Abstract
Background. Little is known about the associations between CD4+ cell counts, human immunodeficiency virus (HIV) load, and human papillomavirus “low-risk” types in noncancerous clinical outcomes. This study examined whether CD4+ count and HIV load predict the size of the largest anal warts in 976 HIV-infected women in an ongoing cohort.
Methods. A linear mixed model was used to determine the association between size of anal wart and CD4+ count and HIV load.
Results. The incidence of anal warts was 4.15 cases per 100 person-years (95% confidence interval [CI], 3.83–4.77) and 1.30 cases per 100 person-years (95% CI, 1.00–1.58) in HIV-infected and HIV-uninfected women, respectively. There appeared to be an inverse association between size of the largest anal warts and CD4+ count at baseline; however, this was not statistically significant. There was no association between size of the largest anal warts and CD4+ count or HIV load over time.
Conclusions. There was no evidence for an association between size of the largest anal warts and CD4+ count or HIV load over time. Further exploration on the role of immune response on the development of anal warts is warranted in a larger study.
Human papillomavirus (HPV) is a necessary cause of cervical cancer [1, 2] and has also been shown to be strongly associated with anal cancer [3]. Although the high-risk HPV types 16 and 18 account for the majority of cancers [1, 2], low-risk HPV genotypes (eg, 6 and 11) are responsible for the development of anogenital warts [4, 5]. Recent estimates predict that approximately 1% of sexually active adults in the United States have visible genital warts [6], and the prevalence may be much higher among individuals infected with human immunodeficiency virus (HIV). A previous study reported that HIV-infected women were 9.32 times (95% confidence interval [CI], 3.04–38.00) more likely to have genital warts than HIV-uninfected women [7]. Furthermore, immunocompromised patients were found to have more recurrence of anogenital warts than immunocompetent persons [8].
Among risk factors shown to be associated with HPV infections and their clinical outcomes, CD4+ cell count and HPV load have been studied extensively, particularly in HIV-infected populations. Accordingly, several studies have reported that a person with a high CD4+ cell count and a low HIV load is less likely to be infected with HPV than a person with a low CD4+ cell count and a high HIV load [9–14]. Although there are numerous studies on how HPV high-risk types, CD4+ cell counts, and HIV loads collectively impact certain clinical outcomes (eg, cervical cancer), little is known about the associations with HPV low-risk types and important noncancerous clinical outcomes (ie, anogenital warts). As a result, the factors that predict changes in size of anal warts have not been identified. In 2002, Conley et al [15] reported that compared with an HIV-infected woman with CD4+ cell count >500 cells/mm3, a person with a CD4+ cell count <200/mm3 is 1.66 times (95% CI, 1.03–2.69) more likely to have incident perianal warts. Although this study lends support to the hypothesis that CD4+ cell count is associated with the risk of anal wart development, a longitudinal study with enough power to examine temporal patterns in the way CD4+ cell count, HIV load, and other factors influence the development and progression of anal warts is warranted.
Anal warts pose a major problem for immunocompromised individuals, but they have not been studied separately from genital warts in previous research. This is tremendously problematic given the evidence that the prevalence of anal warts may be higher than that of intravaginal warts among women [16]. Additionally, a person infected with low-risk HPV types, which result in warts, is more likely to be infected with high-risk HPV types, and there is a strong indication that the presence of anal warts is associated with the development of anal intraepithelial neoplasia, which can lead to anal cancer [17, 18]. For these reasons, in addition to the economic and psychosocial burdens associated with this condition, it is important to determine which factors drive changes in size of anal warts.
The purpose of the current study was to determine, using data from the Women’s Interagency HIV Study (WIHS), an ongoing longitudinal study of HIV-infected and HIV-uninfected women in the United States, whether CD4+ cell count and HIV load predict the size of the largest anal warts in HIV-infected women.
MATERIALS AND METHODS
Study Population
Data used for this study were obtained from the public dataset (release P09) provided by WIHS. WIHS is an ongoing prospective cohort study and has been described in detail elsewhere [19, 20]. In brief, WIHS included clinical consortia in 6 locations across the United States: Bronx/Manhattan, New York; Brooklyn, New York; Washington, District of Columbia; Los Angeles/Southern California/Hawaii; San Francisco/Bay Area, California; and Chicago, Illinois. WIHS has 2 enrollment phases: The first enrollment phase was between October 1994 and November 1995, during which 2059 HIV-infected and 569 HIV-uninfected women were recruited; the second enrollment phase was between October 2001 and September 2002, during which 1143 HIV-infected and 406 HIV-uninfected women were recruited.
