161/01.cir.102.2.238. [DOI] [PubMed] [Google Scholar]
- 10.Suchkova VN, Baggs RB, Francis CW. Effect of 40-kHz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombus model. Circulation. 2000;101:2296–2301. doi: 10.1161/01.cir.101.19.2296. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RJ, Atar S, Fishbein MC, Brasch AV, Peterson TM, Nagi T, et al. Noninvasive, transthoracic, low frequency ultrasound augments thrombolysis in a canine model of acute myocardial infarction. Circulation. 2000;101:2026–2029. doi: 10.1161/01.cir.101.17.2026. [DOI] [PubMed] [Google Scholar]
- 12.Fatar M, Stroick M, Griebe M, Alonso A, Kreisel S, Kern R, et al. Effect of combined ultrasound and microbubbles treatment in an experimental model of cerebral ischemia. Ultrasound Med Biol. 2008;34:1414–1420. doi: 10.1016/j.ultrasmedbio.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Meairs S, Culp W. Microbubbles for thrombolysis of acute ischemic stroke. Cerebrovas Dis. 2009;27 supp 2:55–65. doi: 10.1159/000203127. [DOI] [PubMed] [Google Scholar]
- 14.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 15.Porter TR, LeVeen RF, Fox R, Kircsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am Heart J. 1996;132:964–968. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- 16.Birnbaum Y, Luo H, Nagi T, Fishbein MC, Peterson TM, Li S, et al. Noninvasive in vivo clot dissolution without a thrombolytic drug. Circulation. 1998;97:130–134. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- 17.Culp WC, Porter TR, Xie F, Goertzen TC, McCowan TC, Vonk BN, et al. Microbubble potentiated ultrasound as a method of declotting thrombosed dialysis grafts: experimental study in dogs. Cardiovasc Interv Radiol. 2001;24:407–412. doi: 10.1007/s00270-001-0052-4. [DOI] [PubMed] [Google Scholar]
- 18.Culp WC, Porter TR, McCowan TC, Roberson PK, James CA, Matchett WJ, et al. Microbubble-augmented ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs. J Vasc Interv Radiol. 2003;14:343–347. doi: 10.1097/01.rvi.0000058409.01661.b4. [DOI] [PubMed] [Google Scholar]
- 19.Culp WC, Erdem E, Roberson PK, Husain MM. Microbubble potentiated ultrasound as a method of stroke therapy in a pig model: preliminary findings. J Vasc Interv Radiol. 2003;14:1433–1436. doi: 10.1097/01.rvi.0000096767.47047.fa. [DOI] [PubMed] [Google Scholar]
- 20.Culp WC, Porter TR, Lowery J, Xie F, Roberson PK, Marky L. Intracranial clot lysis with intravenous microbubbles and transcranial ultrasound in swine. Stroke. 2004;35:2407–2411. doi: 10.1161/01.STR.0000140890.86779.79. [DOI] [PubMed] [Google Scholar]
- 21.Zivin JA, Fisher M, DeGirolami U, Hemenway CC, Stashak JA. Tissue plasminogen activator reduces neurological damage after cerebral embolism. Science. 1985;230:1289–1292. doi: 10.1126/science.3934754. [DOI] [PubMed] [Google Scholar]
- 22.Culp BC, Brown AT, Erdem E, Lowery J, Culp WC. Selective intracranial magnification angiography of the rabbit: basic techniques and anatomy. J Vasc Interv Radiol. 2007;18:187–192. doi: 10.1016/j.jvir.2006.12.720. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AC, Campbell JH. Targeted delivery of heparin and LMWH using a fibrin antibody prevents restenosis. Atherosclerosis. 2004;176:73–81. doi: 10.1016/j.atherosclerosis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Brown AT, Skinner RD, Flores R, Hennings L, Borrelli MJ, Lowery J, Culp W. Stroke location and brain function in an embolic rabbit stroke model. J Vasc Interv Radiol. 2010;21:903–909. doi: 10.1016/j.jvir.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel RJ, Suchkova VN, Miyamoto T, Luo H, Baggs RB, Neuman Y, et al. J Am Col Cardiol. 2004;44:1454–1458. doi: 10.1016/j.jacc.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 26.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011 doi: 10.1161/STROKEAHA.110.595256. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;17:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR for the NINDS rt-PA Stroke Study Group. Association of Serial Biomarkers With Acute Ischemic Stroke: The National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 29.Foerch C, Singer OC, Neumann-Haefelin T, du Mesnil de Rochemont R, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol. 2005;62:1130–1134. doi: 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- 30.Stroick M, Fatar M, Ragoschke-Schumm A, Fabender K, Bertsch T, Hennerici MG. Protein S-100B- a prognostic marker for cerebral damage. Current Medicinal Chemistry. 2006;13:3053–3060. doi: 10.2174/092986706778521751. [DOI] [PubMed] [Google Scholar]
- 31.Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36:1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 32.Alexandrov AV, Mikulik R, Ribo M, Sharma VK, Lao AY, Tsivgoulis G, et al. A pilot randomized clinical safety study of sonothrombolysis augmentation with ultrasound-activated perflutren-lipid microspheres for acute ischemic stroke. Stroke. 2008;39:1464–1469. doi: 10.1161/STROKEAHA.107.505727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina CA, Barreto AD, Tsivgoulis G, Sierzenski P, Malkoff MD, Rubiera M, et al. Transcranial ultrasound in clinical sonothrombolysis (TUCSON) trial. Ann Neurol. 2009;66:28–38. doi: 10.1002/ana.21723. [DOI] [PubMed] [Google Scholar]
- 34.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Sabin JA, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 35.Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Invest Radiol. 2010 doi: 10.1097/RLI.0b013e318200757a. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomsik T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Interventional Management of Stroke II Investigators. Revascularization results in the Interventional Management of Stroke II trial. AJNR. 2008;20:582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raabe RD. Ultrasound-accelerated thrombolysis in arterial and venous peripheral occlusions: fibrinogen level effects. J Vasc Interv Radiol. 2010;21:1165–1172. doi: 10.1016/j.jvir.2010.03.020. [DOI] [PubMed] [Google Scholar]