Abstract
In a prospective infant cohort, 21 infants developed Plasmodium vivax malaria during their first year. Twelve of their mothers also had vivax malaria in the corresponding pregnancies or postpartum period. The genotypes of the maternal and infant infections were all different. Eight of the 12 mothers and 9 of the 21 infants had recurrent infections. Relapse parasite genotypes were different to the initial infection in 13 of 20 (65%) mothers compared with 5 of 24 (21%) infants (P = .02). The first P. vivax relapses of life are usually genetically homologous, whereas relapse in adults may result from activation of heterologous latent hypnozoites acquired from previous inoculations.
Plasmodium vivax is a major cause of morbidity in Asia and the Americas. In tropical areas, a high proportion of acute vivax malaria infections are followed soon afterward by a relapse if radical treatment with primaquine is not given [1–3]. The interval to the first relapse averages 3 weeks if rapidly eliminated drugs are given for acute treatment and 7 weeks with slowly eliminated drugs. There is also a high rate of P. vivax recurrence following falciparum malaria (30%–50%) [4, 5]. The intervals between acute vivax malaria and the first relapse and between falciparum malaria and the subsequent vivax episode are very similar, suggesting that this too is a relapse. The relapse patterns in malaria-naive volunteers infected with the Chesson strain of P. vivax [6] and the rhesus monkeys infected with Plasmodium cynomolgi [7] are very similar to those observed in natural infections. It had therefore been assumed that vivax relapses would derive from the same infection that caused the acute illness. Three studies—one in patients in Thailand [8], another in Australian soldiers who acquired vivax malaria in East Timor [9], and a third more recent investigation in Colombia [10]—all showed this was not the case. Approximately two-thirds of the relapses in these studies derived from parasites that could not be detected in the initial acute infection. There were several possible explanations. Perhaps there was suppression of some genotypes in a mixed-genotype infection or significant differences in the incubation periods of inoculated parasite genotype mixtures, or, alternatively, the relapses derived from a different previous mosquito inoculation. We reasoned that although a primary asymptomatic infection can occur in infancy, this is relatively unusual [11], so there should generally be no hypnozoites from previous infections in the first P. vivax infection of life. We therefore genotyped the first P. vivax infection of life and subsequent recurrences in a cohort of infants followed prospectively in a malaria-endemic area on the northwestern border of Thailand.
METHODS
The study took place from November 2007 until January 2011 in the Shoklo Malaria Research Unit (SMRU) clinics on the northwestern border of Thailand, an area of low and seasonal malaria transmission (entomological inoculation rate for P. vivax <0.5 per person per year). Mothers were enrolled after giving written consent in a prospective study to investigate the effects of malaria in pregnancy, postpartum, and in infancy. From enrollment until 12 weeks after delivery, weekly blood smears and polymerase chain reaction (PCR) spots were taken from the mothers. Their infants were sampled every 2 weeks, and after 3 months every 4 weeks until 1 year of age.
Plasmodium vivax was diagnosed by microscopy and treated in mothers and infants with chloroquine (25 mg base/kg; Government Pharmaceutical Organisation [GPO], Thailand). Radical treatment is not given routinely in this population. However, if an infant had >2 recurrent infections of P. vivax, directly observed treatment with primaquine (0.5 mg base/kg for 14 days) was given after the infant was tested G6PD negative. Primaquine was not given to the mothers during pregnancy.
Ethical Review
Ethical approval was obtained from the Faculty of Tropical Medicine, Mahidol University Bangkok, Thailand (MUTM 2007-023) and the Oxford Tropical Medicine Ethical Committee, Oxford University, United Kingdom (code 002-07).
Laboratory
Blood smears (thin and thick films) were stained with Giemsa and read for 200 fields before declaring a negative. Blood spots for PCR analysis were collected on Whatman filter paper and stored in plastic bags with silica gel.
Genotyping
For P. vivax genotyping, PCR amplification of 8 microsatellite markers was performed following previously described protocols [12, 13]. The microsatellite markers were PV 3.27, PV 3.502, PV ms1, PV ms5, PV ms 6, PV ms7, PV ms8, and PV ms16. Measurement of allele length and quantification of peak heights were performed using an ABI 3130 Genetic Analyzer and GeneMapper software version 4.0 (Applied Biosystems). The samples were reanalyzed when amplification was poor for particular loci (maximum peak height <200 fluorescence units). Samples were scored as indeterminate if ≥5 markers (loci) failed to amplify on repeat testing. Blood stage malaria parasites are haploid, and mixed infection with ≥2 or more haploid clones is frequent. The presence of ≥1 additional alleles at a locus could result either from a mixed-genotype infection or mutation (strand slippage) of the microsatellite repeat during asexual division within the blood stage infection producing a mixture. We scored multiple alleles per locus if minor peaks were more than half of the height of the predominant allele present for each locus. To estimate the likelihood of strand slippage during the amplification process (ie, as an ex vivo artifact) we conducted amplifications of 6 microsatellite loci for 5 different samples containing a single P. vivax genotype on 10 separate occasions.
