Abstract
It is now well accepted that stress can precipitate mental and physical illness. However, it is becoming clear that given the same stress, some individuals are very vulnerable and will succumb to illness while others are more resilient and cope effectively, rather than becoming ill. This difference between individuals is called stress sensitivity. Stress-sensitivity of an individual appears to be influenced by genetically inherited factors, early life (even prenatal) stress, and by the presence or absence of factors that provide protection from stress. In comparison to other stress-related diseases, the concept of sensitivity versus resilience to stress-induced reproductive dysfunction has received relatively little attention. The studies presented herein were undertaken to begin to identify stable characteristics and the neural underpinnings of individuals with sensitivity to stress-induced reproductive dysfunction. Female cynomolgus macaques with normal menstrual cycles either stop ovulating (Stress Sensitive) or to continue to ovulate (Stress Resilient) upon exposure to a combined metabolic and psychosocial stress. However, even in the absence of stress, the stress sensitive animals have lower secretion of the ovarian steroids, estrogen and progesterone, have higher heart rates, have lower serotonin function, have fewer serotonin neurons and lower expression of pivotal serotonin-related genes, have lower expression of 5HT2A and 2C genes in the hypothalamus, have higher gene expression of GAD67 and CRH in the hypothalamus and have reduced GnRH transport to the anterior pituitary. Altogether, the results suggest that the neurobiology of reproductive circuits in stress sensitive individuals is compromised. We speculate that with the application of stress, the dysfunction of these neural systems becomes exacerbated and reproductive function ceases.
Keywords: reproduction, stress, serotonin, corticotropin releasing hormone, pro-opiomelanocortin, beta-endorphin, paraventricular nucleus, thalamus, amygdala, cynomolgus macaque
Overview
Exposure to stressful stimuli can lead to a variety of secondary diseases such as anxiety, depression, cardiovascular disease, and immune suppression (McEwen, 2002; McEwen, 2008). Reproductive dysfunction has been recently added to this growing list of stress-related disorders (Cameron, 2000). A significant body of literature has focused upon the application of stress and its consequences on reproductive cyclicity and the related neuroendocrinology. Early in the 1970’s, it was recognized that the stress of population density inhibited estrous cycles in mice (Champlin, 1971), and a great deal of effort has been devoted to understanding the effects of maternal stress during pregnancy on offspring physiology and behavior in rodent species (Gos et al., 2006; Kajantie, 2006; Fumagalli et al., 2007).
Luteinizing hormone (LH) and follicle stimulating hormone (FSH) are the gonadotropins that drive ovarian function, estrogen (E) and progesterone secretion (P), menstrual or estrous cyclicity and ultimately ovulation. In ovariectomized animals, LH secretion becomes elevated and pulsatile. To understand the effect of stress on LH secretion, the ovariectomized pulsatile secretory mode has been utilized. Restraint stress, or activation of the corticotropin releasing hormone (CRH) receptor type 2 with intracerebroventricular urocortin, suppressed luteinizing hormone (LH) pulses in ovariectomized rats (Li et al., 2005). These effects may be mediated via the raphe serotonin system (Ruggiero et al., 1999; Pernar et al., 2004; Clark et al., 2007; Mo et al., 2008) or brainstem noradrenergic systems (Mitchell et al., 2005; Dunn and Swiergiel, 2008), as well as via hypothalamic circuits (MacLusky et al., 1988; Dobson et al., 2003).
Stress and reproduction are important factors in the farming industry. Stresses such as fever, lameness and transportation can significantly decrease fertility in cows and sheep (Dobson and Smith, 2000). Modeling of stress in ewes with endotoxin has enabled the analysis of the neural pathways mediating stress-induced suppression of ovulation. Evidence has been well reviewed indicating that the balance of numerous neurotransmitters such as norepinephrine, serotonin, glutamate and GABA; and the neuropeptides CRH, arginine vasopressin (AVP), and neuropeptide Y impinge directly or indirectly on GnRH neurons to activate or suppress their function depending on the environment (Tilbrook et al., 2002; Dobson et al., 2003; Smith et al., 2003; von Borell et al., 2007). AVP plays a greater role that CRH in sheep, but the reverse is true in rodents (Dobson et al., 2003). Moreover, the medial preoptic region in sheep and rodents contains pivotal GnRH neurons that are not found in humans or primates.
Extreme exercise is considered to be a metabolic stress, and with the advent of greater participation of women in sports, reports emerged that intense athletic participation disrupted menstrual cycles (Arena et al., 1995). Further study indicated that there was a suppression of estrogen secretion during the follicular phase and less progesterone secretion during the luteal phase and blunted FSH secretion during the follicular luteal transition in recreational women runners (De Souza et al., 1998).
A clinical syndrome called Functional Hypothalamic Amenorrhea (FHA), characterized by menstrual cycle abnormalities and infertility, is found in a proportion of women who present at infertility clinics (Berga and Girton, 1989; Berga et al., 1997). Research indicates that FHA occurs in women with combined moderate psychological and metabolic stress (Marcus et al., 2001) and eating disorders are also common in this population (Warren et al., 1999). Moreover, new treatment therapies for FHA that both target strategies for coping with psychological stress and removal of metabolic stresses look very promising (Berga et al., 2003).
Stress models in nonhuman primates have employed endotoxin administration (Xiao et al., 1998; Xiao et al., 1999), interleukin -1 administration (Feng et al., 1991; Ferin, 1995), CRH administration (Xiao and Ferin, 1988), exercise (Williams et al., 2001a; Williams et al., 2001b), diet (Cameron and Nosbisch, 1991), psychosocial stress (relocation to a new room with new neighbors) (Cameron et al., 1998)or combinations of these stresses as found in FHA (Williams et al., 1997; Williams et al., 2007). Administration of endotoxin, interleukin -1 or CRH activated the hypothalamic-pituitary-adrenal axis, increased cortisol secretion and suppressed LH and FSH secretion, which could be reversed with the administration of a CRH antagonist (Xiao et al., 1996) or the opiate antagonist, naloxone (Gindoff and Ferin, 1987). A recent study with rhesus monkeys employing the combination of surgery and relocation showed that inadequate LH and progesterone secretion during the luteal phase is the initial defect leading to abnormal menstrual cycle parameters. This study suggested that secretory inadequacy of the corpus luteum represents the first clinical stage in the damage that stress inflicts on the normal menstrual cycle (Xiao et al., 2002).
A pivotal factor in many studies is that stress was applied and results were obtained in a fashion suggesting that all animals respond equally to the stress. However, it is now becoming apparent that certain individuals are more sensitive to stress than others. This is clearly evident in human populations where some individuals succumb to psychiatric and somatic disease after trauma or stress, but other individuals thrive. In animal models, similar results have been obtained by selective breeding in which stress sensitive and stress resilient lines are produced (Baer and Crumpacker, 1977; Osterlund et al., 1999; Li et al., 2004; Henn and Vollmayr, 2005). Our group has used cynomolgus monkeys and a combination of diet, exercise and relocation to study the effects of stress on reproductive function. When this paradigm is applied to small populations of monkeys, we observed individual differences in reproductive dysfunction with stress. This chapter reviews our investigations and shows that the activity or gene expression in neural circuits mediating stress and reproduction are significantly different in stress sensitive and stress resilient individuals in the absence of stress.
I. The Model
Introduction
In many areas of medicine it is recognized that there are striking individual differences in sensitivity to stress, in that some individuals show marked physiological responses to stressful stimuli and are prone to the development of diseases that occur secondary to chronic stress exposure (i.e., anxiety, depression, cardiovascular disease, immune suppression), while others are stress-resilient and show less physiological response to stressful stimuli and are less likely to develop diseases secondary to chronic stress exposure. Stress-sensitivity of an individual appears to be influenced by genetically inherited factors, prior stress exposure (particularly stress exposure in prenatal or early post-natal development), and by the presence or absence of factors that provide protection from stress (McEwen, 2002). In comparison to other stress-related diseases, the concept of sensitivity versus resilience to stress-induced reproductive dysfunction has received relatively little attention to date. However, a comprehensive review of the effects of psychosocial stresses on reproductive function in humans and nonhuman primates suggests that a number of factors can influence the sensitivity of the reproductive axis to psychosocial stresses, including the perception of stress, the magnitude and duration of stress, social status, and the level of activity within the reproductive axis prior to stress exposure (Cameron, 2000). In addition, several studies also documented individual differences in sensitivity of the reproductive axis to immune stresses (Xiao et al., 1999).
We have undertaken a series of studies in which female cynomolgus macaques were exposed to a combination stress paradigm and their reproductive function was monitored. We found marked differences between individuals in the response of the reproductive system to stress. Following the in vivo characterization, postmortem studies of brain function were executed. These studies revealed that pivotal neural systems in the brain that are involved in stress responsivity were altered in stress sensitive individuals. Following are studies describing the model, the in vivo characterization and the postmortem analysis of the brains of animals with differential sensitivity to stress.
Methods
Animals
All studies were reviewed and approved by the Institutional Animal Care and Use Committee of the ONPRC and performed according to federal guidelines. Fifteen adult female cynomolgus monkeys (Macaca fascicularis) were housed in single stainless steel cages in a temperature-controlled room (24 ± 2 C), with lights on for 12 h a day (0700–1900 h). Monkeys were imported in 1993 and approximate ages established by dental examination. At the time of this study the monkeys were 11–14 years of age. Monkeys were provided with two meals a day at 0930 h and 1500 h. At each meal they received 6 high protein monkey chow biscuits (no. 5047, jumbo biscuits, Ralston Purina Co., St. Louis, MO; approximately 16.5 g each, 3.11 metabolizable Cal/g, 308 Cal/meal). In addition, one-quarter apple was provided with the morning meal. Water was available ad libitum. Animals also received non-caloric treats (ice cubes) and toys in their cages, as well as occasional access to television viewing, as part of the Oregon National Primate Research Center (ONPRC) primate enrichment program. Monkeys had been adapted to these conditions for two years prior to the initiation of this study.
Blood Sample Collection
Blood samples for the measurement of serum estradiol and progesterone were collected from unanesthetized animals every day before the animals were exercised. For collection of blood samples, each monkey was trained to jump from its cage into a transport box and enter a specially designed cage that allowed immobilization of the monkey’s leg, so a blood sample could be obtained from the femoral region by venipuncture, using previously published techniques (Williams et al., 2001a; Williams et al., 2001b). Blood was collected into sterile syringes, transferred into glass tubes, and allowed to clot. Samples were then centrifuged at 2500 rpm for 10 min, and serum was collected and stored at −20 C in plastic vials until assays were performed. Every 4 wk, hematocrit was measured. Hematocrits were maintained within the normal range in all monkeys throughout the study. Monkeys were weighed each day at the time of blood sample collection. Hormone assays were conducted as previously described (Williams et al., 2007).
Monitoring Reproductive Function
Before the study all animals were accustomed to blood sampling procedures and daily checks for menses, which involved swabbing the vaginal area with a cotton-tipped applicator. The occurrence of several normal menstrual cycles was documented in each monkey before the initiation of the study. The first day of menses was designated the first day of the menstrual cycle. A menstrual cycle was considered normal if it was 25–38 days in length, and exhibited typical cyclic changes in reproductive hormones, including a midcycle rise in circulating estradiol followed by a rise in serum progesterone concentrations to levels greater than 2 ng/ml. A monkey was considered to be amenorrheic if she had a cycle longer than 38 days that also showed no evidence of cyclic rises in estradiol and progesterone.
Exercise Training
Animals were trained to run on standard human size treadmills (model 910e, Precor, Inc., Bothell, WA), using previously published techniques (Williams et al., 2001a; Williams et al., 2001b). Each treadmill was covered by a Plexiglass box, which had numerous air holes in the front and back panels to allow adequate ventilation. Monkeys were slowly adapted to the treadmill in the “learn to run” menstrual cycle by first being allowed to sit on the treadmill and explore it for several days and then being allowed to walk slowly. After about one week of walking monkeys were given a “max” test to establish the maximum rate which they were capable of running (Williams et al., 1997). In the max test, monkeys started running at 0.8 miles/hour and speed was then increased 0.2 miles/hour every two minutes until the monkey failed to be able to keep up with the pace of the treadmill. Our previous studies showed that monkeys reached maximum heart rate by the time they reached maximum speed (Williams et al., 1997).
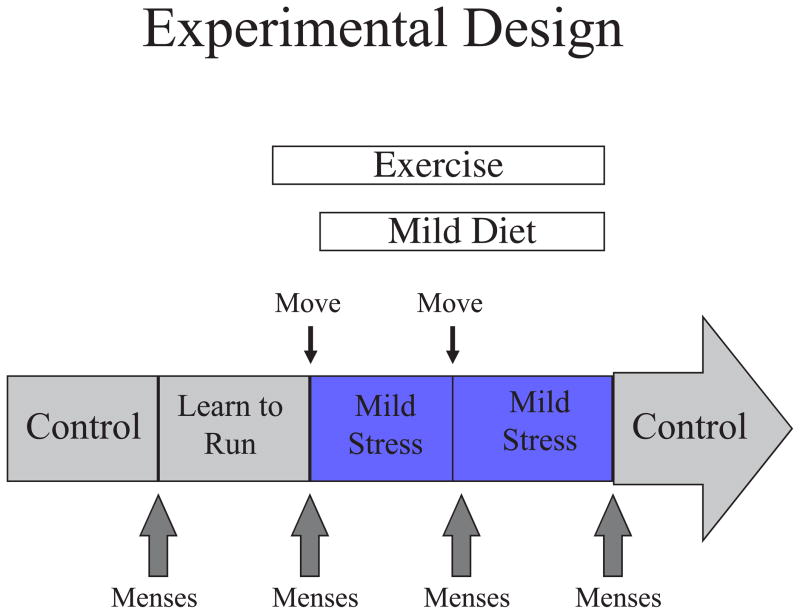
Experimental Design
The experimental model used a combined stress that encompassed mild psychosocial stress + moderate dieting + moderate exercise. The mild psychosocial stress involved moving single-caged monkeys to a new housing room, where they were surrounded by unfamiliar animals. The moderate diet was a 20% decrease in calorie intake, and the moderate exercise was provided by running monkeys on a motorized treadmill at 80% maximum speed (determined for each monkey in the first week of the study) for one hour per day, 5 days per week. The initial study involved a five menstrual cycle design (see Figure 1): Cycle 1- a control menstrual cycle in which blood samples were collected daily to track reproductive hormone secretion, Cycle 2- a learn-to-run cycle in which monkeys were accustomed to the treadmill (first sitting on it, and then walking) while blood sample collection was continued, Cycle 3- stress cycle 1, in which monkeys were moved to a new room on day 1 of the menstrual cycle, calorie intake was decreased by 20% and monkeys initiated running 5 days a week, Cycle 4-stress cycle 2, in which monkeys moved to a second new room on day 1 and calorie restriction and running were continued, and Cycle 5- a recovery cycle in which monkeys were moved back to their home environment, food intake was increased back to ad libitum and exercise was terminated. For monkeys that failed to have a menstrual cycle after initiation of the stress, Cycle 3 was continued for 60 days and then the recovery cycle was initiated. For monkeys that failed mense at the end of a second stress cycle, Cycle 4 was continued for 60 days and then the recovery cycle was initiated.
Figure 1.
Schematic diagram of experimental design.
At the end of the initial study, monkeys were maintained in their home cage with ad libitum food intake and no exercise until they exhibited three normal menstrual cycles. Blood samples were collected daily during this time to determine whether animals displayed consistent peak plasma estradiol concentrations and peak luteal phase progesterone concentrations.
Results
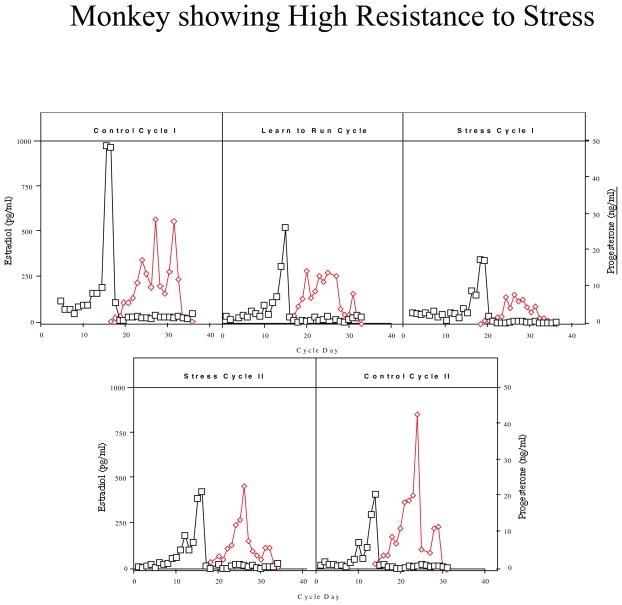
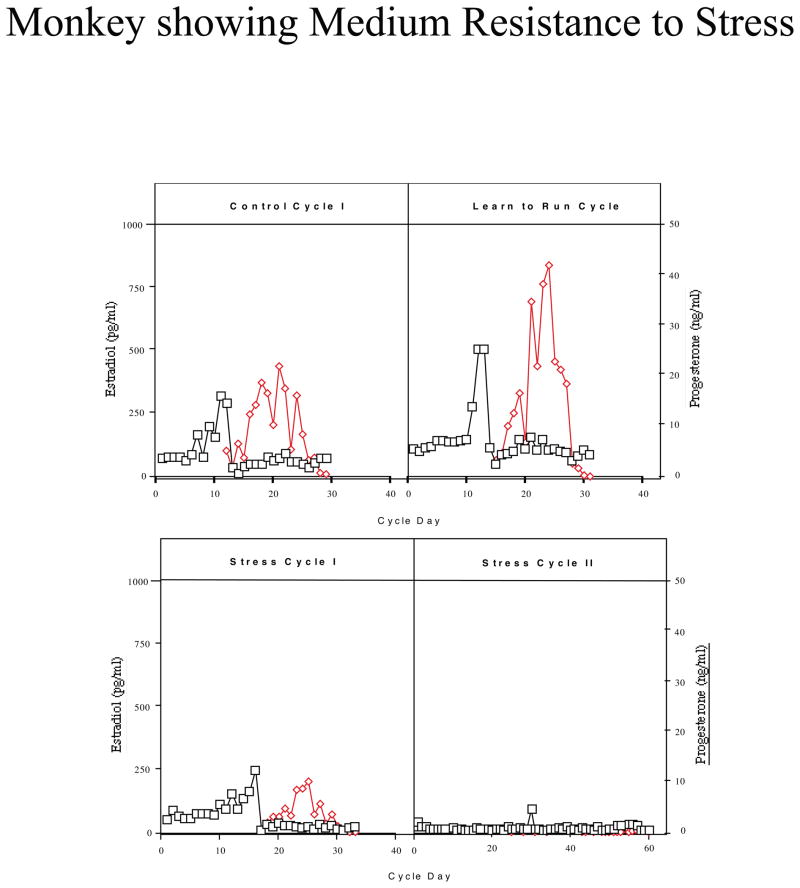
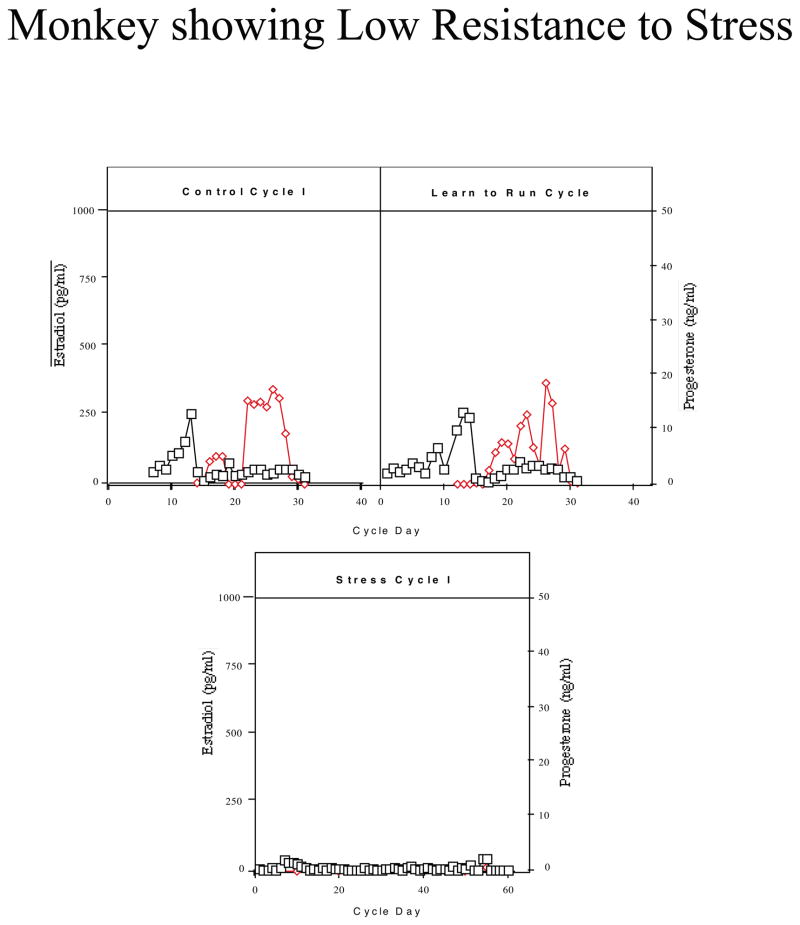
Monkeys were categorized based on their reproductive hormone secretion and menstrual cyclicity during the two stress cycles. About one third of the monkeys continued to have menstrual cycles throughout the stress, retained normal cyclic patterns of ovarian steroid hormones, showed menses within 38 days for each Stress Cycle and continued to ovulate, as judged by the presence of an estradiol surge and progesterone secretion in the luteal phase (n=5; called high stress-resilient, HSR; Figure 2). About one third of the monkeys continued to show cyclic changes in estradiol and progesterone and menses during Stress Cycle 1, and showed menses within 38 days of initiating this cycle, but then showed a suppression of circulating estradiol and progesterone and failed to have a menstrual cycle in Stress Cycle 2 (n=6; called medium stress-resilient, MSR; Figure 3). The last one third of the monkeys showed an immediate suppression of circulating estradiol and progesterone concentrations and failed to have a menstrual cycle within 60 days of initiating stress exposure (n=4; called stress-sensitive, SS; Figure 4). Differences between groups for circulating hormone levels, length of the menstrual cycle, calorie intake, weight, and weight loss were assessed by a one-way analysis of variance, followed by a Student Newman Keuls post-hoc test. A Bonferroni correction was used to account for multiple comparisons. Differences were considered significant for p≤0.05. All data are reported at mean±SEM.
Figure 2.
Circulating levels of plasma estradiol (E; open squares) and progesterone (P; closed diamonds) in a monkey which showed high stress-resilience when exposed to combined psychosocial stress + diet + exercise. This monkey continued to display cyclic patterns of ovarian steroid hormones throughout stress exposure.
Figure 3.
Circulating levels of plasma estradiol (E; open squares) and progesterone (P; closed diamonds) in a monkey which showed medium stress-resilience when exposed to combined psychosocial stress + diet + exercise. This monkey continued to display cyclic patterns of ovarian steroid hormones when initially exposed to stress, but then became amenorrheic with further stress exposure.
Figure 4.
Circulating levels of serum estradiol (E; open squares) and progesterone (P; closed diamonds) in a monkey which showed stress-sensitivity when exposed to combined psychosocial stress + diet + exercise. This monkey immediately became amenorrheic upon stress exposure.
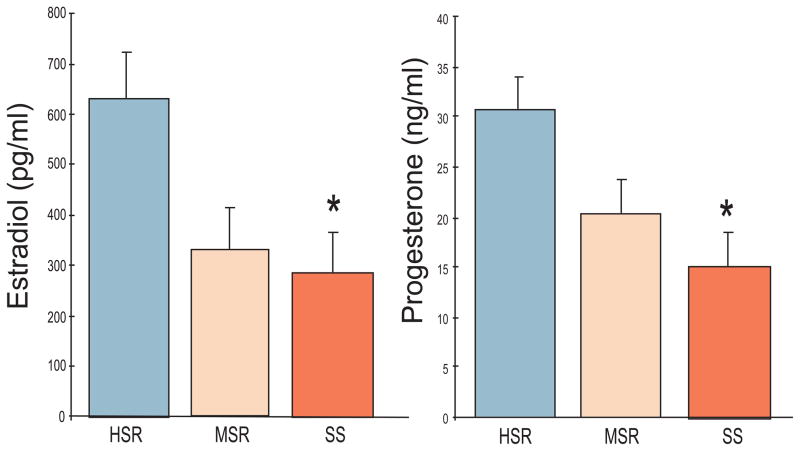
Prior to stress exposure there were significant differences between the SS and HSR groups in peak follicular phase estradiol levels and peak luteal phase progesterone levels (Figure 5), with SS animals showing lower estradiol (SS: 292±86 pg/ml; HSR: 635±90 pg/ml, p=0.01) and progesterone (SS:15.2±4.8 ng/ml; HSR: 31±4.5 ng/ml, p=0.001). There were no significant differences, however, between SS and HSR animals in circulating levels of LH or FSH either at the midcycle surge or during the rest of the cycle in Control Cycle 1. There were also no significant differences in length of the menstrual cycle, length of the follicular phase, and initial body weight or body weight loss between the groups (Table 1). In the second part of the study, significant differences remained throughout three control menstrual cycles in the peak serum estradiol and progesterone concentrations in the HSR and SS groups (Table 2).
Figure 5.
Peak follicular phase plasma estradiol (E) concentrations (left panel) and peak luteal phase plasma progesterone (P) concentrations (right panel) in HSR, MSR and SS monkeys during the Control 1 menstrual cycle. Asterisks indicate a significant difference between groups, p<0.05.
Table 1.
Menstrual cycle and weight parameters by stress-sensitivity category.
| Stress-Sensitivity Category | Menstrual Cycle Length (days) | Follicular Phase Length (days) | Weight (kg) | Weight Change |
|---|---|---|---|---|
| HSR | 31.5±1.9 | 16.2±2.3 | 4.1±0.3 | 0.34±0.6 |
| MSR | 33.9±2.3 | 16.8±3.6 | 3.9±0.4 | 0.3±0.8 |
| SS | 30.7±1.6 | 15.7±4.1 | 4.2±0.4 | 0.32±0.7 |
Table 2.
Peak estradiol and progesterone concentrations in HSR and SS animals across three control menstrual cycles.
| Stress-Sensitivity Category | Cycle 1 E2 (pg/ml) | Cycle 2 E2 (pg/ml) | Cycle 3 E2 (pg/ml) | Cycle 1 P4 (ng/ml) | Cycle 2 P4 (ng/ml) | Cycle 3 P4 (ng/ml) |
|---|---|---|---|---|---|---|
| HSR | 734±91* | 603±98* | 622±54* | 25±3.8* | 23±2.4* | 31±5.6* |
| SS | 452±56 | 431±59 | 367±92 | 14±2.3 | 9.8±4.1 | 13.6±3.3 |
indicates a significant difference, p≤0.05, compared to SS animals in the same cycle
Discussion
The results of this study show striking individual differences in the response of the reproductive axis to a moderate level of combined psychosocial and metabolic stress. About one third of the animals showed a rapid and profound suppression of reproductive hormone secretion (SS) while about one third retained normal menstrual cyclicity throughout two months of stress exposure (HSR). The final one third retained menstrual cyclicity during the initial phases of stress exposure but then lost cyclic secretion of reproductive hormones (MSR). Very interestingly, there was a significant difference in peak follicular phase estradiol concentrations and peak luteal phase progesterone concentrations between the HSR and SS groups, with the MSR group showing intermediate levels of these hormones, even before stress was initiated (in the Control 1 Cycle). The fact that this difference in peak ovarian steroid hormone levels was maintained across three consecutive control menstrual cycles suggests that secretion of ovarian steroid hormones is a stable characteristic of individuals with an increased propensity for sensitivity to stress-induced reproductive dysfunction. On the other hand, characteristics such as body weight, weight loss, menstrual cycle length or length of the follicular phase appear to have no relationship to propensity to develop stress-induced reproductive dysfunction to a moderate, short-term stress exposure.
There would appear to be at least two possible mechanisms that could underlie the link between stress-sensitivity and chronically lower circulating levels of ovarian steroid hormones. One possibility is that stress-sensitive individuals could have less GnRH and LH/FSH stimulation to the ovary resulting from either chronically increased inhibitory input into the GnRH system or chronically decreased stimulatory input into GnRH neurons. In fact, a number of neural systems have been identified whose activity is altered by various forms of stress (e.g., corticotropin-releasing hormone, β-endorphin, neuropeptide Y, and norepinephrine) and each can in turn, alter the central neural drive to the reproductive axis (McEwen, 2007). However, our failure to detect significant differences between HSR and SS animals in serum LH or FSH concentrations across the cycle does not support this hypothesis. But, we note that in this study only a single daily blood sample was collected. Because gonadotropins are released in a pulsatile fashion it is possible that our failure to find differences in serum LH and FSH between HSR and SS groups may reflect the lack of sensitivity in our sampling method. A more sensitive method for examining differences in pulsatile gonadotropin secretion would be to chronically catheterize monkeys, and collect frequent blood samples (at 10 minute intervals) to accurately assess pulsatile LH secretion (Cameron and Nosbisch, 1991). We are currently conducting this study in animals that have been adapted to a vest and tether for remote sampling.
Alternatively, another mechanism potentially linking differential stress-sensitivity to varying levels of circulating ovarian steroid hormones is that low steroid hormone levels may lead to increased anxiety and sensitivity to stress. Estrogen and progesterone regulate many central neural systems that mediate anxiety. The following series of studies examined several pivotal neural systems involved in stress, anxiety and reproductive function. Further studies are needed to determine if increased sensitivity to stress leads to lower ovarian steroid hormone secretion or whether lower ovarian steroid hormone secretion increases sensitivity to stress. It is also possible that there is a circular aspect of this relationship, such that individuals with a predisposition for stress sensitivity are more likely to have lower activity of the hypothalamic-pituitary-gonadal axis, which in turn heightens their sensitivity to stress by further decreasing ovarian steroid hormone levels in the brain.
We believe that it is likely that the results of this study, examining sensitivity to a complex psychosocial and metabolic stress, have direct relevance to a number of forms of stress-induced reproductive dysfunction. Forms of stress-induced reproductive dysfunction that are seen clinically include Functional Hypothalamic Amenorrhea, Anorexia and Bulimia Nervosa, and Exercise-Associated Amenorrhea. Although it was initially believed that each of these syndromes represented a fairly discrete type of stress (i.e., Functional Hypothalamic Amenorrhea: a psychosocial stress, Anorexia Nervosa: a nutritional stress, Exercise-Associated Amenorrhea: an exercise stress) there is a growing recognition that each of these syndromes involves exposure to a combination of stresses. As discussed in the introduction there is strong evidence that Functional Hypothalamic Amenorrhea involves both psychosocial and metabolic stresses. Reproductive dysfunction occurring in patients with Anorexia Nervosa was considered primarily the result of severe nutritional stress; however, it is well documented that a substantial percentage of anorexic patients do not experience normalization of reproductive function with weight restoration (Katz et al., 1978; van Binsbergen et al., 1990). The propensity to exercise intensively (Penas-Lledo et al., 2002) and the significant psychological stress associated with weight gain in anorexic patients (Troop et al., 1998) are likely contributors to the long-term suppression of the reproductive axis. Likewise, in Bulimia Nervosa there is disordered eating, but this is often accompanied by increased exercising, and these patients experience significant psychological stress (Wolff et al., 2000). Recent studies of Exercise-Associated Amenorrhea indicate that within clinical populations that show reproductive dysfunction there is often simultaneous calorie restriction (Loucks et al., 1992; Laughlin and Yen, 1996; Warren and Stiehl, 1999). Thus, clinically relevant forms of stress-induced reproductive dysfunction all involve simultaneous exposure to multiple forms of stress.
In summary, this model established that there are individual differences in sensitivity of the reproductive axis to stress-induced impairment, even for a relatively moderate form of stress. Prior activity of the reproductive axis, in a non-stressed condition can be used to identify individuals that are stress-sensitive versus stress-resilient, in that peak secretion of estradiol and progesterone over a menstrual cycle appears to be a fairly stable individual characteristic. This finding suggests that the development of strategies for recognizing stress-sensitive individuals and then providing targeted therapy to increase stress-resilience may be possible in the future.
The next series of studies questioned whether there are endogenous underlying differences in central neural systems between stress resilient and stress sensitive monkeys which could account for the differences in steroid hormone secretion and the differences in their ability to maintain ovulation in the presence of stress. One of the most important neural systems governing an animal’s state of arousal and ability to cope with stress is the serotonin neural system (Siever et al., 1991).
II. Global Differences in the Serotonin Neural System
Introduction
The serotonin neural system plays a pivotal role in mood and affective regulation, cognition, satiety and in numerous autonomic functions (Van de Kar, 1991; Jacobs and Azmitia, 1992; Mann et al., 1995). Decreased activity of the central serotonin system is found in individuals with increased stress sensitivity and anxiety disorders (Tancer et al., 1994; Ressler and Nemeroff, 2000; Bhagwagar et al., 2002). In addition, stress impacts serotonin function in a variety of ways depending on the intensity and duration of the stress (Botchin et al., 1994; Shively et al., 1995; Filipenko et al., 2002). Although many studies have shown individual responses to stress, we probed the neural function of animals long after the stress was removed and the animals were all cycling normally. We hypothesized that monkeys that show sensitivity of the reproductive axis to stress may have lower activity of the central serotonin system.
Thus, the next goal was to determine whether there are differences in endogenous function of the central serotonergic system in stress sensitive versus stress resistant animals in the absence of stress. We used fenfluramine administration followed by measurement of prolactin and cortisol to access global serotonin availability (O’Keane and Dinan, 1991; Newman et al., 1998). Fenfluramine causes an immediate release of serotonin via transporter reversal and blocks serotonin reuptake thereby causing a rapid elevation of extracellular serotonin. Fenfluramine-induced release of pituitary hormones thus reflects the level of endogenous activity in the serotonergic neurons regulating hypothalamic neuroendocrine systems (Rothman and Baumann, 2002).
Methods
Thirteen female cynomolgus macaques described above were used. Two animals were lost due to clinical reasons. The animals were housed in single cages and monitored daily for menstruation. Upon detection of menstruation, the animals were scheduled for fenfluramine challenge before day 5 of the follicular phase of their cycle in July 2001 and for thyrotropin releasing hormone (TRH) and corticotropin releasing hormone (CRF) challenge in September 2001.
On day 1 of a non-stressed menstrual cycle, animals were scheduled for a fenfluramine challenge before day 5 of the cycle. On the day of the challenge each animal was sedated with ketamine (100 mg) in their home cage and transported to a surgical suite. The monkeys were placed on a temperature-regulated surgical table and connected to vital sign monitors under the supervision of the veterinary surgical staff. Fenfluramine challenges and control challenges of TRH+CRH were administered under propofol anesthesia as previously published (Bethea et al., 2005a).
A two factor ANOVA was conducted on the data with group and time as the dependent variables using the Statistix Analytical Software package (Tallahassee, FL). Specific post-hoc comparisons were made by Tukey’s analysis with a Bonferroni correction for multiple comparisons. The treatment response within a group was analyzed with a nonparametric ANOVA (Freidman’s) for repeated measures followed by Dunn’s post-hoc comparison. When indicated, comparisons were made with Student’s or Welch’s t-test as determined by variances. Differences were considered significant if p < 0.05. Data is presented as mean±SEM.
Results
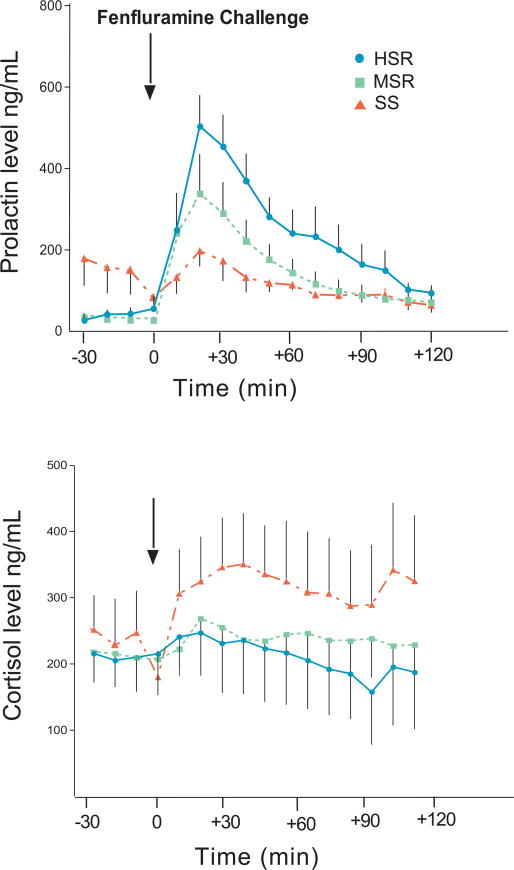
As shown in Figure 6, top panel, prolactin secretion was significantly different between the experimental groups (two way ANOVA, p < 0.0001). Prolactin levels before fenfluramine challenge were higher in the stress sensitive group than in the high or medium stress resistant groups (p < 0.01). However, stress-sensitive animals had a lower response to fenfluramine compared to high stress-resistant animals (post hoc test HSR vs. SS, p < 0.001). The prolactin response of the medium stress-resistant animals did not differ from the other two groups. Cortisol secretion in response to fenfluramine (Figure 6, bottom panel) was also significantly different between the experimental groups (2 way ANOVA, p < 0.0001; post hoc test HSR vs. SS, p < 0.001). Stress-sensitive animals had a greater release of cortisol compared to high stress-resilient animals, with the medium stress-resistant animals again showing no significant difference from the other two groups. In contrast, prolactin secretion in response to the thyrotropin releasing hormone (TRH) challenge was not suppressed in the stress-sensitive group, and the response to CRH challenge was similar between the groups (Bethea et al., 2005a). Thus, a difference in the pituitary stores of prolactin or ACTH cannot explain the results.
Figure 6.
Prolactin (top) and cortisol (bottom) responses to an injection of fenfluramine (5 mg/kg, i.v.) in saline while monkeys were maintained under Propofol anesthesia. In the top panel, there was a significant difference between the groups in the amount of prolactin secreted after fenfluramine (2 way ANOVA, p < 0.0001), with the HSR group secreting significantly more prolactin compared to the SS group (Tukey’s post hoc test, p < 0.001). In the bottom panel, the cortisol response to fenfluramine was significantly different between the experimental groups (2 way ANOVA, p < 0.0001), with the SS group secreting more cortisol compared to the HSR group (Tukey’s post hoc test, p < 0.001). Reprinted from Bethea et al., 2005a.
Discussion
Serotonin appears to be a key neurotransmitter in the regulation of mood, including affective state and anxiety levels (Siever et al., 1991) Moreover, it has been generally hypothesized that a diminished capacity of the serotonergic system may underlie a heightened susceptibility to stress as well as vulnerability to depression and/or drug abuse (Graeff et al., 1996; Summers et al., 1998). Decreases in ovarian steroid hormones also lead to decreases in various aspects of serotonin neural function (Bethea et al., 2002). This data supports the notion that the serotonin system of stress-sensitive individuals has a lower functional capacity than that of stress-resilient individuals even in the absence of stress and it supports the long-standing hypothesis that individuals with heightened sensitivity to stress have diminished serotonin function.