The WIHS protocols include a baseline visit and follow-up every 6 months. During the baseline visit, a standardized in-person questionnaire was administered by trained interviewers. Self-reported information during the interview included general medical history, highly active antiretroviral therapy (HAART) use, obstetric and gynecologic history, alcohol and cigarette use, sexual behaviors, and history of physical and sexual abuse. Medical examination included height/weight/vital signs and examination of skin, abdomen, and breasts. Gynecologic examination included external genitalia, examination of internal vagina and cervix, cervical-vaginal lavage, bimanual and rectal examination, and colposcopy, biopsy, and dysplasia treatment if necessary. Follow-up visits [21] assessed key clinical characteristics such as CD4+ and CD8+ cell counts, HIV serostatus (HIV-uninfected women only), HIV load (HIV-infected women only), and Pap smear. For the current analyses, only those who had at least 1 anal wart over the study course were included.
Variables of Interest and Measurement
Outcome Variable
The outcome variable was the size of the largest anal wart at each visit. Presence of anal warts was recorded on the gynecologic exam form. The length and width (in millimeters) of the largest anal wart was measured and recorded. The physicians and physician assistants were instructed to complete the form and code the lesions and diagnoses. In current analysis, anal warts were defined as warts in one of the following locations: anus upper left, anus lower left, anus upper right, anus lower right, perineum left, and perineum right. The size of the anal wart was calculated by multiplying the width and length of the reported anal wart. In case of multiple warts, we assumed that the largest wart is an anal wart.
Independent Variables
Blood was drawn at each visit for determination of HIV serostatus, CD4+ cell count, and HIV load. The level of CD4+ cell count was quantified using flow cytometry at laboratories certified by the AIDS Clinical Trial Groups [19]. HIV load was measured in serum using a nucleic acid sequence-based amplification assay from Organon Teknika. HIV load tests were done at the National Institute of Allergy and Infectious Diseases AIDS Program, Virology Assurance HIV RNA Proficiency Program, National Institutes of Health [19].
Covariates
Covariates included in the current analysis were race/ethnicity (African American, white, and others); number of sex partners in the past 6 months (0 and ≥1); education level (less than high school education, high school education or general educational development test, some college, and college graduate or graduate school); annual household income (≤$6,000, $6001-$12 000, $12 001–$24 000, and ≥24 001); enrollment (enrollment 1 and enrollment 2); HAART use (yes/no); and marital status (married or living with partners, widowed, separated or divorced, and never married). The HAART use definition in the current analysis was based on the guidelines from the US Department of Health and Human Services, version 2002 [22] and the International AIDS Society Panel Antiretroviral Guidelines [23] and was consistent with previous WIHS analyses [24, 25]. A person was considered on HAART if she met 1 of the following criteria: (1) ≥2 nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least 1 protease inhibitor (PI) or 1 nonnucleoside reverse transcriptase inhibitor (NNRTI); (2) 1 NRTI in combination with at least 1 PI and at least 1 NNRTI; (3) regimen containing ritonavir and saquinavir in combination with 1 NRTI and no NNRTI; and (4) an abacavir- or tenofovir-containing regimen of ≥3 NRTIs in the absence of both PIs and NNRTIs, except for the 3 NRTI regimens consisting of abacavir + tenofovir + lamivudine or didanosine + tenofovir + lamivudine. Combination of zodovudine and stavudine with either a PI or NNRTI were not considered HAART. Monotherapy is considered as taking 1 NRTI, or only PI, or only NNRTI [22, 23].
Statistical Analysis
The distributions of sociodemographic characteristics were examined. For continuous variables, means and their standard deviations were calculated. Incident cases of anal warts were defined as persons who did not have an anal wart at the baseline visit but developed ≥1 anal warts during the follow-up period. Prevalence of anal warts for the entire WIHS cohort and in HIV-infected and HIV-uninfected women at baseline was calculated by taking respective number of persons with warts present at baseline divided by respective total samples. Incidence rates were calculated as the total number of incident cases divided by total follow-up time (ie, person-years) for HIV-infected, HIV-uninfected, and seroconverters separately. The 95% CIs for prevalence and incidence rates were based on a normal distribution if the number of cases was >30 and on the exact Poisson distribution if the number of cases was <30 [26].
CD4+ cell count and HIV load at each visit were provided as continuous variables in the WIHS public data set. During follow-up, some participants had HIV loads suppressed to undetectable levels. We used 10 copies/mL for undetectable level, as it was validated by Notermans et al [27]. In this analysis, CD4+ cell counts were categorized into 3 groups (<200, 200–500, and >500 cells/mm3), and HIV load was categorized into 4 groups (<4000, 4000–20 000, 20 001–100 000, and >100 000 copies/mL). These categories were chosen for consistency with previous WIHS analyses [24, 28–31]. Both CD4+ cell count and HIV load were used in each visit in the longitudinal modeling process.
The linear mixed model was chosen for the current analysis because of its ability to deal with missing values that are common in a longitudinal studies, deal with the highly correlated nature of repeated measurements within individuals and between individuals in a longitudinal study, and account for unbalance measurements (ie, number of visits) of subjects and the time interval between measurements [32]. Unadjusted models were first constructed to determine the total variation in growth velocity [26]. In the adjusted models, time-dependent covariates included number of sex partners in the past 6 months, education level, marital status, annual household income, and HAART use. Each of these variables was entered into the model both as a main effect and as a product with time. The time-independent covariates in the adjusted models were race/ethnicity and enrollment. Those covariates had been identified and used in previous analyses of WIHS [13, 24, 31, 33–35] and were treated as potential confounders in the current analysis. All statistical analyses were performed using command PROC MIXED of the SAS 9.2 statistical package [36]. All tests were 2-sided, and P = .05 was used as the significance level.
RESULTS
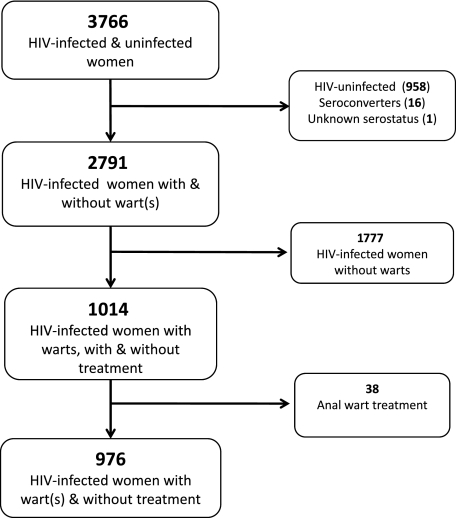
WIHS consisted of 3766 HIV-infected and HIV-uninfected women. Participants were excluded from the current analysis if (1) they were HIV-negative (n = 958); (2) they were seroconverters (n = 16) or had unknown HIV status (n = 1); (3) they did not have any anal warts during follow-up (n = 1777); or (4) they had received treatment for anal warts during the study period (n = 38) (Figure 1). HIV-infected women who received treatment for their anal warts were excluded because these treatments could have greatly influenced the size of the anal wart, many different types of treatment were received, and there were too few participants in the treatment group to perform a meaningful subanalysis.
Figure 1.
Flowchart of inclusion and exclusion of participants in current analysis. Abbreviation: HIV, human immunodeficiency virus.
Of the 1141 women who had at least 1 anal wart during follow-up, 477 women (441 HIV-infected vs 34 HIV-uninfected women) were identified with anal warts at baseline. Therefore, the prevalence of anal warts at baseline was 12.67% (95% CI, 11.61%–13.73%). The respective prevalences of anal warts at baseline in HIV-infected and HIV-uninfected women were 15.80% (95% CI, 14.45%–17.15%) and 3.55% (95% CI, 2.38%–4.72%). These prevalences were significantly different (P < .0001). The incidence rates of anal warts by HIV serostatus for the entire WIHS study are presented in Table 1. Because 724 incident cases of anal warts were identified, the incidence rate was 3.35 cases per 100 person-years (95% CI, 3.11–3.60 cases per 100 person-years). A statistically significant difference (P < .0001) was observed in the incidence rate of anal warts in HIV-infected women (4.15 cases per 100 person-years; 95% CI, 3.83–4.77 cases per person-years) compared with HIV-uninfected women (1.30 cases per 100 person-years; 95% CI, 1.00–1.58 cases per 100 person-years).
Table 1.
Incidence Cases of Anal Warts, Follow-up Time, and Incidence Rate of the Entire Women’s Interagency HIV Study by Serostatus Group
| Serostatus |
|||||
| HIV (+) | HIV (–) | Seroconverterb | Unknownb | Total | |
| No. of incidence cases of anal warts | 644 | 77 | 3 | 0 | 724 |
| Person-years follow-up of anal warts incidence cases | 1938 | 236 | 21 | 2195 | |
| Number of nonwart cases by serostatus group | 1777 | 834 | 13 | 1 | 2625a |
| Person-years follow-up of never developed anal warts | 13 578 | 5706 | 123 | 11 | 19 418 |
| Total person-years follow-up (by groups) | 15 516 | 5942 | 144 | 11 | 21 613 |
| Incidence rate (person-years) | 0.0415 | 0.0130 | 0.0208 | 0.0000 | 0.0335 |
| (95% CI, .0383–.0477)b | (95% CI, .0100–.0158)b | (95% CI, .007–.052)c | (95% CI, .0311–.0360) | ||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
This total already excludes 417 cases of anal warts at baseline visit.
Calculation of 95% CI based on normal distribution.
Calculation of 95% CI based on Poisson distribution.
Table 2 presents the baseline sociodemographic characteristics of study participants included in our analysis. Approximately 20% of participants had a CD4+ cell count of <200 cells/mm3, and 40% had a CD4+ cell count of >500 cells/mm3. Although 33% of participants had an HIV load of <4000 copies/mL, about 50% had an HIV load of >20 000 copies/mL.
Table 2.
Sociodemographic Characteristics of the Women’s Interagency HIV Study HIV-Infected Participants in Current Study
| Characteristics | WIHS (n = 976), No. (%) |
| CD4+ cell count (cells/mm3) | |
| Mean CD4+ cell count ± SD | 324.59 ± 293.04 |
| <200 | 148 (19.79) |
| 200–500 | 328 (43.85) |
| >500 | 272 (36.36) |
| HIV load (copies/mL) | |
| Mean viral load ± SD | 181175 ± 1039797 |
| <4000 | 331 (34.77) |
| 4000–20 000 | 164 (17.23) |
| 20 001–100 000 | 215 (22.58) |
| >100 000 | 242 (25.42) |
| Cigarette smoking status | |
| Current smokers | 565 (65.39) |
| Not current smokers | 299 (34.61) |
| Number of cigarettes smoked per day among current smokers | |
| <10 cigarettes/day | 288 (64.16) |
| 10–20 cigarettes/day | 44 (11.43) |
| ≥20 cigarettes/day | 94 (24.42) |
| Age (median ± SD) | 36.56 ± 7.85 |
| ≤25 | 66 (6.77) |
| 26–35 | 383 (39.28) |
| 36–45 | 407 (41.74) |
| >45 | 119 (12.21) |
| Race/Ethnicity | |
| White | 189 (19.42) |
| African American | 590 (60.64) |
| Others | 194 (19.94) |
| Education | |
| <High school education | 317 (36.35) |
| High school education or GED | 295 (33.83) |
| Some college | 207 (23.74) |
| College graduate or graduate school | 53 (6.08) |
| Household annual income | |
| ≤$6000 | 125 (25.61) |
| $6001–$12 000 | 171 (35.04) |
| $12 001–$24 000 | 118 (24.18) |
| ≥$24 001 | 74 (15.16) |
| Marital status | |
| Married or living with partner | 245 (35.00) |
| Widowed | 55 (7.86) |
| Separated or divorced | 146 (20.86) |
| Never married | 254 (36.29) |
| Number of male sex partners in the past 6 months | |
| 0 | 259 (27.52) |
| ≥1 | 682 (72.48) |
| HAART use at baseline | |
| No | 285 (97.60) |
| Yes | 7 (2.40) |
| Mean size of anal warts (mm2) ± SDa | 13.65 ± 127.71 |
Abbreviations: GED, general educational development test; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; SD, standard deviation.
Among those with anal warts at baseline (n = 344 and n = 73 in enrollment 1 and 2, respectively).
Table 3 presents results on the association between size of anal warts and CD4+ cell count. There was no significant relationship between size of the largest anal warts and CD4+ cell count over time in either the unadjusted or adjusted model. At baseline, women with moderate CD4+ cell counts had, on average, an anal wart size of 1.61 mm2 larger than that of women with the highest CD+ cell count. Similarly, the size of the anal wart of women with the lowest CD4+ cell count was 5.98 mm2 larger than that of women with the highest CD4+ cell count. However, those results were not statistically significant (P = .93 and P = .76, respectively). Interestingly, the growth rate of anal wart size after each visit in women with the lowest and moderate levels of CD4+ cell count were 0.30 mm2 and 0.64 mm2, respectively, less than that of women with the highest CD4+ cell level. However, these differences were not statistically significant.
Table 3.
Linear Mixed Model of Size of Anal Warts and CD4+ Cell Count of the Women’s Interagency HIV Study HIV-Infected Participants in Current Study
| Unadjusted Model |
Adjusted Modele |
|||
| Estimates ± SD | P Value | Estimates ± SD | P Value | |
| Intercept | 59.37 ± 66.69 | .47 | 2.29 ± 46.20 | .96 |
| Visit | −1.35 ± 5.31 | .80 | −1.96 ± 8.22 | .81 |
| CD4+ <200 cells/mm3 | −57.87 ± 78.06 | .46 | 5.98 ± 19.28 | .76 |
| CD4+ 200–500 cells/mm3 | −42.45 ± 77.75 | .59 | 1.61 ± 18.07 | .93 |
| CD4+ >500 cells/mm3 | 0a | … | 0c | … |
| Visit (CD4+ <200 cells/mm3) | 6.41 ± 6.44 | .32 | −0.64 ± 3.65 | .86 |
| Visit (CD4+ 200–500 cells/mm3) | 9.56 ± 6.35 | .13 | −0.30 ± 3.48 | .93 |
| Visit (CD4+ >500 cells/mm3) | 0b | … | 0d | … |
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
Type 3, P = .76.
Type 3, P = .32.
Type 3, P = .91.
Type 3, P = .98.
Model adjusted for no. of sex partners in the last 6 months, race, highly active antiretroviral therapy use, enrollment, marital status, annual household income, and education level.
Table 4 shows estimates on the association between size of anal wart and HIV RNA. Similar to CD4+ cell count, there was no significant association between size of the largest anal wart and HIV load over follow-up time. There appeared to be a significantly larger growth rate of anal wart size among women with HIV load of 20 001–100 000 copies/mL than women in the reference group (HIV load of <4000 copies/mL) (P = .003). However, this difference was diminished in the adjusted model. In the adjusted model, women with an HIV load of >100 000 copies/mL had a larger wart size at baseline (20 mm2) but a slower growth rate of change than those of women with an HIV load of <4000 copies/mL. Again, these differences were not statistically significant in the adjusted model.
Table 4.
Linear Mixed Model of Size of Anal Warts and HIV Load of the Women’s Interagency HIV Study HIV-Infected Participants in Current Study
| Unadjusted Model |
Adjusted Modele |
|||
| Estimates ± SD | P Value | Estimates ± SD | P Value | |
| Intercept | 38.73 ± 18.73 | .03f | 17.92 ± 34.36 | .60 |
| Visit | 0.47 ± 1.58 | .77 | −2.68 ± 7.37 | .71 |
| Viral load <4000 copies/mL | 0a | 0c | ||
| Viral load 4000–20 000 copies/mL | −17.35 ± 33.23 | .60 | −1.22 ± 17.53 | .94 |
| Viral load 20 001–100 000 copies/mL | −58.97 ± 30.15 | .05 | 2.32 ± 15.68 | .88 |
| Viral load >100 000 copies/mL | −41.40 ± 29.05 | .15 | 19.97 ± 15.39 | .20 |
| Visit (Viral load <4000 copies/mL) | 0b | 0d | ||
| Visit (Viral load 4000–20 000 copies/mL copies/mL) | 1.40 ± 3.06 | .65 | −0.61 ± 3.79 | .87 |
| Visit (Viral load 20 001–100 000 copies/mL) | 8.97 ± 2.99 | .003f | 0.53 ± 3.24 | .87 |
| Visit (Viral load >100 000 copies/mL) | 3.67 ± 3.00 | .22 | −3.25 ± 3.51 | .36 |
Abbreviation: SD, standard deviation.
Type 3, P = .21.
Type 3, P = .02.
Type 3, P = .52.
Type 3, P = .76.
Model adjusted for no. of sex partners in the last 6 months, race, highly active antiretroviral therapy use, enrollment, marital status, annual household income, and education level.
Statistically significant at P value < .05.
DISCUSSION
The current analysis examines the possible association between size of anal warts and CD4+ cell count as well as HIV load over time among HIV-infected women using the public dataset from WIHS, an ongoing longitudinal study in the United States. Our study did not provide evidence of an association between the size of the largest anal warts and CD4+ cell count or HIV load over time in HIV-infected women.
However, we did find a higher prevalence of anal warts at baseline among HIV-infected women in the WIHS study than reported elsewhere [6, 32, 37]. For example, the prevalence of anal warts at baseline found in our analysis is >3 times higher than that in the 1996 survey of the Australian Longitudinal Study on Women’s Health, in which 3.1% of 14 762 women aged 18–23 years reported ever having been diagnosed with anal warts. Caution is warranted when comparing our finding with the prevalence of anal warts in Australia because warts were self-reported in that study and their population is younger than that of the WIHS. The incidence rate of anal warts among HIV-infected and HIV-uninfected women found in our analysis is similar to those in other studies [38–41]. For example, in a study also from the WIHS cohort in 2004, Massad et al [41] reported that the incidence of genital warts among HIV-uninfected and HIV-infected women in the WIHS study were 1.30 per 100 person-years, which is comparable to ours, and 5.41 per 100 person-years, which is somewhat higher than ours, respectively. One possible explanation for the higher incidence in their study is that the follow-up in our analysis is longer than the study by Massad et al [41], and as of October 2010, approximately 80% of WIHS participants had received HAART [21]. HAART was proven to be effective in reducing the incidence of anal warts [41], thus possibly explaining the reduced anal wart incidence in HIV-infected women reported herein.
To our knowledge, this is the first study to use the linear mixed model to address whether there is an association between the size of the largest anal warts over time and either CD4+ cell count or HIV load. Therefore, we cannot compare our findings directly with any other study. However, there are a few studies reporting the relationship between presence and incidence of genital warts with CD4+ cell count [39, 42]. In a previous analysis of the WIHS, Greenblatt et al [42] reported a significant inverse association between CD4+ cell count and genital warts. Accordingly, HIV-infected women with a CD4+ cell count of <200 cells/mm3 were 6.78 times more likely to have genital warts than HIV-uninfected women. The difference between our study and the study by Greenblatt et al [42] is that we examined the size of anal warts, whereas they looked at the presence of genital warts. Another difference is that in our study, we investigated the development (ie, progression or regression) of anal warts based on the size of the wart longitudinally, whereas the study by Greenblatt et al [42] was a cross-sectional analysis of baseline visit only.
We also found an inverse association between the size of anal warts and CD4+ cell count at baseline visit with decreasing CD4+ cell count category. One possible explanation is that because a person with a CD4+ cell count of <500 cells/mm3 probably has a larger anal wart than a person with a higher CD4+ cell count, the growth rate would be slower when comparing the measurement of the size of anal warts between 2 visits. Even though we did not find an association between size of anal wart and CD4+ cell count over time, the role of immunity cannot be ruled out because CD4+ cells play an important role in cell-mediated immunity against HPV infection. This is reflected by the increased incidence and progression of HPV infection among immunosuppressed persons. In a cohort of adolescent girls, Moscicki et al [43] observed that the risk for incident Cervical intraepithelial neoplasia (CIN) among HIV-infected adolescents was due to the persistence of Low grade squamous intraepithelial lesions (LSILs). HIV-infected persons are more likely to have multiple recurrences of cervical CIN, chronic condylomatous changes [44], and increased incidence of both cutaneous and genital warts [45]. Additionally, de Jong et al [46] observed a strong proliferative response against 1 or more peptide epitopes derived from HPV 16 E-2 T-cell antigen in peripheral blood mononuclear cell cultures of approximately half of healthy donors. They also found that most of these responses represented reactivity by memory CD4+ T-helper 1–type cells, which are able to secrete interferon-γ on antigenic stimulation.
We were unable to find other studies directly comparing size of anal wart by HIV load, but Dolev et al [47] reported that HIV load was independently associated with the incidence of anogenital warts in the WIHS cohort. It is, however, noted again that the outcome used in our study is different from theirs because we used the size of anal warts and they used the presence of anogenital warts. Although HIV load has been identified as a risk factor for HPV infection [13, 14, 48, 49] and precancerous lesions of the cervix [14] caused by high-risk HPV, it has not been proven to play an important role in the development of anal warts caused by low-risk HPV types.
There are 2 strengths of our study. First, we used the linear mixed model to deal with the aforementioned difficulties inherent to longitudinal data. Second, the use of the linear mixed model allowed us to model the size of anal wart as a continuous outcome variable appropriately. One limitation of this analysis is that by using the size of the largest anal wart present at each visit, we might not be able to follow the same wart over time because the largest wart measured in the first visit might not be the same one that is measured in subsequent visits, especially if there are multiple warts. It is, however, acknowledged that measurement of the same wart over time, especially in a situation of multiple warts, is a not a clinically feasible task. For this reason, a separate study focused on this specific question is warranted.
In summary, we did not find evidence for the association between size of the largest anal warts and CD4+ cell count or HIV load over time. We did, however, find an inverse correlation between the size of anal wart and CD4+ cell count at baseline visit, although this association was not statistically significant. Further exploration on the role of immune function on the development (either progression or regression) of anal warts is warranted because the association between presence of anal wart and immune response has been established.
Notes
Acknowledgments.
Data in this manuscript were collected by the WIHS, Collaborative Study Group with centers (principal investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington, DC Metropolitan Consortium (Mary Young); the Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen Gange).
Financial support.
This work was supported by the Vietnam Education Foundation and National Institutes of Health (NIH)/Fogarty Training Fellowship (D43 TW007669 to H. N. L.) through the Center for International Training and Research, School of Public Health, the University of Texas Health Science Center at Houston, and in part by a Dan L. Duncan Cancer Center Scholar Award (to M. E. S.).
The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders.
Funding is also provided by the National Center for Research Resources (UCSF-CTSI grant UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 3.Palefsky JM. HPV infection in men. Dis Markers. 2007;23:261–72. doi: 10.1155/2007/159137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strand A, Andersson S, Zehbe I, Wilander E. HPV prevalence in anal warts tested with the MY09/MY11 SHARP signal system. Acta Derm Venereol. 1999;79:226–9. doi: 10.1080/000155599750011048. [DOI] [PubMed] [Google Scholar]
- 5.Menton JF, Cremin SM, Canier L, Horgan M, Fanning LJ. Molecular epidemiology of sexually transmitted human papillomavirus in a self referred group of women in Ireland. Virol J. 2009;6:112. doi: 10.1186/1743-422X-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutsky LA, Galloway DA, Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–63. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- 7.Minkoff HL, Eisenberger-Matityahu D, Feldman J, Burk R, Clarke L. Prevalence and incidence of gynecologic disorders among women infected with human immunodeficiency virus. Am J Obstet Gynecol. 1999;180:824–36. doi: 10.1016/s0002-9378(99)70653-8. [DOI] [PubMed] [Google Scholar]
- 8.Beutner KR, Reitano MV, Richwald GA, Wiley DJ. External genital warts: report of the American Medical Association Consensus Conference. AMA expert panel on external genital warts. Clin Infect Dis. 1998;27:796–806. doi: 10.1086/514964. [DOI] [PubMed] [Google Scholar]
- 9.Low A, Didelot-Rousseau MN, Nagot N, et al. Cervical infection with human papillomavirus (HPV) 6 or 11 in high-risk women in Burkina Faso. Sex Transm Infect. 2010;86:342–4. doi: 10.1136/sti.2009.041053. [DOI] [PubMed] [Google Scholar]
- 10.Fife KH, Wu JW, Squires KE, Watts DH, Andersen JW, Brown DR. Prevalence and persistence of cervical human papillomavirus infection in HIV-positive women initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;51:274–82. doi: 10.1097/QAI.0b013e3181a97be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontaine J, Hankins C, Money D, et al. Human papillomavirus type 16 (HPV-16) viral load and persistence of HPV-16 infection in women infected or at risk for HIV. J Clin Virol. 2008;43:307–12. doi: 10.1016/j.jcv.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Strickler HD, Palefsky JM, Shah KV, et al. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J Natl Cancer Inst. 2003;95:1062–71. doi: 10.1093/jnci/95.14.1062. [DOI] [PubMed] [Google Scholar]
- 13.Palefsky JM, Minkoff H, Kalish LA, et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J Natl Cancer Inst. 1999;91:226–36. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 14.Moodley JR, Constant D, Hoffman M, et al. Human papillomavirus prevalence, viral load and pre-cancerous lesions of the cervix in women initiating highly active antiretroviral therapy in South Africa: a cross-sectional study. BMC Cancer. 2009;9:275. doi: 10.1186/1471-2407-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conley LJ, Ellerbrock TV, Bush TJ, Chiasson MA, Sawo D, Wright TC. HIV-1 infection and risk of vulvovaginal and perianal condylomata acuminata and intraepithelial neoplasia: a prospective cohort study. Lancet. 2002;359:108–13. doi: 10.1016/S0140-6736(02)07368-3. [DOI] [PubMed] [Google Scholar]
- 16.Palefsky J, del Rio C. Is high-grade dysplasia on anal pap a high-grade problem? AIDS Clin Care. 2002;14:44–5. [PubMed] [Google Scholar]
- 17.Lacey CJ. Therapy for genital human papillomavirus-related disease. J Clin Virol. 2005;32:S82–90. doi: 10.1016/j.jcv.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Vandepapeliere P, Barrasso R, Meijer CJ, et al. Randomized controlled trial of an adjuvanted human papillomavirus (HPV) type 6 L2E7 vaccine: infection of external anogenital warts with multiple HPV types and failure of therapeutic vaccination. J Infect Dis. 2005;192:2099–107. doi: 10.1086/498164. [DOI] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–25. [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WIHS Data Management and Analysis Center (WDMAC) Women’s Interagency HIV Study (WIHS) dossier. Baltimore, MD: WIHS Data Management and Analysis Center (WDMAC); https://statepiaps.jhsph.edu/wihs/invest-info/dossier.pdf. Accessed 1 May 2011. [Google Scholar]
- 22.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK Panel on Clinical Practices for Treatment of HIV. Guidelines for the use of antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137:381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 23.Yeni PG, Hammer SM, Carpenter CC, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society—USA Panel. JAMA. 2002;288:222–35. doi: 10.1001/jama.288.2.222. [DOI] [PubMed] [Google Scholar]
- 24.Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201:681–90. doi: 10.1086/650467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahdieh-Grant L, Li R, Levine AM, et al. Highly active antiretroviral therapy and cervical squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2004;96:1070–6. doi: 10.1093/jnci/djh192. [DOI] [PubMed] [Google Scholar]
- 26.Fitzmaurice GM, Laird NM, Ware JH. Review of generalized linear models. In: Fitzmaurice GM, Laird NM, Ware JH, editors. Applied longitudinal analysis. Hoboken, NJ: Wiley-Interscience, John Wiley & Sons.; 2004. pp. 257–90. [Google Scholar]
- 27.Notermans DW, de Wolf F, Oudshoorn P, et al. Evaluation of a second-generation nucleic acid sequence-based amplification assay for quantification of HIV type 1 RNA and the use of ultrasensitive protocol adaptations. AIDS Res Hum Retroviruses. 2000;16:1507–17. doi: 10.1089/088922200750006038. [DOI] [PubMed] [Google Scholar]
- 28.Palefsky JM. Anal squamous intraepithelial lesions: relation to HIV and human papillomavirus infection. J Acquir Immune Defic Syndr. 1999;21:S42–8. [PubMed] [Google Scholar]
- 29.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–91. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 30.Holly EA, Ralston ML, Darragh TM, Greenblatt RM, Jay N, Palefsky JM. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843–9. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 31.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 32.Schofield MJ, Minichiello V, Mishra GD, Plummer D, Savage J. Sexually transmitted infections and use of sexual health services among young Australian women: Women’s Health Australia Study. Int J STD AIDS. 2000;11:313–23. doi: 10.1177/095646240001100507. [DOI] [PubMed] [Google Scholar]
- 33.Cook JA, Cohen MH, Grey D, et al. Use of highly active antiretroviral therapy in a cohort of HIV-seropositive women. Am J Public Health. 2002;92:82–7. doi: 10.2105/ajph.92.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowicki MJ, Karim R, Mack WJ, et al. Correlates of CD4+ and CD8+ lymphocyte counts in high-risk immunodeficiency virus (HIV)-seronegative women enrolled in the Women’s Interagency HIV Study (WIHS) Hum Immunol. 2007;68:342–9. doi: 10.1016/j.humimm.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Hessol NA, Holly EA, Efird JT, et al. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS. 2009;23:59–70. doi: 10.1097/QAD.0b013e32831cc101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SAS Institute Inc. SAS version 9.2. Cary, NC: SAS Institute; 2008. [Google Scholar]
- 37.Dinh TH, Sternberg M, Dunne EF, Markowitz LE. Genital warts among 18- to 59-year-olds in the United States, National Health and Nutrition Examination Survey, 1999–2004. Sex Transm Dis. 2008;35:357–60. doi: 10.1097/OLQ.0b013e3181632d61. [DOI] [PubMed] [Google Scholar]
- 38.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 39.Low AJ, Clayton T, Konate I, et al. Yérélon Cohort Study Group. Genital warts and infection with human immunodeficiency virus in high-risk women in Burkina Faso: a longitudinal study. BMC Infect Dis. 2011;11:20. doi: 10.1186/1471-2334-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chirgwin KD, Feldman J, Augenbraun M, Landesman S, Minkoff H. Incidence of venereal warts in human immunodeficiency virus-infected and uninfected women. J Infect Dis. 1995;172:235–8. doi: 10.1093/infdis/172.1.235. [DOI] [PubMed] [Google Scholar]
- 41.Massad LS, Silverberg MJ, Springer G, et al. Effect of antiretroviral therapy on the incidence of genital warts and vulvar neoplasia among women with the human immunodeficiency virus. Am J Obstet Gynecol. 2004;190:1241–8. doi: 10.1016/j.ajog.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 42.Greenblatt RM, Bacchetti P, Barkan S, et al. Lower genital tract infections among HIV-infected and high-risk uninfected women: findings of the Women’s Interagency HIV Study (WIHS) Sex Transm Dis. 1999;26:143–51. doi: 10.1097/00007435-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190:37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 44.Fruchter RG, Maiman M, Sedlis A, Bartley L, Camilien L, Arrastia CD. Multiple recurrences of cervical intraepithelial neoplasia in women with the human immunodeficiency virus. Obstet Gynecol. 1996;87:338–44. doi: 10.1016/0029-7844(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 45.Fennema JS, van Ameijden EJ, Coutinho RA, van den Hoek AA. HIV, sexually transmitted diseases and gynaecologic disorders in women: increased risk for genital herpes and warts among HIV-infected prostitutes in Amsterdam. AIDS. 1995;9:1071–8. [PubMed] [Google Scholar]
- 46.de Jong A, van der Burg SH, Kwappenberg KM, et al. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62:472–9. [PubMed] [Google Scholar]
- 47.Dolev JC, Maurer T, Springer G, et al. Incidence and risk factors for verrucae in women. AIDS. 2008;22:1213–9. doi: 10.1097/QAD.0b013e3283021aa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)–positive and HIV-negative women. J Infect Dis. 2001;184:682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 49.Luque AE, Demeter LM, Reichman RC. Association of human papillomavirus infection and disease with magnitude of human immunodeficiency virus type 1 (HIV-1) RNA plasma level among women with HIV-1 infection. J Infect Dis. 1999;179:1405–9. doi: 10.1086/314754. [DOI] [PubMed] [Google Scholar]