Statistical Analysis
The individual frequency distribution of each allele at each microsatellite locus was calculated, and the probabilities of identical alleles occurring at each locus in 2 independent parasite isolates was thereby derived. The probability of finding a complete match at each locus for the most common genotype was 8.45 × 10−6, and the probability of finding a match at 7 of 8 loci for the most common genotype was 5.63 × 10−5. When comparing parasite genotypes, some parasites were observed that were identical at ≥6 loci and showed either a mixture of 2 adjacent microsatellite alelles at 1 locus (one of which was present in the primary infection) or had alleles that differed by 1 tandem repeat at the remaining 1 or 2 loci. These were considered related and thus likely to be derived from the same mosquito inoculation (Table 1).
Table 1.
Plasmodium vivax Recurrences in Mothers and Infants
| Mothers (n = 12) | Infants (n = 21) | P Value | |
| No. of recurrent Plasmodium vivax infections | 8 | 9 | >.2 |
| No. of recurrences documented, median (range) | 2.5 (1–5) | 2.5 (1–6) | >.2 |
| Interval of recurrence, days, median (range) | 45 (21–82) | 37 (11–168) | |
| First recurrence similar to initial infection | 2/8 A | 7/9 A | .02 |
| 1 indeterminate | 1 indeterminate | ||
| Second recurrence similar to initial infection | 1/6 A | 3/6 A | >.1 |
| 1 indeterminate | 1/6 B | ||
| Third recurrence similar to initial infection | 1/4 B | 2/5 A | .5 |
| 1 indeterminate | 1 indeterminate | ||
| Fourth recurrence similar to initial infection | 0/1 | 2/2 A | .3 |
| Fifth recurrence similar to initial infection | 0/1 | 1/1 B | .5 |
| Sixth recurrence similar to initial infection | … | 1/1 A | … |
| Proportion of recurrences that were genotypically similar | 4/20 | 17/24 | .02 |
| 3 indeterminate | 2 indeterminate | ||
| In genotypically different recurrences, median proportion of different alleles (range) | 80% (50%–100%) | 63% (57%–86%) | .008 |
Criteria for interpretation: A = 0, 1 marker (loci) different or 2 markers (loci) different with ≤1 possible stepwise mutation; B = 3 markers (loci) different but ≤1 repeat unit, different with ≤1 possible stepwise mutation; indeterminate: ≥5 markers (loci) failed to amplify.
RESULTS
Clinical Features
In this prospective cohort, 753 infants were followed for a median of 12 (range, 0–12) months. Over the 39-month study period, 7 infants had P. falciparum and 23 infants had 51 P. vivax infections, with a median of 2.5 (range, 1–6) episodes per infected infant. The median age at first vivax malaria was 31 (range, 4–51) weeks. The median age at first infection in the 9 infants whose infections recurred was 26 (range, 4–40) weeks compared with 36 (range, 4–51) weeks in the 11 infants who did not have a recurrence (P > .2). Of the 51 infant infections, 40 (78%) were symptomatic, presenting with fever, and the remaining 11 were detected actively in routine follow-up. Twelve of the infants’ mothers had vivax malaria in the corresponding pregnancies or postpartum period. Within each mother-infant pairing, the median number of episodes in the mothers was 2.5 (range, 1–5). Blood spots for PCR were available from 8 of these mothers who experienced 20 recurrent infections. All patients responded initially to chloroquine. Four infants received radical curative treatment with primaquine after they had experienced their second recurrent infection.
Genotyping Results
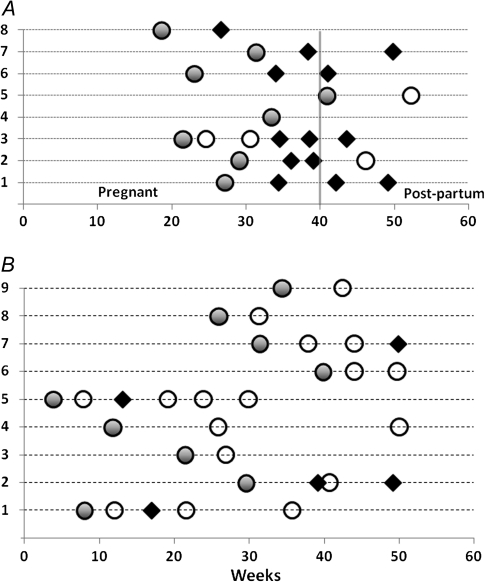
Of the 23 infants, 21 provided blood spots for genotyping, of whom 9 had recurrent infections (in total, 24 recurrences). Mixed-genotype infections were found in 55% of the mothers and 43% of infants. The genotypes of the maternal and corresponding infant infections were different in all cases. Placental samples taken during delivery (n = 5) were negative by microscopy. The genotypes were different to the initial infection for 13 of the 20 recurrences in the 8 mothers, were the same in 4 (in 1 mother the primary infection and 2 subsequent recurrences were similar), and 3 pairs were indeterminate (Figure 1). In contrast, in the infants, most of the presumed relapses were of similar genotypes to the primary infection. Only 5 of 24 recurrences in the infants were with clearly different genotypes (Table 1). The relative risk of having a relapse caused by a different genotype in mothers compared with their infants, in satisfactorily genotyped infections, was 3.5 (95% confidence interval [CI],1.6–8.0; P = .02).
Figure 1.
Temporal pattern and genotyping of recurrences of Plasmodium vivax malaria in 8 mothers (A) and 9 infants (B). Each number on the y-axis represents a patient. Filled circles denote the initial infection. An open circle represents a genetically homologous recurrence, and a black diamond represents a genetically heterologous recurrence. Recurrences where genotyping was indeterminate (2 in infants, 3 in mothers) are not shown. One mother’s sole relapse was indeterminate.
Interrecurrence Intervals
In the mothers the median interrecurrence interval was 45 (range, 21–82) days. The intervals between genetically homologous (median, 45 days; range, 21–82 days) and heterologous recurrences (median, 49 days; range, 21–56 days) were similar. In the infants, the median interrecurrence interval was 37 (range, 11–168) days, and the intervals were also not different between genetically homologous (median, 35 days; range, 11–168 days) and heterologous recurrences (median, 41 days; range, 30–73 days). All recurrences in the infants with intervals <5 weeks (n = 8) were genetically homologous. Of the 18 homologous genotype recurrences, 11 occurred following an interval of 28–44 days. None of the 7 P. falciparum infections were followed by P. vivax within 3 months.
DISCUSSION
This small series suggests that the pattern of P. vivax genotypes in pregnant women with presumed relapses of vivax malaria is similar to that observed previously in Thailand [8]. The majority were with genotypes different to those of the initial infection (which too may have been a relapse). The pattern was different in their infants. The presumed relapses that followed the first vivax malaria episode of life were usually with genotypes similar to those that caused the initial infection. These studies were conducted in an endemic area so reinfection cannot be excluded, although transmission intensity in this area was low (3.7 infections per 100 person-years in infancy). Reinfection probably caused some or all of the 5 genetically heterologous recurrences in the infants but cannot account for the similarity of most recurrences (72%). Chloroquine efficacy is declining in the area but still provides 28-day cure rates of 88%, so recrudescence cannot be excluded. One infant had a second P. vivax recurrence only 11 days after the first recurrence (61 days after the primary infection); the first recurrence was heterologous, but the second was genetically identical to the initial infection, so it was not a recrudescence of the preceding infection but a relapse of the primary infection. Most initial recurrences were 1–2 months following treatment, the time when most relapses occur following chloroquine treatment in this area [1–3].
The assessment of genotypic similarity from this microsatellite typing is imprecise. Recombination between genetically different parasites in the anopheline mosquito vector results in related progeny, which may become very highly related through subsequent interbreeding in serial passage. Microsatellite mutation rate estimates have not been made for P. vivax, but for P. falciparum, they have been estimated at 1.59 × 10−4 (95% CI, 6.98 × 10−5–3.7 × 10−4) [14]. These usually result in single-pair increases or decreases in the number of tandem repeats at each locus, termed “slippage.” Therefore, infections may comprise closely related genotypes, and different genotypes may emerge during the course of infection. Infections that have a common origin may, therefore, differ at ≥1 loci. Slippage might also occur as an ex vivo artifact during the PCR amplification, but repeat testing in this study suggested that this did not occur frequently. We did not attempt to distinguish between these various sources of genetic difference because our primary objective was to distinguish between a single or separate parasite inoculations. We therefore included together parasites with 1 or 2 different alleles as originating from the same inoculation because it was statistically highly improbable that they originated separately.
The parasite genotypes causing maternal and corresponding infant infections were different in each case, which suggests that there was no congenital transmission. Blood transfer of infection should give similar genotypes in donor and recipient. These results suggest that a substantial proportion of relapses in adult patients do not derive from the mosquito inoculation of parasites that caused the preceding infection. In contrast, the infants had similar genotypes in their relapses, suggesting that their relapses did derive from the same inoculation of parasites that caused the initial infection. Because there is no reason why mosquito inoculation should differ substantially between adults and infants, the most likely explanation for this marked difference is activation of heterologous latent hypnozoites in adults from previous inoculations. More complex explanations involving inoculation of multiple genotypes with differences in incubation periods or signaling between genotypes are unnecessary. These data may also help explain the origin of the presumed P. vivax relapses that follow treatment of P. falciparum infections in between one-third and one-half of patients with falciparum malaria in this region [4, 5]. Simultaneous inoculation seems unlikely to account for these because the anopheline vectors rarely carry both parasite species [15]. The P. vivax infections that follow falciparum malaria could result from activation of hypnozoites derived from previous inoculations. Interestingly, none of the 7 P. falciparum infections in infants were followed by P. vivax. Taken together, all the data point to the accumulation of latent hypnozoites in a significant proportion of the population in malaria-endemic areas of Asia.
Notes
Acknowledgments.
We would like to thank all the patients and staff at all the SMRU clinics who participated in this study. SMRU is attached to the Faculty of Tropical Medicine, Mahidol University.
Financial support.
This investigation was part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme, supported by the Wellcome Trust of Great Britain. M. I. is supported by the National Science and Technology Development Agency, the Office of the Higher Education Commission, and Mahidol University under the National Research Universities Initiative; and a Wellcome Trust Intermediate Fellowship grant (080867/Z/06/Z).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Looareesuwan S, Wilairatana P, Krudsood S, et al. Chloroquine sensitivity of Plasmodium vivax in Thailand. Ann Trop Med Parasitol. 1999;93:225–30. [PubMed] [Google Scholar]
- 2.Luxemburger C, van Vugt M, Jonathan S, et al. Treatment of vivax malaria on the western border of Thailand. Trans R Soc Trop Med Hyg. 1999;93:433–8. doi: 10.1016/s0035-9203(99)90149-9. [DOI] [PubMed] [Google Scholar]
- 3.Silachamroon U, Krudsood S, Treeprasertsuk S, et al. Clinical trial of oral artesunate with or without high-dose primaquine for the treatment of vivax malaria in Thailand. Am J Trop Med Hyg. 2003;69:14–18. [PMC free article] [PubMed] [Google Scholar]
- 4.Smithuis F, Kyaw MK, Phe O, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–81. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas NM, Nosten F, Ashley EA, et al. Plasmodium vivax recurrence following falciparum and mixed species malaria: risk factors and effect of antimalarial kinetics. Clin Infect Dis. 2011;52:612–20. doi: 10.1093/cid/ciq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coatney GR, Cooper WC, Young MD. Studies in human malaria. XXX. A summary of 204 sporozoite-induced infections with the Chesson strain of Plasmodium vivax. J Natl Malar Soc. 1950;9:381–96. [PubMed] [Google Scholar]
- 7.Schmidt LH, Fradkin R, Genther CS, Rossan RN, Squires W, Hughes HB. Plasmodium cynomolgi infections in the rhesus monkey. Am J Trop Med Hyg. 1982;31:609–703. doi: 10.4269/ajtmh.1982.31.609. [DOI] [PubMed] [Google Scholar]
- 8.Imwong M, Snounou G, Pukrittayakamee S, et al. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;195:927–33. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- 9.Chen N, Auliff A, Rieckmann K, Gatton M, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis. 2007;195:934–41. doi: 10.1086/512242. [DOI] [PubMed] [Google Scholar]
- 10.Restrepo E, Imwong M, Rojas W, Carmona-Fonseca J, Maestre A. High genetic polymorphism of relapsing P. vivax isolates in northwest Colombia. Acta Trop. 2011;119:23–9. doi: 10.1016/j.actatropica.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swellengrebel NH, De Buck A. Malaria in the Netherlands. Amsterdam: Scheltema & Holkema; 1938. [Google Scholar]
- 12.Gunawardena S, Karunaweera ND, Ferreira MU, et al. Geographic structure of Plasmodium vivax: microsatellite analysis of parasite populations from Sri Lanka, Myanmar, and Ethiopia. Am J Trop Med Hyg. 2010;82:235–42. doi: 10.4269/ajtmh.2010.09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imwong M, Nair S, Pukrittayakamee S, et al. Contrasting genetic structure in Plasmodium vivax populations from Asia and South America. Int J Parasitol. 2007;37:1013–22. doi: 10.1016/j.ijpara.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Anderson TJ, Haubold B, Williams JT, et al. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol Biol Evol. 2000;17:1467–82. doi: 10.1093/oxfordjournals.molbev.a026247. [DOI] [PubMed] [Google Scholar]
- 15.Imwong M, Nakeesathit S, Day NP, White NJ. A review of mixed malaria species infections in anopheline mosquitoes. Malar J. 2011;10:253. doi: 10.1186/1475-2875-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]