Nonetheless, the stress-sensitive group had a higher release of cortisol than the stress- resilient group. This is confounding if cortisol release also reflects the serotonin capacity. There are two potential explanations. One possibility is that in the stress sensitive individuals, the CRH neurons driving the hypothalamic-adrenal axis are super-sensitive to serotonin. Earlier work demonstrated that serotonergic denervation increases the functional neuroendocrine response of the HPA axis to serotonin agonists (Van de Kar et al., 1989). Hence, in the stress-sensitive group, even the lower amount of serotonin that was released by fenfluramine may have acted upon super-sensitive CRF neurons. This line of reasoning is supported by a report that different pathways from the mediobasal hypothalamus mediate serotonergic stimulation of prolactin and corticosterone secretion in rodents (Van de Kar et al., 1985a; Bagdy and Makara, 1994). The cellular or molecular mechanism involved in the switch of axis sensitivity between stress-sensitive and stress-resilient animals could be of great interest. As described below, we found that indeed, CRH is higher in stress sensitive individuals in the absence of stress.
Rodent studies also suggest that the effect of fenfluramine on corticosterone is not mediated by serotonin release (Van de Kar et al., 1985b). If this is true in primates, then perhaps the non-serotonin mediated effect of fenfluramine on cortisol is greater in stress-sensitive than in stress-resilient animals. However, the nature of the non-serotonin mechanism remains unresolved. In a similar manner, fenfluramine challenges in alcoholic patients produce lower prolactin and higher cortisol secretion than in nonalcoholic controls (Anthenelli et al., 2001; Weijers et al., 2001).
Exactly how stressors are transduced by the brain into perceptions of stress, physiological stress responses, and deleterious effects on mood and many other health outcomes is not understood. However, this study probed neural function in non-stressed animals that had a previously documented difference in reproductive function under stress; and we show that there are endogenous differences in serotonin capacity even in the non-stressed state. It is attractive to speculate that the lower endogenous serotonin makes the individual more sensitive to stress. Thus, the “stressfulness” of a stimulus may reside more in the individual nervous system than in the stimulus. Our animals were individually housed and not stressed at the time of the fenfluramine challenge, so the basal cortisol secretion was not a variable between the groups. Thus, the differences observed in stress sensitivity may have resulted from differences in genetic predisposition or early rearing experiences, factors known to influence activity of the hypothalamic-pituitary-adrenal axis (Meaney, 2001). A relationship between low socioeconomic status and low serotonergic activity, also measured by the prolactin response to fenfluramine, has been observed in a study of men and women (Matthews et al., 2000).
From the earlier study in which reproductive function was characterized, we know that the stress-sensitive animals have lower peak and lower average estradiol and progesterone levels across 3 non-stressed menstrual cycles. Indeed, ovarian steroid production appears to be a stable endogenous and individual state. QTL studies in baboons have found a microsatellite polymorphism that has a significant effect on estrogen (Martin et al., 2001). The corpus luteum is the major source of progesterone and the health and function of the corpus luteum depends largely on the viability of the ovulatory follicle (Collins et al., 1984a, b). Thus, it is not surprising that levels of estrogen and progesterone during a non-stressed menstrual cycle correlated with each other, and that both reflect the magnitude of sensitivity of the reproductive axis to stress.
In summary, the stress sensitivity of cynomolgus macaques correlates with prolactin secretion in response to the serotonin releaser, fenfluramine, suggesting that highly stress resistant animals have higher levels of endogenous serotonin than stress sensitive animals even in the absence of stress. In the next study, we examined gene expression related to serotonin neural function in stress sensitive versus stress resilient individuals.
III. Serotonin Gene Expression
Introduction
Serotonin neurotransmission is generally thought of as a combination of synthesis, release, turnover, neural activity and degradation. Pivotal proteins governing these functions are translated from mRNAs coding tryptophan hydroxylase 2 (TPH2), the serotonin reuptake transporter (SERT), the 5HT1A autoreceptor, and the monoamine oxidases A and B (MAO-A, MAO-B).
Therefore, we questioned whether the difference in the functional capacity of the serotonin system could be due to differences in the expression of 4 genes (SERT, 5HT1A, MAO-A, MAO-B) within serotonin neurons of the dorsal raphe nucleus or due to differences in serotonin cell number. We also questioned the expression of tryptophan hydroxylase (TPH), but at the time only TPH-1 was known and the gene for TPH-2 had not been discovered (Walther and Bader, 2003; Walther et al., 2003). When TPH-2 was discovered, the sections of the dorsal raphe from these animals were depleted.
Methods
After completion of all in vivo protocols, the animals were monitored daily for menstruation. Upon detection of menstruation, the animals were scheduled for euthanasia before day 5 of the follicular phase of their cycle during November/December 2001.
The monkeys were euthanized according to the procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was sedated with ketamine, given an overdose of pentobarbital (25 mg/kg, i.v.), and exsanguinated by severance of the descending aorta. A blood sample was obtained at necropsy for determination of estrogen and progesterone concentrations at the time of death. The brain was perfused and 25 μm sections through the midbrain raphe region were obtained as previously described (Bethea et al., 2005b). RT-PCR for a polymorphism in the serotonin reuptake transporter promoter region (5HTTLPR) was conducted on genomic DNA extracted from whole blood as previously described (Bethea et al., 2005b).
In situ hybridization for SERT, 5HT1A, MAO-A and MAO-B, densitometric analysis of the autoradiograms and analysis of the data was previously published (Bethea et al., 2005b). Six anatomical levels of the dorsal raphe nucleus were examined in a rostral to caudal direction at 250μ intervals. All statistical analyses were conducted using the Prism Statistic Program (GraphPad, San Diego, CA). A confidence level of p<0.05 was considered significant.
Results
Specific signals for SERT, 5HT1A autoreceptor, MAO-A and MAO-B mRNAs were detected in the dorsal raphe and representative photomicrographs of the autoradiographic signals for each transcript are shown in (Bethea et al., 2005b).
SERT
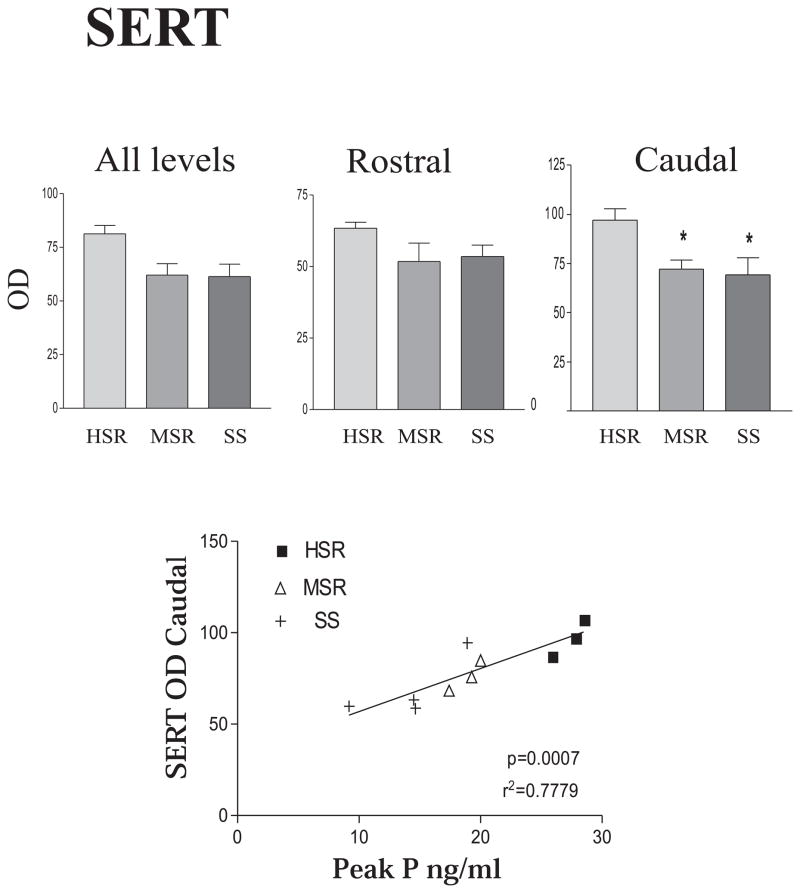
Examination of SERT across 6 levels of the dorsal raphe indicated that there was a significant decrease in expression in the caudal levels. Hence, the overall mean optical density (OD) was calculated and then, mean was obtained for the rostral 4 levels and the caudal 2 levels. As shown in Figure 7, top panel, the stress-sensitive group exhibited a significant decrease in the SERT OD in the caudal levels of the dorsal raphe, but not in the rostral levels. In the overall mean of all levels SERT expression tended to decrease in the MSR and SS groups, but it did not reach statistical significance. Analysis of positive pixel area yielded similar results. SERT OD in the dorsal raphe was also significantly correlated with serum P concentrations measured during the pre-stress control menstrual cycle, i.e. higher SERT mRNA signal was observed in animals with higher serum P concentrations (Figure 7, bottom panel). These animals also had the highest degree of stress resilience.
Figure 7.
Top panel, far left. The mean optical density for SERT signal for all 6 levels of the DRN was obtained for each animal. Then the mean of the animals in each group was obtained so the SEM represents the variance between animals. There was no significant difference in the mean OD (all levels 1–6) for SERT between the groups. Then, the DRN was parsed into rostral (levels 1–3) and caudal (levels 4–6) segments and the mean OD was obtained (center and right panels). The SEM again represents the variance between animals. In this analysis, there was a significant decrease in caudal DRN SERT expression in the medium stress resistant and stress-sensitive groups compared to the high stress resistant group (p < 0.05, ANOVA followed by Tukey’s). Bottom panel. A regression analysis was performed with the mean SERT OD of each individual animal (average across 6 levels) versus the peak serum progesterone concentration of the same animal obtained 3 years earlier during a control, non-stressed menstrual cycle. There was a significant positive correlation between SERT mRNA OD and peak progesterone levels from a non-stressed cycle (p=0.0007). Reprinted from Bethea et al., 2005b.
5HT1A
There was no difference between the groups in 5HT1A mRNA. Nonetheless, the 5HT1A OD in the entire raphe was highly correlated with serum P concentrations measured during the pre-stress control menstrual cycle wherein the animals with the greatest 5HT1A OD exhibited the highest serum P concentrations [r2=0.5917; p=0.009].
MAO-A
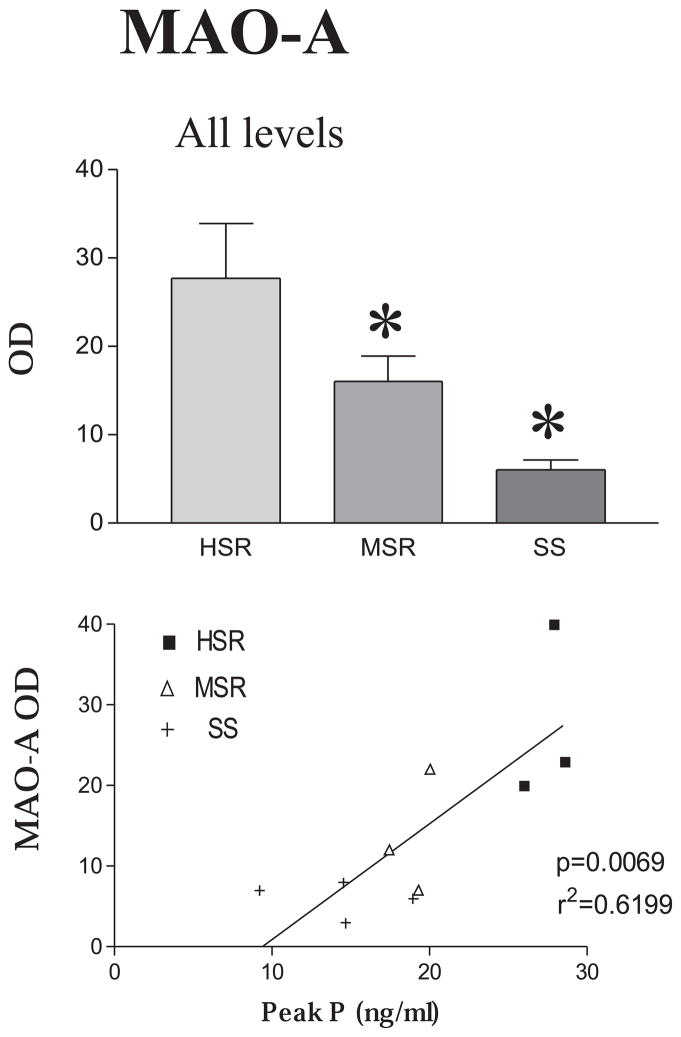
MAO-A mRNA, which codes for the serotonin degradative enzyme, is a low abundance mRNA in the dorsal raphe. However, MAO-A mRNA decreased significantly across all 6 levels of the dorsal raphe as stress resilience decreased. There was markedly less overall MAO-A OD in stress-sensitive and medium-stress resilient animals than in highly stress-resilient animals (Figure 8, top panel). The positive pixel area for MAO-A signal also decreased significantly in the stress-sensitive animals. MAO-A OD was also significantly correlated with serum P concentrations during the pre-stress control menstrual cycle with the highest expression of MAO-A observed in the animals with the highest serum P concentrations (Figure 8, bottom panel).
Figure 8.
Top panel The mean optical density for MAO-A mRNA signal for all 6 levels of the DRN was obtained for each animal. Then, the mean of the animals in each group was obtained so the SEM represents the variance between animals. There was a significant decrease in MAO-A signal in the medium stress resistant and stress-sensitive groups compared to the high stress resistant group (p < 0.007, ANOVA followed by Tukey’s). Bottom panel. A regression analysis was performed with the mean OD for MAO-A of each individual animal (average across 6 levels) versus the peak serum progesterone concentration of the same animal obtained 3 years earlier during a control, non-stressed menstrual cycle. There was a significant positive correlation between MAO-A mRNA OD and peak progesterone levels from a non-stressed cycle (p=0.0069). Reprinted from Bethea et al., 2005b.
MAO-B
MAO-B mRNA codes for the enzyme that preferentially degrades catecholamines and it is expressed abundantly in the dorsal raphe. MAO-B mRNA exhibited a decrease in both OD and pixel areas in stress sensitive groups, in a manner similar to MAO-A, but the differences were not statistically significant. However, MAO-B OD signal was also highly correlated with serum P concentrations during the pre-stress control menstrual cycle, with the highest expression of MAO-B occurring in animals with the highest peak of serum P during a normal control cycle [r2=0.36733; p=0.0036]
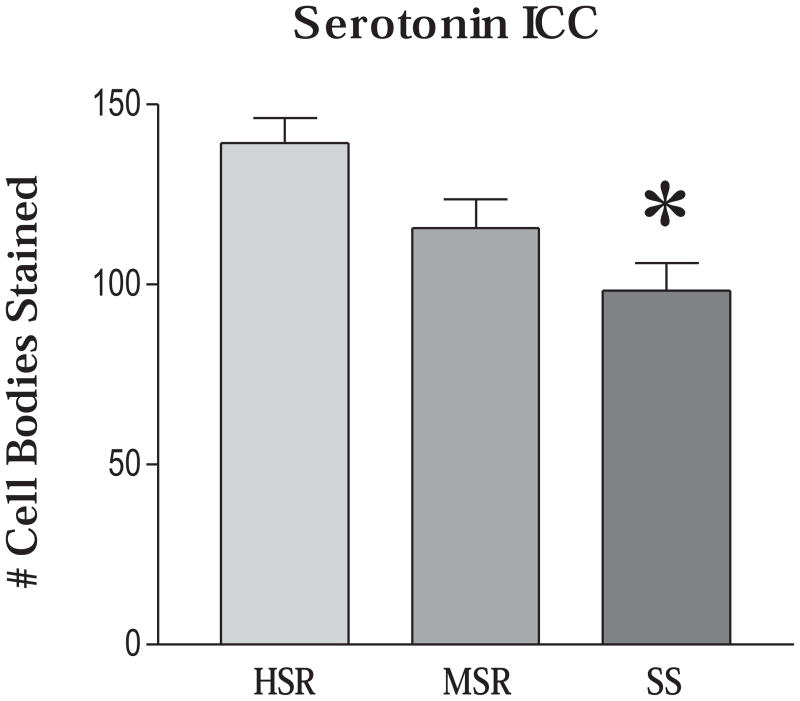
Three levels of the dorsal raphe were chosen for antigen retrieval, serotonin immunocytochemistry (ICC) and counting as previously described (Bethea et al., 2005b). After combining the cell number for all levels for each animal and then obtaining the mean for each group, there was a significant difference across the groups (ANOVA p = 0.0243). The SS group had significantly fewer cells than the HSR group (p, 0.05, Tukey’s), and the MSR group was in between the HSR and SS group (Figure 9)
Figure 9.
The number of serotonin-positive neurons on the right side of the dorsal raphe nucleus was obtained at 3 anatomical levels. The mean of all of the levels was obtained for each animal and then the overall mean was obtained for the group so that the SEM represents the variance between animals. There were significantly fewer serotonin neurons in the SS group (ANOVA, p < 0.03). * Significantly different from HSR with Tukey’s posthoc pairwise comparison, p < 0.05. Reprinted from Bethea et al., 2005b.
RT-PCR analysis of the polymorphic locus 2 in the promoter region of the serotonin reuptake transporter gene, 5HTTLPR, indicated that all of the monkeys in this study contained only the long allele of this polymorphism. Subsequent reports indicate that cynomolgus macaques are not polymorphic at this locus.
Discussion
We followed up our initial fenfluramine responsiveness findings by assessing the level of expression for 4 genes in serotonin neurons and then counting the number of serotonin-positive neurons at 3 levels of the DRN. We found that overall expression for this group of genes in the dorsal raphe is compromised to a lesser or greater extent in stress-sensitive monkeys, even in the absence of stress, and that the decrease in gene expression may reflect the decrease in serotonin cell number in stress-sensitive animals. This study reinforces the notion that serotonin neural function is compromised in stress-sensitive individuals, and shows, for the first time in primates, that there are fewer serotonin neurons and less overall gene expression, in stress-sensitive individuals. Two mRNAs pivotal for serotonin function (SERT and MAO-A) are significantly lower in stress-sensitive compared to stress-resilient animals. Two other mRNAs that play a role in serotonin function (5-HT1A and MAO-B) show a decremental trend in the stress-sensitive animals compared to the stress-resilient animals. More importantly, all 4 of these mRNAs show a significant correlation with serum levels of progesterone during a non-stressed control cycle, which were significantly lower in stress-sensitive animals. None of the variables examined had any correlation to the serum concentrations of E or P at the time of euthanasia. We have no method for integrating changes across different genes, but it seems clear that gene expression in the serotonin system is compromised in stress-sensitive animals in the absence of stress. Moreover, analysis of these data as a population continuum is more reflective of physiology than dividing them into discrete groups.
Subsequent to the conduct of these experiments, a second isoform of TPH was discovered called TPH-2 that is responsible for the synthesis of brain serotonin. We examined sections from our animals for TPH mRNA, but at the time only TPH-1 was known (Pecins-Thompson et al., 1996). Of the 251bp in our TPH-1 cDNA, a 230bp stretch is 65% homologous to TPH-2. We cannot rule out the possibility that it was hybridizing to TPH-2 under the stringency conditions employed. Due to this uncertainty, we cannot report the results with confidence and the raphe sections from these animals are depleted. Therefore, TPH2 was recently examined in new groups of animals. TPH2 was significantly lower in stress sensitive animals than in highly stress resilient animals (unpublished and data not shown).
There is a body of literature showing that stress can lead to secondary disease including reproductive dysfunction in sensitive individuals (Xiao et al., 1999; Cameron, 2000; McEwen, 2007). Decreased activity of central serotonin pathways is linked to a number of stress-sensitive psychiatric disorders including anxiety and depression (Mann et al., 1996; Cleare et al., 1998; Arango et al., 2002; Bhagwagar et al., 2002), bipolar disorder (Sobczak et al., 2002), alcoholism (Weijers et al., 2001), anorexia nervosa (Monteleone et al., 1998), and premenstrual syndrome (Fitzgerald et al., 2003). Both stress and circulating levels of ovarian steroid hormones can influence serotonin neurotransmission (Bethea et al., 2002; Shively et al., 2003). The serotonin neural system also contributes to aspects of cognition, learning and memory (Meltzer et al., 1998; Terry et al., 2008; Thomas and O’Brien, 2008). Hence, it has become an important site for pharmacologic intervention in psychiatric disorders, and increasingly in stress-related physiological disorders. Moreover, the same risk factors that lead to an increased incidence of anxiety disorders also lead to an increased incidence of other stress-related problems including growth retardation (Montgomery et al., 1997), type 2 diabetes (Russak and Schwartz, 1997), and rheumatoid arthritis (Wallace, 1987). Sensitivity to stress-induced reproductive dysfunction may fall in this same category.
The observation that stress-sensitive animals have fewer serotonin neurons was unexpected. We previously reported that there were no differences in serotonin cell number in spayed rhesus macaques with or without one month of hormone therapy (HT) (Bethea, 1994) and human studies have not observed differences in serotonin cell number in depressed suicides versus controls (Underwood et al., 1999). However, other studies found fewer DRN neurons in diseases such as Alzheimer’s (Aletrino et al., 1992) and alcoholism (Halliday et al., 1993). A long-term experiment is underway in our Japanese macaque troop to test the hypothesis that ovarian hormones protect serotonin neurons from apoptosis.
The data from the stress-sensitive animals do not directly reflect the earlier studies with hormone replacement (Bethea et al., 1999; Bethea et al., 2002) because of the differences in serotonin neuron number. We previously found that estrogen (with or without progesterone) treatment of spayed rhesus macaques decreases SERT, 5HT1A and MAO-A mRNA expression in the DRN. In this study, we observed the highest expression of SERT, 5HT1A and MAO-A on autoradiograms in the animals with the greatest number of serotonin neurons and highest serum progesterone levels. We found lower gene expression signals in animals with fewer neurons. Thus, although estrogen and progesterone regulate serotonin gene expression, they not be directly or wholly responsible for the changes in gene expression in the different stress sensitive groups. Rather, we believe that the lifetime secretion of ovarian steroids may be preventing cell death or altering the set point of function in the serotonin system.
We speculate that when animals of similar temperament and serotonin cell number, like the spayed rhesus monkeys, are acutely treated with ovarian hormones, then the mRNA regulation previously observed will be manifested, that is downregulation of SERT, 5HT1A and MAO-A. In this study, the animals were all euthanized in the early follicular phase of a non-stressed menstrual cycle when estrogen and progesterone levels were similar across the groups. Retrospectively, the animals with the highest cyclic secretion of progesterone had the most serotonin neurons, and they yielded higher gene expression signals on autoradiograms, i.e. elevated SERT, 5HT1A and MAO-A compared to stress sensitive animals. Hence, in this model the gene expression detected on the films may be a consequence of the serotonin cell number or viability and not acute hormone levels.
Neurodegenerative diseases have become a major target for the development of pharmacotherapies. These diseases share synaptic loss, neuronal atrophy and death as common pathological hallmarks. Recent data suggests that depression, mood disorders and other mental illnesses may have a degenerative component and that antidepressants contribute to neural plasticity and cellular resilience (Kempermann and Kronenberg, 2003; Manji et al., 2003). Estrogen has been shown to be neuroprotective in other brain regions (Yang et al., 2003; Zhao et al., 2004), and so it is possible that ovarian hormones are neuroprotective for serotonin neurons as well. Therefore, we hypothesize that animals with higher lifetime secretion of ovarian steroids will experience decreased serotonin neuronal death and greater cellular resilience. The maintenance of a larger population of serotonin neurons will promote stress-resilience due to greater overall serotonin availability and neurotransmission. It is attractive to speculate further that the lower serotonin cell number and gene expression makes the individual more sensitive to stress and stress induced reproductive dysfunction.
In summary, the number of serotonin neurons and overall expression of pivotal genes in serotonin neurons correlates strongly with the stress sensitivity of the individual and with the peak of progesterone secretion during non-stressed control cycles. That is, animals with high stress resistance have the highest lifetime secretion of progesterone, the greatest number of serotonin neurons and the highest expression of serotonin-related genes in the dorsal raphe nucleus. These strong associations between progesterone, degree of stress-sensitivity and serotonin warrant further investigation to determine causal relationships within these systems.
Next, we questioned whether serotonin receptors and other systems that impact GnRH release are altered in stress sensitive individuals. Therefore, we began our exploration of the hypothalamic systems that impact GnRH release and the control of the reproductive axis. First, we examined the serotonin receptors, and then we examined the expression of GAD67, the enzyme responsible for the synthesis of the major inhibitory neurotransmitter, GABA. Subsequently, we examined CRH and POMC gene expression as two additional systems believed to inhibit GnRH.
IV. Hypothalamic Systems: Serotonin Receptors and GABA
Introduction
The hypothalamus receives abundant innervation from the raphe serotonergic system (Azmitia and Segal, 1978; Azmitia and Gannon, 1986) and expresses serotonin 5HT1A, 2A and 2C receptors (Wright et al., 1995; Gundlah et al., 1999). Hypothalamic serotonin is involved in the regulation of GnRH secretion through systems that regulate GnRH neurons and that are influenced by stress. Among them, gamma-aminobutyric acid (GABA) provides the major input to GnRH neurons (Robinson, 1995; Sim et al., 2000; Jansen et al., 2003). There is evidence that serotonin inhibits LH secretion in rats through the stimulation of GABA neurons (Morello et al., 1989). In the monkey, GAD67 (the rate-limiting enzyme in the synthesis of GABA) expressing neurons localized in the infundibulum co-express the serotonin 5HT2C receptor. Estrogen treatment suppressed both GAD67 (Mirkes and Bethea, 2001), 2001) and 5HT2C receptor (Gundlah et al., 1999) gene expression, suggesting the participation of these neurons in the control of ovulation in monkeys.
We hypothesized that the lower serotonergic input from the dorsal raphe nucleus to the hypothalamus, or the lower concentrations of estrogen and progesterone in the stress-sensitive animals may affect the expression of 5HT receptors in the hypothalamus, as well as the expression of GAD67 in GABA neurons that are regulated by serotonin. The same animals from which the midbrain raphe was obtained also provided the hypothalami for the following studies.
Methods
In situ hybridization for 5HT1A, 5HT2A and 5HT2C receptors and GAD67, densitometric analysis of the autoradiograms and analysis of the data was previously published (Centeno et al., 2007a). Prehybridization, hybridization and wash temperatures were empirically optimized for each probe. The development, sequence and characterization of the monkey specific 5HT1A, 5HT2A and 5HT2C receptors and GAD67 cDNAs and riboprobe hybridization were published previously (Pecins-Thompson and Bethea, 1998; Gundlah et al., 1999; Mirkes and Bethea, 2001). All statistical analyses were conducted using the Prism Statistic Program (GraphPad, San Diego, CA). A confidence level of p<0.05 was considered significant.
Results
5HT1A mRNA expression in the VMN of HSR, MSR and SS monkeys
In macaque hypothalamus, the ventromedial nucleus (VMN) exhibited the densest expression of 5HT1A receptor mRNA (Gundlah et al., 1999), and also expressed estrogen and progestin receptor mRNA (Bethea et al., 1996). Therefore, 4 levels of the VMN were examined for 5HT1A mRNA expression in the characterized groups. There were no differences between groups in either 5HT1A pixel area or optical density (OD) at any level of the VMN [data published in (Centeno et al., 2007a)].
5HT2A mRNA expression in the PVN of HSR, MSR and SS monkeys
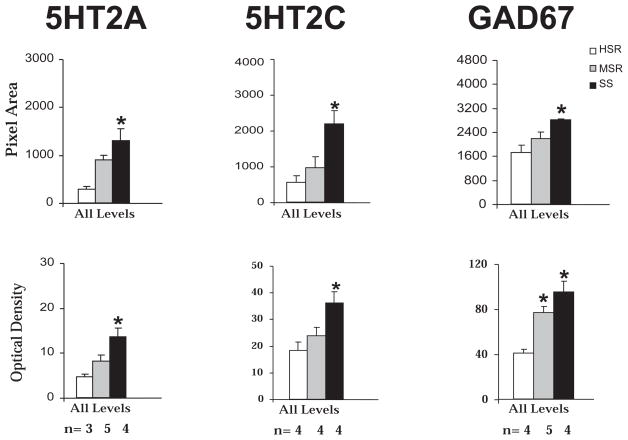
We showed that 5HT2A receptor mRNA expression was most prominent in the hypothalamic paraventricular nucleus (PVN) and mammillary nuclei (Gundlah et al., 1999). Since the PVN plays a pivotal role in neuroendocrine regulation, we examined 5HT2A mRNA expression in 5 levels of the PVN of the characterized groups. Figure 10, left panel, shows the overall mean positive pixel area and OD of 5HT2A ISH signal in the PVN. The average 5HT2A pixel area and OD were significantly higher in the SS animals (p<0.05).
Figure 10. 5HT2A, 5HT2C and GAD67 mRNA expression in hypothalamus of HSR, MSR and SS monkeys.
Left panel. 5HT2A optical density (OD) and pixel area were obtained at 5 levels of the PVN of HSR, MSR and SS monkeys. The mean of all of the levels was obtained for each animal and then the overall mean was obtained for the group so that the SEM represents the variance between animals. There was significantly more 5HT2A mRNA in the PVN of SS animals compared to HSR animals
Middle panel. 5HT2C optical density (OD) and pixel area were obtained at 3 levels of the infundibulum of HSR, MSR and SS monkeys. The mean of all of the levels was obtained for each animal and then the overall mean was obtained for the group so that the SEM represents the variance between animals. There was significantly more 5HT2C mRNA in the infundibulum of SS animals compared to HSR animals
Right panel. GAD67 optical density (OD) and pixel area were obtained at 3 levels of the infundibulum of HSR, MSR and SS monkeys. The mean of all of the levels was obtained for each animal and then the overall mean was obtained for the group so that the SEM represents the variance between animals. There was significantly more GAD67 mRNA in the infundibulum of SS animals compared to HSR animals. *p<0.05, Kruskal-Wallis one-way ANOVA, Dunn’s posthoc test compared with HSR animals. The number of animals used in each group is shown below the histograms. Reprinted from Centeno et al., 2007a.
5HT2C mRNA expression in the infundibulum of HSR, MSR and SS monkeys
We showed that the 5HT2C receptor mRNA was densely expressed and regulated by ovarian steroid hormone replacement in the infundibular region of macaques (Gundlah et al., 1999). This region contains dense populations of estrogen and progestin receptor expressing neurons as well (Bethea et al., 1996) and it plays a crucial role in the regulation of reproductive function. Therefore, we examined 5HT2C mRNA expression in 3 levels of the infundibular nucleus of the characterized groups. Figure 10, middle panel, shows that SS animals exhibited significantly higher 5HT2C positive pixel area and OD in the overall average of all levels of the infundibulum, compared with HSR animals (p<0.05; Kruskal-Wallis ANOVA, followed by Dunn’s posthoc test).
GAD67 mRNA expression in the infundibulum of HSR, MSR and SS monkeys
We previously showed that GAD67 mRNA expression was also robust in the macaque infundibulum and infundibular GABA neurons expressed 5HT2C mRNA (Mirkes and Bethea, 2001). Moreover, the infundibular nucleus contains dense populations of estrogen and progestin receptor containing neurons and GAD67 was suppressed by ovarian steroid hormone replacement. Therefore, we examined GAD67 mRNA expression at 3 levels of the infundibular nucleus of the characterized groups. As illustrated in Figure 10, right panel, SS monkeys exhibited significantly higher average GAD67 positive pixel area and OD, compared with HSR individuals (p<0.05; Kruskal-Wallis ANOVA followed by Dunn’s post hoc test). There was no significant difference in the levels of GAD67 mRNA in the infundibulum of MSR monkeys, compared with HSR animals. The same pattern of expression was observed in the posterior hypothalamus (PH) (Centeno et al., 2007a). In addition, infundibular GAD67 mRNA was positively correlated with 5HT2C mRNA (R=0.644, P=0.019), and negatively correlated with luteal phase serum progesterone (R=0.732, P=0.016).
Discussion
These experiments show that stress-sensitive monkeys exhibited higher levels of 5HT2A receptor mRNA in the PVN, higher levels of 5HT2C receptor mRNA in the hypothalamic infundibular region, as well as higher levels of GAD67 mRNA in the infundibulum and the PH, compared with stress-resilient individuals, but there was no change in the expression of 5HT1A receptor mRNA levels in the VMN.
It is notable that the expression of 5HT1A receptor in the hypothalamic VMN is not different between stress-sensitive, medium stress-resilient and highly stress-resilient monkeys. The dense expression of 5HT1A in the VMN is consistent with our previous observations (Gundlah et al., 1999). However, 5HT1A mRNA in the VMN was not regulated by ovarian steroid hormone replacement, in spite of a robust population of steroid hormone receptor expressing neurons. Therefore, neither the difference in ovarian hormone concentrations nor the predicted difference in serotonin production between stress-sensitive and stress-resilient animals impacted the expression of the 5HT1A receptor in the VMN.
The serotonin 5HT1A receptor is coupled to protein Gi, reducing adenylyl cyclase and/or increasing the opening of K+ channels (Fargin et al., 1991; Albert et al., 1996). The hypothalamic 5HT1A receptor is involved in the inhibitory action of serotonin on female rodent sexual behavior. Administration of 5HT1A agonists into the VMN inhibits lordosis behavior in rats (Aiello-Zaldivar et al., 1992; Uphouse et al., 1992). In addition, serotonin in the VMN plays a modulatory role in the regulation of feeding behavior (Blundell and Hill, 1987; Schwartz et al., 1990). The VMN contains few if any neuroendocrine neurons (neurons that project to the median eminence and regulate anterior pituitary hormone secretion). Our indicator of stress sensitivity is ovulation, which is controlled by neuroendocrine areas outside of the VMN. Along this line of reasoning, serotonin circuits that are not involved in mediating stress to neuroendocrine neurons may not be different between the groups.
The dense concentration of 5HT2A mRNA in the PVN is in agreement with our previous observations in pigtail macaques (Gundlah et al., 1999). In this study, stress-sensitive monkeys exhibited higher levels of 5HT2A mRNA in this nucleus, compared with stress-resilient individuals. In contrast, ovarian steroid hormone replacement had no effect on 5HT2A expression (Gundlah et al., 1999). Thus, it is attractive to speculate that the decrease in the activity of the serotonin system in stress-sensitive animals, and not the difference in ovarian hormone secretion, led to an upregulation of the 5HT2A receptor in the stress-sensitive animals.
The 5HT2A receptor is coupled to a protein Gq, activating a phospholipase C (PLC) that produces a mobilization of intracellular calcium and activation of protein kinase C (PKC) and neuronal excitability (Sanders-Bush and Canton, 1995). Activation of PVN 5HT2A receptors by specific agonists produces an increase in the secretion of hormones related to the stress system, like ACTH, corticosterone, oxytocin, renin, and prolactin, as well as an activation of CRH- and oxytocin-expressing neurons (Van de Kar et al., 2001). Increased 5HT2A receptor expression has been reported in individuals with reduced serotonergic tone, as is the case for obsessive-compulsive disorder patients (Adams et al., 2005), suicide victims (Escriba et al., 2004) and in anorexia nervosa (Kaye et al., 2005). In addition, the 5HT 2A receptor expression is downregulated after serotonin or antidepressant treatment (Peroutka and Snyder, 1980; Blackshear and Sanders-Bush, 1982; Saucier et al., 1998). Taken together, these reports suggest that a decrease in serotonergic input is related to 5HT2A receptor upregulation. Stress-sensitive animals exhibited lower serotonergic activity as well as fewer serotonin cells and lower expression of genes related to serotonin function in the dorsal raphe nucleus. Therefore, the higher expression of the 5HT2A receptor mRNA observed in stress-sensitive monkeys in the present study may be a compensatory mechanism for the lower dorsal raphe serotonergic tone to the PVN and it may be contributing, in part, to the increased sensitivity to stress observed in these animals.
The dense localization of the 5HT2C receptor in the infundibulum is consistent with our previous observations. Moreover, the 5HT2C receptor was decreased by ovarian steroid hormone replacement in the infundibular nucleus (Gundlah et al., 1999). In this study, we found that the 5HT2C receptor was increased in stress-sensitive animals, which also have the lowest amount of serotonergic tone and the lowest concentrations of estrogen and progesterone during normal menstrual cycles. Either of these deficits could have led to upregulation of the 5HT2C receptor in the infundibulum, a region with a large concentration of neurons that express estrogen and progestin receptors (Bethea et al., 1996).
Like the 5HT2A receptor, the 5HT2C receptor is coupled to a protein Gq, activating the PLC and PKC systems and increasing neuronal excitability (Boess and Martin, 1994). It was recently reported that depletion of serotonin increases expression of 5HT2C mRNA isoforms encoding receptors with higher sensitivity to serotonin (Gurevich et al., 2002). In addition, repeated administration of 5HT2C agonists down regulates 5HT2C receptors (Pranzatelli et al., 1993). These results suggest that serotonin levels affect the expression and activity of the 5HT2C receptor. Therefore, we speculate that the lower levels of serotonin in stress-sensitive monkeys, together with the lower levels of estrogen and progesterone, contribute to the upregulation of 5HT2C receptor observed in the present study.
GAD67 mRNA was robustly expressed in the infundibulum of cynomolgus macaques, which is consistent with our previous report of GAD67 expression in pigtail macaques. Moreover, we previously found that GAD67 mRNA expression was decreased by ovarian steroid hormone replacement and that 5HT2C receptor mRNA colocalizes with GAD67 mRNA in infundibular neurons (Mirkes and Bethea, 2001). In the present study, we found that stress-sensitive monkeys expressed higher levels of GAD67 mRNA in the infundibular region, compared with highly stress-resilient animals. Moreover, there was a positive correlation between GAD67 and 5HT2C receptor mRNA.
GABA has been proposed as a mediator in the steroid feedback that modulates GnRH secretion (Sullivan and Moenter, 2005), and it may play a role in some forms of hypothalamicinfertility, like in the polycystic ovarian syndrome, as well as in infertility related to negative energy balance (Sullivan and Moenter, 2004b, a). Increased GAD67 and GAD65 expression is observed in long-term feed-restricted male rats and is involved in the reduction of LH secretion observed in these animals (Leonhardt et al., 1999). There is evidence that serotonin inhibits LH secretion in rats through the stimulation of GABA neurons (Morello et al., 1989). A body of literature suggests that GABA acts through GABAA receptors within the vicinity of theGnRH neuron soma to inhibit LH secretion (Herbison et al., 1991; Jarry et al., 1991; Scott and Clarke, 1993; Mitsushima et al., 1994). Furthermore, GnRH neurons express functional GABAA receptors that may inhibit GnRH neuronal excitability (Spergel et al., 1999; Sim et al., 2000; Han et al., 2004).
It is attractive to speculate that the lower levels of ovarian steroids in stress-sensitive monkeys and the higher levels of 5HT2C may be responsible for the increased levels of GAD67 observed in stress-sensitive animals. In turn, this could lead to an increase in GABA synthesis and secretion. GABA may be suppressing GnRH and LH secretion, which would affect estrogen and progesterone secretion by the ovary, as indicated by the negative correlation between GAD67 expression and luteal phase progesterone levels obtained in the present study.
In summary, we found that in non-stressed conditions, monkeys that were previously characterized as stress-sensitive, exhibited higher expression of 5HT2A receptor mRNA in the PVN, an area strongly implicated in governance of neuroendocrine stress responses. Stress-sensitive monkeys also exhibited higher 5HT2C receptor and GAD67 mRNAs in the infundibulum, an area crucial for ovulation. GABA neurons in the infundibulum express steroid hormone receptors and may directly respond to the lower levels of estrogen and progesterone in stress-sensitive monkeys. Alternatively, the lower serotonin tone in stress-sensitive animals may lead to upregulation of 5HT2A and 2C receptors, upregulation of GAD67, a decrease in pituitary LH and ultimately lower serum estrogen and progesterone levels. However, 5HT1A receptor mRNA in the VMN was not different between the stress-characterized groups. The VMN has been implicated in the regulation of sexual behavior and food intake, but not neuroendocrine function. This further suggests that the serotonin and GABAergic systems may be selectively altered in the stress-sensitive and reproductive-related circuits of stress-sensitive monkeys, and may be participating in altering the sensitivity of the reproductive system to stress in these individuals, although non-stress related circuits may be unaffected. Other regulatory neural systems that are activated in conditions of stress and that impinge upon GnRH neurons include corticotropin releasing hormone (CRH) and β-endorphin, derived from pro-opiomelanocortin (POMC).
V. Hypothalamic systems: CRH and POMC (β-endorphin)
Introduction
In many stressful situations the hypothalamic-pituitary-adrenal (HPA) axis becomes activated and CRH, ACTH and cortisol increase. CRH was originally isolated from the hypothalamus (Vale et al., 1981) and its neuroendocrine role in the HPA response to stress is well characterized (Morimoto et al., 1993; Nemeroff, 2004). CRH also plays a role in the integration of autonomic and behavioral responses to stress. Within the limbic system there is evidence that the CRH system modulates behavioral traits such as locomotor activity, sleep, addictive behavior and in particular, anxiety related behaviors (Dunn and Berridge, 1990; Liebsch et al., 1995). Moreover, CRH neurons and fibers are found in numerous limbic structures outside of the hypothalamus (Frim et al., 1990; Delville et al., 1992; Keegan et al., 1994). Therefore, we questioned whether CRH expression differed between individuals who show differential sensitivity to stress-induced reproductive dysfunction.
Another neuropeptide involved in the regulation of ovulation is β-endorphin, which is derived from pro-opiomelanocortin (POMC) (Leadem and Kalra, 1985; Petraglia et al., 1986). In rodents, the synthesis of β-endorphin is linked to the transcription of POMC mRNA (Millington et al., 1986). β-endorphin-containing neurons inhibit GnRH neuronal activity under certain circumstances, in particular during the progestin-dominated luteal phase of the menstrual cycle (Ferin and Van de Wiele, 1984). Stress-sensitive animals cease ovulation immediately upon exposure to stress, which could potentially involve rapid activation of POMC neurons and release of β-endorphin. Therefore, we speculated that β-endorphin expression might differ between individuals with sensitivity versus resilience to stress-induced reproductive dysfunction.
In the following study, we examined the expression of CRH in the hypothalamic paraventricular nucleus (PVN), the centrum medianum-subfascicularis complex of the thalamus (CM-Sf) and in the central nucleus of the amygdala; and we examined the expression of POMC, the precursor mRNA for β-endorphin, in the medial basal hypothalamus of cynomolgus monkeys that had been previously shown to exhibit different sensitivities to stress-induced reproductive.
Methods
CRH mRNA expression was examined with in situ hybridization (ISH) at 5 levels of the hypothalamic paraventricular nucleus (PVN) and at 5 levels of the centrum-medianum-subfascicularis complex of the thalamus (CM-Sf). CRH protein expression was examined with immunocytochemistry (ICC) at 5 levels of the hypothalamic PVN and at 4 levels of the central nucleus of the amygdala. POMC mRNA expression was examined with ISH at 6 levels of the hypothalamic infundibular nucleus. Protocols for immunocytochemistry, in situ hybridization, image analysis with NIH Image and statistical analysis, were previously published (Centeno et al., 2007c).
Results
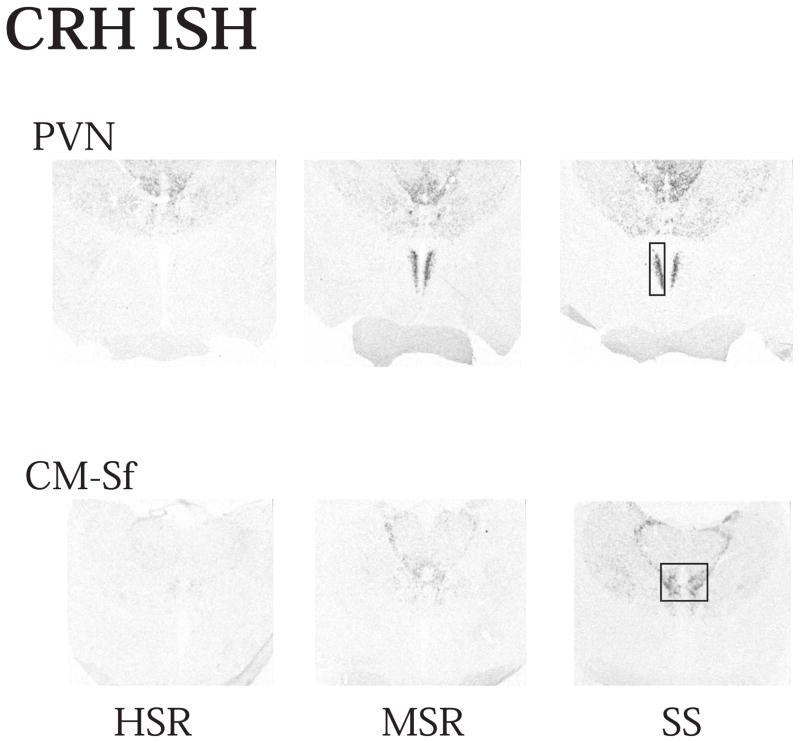
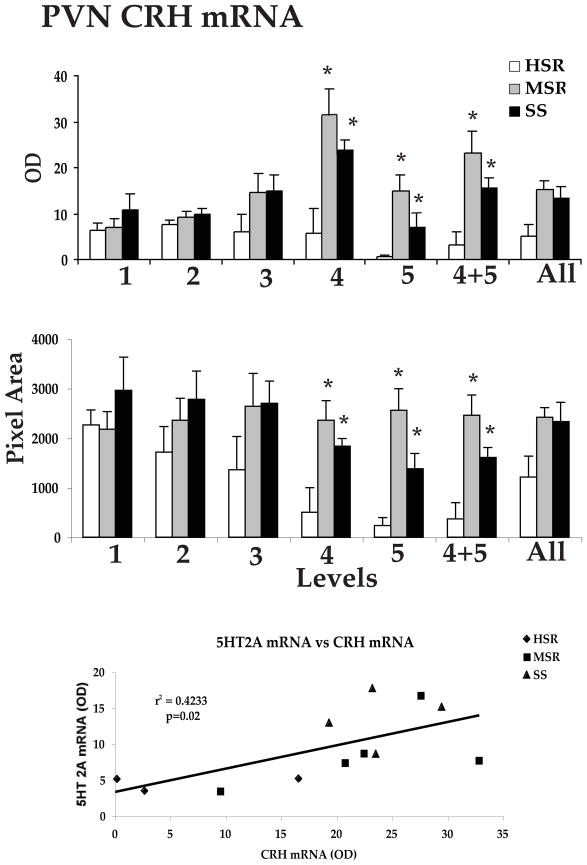
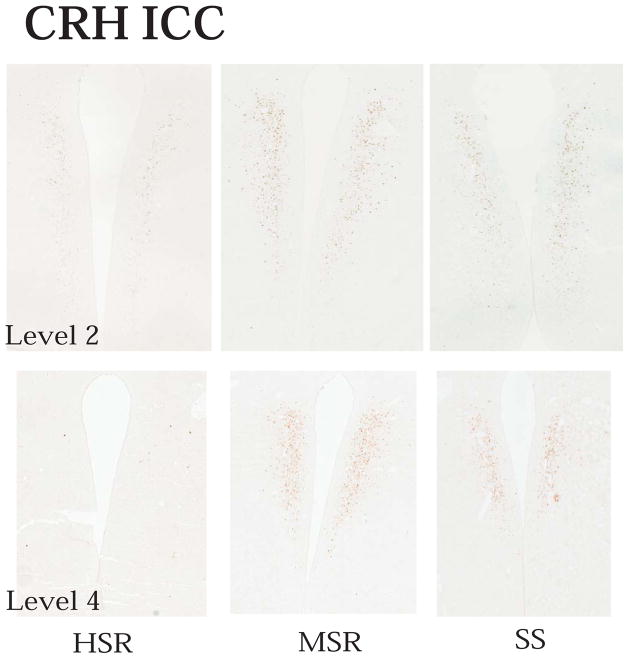
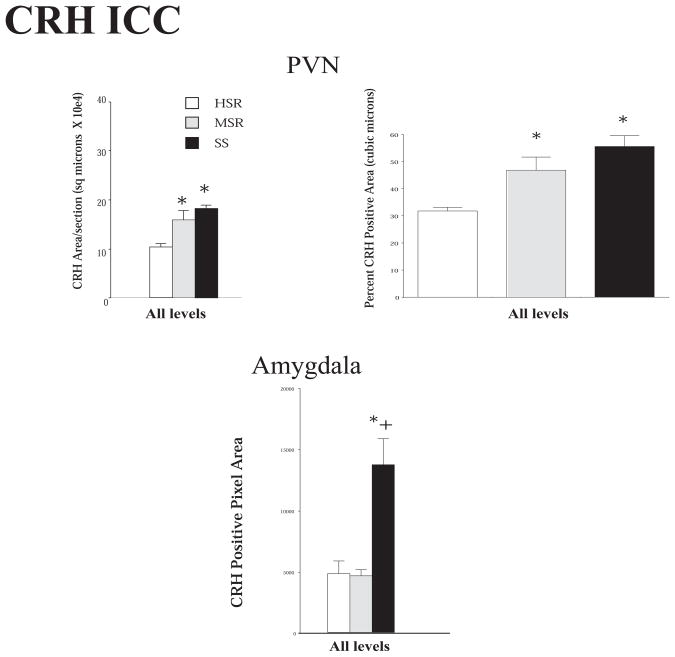
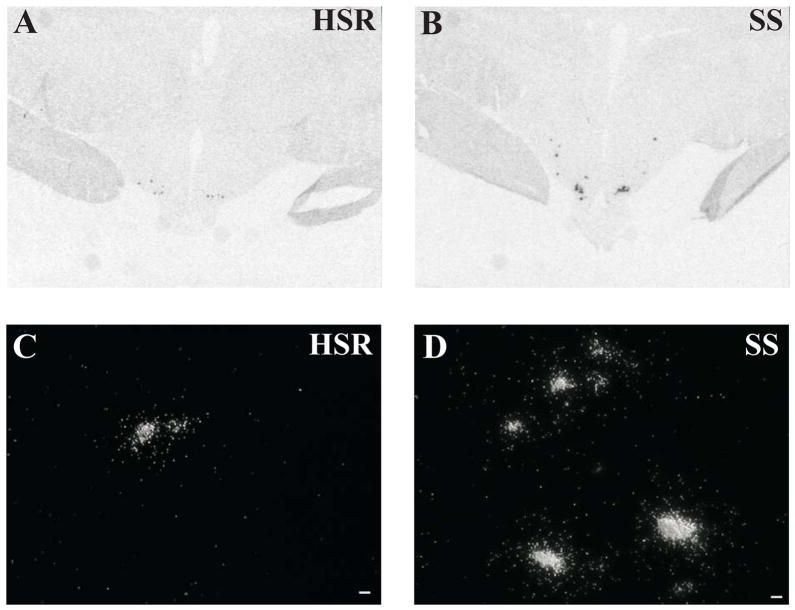
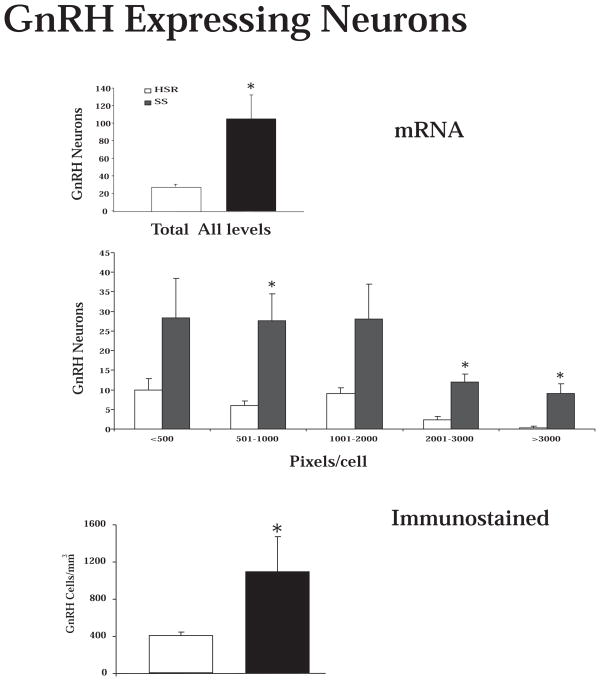
Representative autoradiographs of CRH mRNA signal in the PVN and CM-Sf complex from HSR, MSR and SS individuals are shown in Figure 11. The CRH signal is robust in both areas of the SS animals and declines to nearly undetectable in the HSR animals. As shown in Figure 12, top and middle panels, CRH positive pixel area and OD were significantly elevated in the caudal 2 levels of the PVN in the MSR and SS animals compared to HSR animals. There was no difference between groups in the rostral 3 levels, which masked the difference in the caudal levels in the overall average. Nonetheless, the average of levels 4 and 5 demonstrated a significant difference between the groups (p < 0.05). Upon examination of the PVN in hematoxylin stained sections, it appeared that the PVN was larger and extended further in a caudal direction in the MSR and SS groups (not shown). Figure 12, bottom panel shows there was a significant positive correlation between the expression of CRH mRNA in the PVN and 5HT2A mRNA in the PVN (r2=0.4233; p=0.02). CRH mRNA expression in the CM-Sf of the thalamus was also higher in stress sensitive animals (Centeno et al., 2007c).
Figure 11.
Autoradiographs of CRH mRNA signal at level 2 of the PVN and CM-Sf of a representative animal from each experimental group (HSR, MSR and SS). CRH mRNA expression is barely detectable in the HSR animal, but it increases markedly in the MSR and SS animals. Reprinted from Centeno et al., 2007c.
Figure 12.
Top and Middle Optical density and positive pixel area of the CRH mRNA signal in the PVN at each of 5 rostral to caudal levels, in levels 4 and 5 combined, and in all levels combined. There is a significant difference between the groups at caudal levels 4 and 5 and in the average of levels 4 and 5 (p < 0.05, ANOVA in both analyses). CRH mRNA expression is significantly higher in the MSR and SS groups compared to the HSR group (p < 0.05, SNK). Bottom. The average CRH mRNA expression in the PVN was significantly correlated with 5HT2A mRNA expression in the PVN. Reprinted from Centeno et al., 2007c.
The differences in CRH mRNA expression in the PVN were manifested at the protein level as well. Figure 13 contains representative montages of the CRH immunostaining at levels 2 and 4 in the PVN of HSR, MSR and SS individuals. At matching levels of the PVN, the CRH immunostaining is darker and more widespread in the MSR and SS individuals than in the HSR individuals. To quantify the immunostaining, Slidebook 4.2 was employed to segment positive immunostained pixels. As shown in Figure 14, left panel, there was a significant increase in the overall average CRH positive area/section from all 5 levels in MSR and SS groups compared to the HSR group (p < 0.05). The percent of the regional volume occupied by CRH immunostaining is shown in Figure 14, right panel. There was a significant increase in CRH volume in the MSR and SS groups compared to the HSR group (p < 0.05).
Figure 13.
Photomicrographs of CRH immunostaining at level 2 and level 4 of a representative animal from each group. The immunostaining is darker and somewhat more extensive in the MSR and SS animals at level 2. At level 4, the PVN has disappeared in this HSR animal, but CRH-positive neurons are still prevalent in the MSR and SS animals. By level 5, the PVN was absent in all HSR animals. Reprinted from Centeno et al., 2007c.
Figure 14.
Histograms illustrating the quantity of CRH immunostaining in 5 levels of the PVN and in 4 levels of the central nucleus of the amygdala of the HSR, MSR and SS groups. Top left panel, shows the overall average CRH positive area (μm2)/section in each group. There was a significant difference between the groups with MSR and SS groups exhibiting significantly higher expression of CRH protein than the HSR group Top right panel shows the volume of CRH immunostaining expressed as a percent of the volume of the region (μm3). There was a significant difference between the groups with MSR and SS groups exhibiting significantly higher expression of CRH protein than the HSR group. Bottom panel illustrates the density of CRH fibers (positive pixel area covered by fibers) in the central nucleus of the amygdala from HSR, MSR and SS groups. There was a significant difference between the groups with the SS group exhibiting significantly higher CRH fiber density than the MSR and HSR groups * different from HSR; + different from MSR; ANOVA followed by Student-Newman-Keuls posthoc pairwise comparison. Reprinted from Centeno et al., 2007c.
Immunostaining of the amygdala for CRH revealed a dense fiber plexis in the central nucleus. As shown in Figure 14, bottom panel, there was a significant difference in CRH positive pixel area between the groups, and the pixel area of CRH fiber staining was significantly higher in the SS group than in the MSR or HSR groups (p < 0.05).
There was no difference in POMC mRNA expression in the infundibular nucleus of the mediobasal hypothalamus as determined with in situ hybridization.
Discussion
The CRH system has been strongly implicated in stress-related disorders (Holsboer, 1999). Moreover, CSF CRH is elevated in individuals with major depressive disorder (Dunn and Berridge, 1990; Carpenter et al., 2004) and in individuals who suffer from adult affective disorders due to childhood trauma and concurrent stress (Gutman and Nemeroff, 2003; Bao et al., 2005).
These data suggest that ongoing CRH production is higher in SS animals in several areas within the central nervous system. Whether this would translate to a greater release of CRH under stress is unknown. Female rats have higher CRH mRNA expression than males, but they do not release more CRH under stress (Duncko et al., 2001). Moreover, the areas in which CRH was higher are not directly involved in the regulation of the HPA axis. The rostral regions of the PVN contain the neuroendocrine CRH neurons, i.e., the neurons that project to the median eminence and control the secretion of ACTH (Swanson and Sawchenko, 1983). The neurons that project to other areas of the brain, particularly to the midbrain and periaquaductal gray regions are present in the caudal two-thirds of the PVN (Luiten et al., 1985; Portillo et al., 1998). The CRH produced in the amygdala and CM-Sf of the thalamus probably does not directly regulate ACTH either. Rather these neurons play a role in communication within the greater limbic system. Outside of the neuroendocrine CRH system, CRH administration produces hypertension, tachycardia and an elevated oxygen consumption, induces a reduction in food intake, increases grooming behavior, locomotor activity, vocalization and induces an aroused state, but decreases sexual receptivity (Lenz, 1987). CRH can act at sites distant from release via convection through CSF (Bittencourt and Sawchenko, 2000), and CSF CRH levels are elevated in monkeys with a fearful temperament (Kalin et al., 2000).
Previous studies have shown that CRH and activation of the CRH system inhibits LH secretion and in turn, ovulation, via CRH-R2 receptors (Rivier et al., 1986; Ferin, 1993; Polkowska and Przekop, 1997; Li et al., 2005). Also, CRH fibers have been shown to be juxapositioned on GnRH neurons in humans (Dudas and Merchenthaler, 2002). However, stress suppressed reproductive function in CRH knockout mice similarly to wild type mice (Jeong et al., 1999). In addition, CRH from the PVN did not suppress pulsatile LH secretion in lactating rats (Tsukamura et al., 1993) and neuroanatomical studies do not find direct connections between CRH and GnRH neurons in rats (Hahn et al., 2003). We have preliminary data suggesting that there is little difference in the diurnal release of cortisol or the cortisol response to acute stress between SS, MSR or HSR monkeys (Herod and Cameron, 2007). This information leads to the speculation that the lack of difference in CRH expression in the rostral PVN may underlie the lack of difference in normal diurnal HPA axis activity or the HPA axis activation in response to acute stress in monkeys that differ in responsiveness to stress-induced reproductive dysfunction. Instead, the elevation in CRH expression in the caudal PVN, thalamus and amygdala of SS animals may be communicating with other brain areas.
Our observation of more CRH neurons in the caudal region of the PVN in SS animals is consistent with the report that depressed patients exhibit 4 times more CRH neurons than controls (Raadsheer et al., 1994). Although careful volumetric studies are needed, it appears that the PVN extends further in the caudal direction in the SS animals. This observation is supported by an earlier study reporting a larger PVN in depressed patients (Bao et al., 2005). The caudal PVN CRH neurons may play a role in the regulation of the serotonin system, which is compromised in SS animals.
The amygdala is a pivotal site of extrahypothalamic production of CRH, and CRH fibers, which project to the bed nucleus of the stria terminalis, are prominent in the central nucleus of the amygdala (Bassett and Foote, 1992). The central nucleus plays a crucial role in mediating fear and anxiety in primates (Kalin et al., 2004) and an oral CRH-R1 antagonist decreases stereotypical mouth movements in a behavioral test of anxiety in macaques (He et al., 2000). Also, infusion of a CRH-R1 antisense oligonucleotide into the central nucleus has been shown to reduce anxiety behaviors in rats (Liebsch et al., 1995). Moreover, the amygdala sends a CRH projection to the dorsal raphe (Peyron et al., 1998), which regulates serotonin neurons (Summers et al., 2003).
CRH and the urocortins influence several aspects of serotonergic neurotransmission, including the release and synthesis of serotonin as well as the firing rate of serotonin neurons (Oshima et al., 2003; Linthorst, 2005). Knockout of CRH-R1 results in enhanced synthesis of serotonin, suggesting that CRH decreases serotonin through the R1 receptor (Penalva et al., 2002). It was recently reported that blocking CRH action in the median raphe nucleus decreased c-fos expression in the central nucleus of the amygdala, further emphasizing the reciprocal connectivity of these regions (Funk et al., 2003). Thus, our observation that CRH is higher in SS animals than in MSR or HSR animals suggests that there would be an increase in CRH release in the raphe nucleus, which could decrease serotonin function, leading to dysfunction of numerous autonomic systems, including reproduction.
CRH mRNA was elevated in the CM-Sf of the thalamus of SS animals. This area corresponds to the center median-parafascicular complex in humans. A body of literature suggests that CRH neurons in the CM-Sf complex may participate in processing somatosensory and visceral information related to stress. (Kaelber and Mitchell, 1975; Kaelber et al., 1975; Fuchs and Siegel, 1984; Hsu et al., 2001; Parent and Parent, 2005).
Another important neuropeptide that inhibits GnRH neurons is β-endorphin (Van Vugt et al., 1984; Ferin, 1987). Moreover, the suppressive effect of CRH on LH secretion is abolished by the opiate antagonist, naloxone, indicating that CRH increasesβ-endorphin, which in turn, inhibits LH secretion (Gindoff and Ferin, 1987). Therefore, we hypothesized that SS animals may have elevated expression of POMC mRNA, which codes for the precursor protein of β-endorphin. However, the data render this hypothesis null. There was no significant difference in POMC mRNA expression between the SS, MSR and HSR groups. Nonetheless, we cannot rule out a difference in post-translational processing of the POMC protein, which could lead to differences in the release of β-endorphin peptide. Also, this observation does not rule out differences in the release of β-endorphin under stress, and we are currently examining this possibility.
In summary, under non-stressed conditions, animals that have previously exhibited increased sensitivity to the suppression of reproductive function during stress have significant differences in their brains compared to animals that maintain reproductive function during stress. They have fewer serotonin neurons and reduced expression of serotonin-related genes, they have higher expression of 5HT2A and 5HT2C receptor mRNA in hypothalamic terminal fields, they have higher expression of GAD67 in GABA neurons adjacent to GnRH neurons, they have higher levels of CRH mRNA and protein in the caudal PVN, higher levels of CRH mRNA in the CM-Sf and a greater density of CRH fibers in the central nucleus of the amygdala. However, they show no difference in 5HT1A mRNA expression in the hypothalamic ventromedial nucleus, which is not a neuroendocrine area, and they show no difference in POMC expression in the arcuate/infundibular region where GnRH neurons reside in macaques. Our next goal was to examine the GnRH system, the final link in the control of reproductive function.
VI. Hypothalamic GnRH neuron function
Introduction
GnRH is the neuropeptide that controls the secretion of pituitary LH and FSH, which in turn regulate the production of ovarian estrogen and progesterone, and promote ovulation. The regulation of GnRH is complex, involving both negative and positive feedback through estrogen. Negative feedback involves estrogenic suppression of GnRH, thus removal of the ovaries results in elevated LH. Positive feedback involves the ability of high levels of estrogen to stimulate GnRH, thus producing the preovulatory surge of LH. However, estrogen may act on GnRH neurons via ERβ and act on upstream neural circuits, which impinge on GnRH neurons to achieve the different regulatory components (Herbison, 1998). Since ovulation is the endpoint by which we determined stress sensitivity, and since GnRH is the pivotal neuropeptide governing the HPG axis, we questioned whether the GnRH system was also showing suboptimal function in SS monkeys. To do this, we examined the expression of GnRH mRNA by in situ hybridization, and the expression of GnRH peptide by immunohistochemistry in the mediobasal hypothalamus of SS and HSR monkeys.
Methods
Only 3 animals per group had complete representation of the median eminence and were considered for this analysis. GnRH riboprobe preparation, in situ hybridization, GnRH immunocytochemistry and image analysis were previously described (Centeno et al., 2007b). Student’s t test was used for statistical analysis.
Results
Figure 15A and B show autoradiograms of GnRH ISH signal in the mediobasal hypothalamus of one representative HSR and one SS monkey, respectively. Because GnRH cells are diffusely located in the mediobasal hypothalamus, the type of discrete signal observed in the autoradiogram cannot be subjected to densitometry film analysis. Therefore, the sections were exposed with emulsion and individual cells, as well as the number of grains per cell expressed as positive pixels, were counted in the dark field images as shown in Figures 15C and D. As illustrated in Figure 16, top and middle panels, SS macaques exhibited more GnRH neurons compared with HSR animals (p<0.05). In addition, SS animals exhibited significantly more neurons with 501–1000 positive pixels per cell and with greater than 2001 positive pixels per cell (*p<0.05).
Figure 15. GnRH mRNA expression in the MBH of high stress-resilient and stress-sensitive monkeys.
(A) Autoradiogram of GnRH in situ hybridization signal in the arcuate nucleus and median eminence of one high stress-resilient (HSR) monkey
(B) Autoradiogram of GnRH in situ hybridization signal in the arcuate nucleus and median eminence of one stress-sensitive (SS) monkey.
(C) Dark field image of GnRH ISH signal after exposure with emulsion in the ARC of one high stress-resilient (HSR) monkey (scale bar = 10 μm).
(D) Dark field image of GnRH in situ hybridization signal after exposure with emulsion in the arcuate nucleus of one stress-sensitive (SS) monkey (scale bar = 10 μm).
Reprinted from Centeno et al., 2006b.
Figure 16. Analysis of the total number of cells expressing GnRH mRNA in the arcuate nucleus and median eminence of HSR and SS monkeys (n=3/group).
Top. The number of neurons expressing GnRH mRNA grains at 3x background was counted in 6 sections through the arcuate nucleus and median eminence. The histogram illustrates the mean (± SEM) of the total number of neurons that express GnRH mRNA.
Middle. The grain over each GnRH neurons were segmented into positive pixels. The histogram illustrates the mean (± SEM) of the total number of GnRH neurons exhibiting different numbers of positive pixels over grains per cell.
Bottom. Histogram of the mean (± SEM) density of GnRH-immunopositive cell bodies in the arcuate nucleus and median eminence of HSR and SS monkeys (n=3/group) expressed as the number of cells per mm3. Six sections at 250μ intervals through the mediobasal hypothalamus were counted and the total number of neurones was obtained for each animal/mm3 (density). The group means were then computed and statistically compared. *p<0.05, Student’s t-test. Reprinted from Centeno et al., 2006b.
GnRH-immunoreactive cells were counted in a montage of the arcuate nucleus and the median eminence using the Marianas Stereology workstation and the Slidebook 4.1 software. The number of GnRH positive neurons per cubic mm was calculated and the results are shown in Figure 16, bottom panel. SS animals exhibited significantly more GnRH-positive cell bodies than HSR animals. Due to the 250-micron distance between the sections, it is unlikely that a GnRH cell body would be present in two sections.
The median eminence was well represented in 3 SS animals and in 3 HSR animals, with each animal exhibiting intact median eminence in 2–4 sections. Individual GnRH beaded fibers were prominent in the median eminence and were segmented into positive pixels by NIH Image software. As shown in Figure 17, HSR animals had a significantly greater number of pixels representing GnRH fiber immunostaining than did SS animals (p<0.05).
Figure 17.
Histogram illustrating the average of the positive pixels in a sample area of GnRH fibres in the median eminence of HSR and SS monkeys (SS). (n=3/group; *p<0.05, Student’s t-test). Reprinted from Centeno et al., 2006b.
Of note, the average cycle length for the 8 months preceding euthanasia was obtained for each animal. Each animal had exhibited 6 or more menses in the 8-month period preceding euthanasia. The three animals in the HSR group exhibited cycle lengths of 31.8±0.6, 31.5±4.0 and 28.9±0.2, respectively. The three animals in the SS group exhibited cycle lengths of 38.2±9.3, 34.0±4.02 and 34.4±1.3, respectively (not different between groups).
Discussion
In our model of hypothalamic amenorrhea, the end point of our stress assessment is ovulation, which is dependent upon proper function of the HPG axis. The pivotal peptide controlling this axis is GnRH. Therefore, we questioned whether the GnRH system was altered in SS versus HSR individuals, either with regard to synthesis of GnRH or secretion of GnRH. We hypothesized that both synthesis and secretion may differ between SS and HSR groups or that synthesis may be unimpaired in SS animals but that GnRH secretion could be impaired as a result of differences in neural input to GnRH neurons.
In the present study, we found that the mediobasal hypothalamus of SS monkeys during the early follicular phase of a non-stressed menstrual cycle (before day 5), exhibited an increased number and density of detectable GnRH cell bodies compared to HSR individuals. In addition, the numbers of GnRH neurons with very high GnRH expression were significantly increased in SS animals compared to HSR animals. Lack of detection of GnRH mRNA or protein in soma expressing low levels of GnRH may be the cause of fewer numbers of GnRH neurons in the HSR animals. Regardless of whether there are more GnRH neurons or simply more highly expressing GnRH neurons in SS animals, clearly SS animals have higher overall GnRH mRNA and protein in GnRH cell soma. However, SS monkeys showed a significantly lower density of GnRH-positive fibers in the median eminence suggesting that (a) either the peptide accumulated in the cell bodies and was not transported down the axons or (b) that SS animals were producing and releasing more GnRH, thereby depleting the GnRH fibers. As discussed below, we have evidence to support the first explanation, rather than the second. Moreover, we speculate that with stress, these differences become even more exaggerated.
Earlier studies have reported similar changes in GnRH neurons after stress exposure in rats and sheep. Intermittent electric shocks suppressed GnRH secretion and ovulation in ewes (Przekop et al., 1988). The hypothalamus of those animals showed a reduction of GnRH-immunoreactive fibers in the median eminence, but an increased density of GnRH–immunoreactive cell bodies in the preoptic area (Przekop et al., 1988). The same result was obtained after nutritional stress (Polkowska, 1996) or CRF treatment (Polkowska and Przekop, 1997). Moreover, increased activity of the amygdala reduced the density of hypothalamic GnRH-immunoreactive fibers in rats (Amado et al., 1993; Friedman et al., 2002). All of these reports suggested that reduced GnRH transport might be responsible for the higher concentration of the peptide in the cell bodies, but lower density in median eminence fibers, as we observed in SS monkeys.
There are other important physiological states in which there is discordance between hypothalamic GnRH and LH secretion. For example, LH is high in neonatal monkeys and then becomes suppressed in juvenile monkeys until the onset of puberty. However, there is no difference in hypothalamic GnRH mRNA or protein when neonatal and juvenile male or female macaques have been compared, although pituitary and serum LH are markedly reduced in juveniles (Fraser et al., 1989; Ma et al., 1994; El Majdoubi et al., 2000).
The observed association between lower peak E production and higher GnRH mRNA in the SS monkeys is also consistent with other reports. Estradiol treatment of gonadectomized macaques reduced GnRH mRNA expression (El Majdoubi et al., 1998), suggesting that the lower secretion of E in the SS animals may contribute to the higher expression of GnRH. Krajewski et al. reported three subtypes of GnRH neurons in cynomolgus monkeys, and estradiol treatment of ovariectomized cynomolgus monkeys reduced GnRH mRNA expression in type I GnRH neurons (Krajewski et al., 2003). Higher levels of GnRH gene expression in type I GnRH neurons were also reported by this same group in postmenopausal women (Rance and Uswandi, 1996). In the present study, we found significantly more cells with greater than 2000 GnRH pixels/cell in SS animals, compared with HSR animals. Based on the size, mRNA content and localization, we speculate that the cells with greater than 2000 GnRH pixels/cell represent the type I GnRH neurons. Thus, the lower levels of E production in the SS monkeys may contribute to the higher levels of GnRH mRNA expression observed in this study. In turn, dysregulation of transport could result in decreased GnRH release; decreased LH and FSH release and compromised ovarian estradiol secretion, thereby establishing a minimally effective HPG axis.
Alternatively, the GnRH neurons of the SS individuals may be producing and secreting more GnRH which would also be manifested by higher GnRH mRNA and lower GnRH fiber content. We are currently conducting studies of pulsatile LH secretion in monkeys with different sensitivity to stress and our preliminary data indicate that during an 8-hour period in the follicular phase, the SS animals have fewer than half the number of LH pulses than do the HSR animals (Herod and Cameron, 2007). Thus, it is likely that the lower GnRH fiber staining in SS animals is due to reduced transport and release of GnRH.
Although it has been reported that GnRH neurons express ERβ (Skynner et al., 1999), the effects of E on GnRH neurons have been mainly attributed to actions via interneurons. GABA has been proposed as a pivotal mediator in the steroid feedback that modulates GnRH secretion (Leonhardt et al., 1999; Sullivan and Moenter, 2005). A body of evidence shows thatGABA acts through GABAA receptors within the vicinity of theGnRH neuron soma to inhibit LH secretion (Herbison et al., 1991; Jarry et al., 1991; Scott and Clarke, 1993; Mitsushima et al., 1994) and GnRH neurons express GABAA receptors that may inhibit GnRH neuronal excitability (Spergel et al., 1999; Han et al., 2004). Our observation of increased GAD67 is consistent with an inhibitory effect of GABA on GnRH processing.
The CRF system is also a potential candidate mediating the stress-related suppression of the reproductive axis. Activity of the hypothalamic-pituitary-adrenal (HPA) axis and cortisol are elevated in many stress conditions that are associated with reproductive dysfunction, including Functional Hypothalamic Amenorrhea (Barbarino et al., 1989; Biller et al., 1990; Berga et al., 1997; Brundu et al., 2006). Antagonism of CRF has been reported to prevent reproductive failure in stressed male rats (Rivier et al., 1986). However, other studies have found no causality between cortisol or CRF and LH secretion in various stress models (Helmreich et al., 1993; Tsukamura et al., 1993; Jeong et al., 1999) and evidence of direct synaptic contact between CRF and GnRH neurons is lacking (Hahn et al., 2003). In studies underway, we find little relation between basal cortisol or acute stress-induced cortisol and stress sensitivity status (Herod and Cameron, unpublished data).
In summary, we found that monkeys sensitive to stress-induced reproductive dysfunction showed a higher number and density of GnRH cell bodies and a greater number of GnRH neurons with robust GnRH expression, but a lower density of GnRH fibers in the median eminence, compared with stress-resilient individuals. The mechanisms leading to these observations remain to be defined. However, it is possible that the lower preovulatory estrogen surge leads to greater GnRH expression in the soma, but inhibitory neurotransmitters decrease action potentials within GnRH neurons, which may be coupled to peptide transport (Chytrova and Johnson, 2004).
VII. Summary
Individual monkeys who exhibit an immediate cessation of ovulation upon exposure to a combined metabolic and psychosocial stress have altered neural expression of pivotal genes in pathways integrating stress and reproductive function. These alterations are present when the individuals are not stressed and probably play a role in the rapid shutdown of the reproductive system when stress is administered. We found that SS animals have compromised serotonin function. They release less serotonin as indicated by prolactin secretion when administered the serotonin-releasing agent, fenfluramine. Upon examination of serotonin related gene expression in the dorsal raphe nucleus, we found that compared to HSR animals, SS animals express significantly lower amounts of TPH2, SERT, and MAO-A mRNAs with a similar trend in 5HT1A and MAO-B. Moreover, the expression of each of these transcripts is positively correlated with the peak of progesterone during a control, non-stressed menstrual cycle. That is, the higher the peak of progesterone, the higher the expression of each transcript. Gene expression for the serotonin 2A and 2C receptors was increased in SS animals compared to HSR animals, which is consistent with the presumed lower serotonin afferent input to the PVN and infundibular nucleus. However, 5HT1A receptor expression in the VMN, a non-neuroendocrine nucleus, was not different between sensitivity groups. In addition, GAD67 mRNA expression was significantly higher in the infundibulum of SS animals suggesting that inhibitory GABAergic output within this reproductive center is higher in SS animals than HSR animals. Examination of CRH gene and protein expression revealed some remarkable differences between SS and HSR animals. SS animals apparently have a larger PVN that extends further in the caudal direction. In this PVN extension, CRF neurons are prevalent and there is higher expression of CRH mRNA and protein in the PVN due to this extension. The caudal PVN projects to the amygdala and midbrain, but not to the median eminence. Indeed, there were more CRH fibers in the central nucleus of the amygdala in SS animals compared to HSR animals. Reasoning from this information, it appears that SS animals have chronic excess CRH, which may impact serotonergic and amygdalar functions. No difference was observed in POMC mRNA expression in the infundibulum under non-stress conditions. The GnRH system was the final common pathway leading to cessation of ovulation. Curiously, there were more GnRH neurons detected in the SS animals than in the HSR animals. However, there were fewer GnRH positive fibers in the median eminence of SS animals. Together the data suggest that GnRH transport is compromised in SS animals, but how this is accomplished requires further investigation. Altogether, this knowledge suggests that there are profound differences in the function of neural systems in stress sensitive individuals. They appear to be primed for robust physiological and neurobiological responses to stress, which in our model, translates to rapid cessation of reproductive function. Studies are underway to determine gene expression in these systems during stress and to determine if selective serotonin inhibitors or CRH antagonists can prevent the stress sensitive response of the reproductive system.
Acknowledgments
This research was supported by Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD 18185 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, NIH/MH62677 to CLB and NIH/RR00163 for the operation of ONPRC.
The authors are indebted to the Division of Animal Resources at ONPRC for the expert care of the animals and to the Department of Pathobiology for performing the necropsies. In addition, we would like to acknowledge Mr. Sam Fox who conducted the fenfluramine, CRH and TRH challenge experiments; Dr. Nick Lu who perfused the brains, Mr. John Streicher who sectioned the midbrains, Ms. Rachel Sanchez who performed the immunocytochemical analysis of the CRH fibers in the amygdala and Ms. Arubala Reddy who assisted with the construction of the CRH cDNA.
Literature Cited
- Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, Bolwig TG, Knudsen GM. Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8:391–401. doi: 10.1017/S1461145705005055. [DOI] [PubMed] [Google Scholar]
- Aiello-Zaldivar M, Luine V, Frankfurt M. 5,7-DHT facilitated lordosis: effects of 5-HT agonists. Neuroreport. 1992;3:542–544. doi: 10.1097/00001756-199206000-00024. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lembo P, Storring JM, Charest A, Saucier C. The 5-HT1A receptor: signaling, desensitization, and gene transcription. Neuropsychopharmacology. 1996;14:19–25. doi: 10.1016/S0893-133X(96)80055-8. [DOI] [PubMed] [Google Scholar]
- Aletrino MA, Vogels OJM, Van Domburg PHMF, Ten Donkelaar HJ. Cell loss in the nucleus raphes dorsalis in Alzeheimer’s disease. Neurobiol Aging. 1992;13:461–468. doi: 10.1016/0197-4580(92)90073-7. [DOI] [PubMed] [Google Scholar]
- Amado D, Cavalheiro EA, Bentivoglio M. Epilepsy and hormonal regulation: the patterns of GnRH and galanin immunoreactivity in the hypothalamus of epileptic female rats. Epilepsy Res. 1993;14:149–159. doi: 10.1016/0920-1211(93)90019-4. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Maxwell RA, Geracoti TDJ, Hauger R. Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls. Alcoholism: Clinical and Experimental Research. 2001;25:692–703. [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Arena B, Maffulli N, Maffulli F, Morleo MA. Reproductive hormones and menstrual changes with exercise in female athletes. Sports Med. 1995;19:278–287. doi: 10.2165/00007256-199519040-00005. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Advances in Neurology. 1986;43:407–468. [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal raphe and median raphe nuclei in the rat. Journal of Comparative Neurology. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Baer DS, Crumpacker DW. Fertility and offspring survival in mice selected for different sensitivities to alcohol. Behav Genet. 1977;7:95–103. doi: 10.1007/BF01065999. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Makara GB. Hypothalamic paraventricular nucleus lesions differentially affect serotonin-1A (5HT-1A) and 5HT2 receptor agonist-induced oxytocin, prolactin and corticosterone responses. Endocrinology. 1994;143:1127–1131. doi: 10.1210/endo.134.3.8119151. [DOI] [PubMed] [Google Scholar]
- Bao AM, Hestiantoro A, Van Someren EJ, Swaab DF, Zhou JN. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain. 2005;128:1301–1313. doi: 10.1093/brain/awh448. [DOI] [PubMed] [Google Scholar]
- Barbarino A, De Marinis L, Folli G, Tofani A, Della Casa S, D’Amico C, Mancini A, Corsello SM, Sambo P, Barini A. Corticotropin-releasing hormone inhibition of gonadotropin secretion during the menstrual cycle. Metabolism. 1989;38:504–506. doi: 10.1016/0026-0495(89)90208-4. [DOI] [PubMed] [Google Scholar]
- Bassett JL, Foote SL. Distribution of corticotropin-releasing factor-like immunoreactivity in squirrel monkey (Saimiri sciureus) amygdala. J Comp Neurol. 1992;323:91–102. doi: 10.1002/cne.903230108. [DOI] [PubMed] [Google Scholar]
- Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertility & Sterility. 1997;67:1024–1030. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- Berga SL, Girton LG. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatric Clinics of North America. 1989;12:105–116. [PubMed] [Google Scholar]
- Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–981. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- Bethea CL. Regulation of progestin receptors in raphe neurons of steroid-treated monkeys. Neuroendocrinology. 1994;60:50–61. doi: 10.1159/000126719. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Brown NA, Kohama SG. Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology. 1996;137:4372–4383. doi: 10.1210/endo.137.10.8828498. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005a;83:148–155. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Pecins-Thompson M, Schutzer WE, Gundlah C, Lu ZN. Ovarian steroids and serotonin neural function. Molecular Neurobiology. 1999;18:87–123. doi: 10.1007/BF02914268. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005b;132:151–166. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Whale R, Cowen PJ. State and trait abnormalities in serotonin function in major depression. British Journal of Psychiatry. 2002;180:24–28. doi: 10.1192/bjp.180.1.24. [DOI] [PubMed] [Google Scholar]
- Biller BMK, Federoff HJ, Koenig JI, Klibanski A. Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. Journal of Clinical Endocrinology and Metabolism. 1990;70:311–317. doi: 10.1210/jcem-70-2-311. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear MA, Sanders-Bush E. Serotonin receptor sensitivity after acute and chronic treatment with mianserin. J Pharmacol Exp Ther. 1982;221:303–308. [PubMed] [Google Scholar]
- Blundell JE, Hill AJ. Serotoninergic modulation of the pattern of eating and the profile of hunger-satiety in humans. Int J Obes. 1987;11(Suppl 3):141–155. [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Botchin MB, Kaplan JR, Manuck SB, Mann JJ. Neuroendocrine responses to fenfluramine challenge are influenced by exposure to chronic social stress in adult male cynomolgus macaques. Psychoneuroendocrinology. 1994;19:1–11. doi: 10.1016/0306-4530(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006;91:1561–1565. doi: 10.1210/jc.2005-2422. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Reproductive dysfunction in primates, behaviorally induced. In: Fink G, editor. Encyclopedia of Stress. New York: Academic Press; 2000. pp. 366–372. [Google Scholar]
- Cameron JL, Bridges MW, Graham RE, Bench L, Berga SL, Matthews K. Basal heart rate predicts development of reproductive dysfunction in response to psychological stress. 80th Annual Meeting of the Endocrine Society; 1998. pp. 1–76. [Google Scholar]
- Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 1991;128:1532–1540. doi: 10.1210/endo-128-3-1532. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29:777–784. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic expression of serotonin 1A, 2A and 2C receptors and GAD67 in female cynomolgus monkeys with different sensitivity to stress. Brain Research. 2007a;1142:1–12. doi: 10.1016/j.brainres.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, CLB Hypothalamic GnRH expression in female monkeys with different sensitivity to stress. J Neuroendocrinology. 2007b;19:1–11. doi: 10.1111/j.1365-2826.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology. 2007c;86:277–288. doi: 10.1159/000109877. [DOI] [PubMed] [Google Scholar]
- Champlin AK. Suppression of oestrus in grouped mice: the effects of various densities and the possible nature of the stimulus. J Reprod Fertil. 1971;27:233–241. doi: 10.1530/jrf.0.0270233. [DOI] [PubMed] [Google Scholar]
- Chytrova G, Johnson JE. Spontaneous retinal activity modulates BDNF trafficking in the developing chick visual system. Mol Cell Neurosci. 2004;25:549–557. doi: 10.1016/j.mcn.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Hoplight BJ, Neumaier JF. Chronic low dose ovine corticotropin releasing factor or urocortin II into the rostral dorsal raphe alters exploratory behavior and serotonergic gene expression in specific subregions of the dorsal raphe. Neuroscience. 2007;146:1888–1905. doi: 10.1016/j.neuroscience.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare AJ, Murray RM, O’Keane V. Assessment of sertonergic function in major depression using d-fenfluramine: relation to clinical variables and antidepressant response. Biological Psychiatry. 1998;44:555–561. doi: 10.1016/s0006-3223(98)00018-3. [DOI] [PubMed] [Google Scholar]
- Collins RL, Williams RF, Hodgen GD. Endocrine consequences of prolonged ovarian hyperstimulation: hyperprolactinemia, follicular atresia, and premature luteinization. Fertility and Sterility. 1984a;42:436–445. [PubMed] [Google Scholar]
- Collins RL, Williams RF, Hodgen GD. Human menopausal gonadotropin/human chorionic gonadotropin-induced ovarian hyperstimulation with transient hyperprolactinemia: Steroidogenesis enhanced during bromocriptine therapy in monkeys. Journal of Clinical Endocrinology & Metabolism. 1984b;55:727–733. doi: 10.1210/jcem-59-4-727. [DOI] [PubMed] [Google Scholar]
- De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, Lasley BL. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- Delville Y, Stires C, Ferris CF. Distribution of corticotropin-releasing hormone immunoreactivity in golden hamster brain. Brain Res Bull. 1992;29:681–684. doi: 10.1016/0361-9230(92)90138-n. [DOI] [PubMed] [Google Scholar]
- Dobson H, Ghuman S, Prabhakar S, Smith R. A conceptual model of the influence of stress on female reproduction. Reproduction. 2003;125:151–163. doi: 10.1530/rep.0.1250151. [DOI] [PubMed] [Google Scholar]
- Dobson H, Smith RF. What is stress, and how does it affect reproduction? Anim Reprod Sci. 2000;60–61:743–752. doi: 10.1016/s0378-4320(00)00080-4. [DOI] [PubMed] [Google Scholar]
- Dudas B, Merchenthaler I. Close juxtapositions between luteinizing hormone-releasing hormone-immunoreactive neurons and corticotropin-releasing factor-immunoreactive axons in the human diencephalon. J Clin Endocrinol Metab. 2002;87:5778–5784. doi: 10.1210/jc.2002-020996. [DOI] [PubMed] [Google Scholar]
- Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26:77–89. doi: 10.1016/s0306-4530(00)00040-8. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583:186–193. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Majdoubi M, Sahu A, Plant TM. Effect of estrogen on hypothalamic transforming growth factor alpha and gonadotropin-releasing hormone gene expression in the female rhesus monkey. Neuroendocrinology. 1998;67:228–235. doi: 10.1159/000054318. [DOI] [PubMed] [Google Scholar]
- El Majdoubi M, Sahu A, Plant TM. Changes in hypothalamic gene expression associated with the arrest of pulsatile gonadotropin-releasing hormone release during infancy in the agonadal male rhesus monkey (Macaca mulatta) Endocrinology. 2000;141:3273–3277. doi: 10.1210/endo.141.9.7687. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- Fargin A, Yamamoto K, Cotecchia S, Goldsmith PK, Spiegel AM, Lapetina EG, Caron MG, Lefkowitz RJ. Dual coupling of the cloned 5-HT1A receptor to both adenylyl cyclase and phospholipase C is mediated via the same Gi protein. Cell Signal. 1991;3:547–557. doi: 10.1016/0898-6568(91)90031-o. [DOI] [PubMed] [Google Scholar]
- Feng YJ, Shalts E, Xia LN, Rivier J, Rivier C, Vale W, Ferin M. An inhibitory effects of interleukin-1a on basal gonadotropin release in the ovariectomized rhesus monkey: reversal by a corticotropin-releasing factor antagonist. Endocrinology. 1991;128:2077–2082. doi: 10.1210/endo-128-4-2077. [DOI] [PubMed] [Google Scholar]
- Ferin M. A role for the endogenous opioid peptides in the regulation of gonadotropin secretion in the primate. Horm Res. 1987;28:119–125. doi: 10.1159/000180935. [DOI] [PubMed] [Google Scholar]
- Ferin M. Neuropeptides, the stress response, and the hypothalamo-pituitary-gonadal axis in the female rhesus monkey. Ann N Y Acad Sci. 1993;697:106–116. doi: 10.1111/j.1749-6632.1993.tb49927.x. [DOI] [PubMed] [Google Scholar]
- Ferin M. The antireproductive role of corticotropin releasing hormone and interleukin-1 in the female rhesus monkey. Ann Endocrinol (Paris) 1995;56:181–186. [PubMed] [Google Scholar]
- Ferin M, Vande Wiele R. Endogenous opioid peptides and the control of the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 1984;18:365–373. doi: 10.1016/0028-2243(84)90059-5. [DOI] [PubMed] [Google Scholar]
- Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Increase in expression of brain serotonin transporter and monoamine oxidase genes induced by repeated experience of social defeats in male mice. Biochemistry. 2002;67:451–455. doi: 10.1023/a:1015238124000. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Malone KM, Li S, Harrison WM, McBride PA, Endicott J, Cooper T, Mann JJ. Blunted serotonin response to fenfluramine challenge in premenstrual dysphoric disorder. American Journal of Psychiatry. 2003;154:556–558. doi: 10.1176/ajp.154.4.556. [DOI] [PubMed] [Google Scholar]
- Fraser MO, Pohl CR, Plant TM. The hypogonadotropic state of the prepubertal male rhesus monkey (Macaca mulatta) is not associated with a decrease in hypothalamic gonadotropin-releasing hormone content. Biol Reprod. 1989;40:972–980. doi: 10.1095/biolreprod40.5.972. [DOI] [PubMed] [Google Scholar]
- Friedman MN, Geula C, Holmes GL, Herzog AG. GnRH-immunoreactive fiber changes with unilateral amygdala-kindled seizures. Epilepsy Res. 2002;52:73–77. doi: 10.1016/s0920-1211(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Frim DM, Robinson BG, Pasieka KB, Majzoub JA. Differential regulation of corticotropin-releasing hormone mRNA in rat brain. Am J Physiol. 1990;258:E686–692. doi: 10.1152/ajpendo.1990.258.4.E686. [DOI] [PubMed] [Google Scholar]
- Fuchs SA, Siegel A. Neural pathways mediating hypothalamically elicited flight behavior in the cat. Brain Res. 1984;306:263–281. doi: 10.1016/0006-8993(84)90376-7. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Shaham Y, Le AD. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122:1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Gindoff PR, Ferin M. Endogenous opioid peptides modulate the effect of corticotropin-releasing factor on gonadotropin release in the primate. Endocrinology. 1987;121:837–842. doi: 10.1210/endo-121-3-837. [DOI] [PubMed] [Google Scholar]
- Gos T, Becker K, Bock J, Malecki U, Bogerts B, Poeggel G, Braun K. Early neonatal and postweaning social emotional deprivation interferes with the maturation of serotonergic and tyrosine hydroxylase-immunoreactive afferent fiber systems in the rodent nucleus accumbens, hippocampus and amygdala. Neuroscience. 2006;140:811–821. doi: 10.1016/j.neuroscience.2006.02.078. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5HT in stress, anxiety and depression. Pharmacology, Biochemistry and Behavior. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Pecins-Thompson M, Schutzer WE, Bethea CL. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Molecular Brain Research. 1999;63:325–339. doi: 10.1016/s0169-328x(98)00295-2. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci. 2002;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Nemeroff CB. Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav. 2003;79:471–478. doi: 10.1016/s0031-9384(03)00166-5. [DOI] [PubMed] [Google Scholar]
- Hahn JD, Kalamatianos T, Coen CW. Studies on the neuroanatomical basis for stress-induced oestrogen-potentiated suppression of reproductive function: evidence against direct corticotropin-releasing hormone projections to the vicinity of luteinizing hormone-releasing hormone cell bodies in female rats. J Neuroendocrinol. 2003;15:732–742. doi: 10.1046/j.1365-2826.2003.01056.x. [DOI] [PubMed] [Google Scholar]
- Halliday G, Ellis J, Heard R, Caine D, Harper C. Brainstem serotonergic neurons in chronic alcoholics with and without the memory impairment of Korsakoff’s psychosis. J Neuropathol Exp Neurol. 1993;52:567–579. doi: 10.1097/00005072-199311000-00003. [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE. Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology. 2004;145:495–499. doi: 10.1210/en.2003-1333. [DOI] [PubMed] [Google Scholar]
- He L, Gilligan PJ, Zaczek R, Fitzgerald LW, McElroy J, Shen HS, Saye JA, Kalin NH, Shelton S, Christ D, Trainor G, Hartig P. 4-(1,3-Dimethoxyprop-2-ylamino)-2,7-dimethyl-8-(2, 4-dichlorophenyl)pyrazolo[1,5-a]-1,3,5-triazine: a potent, orally bioavailable CRF(1) receptor antagonist. J Med Chem. 2000;43:449–456. doi: 10.1021/jm9904351. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Mattern LG, Cameron JL. Lack of a role of the hypothalamic-pituitary-adrenal axis in the fasting-induced suppression of luteinizing hormone secretion in adult male rhesus monkeys (Macaca mulatta) Endocrinology. 1993;132:2427–2437. doi: 10.1210/endo.132.6.8389280. [DOI] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocrine Reviews. 1998;3:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Chapman C, Dyer RG. Role of medial preoptic GABA neurones in regulating luteinising hormone secretion in the ovariectomised rat. Exp Brain Res. 1991;87:345–352. doi: 10.1007/BF00231851. [DOI] [PubMed] [Google Scholar]
- Herod S, Cameron JL. Female monkeys sensitive to stress-induced reproductive dysfunction have low central drive to the reproductive axis in nonstressed conditions. Annual Meeting of the Endocrine Society; Toronto, Canada. 2007. [Google Scholar]
- Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Lombardo KA, Herringa RJ, Bakshi VP, Roseboom PH, Kalin NH. Corticotropin-releasing hormone messenger RNA distribution and stress-induced activation in the thalamus. Neuroscience. 2001;105:911–921. doi: 10.1016/s0306-4522(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiolog Rev. 1992;72:165–231. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144:3663–3676. doi: 10.1210/en.2002-0188. [DOI] [PubMed] [Google Scholar]
- Jarry H, Leonhardt S, Wuttke W. Gamma-aminobutyric acid neurons in the preoptic/anterior hypothalamic area synchronize the phasic activity of the gonadotropin-releasing hormone pulse generator in ovariectomized rats. Neuroendocrinology. 1991;53:261–267. doi: 10.1159/000125727. [DOI] [PubMed] [Google Scholar]
- Jeong KH, Jacobson L, Widmaier EP, Majzoub JA. Normal suppression of the reproductive axis following stress in corticotropin-releasing hormone-deficient mice. Endocrinology. 1999;140:1702–1708. doi: 10.1210/endo.140.4.6669. [DOI] [PubMed] [Google Scholar]
- Kaelber WW, Mitchell CL. Alteration in escape responding in the cat. A lesion and degeneration and comparison following stimulation studies. Brain Behav Evol. 1975;12:137–150. doi: 10.1159/000124399. [DOI] [PubMed] [Google Scholar]
- Kaelber WW, Mitchell CL, Yarmat AJ, Afifi AK, Lorens SA. Centrum-medianum--parafascicularis lesions and reactivity to noxious and non-noxious stimuli. Exp Neurol. 1975;46:282–290. doi: 10.1016/0014-4886(75)90135-1. [DOI] [PubMed] [Google Scholar]
- Kajantie E. Fetal origins of stress-related adult disease. Ann NY Acad Sci. 2006;1083:11–27. doi: 10.1196/annals.1367.026. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Biol Psychiatry. 2000;47:579–585. doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Boyar R, Roffwarg H, Hellman L, Weiner H. Weight and circadian luteinizing hormone secretory pattern in anorexia nervosa. Psychosom Med. 1978;40:549–567. doi: 10.1097/00006842-197811000-00003. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Bailer UF, Frank GK, Wagner A, Henry SE. Brain imaging of serotonin after recovery from anorexia and bulimia nervosa. Physiol Behav. 2005;86:15–17. doi: 10.1016/j.physbeh.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Keegan CE, Herman JP, Karolyi IJ, O’Shea KS, Camper SA, Seasholtz AF. Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology. 1994;134:2547–2555. doi: 10.1210/endo.134.6.8194481. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons-adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biological Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Abel TW, Voytko ML, Rance NE. Ovarian steroids differentially modulate the gene expression of gonadotropin-releasing hormone neuronal subtypes in the ovariectomized cynomolgus monkey. J Clin Endocrinol Metab. 2003;88:655–662. doi: 10.1210/jc.2002-020887. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Yen SS. Nutritional and endocrine-metabolic aberrations in amenorrheic athletes. J Clin Endocrinol Metab. 1996;81:4301–4309. doi: 10.1210/jcem.81.12.8954031. [DOI] [PubMed] [Google Scholar]
- Leadem CA, Kalra SP. Reversal of beta-endorphin-induced blockade of ovulation and luteinizing hormone surge with prostaglandin E2. Endocrinology. 1985;117:684–689. doi: 10.1210/endo-117-2-684. [DOI] [PubMed] [Google Scholar]
- Lenz HJ. Extrapituitary effects of corticotropin-releasing factor. Hormone and Metabolic Research. 1987;16(Suppl):17–23. [PubMed] [Google Scholar]
- Leonhardt S, Shahab M, Luft H, Wuttke W, Jarry H. Reduction of luteinizing hormone secretion induced by long-term feed restriction in male rats is associated with increased expression of GABA-synthesizing enzymes without alterations of GnRH gene expression. J Neuroendocrinol. 1999;11:613–619. doi: 10.1046/j.1365-2826.1999.00377.x. [DOI] [PubMed] [Google Scholar]
- Li XF, Bowe JE, Lightman SL, O’Byrne KT. Role of corticotropin-releasing factor receptor-2 in stress-induced suppression of pulsatile lutenizing hormone secretion in the rat. Endocrinology. 2005;146:318–322. doi: 10.1210/en.2004-0950. [DOI] [PubMed] [Google Scholar]
- Li XF, Edward J, Mitchell JC, Shao B, Bowes JE, Coen CW, Lightman SL, O’Byrne KT. Differential effects of repeated restraint stress on pulsatile lutenizing hormone secretion in female Fischer, Lewis and Wistar rats. J Neuroendocrinol. 2004;16:620–627. doi: 10.1111/j.1365-2826.2004.01209.x. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- Linthorst AC. Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol. 2005:181–204. doi: 10.1007/3-540-28082-0_7. [DOI] [PubMed] [Google Scholar]
- Loucks AB, Laughlin GA, Mortola JF, Girton L, Nelson JC, Yen SS. Hypothalamic-pituitary-thyroidal function in eumenorrheic and amenorrheic athletes. J Clin Endocrinol Metab. 1992;75:514–518. doi: 10.1210/jcem.75.2.1639953. [DOI] [PubMed] [Google Scholar]
- Luiten PG, ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res. 1985;329:374–378. doi: 10.1016/0006-8993(85)90554-2. [DOI] [PubMed] [Google Scholar]
- Ma YJ, Costa ME, Ojeda SR. Developmental expression of the genes encoding transforming growth factor alpha and its receptor in the hypothalamus of female rhesus macaques. Neuroendocrinology. 1994;60:346–359. doi: 10.1159/000126769. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, Leranth C. Immunocytochemical evidence for direct synaptic connections between corticotrophin-releasing factor (CRF) and gonadotrophin-releasing hormone (GnRH)-containing neurons in the preoptic area of the rat. Brain Res. 1988;439:391–395. doi: 10.1016/0006-8993(88)91501-6. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, NAG, Zarate CA, Jr, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry. 1996;153:174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- Mann JJ, McBride PA, Malone KM, DeMeo M, Keilp J. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertility & Sterility. 2001;76:310–316. doi: 10.1016/s0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blangero J, Rogers J, Mahaney MC, Hixson JE, Carey KD, Morin PA, Comuzzie AG. A quantitative trait locus influencing estrogen levels maps to a region homologous to human chromosome 20. Physiological Genomics. 2001;5:75–80. doi: 10.1152/physiolgenomics.2001.5.2.75. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Flory JD, Muldoon MF, Manuck SB. Does socioeconomic status relate to central serotonergic responsivity in healthy adults? Psychosom Med. 2000;62:231–237. doi: 10.1097/00006842-200003000-00015. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The End of Stress As We Know It. Joseph Henry Press; Washington, DC: 2002. [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF., III Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology. 1998;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Millington WR, Blum M, Knight R, Mueller GP, Roberts JL, O’Donohue TL. A diurnal rhythm in proopiomelanocortin messenger ribonucleic acid that varies concomitantly with the content and secretion of beta-endorphin in the intermediate lobe of the rat pituitary. Endocrinology. 1986;118:829–834. doi: 10.1210/endo-118-2-829. [DOI] [PubMed] [Google Scholar]
- Mirkes SJ, Bethea CL. Oestrogen, progesterone and serotonin converge on GABAergic neurones in the monkey hypothalamus. Journal Neuroendocrinology. 2001;13:182–192. doi: 10.1046/j.1365-2826.2001.00612.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JC, Li XF, Breen L, Thalabard JC, O’Byrne KT. The role of the locus coeruleus in corticotropin-releasing hormone and stress-induced suppression of pulsatile luteinizing hormone secretion in the female rat. Endocrinology. 2005;146:323–331. doi: 10.1210/en.2004-1053. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Hei DL, Terasawa E. gamma-Aminobutyric acid is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci U S A. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res Bull. 2008;76:493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Brambilla F, Bortolotti F, La Rocca A, Maj M. Prolactin response to d-fenfluramine is blunted in people with anorexia nervosa. British Journal of Psychiatry. 1998;172:439–442. doi: 10.1192/bjp.172.5.439. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Bartley MJ, Wilkinson RG. Family conflict and slow growth. Archives of Disorders of Childhood. 1997;77:326–330. doi: 10.1136/adc.77.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morello H, Caligaris L, Haymal B, Taleisnik S. Inhibition of proestrous LH surge and ovulation in rats evoked by stimulation of the medial raphe nucleus involves a GABA-mediated mechanism. Neuroendocrinology. 1989;50:81–87. doi: 10.1159/000125205. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotropin-releasing factor (CRF-41) in psychological stress in rats. Journal of Physiology. 1993;460:221–229. doi: 10.1113/jphysiol.1993.sp019468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CC. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 2004;38:14–20. [PubMed] [Google Scholar]
- Newman ME, Shapira B, Lerer B. Evaluation of central serotonergic function in affective and related disorders by the fenfluramine challenge test: a critical review. Int J Neuropsychopharmcol. 1998;1:49–69. doi: 10.1017/S1461145798001072. [DOI] [PubMed] [Google Scholar]
- O’Keane V, Dinan TG. Prolactin and cortisol responses to d-fenfluramine in major depression: evidence for diminished responsivity of central serotonergic function. American Journal of Psychiatry. 1991;148:1009–1015. doi: 10.1176/ajp.148.8.1009. [DOI] [PubMed] [Google Scholar]
- Oshima A, Flachskamm C, Reul JM, Holsboer F, Linthorst AC. Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology. 2003;28:2148–2159. doi: 10.1038/sj.npp.1300267. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Overstreet DH, Hurd YL. The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta-estradiol. Molecular Brain Research. 1999;74:158–166. doi: 10.1016/s0169-328x(99)00274-0. [DOI] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol. 2005;481:127–144. doi: 10.1002/cne.20348. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of 5HT1A autoreceptor messenger ribonucleic acid expression in the dorsal raphe of rhesus macaques. Neuroscience. 1998;89:267–277. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. The Journal of Neuroscience. 1996;16:7021–7029. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penalva RG, Flachskamm C, Zimmermann S, Wurst W, Holsboer F, Reul JM, Linthorst AC. Corticotropin-releasing hormone receptor type 1-deficiency enhances hippocampal serotonergic neurotransmission: an in vivo microdialysis study in mutant mice. Neuroscience. 2002;109:253–266. doi: 10.1016/s0306-4522(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Penas-Lledo E, Vaz Leal FJ, Waller G. Excessive exercise in anorexia nervosa and bulimia nervosa: relation to eating characteristics and general psychopathology. Int J Eat Disord. 2002;31:370–375. doi: 10.1002/eat.10042. [DOI] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroutka SJ, Snyder SH. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science. 1980;210:88–90. doi: 10.1126/science.6251550. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Di Meo G, De Leo V, Nappi C, Facchinetti F, Genazzani AR. Plasma beta-endorphin levels in anovulatory states: changes after treatments for the induction of ovulation. Fertil Steril. 1986;45:185–190. doi: 10.1016/s0015-0282(16)49152-1. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Polkowska J. Stress and nutritional influences on GnRH and somatostatin neuronal systems in the ewe. Acta Neurobiol Exp (Wars) 1996;56:797–806. doi: 10.55782/ane-1996-1185. [DOI] [PubMed] [Google Scholar]
- Polkowska J, Przekop F. The effect of corticotropin-releasing factor (CRF) on the gonadotropin hormone releasing hormone (GnRH) hypothalamic neuronal system during preovulatory period in the ewe. Acta Neurobiol Exp (Wars) 1997;57:91–99. doi: 10.55782/ane-1997-1216. [DOI] [PubMed] [Google Scholar]
- Portillo F, Carrasco M, Vallo JJ. Separate populations of neurons within the paraventricular hypothalamic nucleus of the rat project to vagal and thoracic autonomic preganglionic levels and express c-Fos protein induced by lithium chloride. J Chem Neuroanat. 1998;14:95–102. doi: 10.1016/s0891-0618(97)10022-9. [DOI] [PubMed] [Google Scholar]
- Pranzatelli MR, Murthy JN, Tailor PT. Novel regulation of 5-HT1C receptors: down-regulation induced both by 5-HT1C/2 receptor agonists and antagonists. Eur J Pharmacol. 1993;244:1–5. doi: 10.1016/0922-4106(93)90052-b. [DOI] [PubMed] [Google Scholar]
- Przekop F, Polkowska J, Mateusiak K. The effect of prolonged stress on the hypothalamic luteinizing hormone-releasing hormone (LHRH) in the anoestrous ewe. Exp Clin Endocrinol. 1988;91:334–340. doi: 10.1055/s-0029-1210766. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab. 1996;81:3540–3546. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science. 1986;231:607–609. doi: 10.1126/science.3003907. [DOI] [PubMed] [Google Scholar]
- Robinson JE. Gamma amino-butyric acid and the control of GnRH secretion in sheep. Journal of Reproduction and Fertility Supplement. 1995;49:221–230. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacology, Biochemistry and Behavior. 2002;71:825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience. 1999;91:1343–1354. doi: 10.1016/s0306-4522(98)00703-9. [DOI] [PubMed] [Google Scholar]
- Russak LG, Schwartz GE. Feelings of parental care predict health status in midlife: A 35 year follow-up of the Harvard Mastery of Stress Study. Journal of Behavioral Medicine. 1997;20:1–11. doi: 10.1023/a:1025525428213. [DOI] [PubMed] [Google Scholar]
- Sanders-Bush E, Canton H. Serotonin receptors: signal transduction pathways. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology, The Fourth Generation of Progress. New York: Raven Press; 1995. pp. 431–442. [Google Scholar]
- Saucier C, Morris SJ, Albert PR. Endogenous serotonin-2A and -2C receptors in Balb/c-3T3 cells revealed in serotonin-free medium: desensitization and down-regulation by serotonin. Biochem Pharmacol. 1998;56:1347–1357. doi: 10.1016/s0006-2952(98)00244-5. [DOI] [PubMed] [Google Scholar]
- Schwartz DH, Hernandez L, Hoebel BG. Serotonin release in lateral and medial hypothalamus during feeding and its anticipation. Brain Res Bull. 1990;25:797–802. doi: 10.1016/0361-9230(90)90173-w. [DOI] [PubMed] [Google Scholar]
- Scott CJ, Clarke IJ. Evidence that changes in the function of the subtypes of the receptors for gamma-amino butyric acid may be involved in the seasonal changes in the negative-feedback effects of estrogen on gonadotropin-releasing hormone secretion and plasma luteinizing hormone levels in the ewe. Endocrinology. 1993;133:2904–2912. doi: 10.1210/endo.133.6.8243318. [DOI] [PubMed] [Google Scholar]
- Shively CA, Fontenot MB, Kaplan JR. Social status, behavior, and central serotonergic responsivity in female cynomolgus monkeys. American Journal of Primatology. 1995;37:333–339. doi: 10.1002/ajp.1350370408. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3:114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Kahn RS, Lawlor BA, Trestman RL, Lawrence TL, Coccaro EF. II. Critical issues in defining the role of serotonin in psychiatric disorders. Pharmacological Reviews. 1991;43:509–525. [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE. Detection of estrogen receptor alpha and beta messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology. 1999;140:5195–5201. doi: 10.1210/endo.140.11.7146. [DOI] [PubMed] [Google Scholar]
- Smith RF, Ghuman SP, Evans NP, Karsch FJ, Dobson H. Stress and the control of LH secretion in the ewe. Reprod Suppl. 2003;61:267–282. [PubMed] [Google Scholar]
- Sobczak S, Honig A, van Duinen MA, Reidel WJ. Serotonergic dysregulation in bipolar disorders: a literature review of serotonergic challenge studies. Bipolar Disorders. 2002;4:347–356. doi: 10.1034/j.1399-5618.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA-and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–2050. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Gamma-aminobutyric acid neurons integrate and rapidly transmit permissive and inhibitory metabolic cues to gonadotropin-releasing hormone neurons. Endocrinology. 2004a;145:1194–1202. doi: 10.1210/en.2003-1374. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004b;101:7129–7134. doi: 10.1073/pnas.0308058101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod. 2005;72:33–41. doi: 10.1095/biolreprod.104.033126. [DOI] [PubMed] [Google Scholar]
- Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of raphe nuclei elicited by prior stress and the neuropeptide corticotropin-releasing factor. J Neuroendocrinol. 2003;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Summers CH, Larson ET, Summers TR, Renner KJ, Greenberg N. Regional and temporal separation of serotonergic activity mediating social stress. Neuroscience. 1998;87:489–496. doi: 10.1016/s0306-4522(98)00144-4. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tancer ME, Mailman RB, Stein MF, Mason GA, Carson SW, Golden RN. Neuroendocrine responsivity to monoaminergic system probes in generalized social phobia. Anxiety. 1994;1:216–223. [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Wilson C. Cognitive dysfunction in neuropsychiatric disorders: Selected serotonin receptor subtypes as therapeutic targets. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, O’Brien JT. Depression and cognition in older adults. Curr Opin Psychiatry. 2008;21:8–13. doi: 10.1097/YCO.0b013e3282f2139b. [DOI] [PubMed] [Google Scholar]
- Tilbrook AJ, Turner AI, Clarke IJ. Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress. 2002;5:83–100. doi: 10.1080/10253890290027912. [DOI] [PubMed] [Google Scholar]
- Troop NA, Holbrey A, Treasure JL. Stress, coping, and crisis support in eating disorders. Int J Eat Disord. 1998;24:157–166. doi: 10.1002/(sici)1098-108x(199809)24:2<157::aid-eat5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Tsukamura H, Ohkura S, Coen CW, Maeda KI. The paraventricular nucleus and corticotrophin-releasing hormone are not critical in suppressing pulsatile LH secretion in ovariectomized lactating rats. J Endocrinol. 1993;137:291–297. doi: 10.1677/joe.0.1370291. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biological Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Caldarola-Pastuszka M, Montanez S. Intracerebral actions of the 5-HT1A agonists, 8-OH-DPAT and buspirone and of the 5-HT1A partial agonist/antagonist, NAN-190, on female sexual behavior. Neuropharmacology. 1992;31:969–981. doi: 10.1016/0028-3908(92)90097-9. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- van Binsbergen CJ, Coelingh Bennink HJ, Odink J, Haspels AA, Koppeschaar HP. A comparative and longitudinal study on endocrine changes related to ovarian function in patients with anorexia nervosa. J Clin Endocrinol Metab. 1990;71:705–711. doi: 10.1210/jcem-71-3-705. [DOI] [PubMed] [Google Scholar]
- Van de Kar L. Neuroendocrine pharmacology of serotonergic (5HT) neurons. Animal Review of Pharmacology and Toxicology. 1991;31:289–320. doi: 10.1146/annurev.pa.31.040191.001445. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Carnes M, Maslowski J, Bonadonna AM, Rittenhouse PA, Kunimoto K, Piechowski RA, Bethea CL. Neuroendocrine evidence for denervation supersensitivity of serotonin receptors: effects of the 5HT agonist RU 24969 on corticotropin, corticosterone, prolactin and renin secretion. Journal of Pharmacology and Experimental Therapeutics. 1989;251:428–433. [PubMed] [Google Scholar]
- Van de Kar LD, Javed A, Zhang Y, Serres F, Raap DK, Gray TS. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21:3572–3579. doi: 10.1523/JNEUROSCI.21-10-03572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kar LD, Karteszi M, Bethea CL, Ganong WF. Serotonergic stimulation of prolactin and corticosterone secretion is mediated by different pathways from the mediobasal hypothalamus. Neuroendocrinology. 1985a;41:380–384. doi: 10.1159/000124205. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Urban JH, Richardson KD, Bethea CL. Pharmacological studies on the serotonergic and nonserotonin-mediated stimulation of prolactin and corticosterone secretion by fenfluramine. Neuroendocrinology. 1985b;41:283–288. doi: 10.1159/000124191. [DOI] [PubMed] [Google Scholar]
- Van Vugt DA, Lam NY, Ferin M. Reduced frequency of pulsatile luteinizing hormone secretion in the luteal phase of the rhesus monkey. Involvement of endogenous opiates. Endocrinology. 1984;115:1095–1101. doi: 10.1210/endo-115-3-1095. [DOI] [PubMed] [Google Scholar]
- von Borell E, Dobson H, Prunier A. Stress, behaviour and reproductive performance in female cattle and pigs. Horm Behav. 2007;52:130–138. doi: 10.1016/j.yhbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Wallace DJ. The role of stress and trauma in rheumatoid arthritis and systemic lupus erythematosus. Seminars in Arthritis and Rheumatology. 1987;16:153–157. doi: 10.1016/0049-0172(87)90018-7. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- Warren MP, Stiehl AL. Exercise and female adolescents: effects on the reproductive and skeletal systems. J Am Med Womens Assoc. 1999;54:115–120. 138. [PubMed] [Google Scholar]
- Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothalamic amenorrhea: hypoleptinemia and disordered eating. Journal of Clinical Endocrinology and Metabolism. 1999;84:873–877. doi: 10.1210/jcem.84.3.5551. [DOI] [PubMed] [Google Scholar]
- Weijers HG, Wiesbeck GA, Jakob F, Boning J. Neuroendocrine responses to fenfluramine and its relationship to personality in alcoholism. J Neural Transm. 2001;108:1093–1105. doi: 10.1007/s007020170027. [DOI] [PubMed] [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Mild metabolic stress potentiates the suppressive effect of psychological stress on reproductive function in female cynomolgus monkeys. Endocrine Society Abstracts. 1997:PI-367. [Google Scholar]
- Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–276. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- Williams NI, Caston-Balderrama AL, Helmreich DL, Parfitt DB, Nosbisch C, Cameron JL. Longitudinal changes in reproductive hormones and menstrual cyclicity in cynomolgus monkeys during strenuous exercise training: abrupt transition to exercise-induced amenorrhea. Endocrinology. 2001a;142:2381–2389. doi: 10.1210/endo.142.6.8113. [DOI] [PubMed] [Google Scholar]
- Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab. 2001b;86:5184–5193. doi: 10.1210/jcem.86.11.8024. [DOI] [PubMed] [Google Scholar]
- Wolff GE, Crosby RD, Roberts JA, Wittrock DA. Differences in daily stress, mood, coping, and eating behavior in binge eating and nonbinge eating college women. Addict Behav. 2000;25:205–216. doi: 10.1016/s0306-4603(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lunfergren KH, Davis BM, Jennes L. Comparative localization of serotonin 1A, 1C and 2 receptor subtype mRNA in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Barth A, Zhu J, Ferin M. Stress and the menstrual cycle: relevance of cycle quality in the short- and long-term response to a 5-day endotoxin challenge during the follicular phase in the rhesus monkey. J Clin Endocrinol Metab. 1998;83:2454–2460. doi: 10.1210/jcem.83.7.4926. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M. Stress and the menstrual cycle: short- and long-term response to a five-day endotoxin challenge during the luteal phase in the rhesus monkey. Journal of Clinical Endocrinology and Metabolism. 1999;84:623–626. doi: 10.1210/jcem.84.2.5448. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Ferin M. Inadequate luteal function is the initial clinical cyclic defect in a 12-day stress model that includes a psychogenic component in the Rhesus monkey. J Clin Endocrinol Metab. 2002;87:2232–2237. doi: 10.1210/jcem.87.5.8500. [DOI] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Thornell D, Shalts E, Ferin M. The luteinizing hormone but not the cortisol response to arginine vasopressin is prevented by naloxone and a corticotropin-releasing hormone antagonist in the ovariectomized rhesus monkey. Neuroendocrinology. 1996;64:225–232. doi: 10.1159/000127121. [DOI] [PubMed] [Google Scholar]
- Xiao EN, Ferin M. The inhibitory action of corticotropin-releasing hormone on gonadotropin secretion in the ovariectomized rhesus monkey is not mediated by adrenocorticotropic hormone. Biol Reprod. 1988;38:763–767. doi: 10.1095/biolreprod38.4.763. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann N Y Acad Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]