Abstract
Background. Children have a lower response rate to antimonial drugs and higher elimination rate of antimony (Sb) than adults. Oral miltefosine has not been evaluated for pediatric cutaneous leishmaniasis.
Methods. A randomized, noninferiority clinical trial with masked evaluation was conducted at 3 locations in Colombia where Leishmania panamensis and Leishmania guyanensis predominated. One hundred sixteen children aged 2–12 years with parasitologically confirmed cutaneous leishmaniasis were randomized to directly observed treatment with meglumine antimoniate (20 mg Sb/kg/d for 20 days; intramuscular) (n = 58) or miltefosine (1.8–2.5 mg/kg/d for 28 days; by mouth) (n = 58). Primary outcome was treatment failure at or before week 26 after initiation of treatment. Miltefosine was noninferior if the proportion of treatment failures was ≤15% higher than achieved with meglumine antimoniate (1-sided test, α = .05).
Results. Ninety-five percent of children (111/116) completed follow-up evaluation. By intention-to-treat analysis, failure rate was 17.2% (98% confidence interval [CI], 5.7%–28.7%) for miltefosine and 31% (98% CI, 16.9%–45.2%) for meglumine antimoniate. The difference between treatment groups was 13.8%, (98% CI, −4.5% to 32%) (P = .04). Adverse events were mild for both treatments.
Conclusions. Miltefosine is noninferior to meglumine antimoniate for treatment of pediatric cutaneous leishmaniasis caused by Leishmania (Viannia) species. Advantages of oral administration and low toxicity favor use of miltefosine in children.
Clinical Trial Registration. NCT00487253.
Internal migration and demographic changes in Latin America have been accompanied by transmission of dermal leishmaniasis in the domestic setting and increased incidence among children [1–5]. Pentavalent antimonial drugs continue to be the first-line treatment for cutaneous leishmaniasis in Latin America [6]. Children have only recently been included in clinical trials, revealing a significantly lower response rate and significantly higher elimination rate of antimony compared with adults [7, 8]. These concerns and the challenge of adherence to treatment requiring daily intramuscular injections over the course of 20 days in remote, resource-poor settings where cutaneous leishmaniasis occurs demand an effective therapeutic alternative for pediatric cutaneous leishmaniasis.
Oral miltefosine is well tolerated and efficacious against Old World visceral leishmaniasis and cutaneous leishmaniasis caused by some but not all species of Leishmania [9–16]. Variable susceptibility of Leishmania species to miltefosine has been suggested by in vitro analyses [17, 18]. Although the efficacy of miltefosine in children has been shown for visceral leishmaniasis caused by Leishmania donovani [19–21], efficacy for pediatric cutaneous leishmaniasis has not been evaluated. Recently reported trials conducted in Brazil included very small numbers of children, few being <7 years of age [14, 15]. Treatment policy for use of miltefosine in pediatric cutaneous leishmaniasis awaits randomized controlled evaluation of efficacy in the target population. We report the results of a multicenter, open-label, randomized, noninferiority clinical trial of miltefosine versus meglumine antimoniate for pediatric cutaneous leishmaniasis caused by species of the Viannia subgenus.
METHODS
Study Populations
The study was conducted in 3 geographic locations in Colombia: the municipalities of Chaparral (Tolima), Tumaco (Nariño), and Cali (Valle). Chaparral, located in the central region of Colombia, experienced an epidemic outbreak of cutaneous leishmaniasis between 2003 and 2007 characterized by domestic transmission of Leishmania guyanensis [5, 22], which is now endemic. Tumaco, on the southern Pacific coast of Colombia, is endemic for Leishmania panamensis and to a lesser extent Leishmania braziliensis. Cali is the capital of the Department of Valle del Cauca, located within the northern Pacific coast region, and is the referral center for leishmaniasis from southwestern Colombia, where both L. braziliensis and L. panamensis are prevalent, with the latter predominating. [23–25].
Eligibility Criteria
Eligible participants included children aged 2–12 years with parasitologically confirmed cutaneous leishmaniasis who were available to receive supervised treatment for up to 28 days and participate in follow-up for 26 weeks. Exclusion criteria were weight <10 kg, mucocutaneous disease, use of anti-Leishmania medications during the month prior to diagnosis, medical history of cardiac, renal, or hepatic disease, menarche, and baseline values for hemoglobin, amylase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and serum urea nitrogen outside the normal range. In case of borderline values, decision to include or exclude was supported by clinical assessment. Patients who were ineligible for the trial, declined to participate, or withdrew consent received standard-of-care treatment in accordance with guidelines of the Colombian Ministry of Social Protection [26].
Study Design
This multicenter, open-label, randomized clinical trial with masked evaluation was designed to determine the noninferiority of miltefosine (Impavido®; Zentaris) compared with standard-of-care treatment with meglumine antimoniate (Glucantime®; Aventis Pharma). The rationale for determining noninferiority was based on the advantage of oral administration and the lower toxicity profile of miltefosine. Double blinding was not undertaken because of the different routes of administration of the study medications and the unjustified and unethical risk of injection placebo. Sample size estimate assumed 20% treatment failure for meglumine antimoniate and 15% for miltefosine and a 15% maximum inferiority of miltefosine. Sixty-two children per group were necessary to demonstrate this difference with an α value of .05 (1 tail) and a power of 90%. The 15% maximum difference was determined by consensus of a panel of physicians experienced in the treatment of leishmaniasis.
The study was approved by the institutional ethical review boards of Centro Internacional de Entrenamiento e Investigaciones Medicas (CIDEIM) and Centro Dermatológico Federico Lleras Acosta, the national reference center for dermatologic disease. Legal guardians of all participants provided written informed consent; patients aged ≥7 years provided written informed assent.
Participants were randomized to receive either meglumine antimoniate (81 mg Sb/mL) at 20 mg Sb/kg/d intramuscular for 20 consecutive days or miltefosine (10 mg miltefosine/capsule) at 1.5–2.5 mg/kg/d by mouth during 28 consecutive days, divided into 2 or 3 daily doses. A computerized balanced block randomization scheme was used to generate group assignment, which was stratified according to study site and age group (2 to < 7 and 7–12 years). To ensure allocation concealment, treatment was assigned by the coordinating center (CIDEIM) via phone call from the study site at subject inclusion. Directly observed treatment was administered daily by study personnel.
Clinical and Laboratory Procedures
Clinical evaluation was conducted at enrollment, daily during treatment, and at 13 and 26 weeks after initiation of treatment. Lesions were measured and standardized photographs were taken at enrollment and 13 and 26 weeks after initiation of treatment or when a therapeutic failure was identified. Rescue treatment was either meglumine antimoniate or miltefosine and was directly observed by study personnel.
Parasitologic diagnosis was established by microscopic examination of tissue smears and culture of aspirates from the lesion border [27]. Leishmania species were identified using a panel of subgenus and species-specific monoclonal antibodies or isoenzyme electrophoresis [23, 25]. Aspirates of lesions were obtained at the end of treatment and cultured as described elsewhere [27]. Hemoglobin, amylase, AST, ALT, alkaline phosphatase, creatinine, and serum urea nitrogen levels were evaluated midway and at the end of treatment to monitor potential drug-related toxicity. Children presenting with abnormal laboratory values were monitored until their normalization.
Outcome Definitions
The primary outcome of the study was therapeutic failure determined at or before 26 weeks after initiation of treatment. During each follow-up visit, lesions were evaluated and classified as follows.
End of Treatment
Improvement was defined as a decrease in lesion size and inflammatory signs (induration, raised borders, redness) of lesions; apparent cure, as complete re-epithelization and absence of inflammatory signs for all cutaneous leishmaniasis lesions; and early treatment failure as an increase of >100% in the size of any lesion compared with its size at baseline or the appearance of new cutaneous leishmaniasis lesions.
13 Weeks
Initial therapeutic response was defined as complete re-epithelization and the absence of inflammatory signs for all cutaneous leishmaniasis lesions, and therapeutic failure at week 13 was defined as incomplete re-epithelization and/or the presence of induration, raised borders, or redness in any lesion or the appearance of new lesions.
26 Weeks (Final Therapeutic Outcome)
Final therapeutic response was defined as an initial clinical therapeutic response attained by week 13 and maintained until week 26 without the appearance of new lesions, and therapeutic failure at week 26 was defined as the presence of inflammatory signs (induration, raised borders, or redness) or ulceration in any of the original lesions or the appearance of new lesions.
When participants met definitions of therapeutic failure (at any of the follow-up visits), follow-up was concluded, and alternative treatment was provided. To eliminate ascertainment bias, treatment outcome was determined by a masked evaluator using standardized photographs of lesions. In case of disagreement between the clinical evaluation by study site physicians and the masked evaluator, the photos were evaluated by a second masked dermatologist. This occurred in 4 cases; outcome assessment by the 2 masked evaluators concurred in all cases.
Adverse events were identified throughout treatment and follow-up by study personnel using a structured questionnaire to record constitutional and gastrointestinal symptoms. Adverse events were classified according to Common Terminology Criteria for Adverse Events, version 3.0 [28].
Parasitologic response, defined as failure to culture parasites from lesion aspirates obtained at the end of treatment, was a secondary outcome. The effect of Leishmania species, study site, and patient age on the efficacy of oral miltefosine in children with cutaneous leishmaniasis was explored in post hoc subgroup analyses.
Statistical Methods
Baseline characteristics were compared using Mann–Whitney, Fisher’s, and χ2 tests. Proportions of patients presenting treatment failure in each group were compared using χ2 test for contingency tables. The 98% confidence interval (CI) of the difference in the proportion of therapeutic failures was estimated. Miltefosine was considered noninferior if the proportion of treatment failures was ≤15% higher than for meglumine antimoniate (1-sided test, α = .01). Logistical regression models were fitted to assess the relation of Leishmania species, patient age, and size and number of lesions with the outcome. Data were entered into the TrialDB database after verification. Analyses were performed using Stata software, release 9.2.
Data were analyzed as intention to treat (ITT) and per protocol (PP). Subjects withdrawn from the study or unavailable for follow-up were considered therapeutic failures for ITT analysis and excluded from PP analysis. The data safety monitoring committee adopted a plan of analysis to estimate the relationship of adverse events and laboratory results with each study medication from initiation to end of treatment, and the committee reviewed reported adverse events and the corresponding patient histories.
Interim analysis was conducted when 80 children (meglumine antimoniate, 41; miltefosine, 39) had completed the final follow-up. The PP and ITT analyses were performed assuming α = .01. Based on this analysis, enrollment was stopped with 116 subjects. Although sample size was estimated assuming α = .05, we report results assuming the more conservative significance level used in the interim analysis (α = .01).
RESULTS
Study Participants
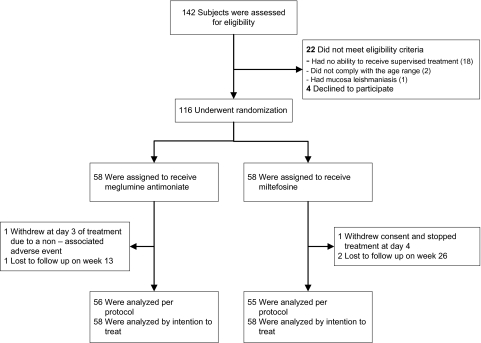
Patients were enrolled from July 2007 to November 2009. The last 26-week follow-up was completed in June 2010. Eligibility was evaluated in 142 children. A total of 116 children, 54 from Tumaco, 50 from Chaparral, and 12 from Cali, were randomized to receive meglumine antimoniate (n = 58) or miltefosine (n = 58) (Figure 1). The dose of miltefosine that was actually administered based on the pediatric presentation as capsules of 10 mg ranged from 1.8–2.5 mg/kg/d (mean, 2.3 mg/kg/d). One patient per group failed to complete the assigned treatment: a serious adverse event (sickle-cell crisis) required withdrawal of a patient from the meglumine antimoniate group; informed consent was withdrawn for a patient in the miltefosine group. Directly observed treatment and follow-up evaluation at 26 weeks were completed by 95.6% of randomized patients (111/116).
Figure 1.
Study enrollment, randomization, and follow-up.
The only significant difference between groups for baseline characteristics was more frequent presence of palpable lymphatic involvement along the trajectory-draining lesions in the miltefosine group (Table 1). Importantly, children aged <7 years, who have been shown to eliminate antimony more rapidly than adults [8], constituted approximately half of the subjects in both treatment groups.
Table 1.
Baseline Characteristics According to Study Group
| Characteristics | Meglumine Antimoniate (n = 58) | Miltefosine (n = 58) | P |
| Age, median (range), y | 7 (2–11) | 7 (2–12) | .91a |
| Male sex, No. (%) | 31 (53.4) | 24 (41.3) | .19b |
| Race, No. (%) | .87b | ||
| Mestizo | 30 (51.7) | 30 (51.7) | |
| Afro-Colombian | 22 (37.9) | 24 (41.4) | |
| Indigenous | 4 (6.9) | 3 (5.2) | |
| White | 1 (1.7) | 1 (1.7) | |
| Mulatto | 1 (1.7) | 0 | |
| Weight, median (range), kg | 20 (10–33.3) | 20.4 (10–51) | .71a |
| Subjects previously treated with meglumine antimoniate for current lesions,c No. (%) | 1 (1.7) | 3 (5.2) | .62d |
| Lesions per subject, median (range), No. | 2 (1–7) | 2 (1–8) | .35a |
| Lesion size, median (range), mm2 | 209 (28–1764) | 277 (2–2441) | .06a |
| Location of lesion, No. (%) | .07b | ||
| Head and neck | 48 (39.0) | 31 (25.8) | |
| Upper limbs | 37 (30.1) | 48 (40.0) | |
| Lower limbs | 23 (18.7) | 31 (25.8) | |
| Trunk | 15 (12.2) | 10 (8.3) | |
| Type of lesion, No. (%) | .29b | ||
| Ulcer | 110 (89.4) | 113 (94.1) | |
| Papule | 4 (3.3) | 4 (3.3) | |
| Plaque | 6 (4.9) | 1 (0.8) | |
| Nodule | 3 (2.4) | 2 (1.7) | |
| Lymphatic tract, No. of subjects (%) | 0 | 5 | .03d |
| Regional lymph nodes, No. of subjects (%) | 10 (17.2) | 11 (19.0) | .80b |
| Duration of older lesion,c median (range), mo | 2 (1–24) | 2 (1–14) | .31a |
| Species identified, No. (%) | .06b | ||
| Leishmania panamensis | 19 (76.0) | 24 (68.6) | |
| