Abstract
Purpose of review
Topical tenofovir gel and oral tenofovir and emtricitabine/tenofovir (FTC/TDF) have been demonstrated to have efficacy in preventing HIV-1 in some populations. Pre-exposure prophylaxis (PrEP) trials and future directions are summarized.
Recent findings
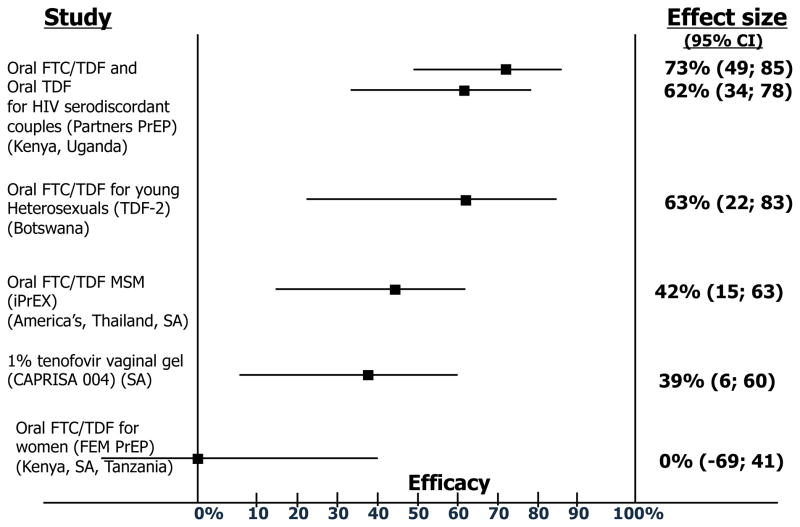
Peri-coital use of 1% tenofovir gel in CAPRISA 004 reduced HIV-1 acquisition by 39% and HSV-2 acquisition by 51%. Daily oral FTC/TDF demonstrated 44% reduction in HIV-1 acquisition among men who have sex with men (MSM) in iPrEx. Both studies showed higher efficacy among those with higher adherence. Efficacy of daily oral TDF and FTC/TDF was 66% and 73%, respectively, among HIV-1 uninfected partners in an HIV-1 serodiscordant partnership in the Partners PrEP Study. Efficacy of daily oral FTC/TDF was 66% in young heterosexuals in Botswana in the TDF-2 trial. The FEM-PrEP and VOICE studies in African women found no efficacy with oral FTC/TDF and TDF, respectively. Safety and tolerability were excellent and limited resistance was observed in seroconverters.
Summary
Topical tenofovir gel showed efficacy in African women and daily oral TDF and FTC/TDF were efficacious in MSM, and African HIV-1 serodiscordant couples and young heterosexuals. The reasons for lack of efficacy of oral FTC/TDF and TDF in two studies in African women are being investigated. Longer-acting formulations, invtravaginal rings, and new candidate antiretrovirals are being evaluated.
Keywords: pre-exposure prophylaxis, HIV prevention, microbicides, tenofovir, emtricitabine-tenofovir
Introduction
In spite of substantial progress in scale-up of antiretroviral therapy (ART) for HIV treatment, new infections outpace the number of infected persons initiating ART [1]. A recent trial, HPTN 052, definitively demonstrated that ART substantially reduces risk of HIV transmission, in the context of intensive counseling on risk-reduction and viral load monitoring [2]. Policymakers are considering implications of translating HPTN 052 findings into programmatic priorities. Studies in diverse settings have highlighted challenges in achieving linkages to HIV care with high uptake and retention, which is essential to achieve the durable viral suppression that results in reduced HIV infectiousness [3,4]. Thus, there remains a need to also identify and implement evidence-based primary HIV prevention strategies, including antiretrovirals (ARVs) for primary HIV prevention as pre-exposure prophylaxis (PrEP). During the past year, studies demonstrating proof-of-concept of topical and oral PrEP have reported results. This review will focus on the rationale for PrEP, design of human PrEP studies, recent findings from PrEP efficacy trials, and remaining questions and uncertainties.
Rationale behind topical and oral PrEP for HIV prevention
The rationale for prevention of sexual HIV acquisition with pre-exposure use of antiretroviral drugs stems from demonstration that ARVs prevent transmission of HIV from an infected mother to infant and from animal model studies. ARVs are the cornerstone of prevention of mother-to-child transmission of HIV, first demonstrated with peripartum zidovudine [5]. Recent studies have shown that post-natal ARVs, provided to infants with ongoing exposure to HIV through breastmilk, can substantially reduce HIV risk [6]. Thus, these infant studies provide proof-of-concept that ARV prophylaxis can be highly efficacious in the context of known and ongoing HIV exposure [7].
The feasibility of use of ARVs for prevention of HIV acquisition from sexual exposure has been a focus of macaque SHIV challenge studies and more recently, humanized mouse HIV challenge studies [8], using tenofovir disoproxyl fumarate (TDF) and emtrictiabine/tenofovir (FTC/TDF), given their action early in the HIV life cycle, high potency, genital tract levels, excellent safety and tolerability, and low incidence of resistance. Animal studies have tested drug dosing and delivery routes that reflect oral dosing in humans and repeat low dose mucosal virus challenges to mimic sexual exposure to HIV. Overall, these studies indicated high levels of protection from topical tenofovir gel [9] and daily oral dosing of TDF and FTC/TDF, potentially greater protection from combination FTC/TDF than TDF alone [10,11], and efficacy when FTC/TDF was dosed intermittently (3 days before and 2 hours after rectal SHIV exposure) [12]. Animal studies also are being used to identify potential new PrEP candidates, including topical maraviroc [13] and raltegravir [14].
Human efficacy trials of topical and oral PrEP
Proof-of-concept clinical trials of the safety and efficacy of topical 1% tenofovir gel and oral TDF and oral FTC/TDF as PrEP have been implemented in African women at risk of heterosexual HIV acquisition (CAPRISA 004, FEM-PrEP, Partners PrEP, TDF2, VOICE, FACTS 001), African heterosexual men (Partners PrEP, TDF2), men who have sex with men (MSM) from the Americas and other settings (iPrEx), and injection drug users (Bangkok Tenofovir Study) (Table 1). These studies are summarized below in chronological order of their reporting of efficacy findings.
Table 1.
Ongoing human efficacy trials of topical and oral PrEP
| Study name (location) | Sponsor/Funder | Population | N | PrEP Agent | Status |
|---|---|---|---|---|---|
| CAPRISA 004 (South Africa) | NIH/BMGF | Women | 889 | Vaginal tenofovir gel (coitally-associated use) | 39% efficacy 15 |
| iPrEx (Brazil, Ecuador, Peru, South Africa, Thailand, US) | NIH/BMGF | Men who have sex with men and transgender women | 2499 | FTC/TDF | 44% efficacy 19 |
| FEM-PrEP (Kenya, South Africa, Tanzania) | USAID/FHI | Higher-risk women | 1950 | FTC/TDF | Stopped for lack of efficacy April 2011 23 |
| Partners PrEP Study (Kenya, Uganda) | UW / BMGF | HIV serodiscordant couples | 4758 | TDF, FTC/TDF | 62% efficacy for TDF 73% efficacy for FTC/TDF 25 |
| TDF2 Study (Botswana) | CDC | Heterosexual men and women, ages 18–35 | 1200 | FTC/TDF | 62% efficacy 31 |
| VOICE / MTN 003 (South Africa, Uganda, Zimbabwe) | MTN / NIH | Women | 5021 | TDF, FTC/TDF, Vaginal tenofovir gel (daily) | Oral TDF stopped for lack of efficacy September 2011 32 |
| Bangkok Tenofovir Study (Thailand) | CDC | Injection drug users | 2400 | TDF | Ongoing 27 |
| FACTS 001 (South Africa) | CONRAD, South Africa, USAID, BMGF | Women | 2600 | Vaginal tenofovir gel (coitally-associated use) | Initiated October 2011 |
BMGF, Bill & Melinda Gates Foundation; CDC, United States Centers for Disease Control and Prevention; FHI, FHI360; MTN, Microbicides Trials Network; NIH, United States National Institutes of Health; USAID, United States Agency for International Development
CAPRISA 004 enrolled 889 HIV-uninfected women, ages 18–44 in KwaZulu-Natal, South Africa, who were randomized to pericoital use of 1% tenofovir gel or placebo, dosed by the BAT24 strategy (for Before and After sex, not to exceed Two doses in 24 hours). The event-driven timing was developed from dosing of nevirapine to HIV exposed infants for HIV prevention. In CAPRISA 004, tenofovir gel reduced the risk of HIV acquisition by 39% (HR 0.61; 95% CI 0.40–0.94 p=0.017) [15]. Notably, HIV incidence was 9.1 per 100 women-years in the placebo arm, an extraordinarily high rate. No significant viral resistance to tenofovir (i.e., the K65R mutation) was detected using population sequencing in viruses obtained from HIV seroconverters. In subgroup analyses, efficacy was 54% in women who reported >80% adherence to gel use with sex acts in the prior month (p=0.025). In a case-control analysis of cervicovaginal tenofovir levels, women with levels >1000ng/mL had a 74% lower risk of HIV infection than those with <1000 ng/mL [16], providing further evidence of an adherence-efficacy relationship. An unexpected ‘bonus’ finding in the study was a 51% reduction in HSV-2 incidence in the tenofovir arm,, which is consistent with in vitro work demonstrating mucosal concentrations achieved with 1% tenofovir gel have direct anti-herpetic activity [17]. Concentrations of tenofovir diphosphate in vaginal tissues are approximately 1000-fold higher with 1% tenofovir gel dosing compared to oral dosing using TDF, which likely explains the efficacy of topical dosing in spite of low systemic absorption [18].
The iPrEx study of 2499 HIV seronegative MSM and transgender women from the Americas, South Africa and Thailand into a placebo-controlled trial of daily oral FTC/TDF showed that FTC/TDF reduced HIV acquisition risk by 44% (95% CI 15–63). Subgroup analyses indicated higher efficacy (73%) among those with ≥90% adherence [19]. Importantly, plasma and intracellular drug levels demonstrated that only 8% of seroconverters and 54% non-seroconverters had detectable tenofovir or emtricitabine; having detectable drug was strongly associated with lower risk of HIV (OR 12.9, 95% CI 1.7–99.3). Resistance to emtricitabine (i.e., M184 I/V mutations) was detected in 2 participants randomized to FTC/TDF who were seroconverting at randomization; these resistance mutations were no longer detectable by ultrasensitive resistance testing six months after seroconversion and withdrawal of PrEP [20]. Safety was high with no substantial differences in the rate of serious adverse events, including laboratory events, by arm. A 1% reduction in bone mineral density was observed in the FTC/TDF arm, compared with placebo, with no difference in fracture rate [21]. No risk compensation was observed based on self-reported condom use during anal sex. Thus, iPrEx indicates excellent safety and moderate efficacy in the context of moderate adherence, as indicated by drug levels [22]. Rare resistance was detected -- among two subjects who initiated PrEP during acute seroconversion. Uptake, adherence, risk behaviors, and efficacy in the context of known efficacy of FTC/TDF PrEP is being studied among former iPrEx participants and a subset of new enrollees in a recently-initiated open-label extension study (iPrEx OLE).
The FEM-PrEP study enrolled 1951 high-risk HIV-uninfected women from Kenya, South Africa, and Tanzania into a placebo-controlled trial of daily oral FTC/TDF. The study was stopped by its Independent Data Monitoring Committee in April 2011 due to futility: an equal numbers of infections (n=28) were seen in the two study arms [23]. Potential explanations for the lack of efficacy include low adherence, which is being evaluated by drug levels, differential adherence by arm, and possibly tolerability or drug-drug interactions with hormonal contraception, given a higher pregnancy rate in the active FTC/TDF arm. Final analyses of the FEM-PrEP trial will be available in early 2012.
The TDF2 study enrolled 1200 heterosexual men and women between the ages of 18 and 40 in Botswana into a placebo-controlled trial of daily oral FTC/TDF; enrollment was terminated early due to larger-than-expected rates of loss-to-follow-up. The study reported results in July 2011: 62.6% efficacy (95% CI 21.5–83.4) for HIV protection due to PrEP. The trial was underpowered to demonstrate sex-based differences with 33 seroconversions, but the point estimates were protective for both men (80.1%, p=0.03) and women (49.4%, p=0.1). Safety and tolerability of FTC/TDF were high. There was no evidence of behavioral risk compensation.
The Partners PrEP Study (for which we are the lead investigators) is an ongoing 3 arm placebo-controlled trial of daily oral TDF and FTC/TDF among 4758 HIV serodiscordant couples from Kenya and Africa among whom the HIV-infected partner is not eligible for ART according to national guidelines; the HIV-uninfected partners are randomized to receive PrEP or placebo [24]. On July 10, 2011, the study’s Data Safety Monitoring Board recommended that the placebo arm be discontinued due to meeting pre-determined stopping guidelines for efficacy. Overall, 62% efficacy of TDF (95% CI 34–78) and 73% efficacy of FTC/TDF (95% CI 49–85) compared to placebo, were observed; the difference between TDF and FTC/TDF was not statistically significant (p=0.18). Both TDF and FTC/TDF significantly reduced HIV risk for both men and women [25]. Adherence to study drug was very high based on clinic-based pill counts of unreturned study medication, and in 3 sites, electronic monitoring and home visits for unannounced pill counts [26]. Drug concentrations and resistance testing in seroconverters is underway. Safety of both drugs was high and no evidence of risk compensation was observed.
The VOICE trial is a 5 arm study among 5028 HIV uninfected women from South Africa, Uganda, and Zimbabwe in which daily 1% tenofovir gel, daily oral TDF, and daily oral FTC/TDF are being evaluated for safety and effectiveness compared to respective gel/oral placebos. The Data Safety Monitoring Committee for the VOICE trial recommended discontinuation of the oral TDF arm in September 2011 due to inability to demonstrate efficacy. Specifics of the numbers of infections in that arm have not been released since the trial is ongoing, so as not comprise the integrity of the trial. The VOICE trial is anticipated to be completed by mid-2012, and will provide comparative efficacy of daily topical 1% tenofovir gel on HIV and HSV-2 acquisition, and daily oral FTC/TDF among at-risk African women.
Lastly, the Bangkok Tenofovir Study trial of daily oral TDF among injection drug users compared to placebo is anticipated to have efficacy results in 2012. A majority of the participants are enrolled in methadone replacement programs, where they receive their study medication, essentially receiving directly-observed PrEP [27].
Current understanding and future directions for PrEP
Efficacy trials have demonstrated high safety and moderate to high efficacy of topical and oral PrEP among South African women (CAPRISA 004), MSM from diverse settings (iPrEx), young Botswana heterosexuals (TDF2), and East African HIV serodiscordant couples (Partners PrEP). Unraveling potential behavioral and biologic explanations for lack of efficacy of oral PrEP in some populations of women -- FTC/TDF in FEM-PrEP and TDF in VOICE -- is essential to understanding whether the lack of efficacy is due to adherence to study product, types of sexual exposure, and/or biologic co-factors. As is often the case with development of new biomedical interventions, including for HIV prevention, the process of scientific discovery is iterative and complex.
The CAPRISA 004 trial demonstrates proof-of-concept that peri-coital use of tenofovir gel significantly reduced HIV risk in a very high incidence population. Adherence measures, including drug levels, indicate that high vaginal concentrations of tenofovir are achieved with vaginal dosing, and are associated with protection from HIV and likely account for the protective effect against HSV-2. The VOICE trial is anticipated to have critical efficacy data on daily 1% tenofovir gel for HIV and HSV-2 acquisition in 2012. The recently-launched FACTS 001 study is a confirmatory study of the peri-coital BAT24 strategy among a more diverse population of South African women than CAPRISA 004. VOICE and FACTS 001 will provide essential data on safety and effectiveness of 1% tenofovir gel in order to hopefully move an effective microbicide forward to licensure and wide-scale sue. Tenofovir gel has recently been reformulated for rectal application and will be evaluated for safety and pharmacokinetics among among MSM in a clinical trial initiating in 2012.
The iPrEx trial demonstrates that daily oral FTC/TDF has moderate efficacy (44%) among young, high-risk MSM who were recruited from diverse settings in the Americas, South Africa and Thailand. Importantly, efficacy appeared to be strongly associated with adherence. The iPrEx-OLE study will determine whether knowledge of efficacy of PrEP significantly increases adherence and alters risk behaviors among MSM – arguably, open-label studies like iPrEx OLE will determine whether PrEP is a viable public health strategy for MSM.
The Partners PrEP and TDF2 trials provide strong evidence for PrEP efficacy among African heterosexual populations, who make up the largest portion of the global epidemic. In Partners PrEP, both TDF and FTC/TDF were highly effective in both men and women. The study enrolled HIV serodiscordant couples who recognize their risk of HIV, and achieved high adherence to daily oral PrEP. HIV serodiscordant couples in Africa account for a substantial proportion of new HIV infections in Africa and ARV-based prevention, including PrEP targeted to the highest-risk couples before the HIV infected partner elects or is able to initiate ART, is an important prevention strategy to be piloted [28].
While awaiting findings from recently-completed and ongoing trials of oral and topical tenofovir-based PrEP in women and injection drug users, it is important to recognize the importance of these initial trials to demonstrating proof-of-concept of ARV-based primary HIV prevention. Much more needs to be understood about targeting of these strategies to those at highest risk in order to be a cost-effective intervention [29], evaluating program delivery models to reduce the risk of PrEP use in acute HIV infection, motivating and monitoring adherence to PrEP, and messaging about PrEP as part of a combined risk reduction strategy given its partial efficacy for HIV prevention. Longer-term safety, adherence, and efficacy needs to be assessed in the context of less frequent visits and briefer counseling than was provided in the intensive proof-of-concept trials.. Importantly, safety of these products needs to be studied in pregnant and breastfeeding women, and adolescent women, who are at high risk of HIV acquisition and need new prevention strategies.
Some persons would prefer to use PrEP intermittently, which requires greater understanding of the pharmacokinetics and adherence to intermittent dosing of tenofovir-based PrEP, as will be assessed in smaller studies being initiated in 2011–2. While macaque studies demonstrate biologic feasibility of intermittent oral PrEP dosing, human studies are needed to determine the extent to which individuals at high risk of HIV are able to anticipate sexual activity and achieve sufficient pre-exposure and post-exposure dosing to confer protection.
The PrEP field is also evaluating new candidate products, including non-nucleoside reverse transcriptase inhibitors (dapivirine and rilpivirine) and maraviroc, as well as sustained release delivery systems to provide more options with less dependence on coitally-dependent or daily dosing. Dapivirine has excellent safety and sustained dispersion in a vaginal ring formulation developed by the International Partnership for Microbicides [30], and will be studied for efficacy in collaboration with the US Microbicides Trials Network (MTN 020, ASPIRE trial), beginning in 2012. Oral maraviroc will be evaluated in a comparative safety and tolerability study in MSM (HPTN 069). Dapivirine and maraviroc are being co-formulated in a vaginal ring formulation. Rilpivirine, a NNRTI recently-licensed for treatment, has been formulated into a parenteral formulation for prolonged plasma exposure, and is being evaluated for pharmacokinetics and dose ranging in early clinical trials. Tenofovir is being developed for a vaginal ring formulation and is currently in pre-clinical evaluation. Other candidate ARV compounds and delivery systems (e.g., a dissolvable film) are in pre-clinical stages of evaluation.
Conclusion
Proof-of-concept has been demonstrated for tenofovir-based topical and oral PrEP for primary prevention. Completion of the remaining trials and analyses are needed to have a more complete understanding of effectiveness in different populations and the relationship between adherence and efficacy. Demonstration projects in populations where PrEP has been found to be effective are needed to evaluate targeted implementation and cost-effectiveness of PrEP. Effective HIV prevention requires choices of primary prevention strategies such as topical and oral PrEP, and scale-up of secondary prevention including ART for HIV-infected persons. While the public health impact of PrEP is anticipated to be greater as products and delivery systems with sustained coverage are identified, targeted provision of daily and oral tenofovir-based PrEP to populations at high-risk of HIV acquisition should be evaluated for including as part of combination HIV prevention programs.
Figure 1. Topical and oral PrEP: HIV prevention efficacy from completed clinical trials.
Figure does not include oral tenofovir data from VOICE, as data have not been released
Reproduced with permission from: Karim SSA and Karim QA; Antiretroviral prophylaxis: a defining moment in HIV control; The Lancet, Early Online Publication, 18 July 2011. doi:10.1016/S0140-6736(11)61136-7
Key points.
Proof of concept has been demonstrated for topical 1% tenofovir gel, dosed within 12 hours before and 12 hours after sex, in significantly reducing the risk of HIV-1 and HSV-2 acquisition among heterosexual women in South Africa (CAPRISA 004) with trials of daily 1% tenofovir gel (VOICE) and a confirmatory study of the peri-coitally dosed 1% tenofovir gel (FACTS) underway.
Proof of concept has been demonstrated for daily oral emtricitabine-tenofovir among men who have sex MSM (iPrEx) young heterosexuals in Botswana (TDF-2) and both emtricitabine/tenofovir and tenofovir among African HIV-1 serodiscordant couples (Partners PrEP).
Lack of efficacy of daily oral emtricitabine/tenofovir and tenofovir among women need to be understood from the FEM-PrEP and VOICE trials, in terms of adherence and biologic factors that may explain differences in efficacy in different populations and settings.
Longer-acting formulations of new candidate products for pre-exposure prophylaxis, and topical delivery systems, such as intra-vaginal rings, are anticipated to be less user adherence dependent and are under clinical evaluation.
Acknowledgments
Funding support: Bill and Melinda Gates Foundation #47674. U.S. National Institutes of Health R01 MH095507.
Gilead provided study drug for the Partners PrEP study but did not provide funding to the authors or the institution.
Footnotes
The authors report no conflict of interest.
References
- 1.UNAIDS/WHO. Joint United Nations Programme on HIV/AIDS. 2010. UNAIDS Report on the Global AIDS Epidemic. [Google Scholar]
- **2.Cohen MS, Shaw GM, McMichael AJ, et al. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. This randomized trial provides definitive evidence of HIV-1 prevention benefits of ART, with 96% reduction in HIV transmission among HIV-1 infected persons with CD4 350–550 in a known HIV serodiscordant relationships who were randomized to immediate ART initiation compared to delayed ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. Systematic review of 28 studies which demonstrate the high drop-off in retention at each step of HIV testing to receipt of CD4 count and clinical staging, from staging to ART eligibility, and ART eligibility to ART initiation. Their synthesis indicates that fewer than 1/3 of persons who test positive for HIV and are not eligible for ART when diagnoses are retained continuously in care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51:725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 6.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mofenson LM. Protecting the next generation--eliminating perinatal HIV-1 infection. N Engl J Med. 2010;362:2316–2318. doi: 10.1056/NEJMe1004406. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Lerma JG, Paxton L, Kilmarx PH, et al. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol Sci. 2010;31:74–81. doi: 10.1016/j.tips.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–911. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 12.Cong ME, Youngpairoj AS, Zheng Q, et al. Protection against rectal transmission of an emtricitabine-resistant simian/human immunodeficiency virus SHIV162p3M184V mutant by intermittent prophylaxis with Truvada. J Virol. 2011;85:7933–7936. doi: 10.1128/JVI.00843-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veazey R, Ketas T, Dufour J, et al. Protection of rhesus macaques from vaginal infection by maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. Presented at 17th Conference on Retroviruses and Opportunistic Infections (CROI); San Francisco, CA. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobard C, Sharma S, Parikh U, et al. High protection against vaginal infection in macaques by PEP with gel containing RAL. Presented at 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, Massachusetts. 2011. p. Abstract 30. [Google Scholar]
- **15.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. This randomized trial of peri-coital use of 1% tenofovir gel demonstrated a 39% reduction in HIV-1 acquisition, and an association between adherence and efficacy in subgroup analyses (54% efficacy in those with >80% gel use with sex in the prior month). This was the first trial of a topical antiretroviral-based microbicide to demonstrate efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Abdool Karim SS, Kashuba AD, Werner L, et al. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–281. doi: 10.1016/S0140-6736(11)60878-7. This study measured cervicovaginal fluid tenofovir levels in HIV-1 seroconverters and non-seroconverters on the active arm (1% tenofovir gel) from CAPRISA 004 to evaluate potential threshold tenofovir concentrations needed for HIV protection. They found a significant association of cervicovaginal fluid tenofovir levels >1000 ng/mL and HIV-1 protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *17.Andrei G, Lisco A, Vanpouille C, et al. Topical Tenofovir, a Microbicide Effective against HIV, Inhibits Herpes Simplex Virus-2 Replication. Cell Host Microbe. 2011;10:379–389. doi: 10.1016/j.chom.2011.08.015. This study evaluated tenofovir for in vitro activity against herpes simplex virus-2, given the demonstration of reduced HSV-2 acquisition with 1% tenofovir gel in CAPRISA 004. The in vitro studies demonstrate direct antiherpetic activity of 1% tenofovir gel with inhibition of HSV replication in cell lines and human lymphoid and cervicovaginal tissues ex vivo, and inhibition of HSV DNA polymerase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix C, Minnis A, Guddera V, et al. MTN-001: A phase 2 cross-over study of daily oral and vaginal TFV in healthy, sexually active women results in significantly different product acceptability and vaginal tissue drug concentrations. Presented at 18th Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2011. p. Abstract 35LB. [Google Scholar]
- **19.Grant RM, Lama JR, Anderson PL, et al. Pre-exposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. This placebo-controlled, randomized efficacy trial of emtricitabine/tenofovir among MSM and transgender women demonstrated 44% reduction in HIV incidence, and a relationship between drug adherence and efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liegler T, Abdel-Mohsen M, Atchison R, et al. Drug resistance and minor drug resistant variants in iPrEx. Presented at 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, Massachusetts. 2011. p. Abstract 97LB. [Google Scholar]
- 21.Liu A, Vittinghoff E, Irby R, et al. BMD loss in HIV– men participating in a TDF PrEP clinical trial in San Francisco. Presented at 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, Massachusetts. 2011. p. Abstract 93. [Google Scholar]
- 22.Amico R, Liu A, McMahon V, et al. Adherence indicators and PrEP drug levels in the iPrEx Study. Presented at 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, Massachusetts. 2011. p. Abstract 95LB. [Google Scholar]
- 23.Family Health International. VOICE HIV prevention trial continues, but researchers suspend oral tenofovir arm because of futility. Vol. 2011 Research Triangle Park, NC: FHI 360; 2011. [Google Scholar]
- 24.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 Serodiscordant Couples Enrolled in a Clinical Trial of Antiretroviral Pre-Exposure Prophylaxis for HIV-1 Prevention. PLoS ONE. 2011;6:e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeten J, Celum C on behalf of the Partners PrEP Study Team. Antiretroviral preexposure prophylaxis for HIV-1 prevention among heterosexual African men and women: the Partners PrEP Study. Presented at 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; July 17–20; Rome, Italy. 2011. p. Abstract MOAX0106. [Google Scholar]
- 26.Haberer J, Baeten J, Celum C, et al. Near perfect early adherence to antiretroviral PrEP against HIV infection among HIV serodiscordant couples as determined by multiple measures: preliminary data from the Partners PrEP Study. Presented at 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, Massachusetts. 2011. p. Abstract 488. [Google Scholar]
- 27.Martin M, Vanichseni S, Suntharasamai P, et al. Enrollment Characteristics and Risk Behaviors of Injection Drug Users Participating in the Bangkok Tenofovir Study, Thailand. PLoS ONE. 2011;6:e25127. doi: 10.1371/journal.pone.0025127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallett T, Baeten J, Heffron R, et al. Modeling the optimal use of antiretrovirals in HIV-1 serodiscordant heterosexual couples in South Africa. PLoS ONE. doi: 10.1371/journal.pmed.1001123. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walensky R. Cost-effectiveness in HIV care: understanding value in a world of limited resources. Presented at 18th Conference on Retroviruses and Opportunistic Infections (CROI); Boston, Massachusetts. 2011. p. Abstract 74. [Google Scholar]
- 30.Romano J, Variano B, Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25:483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]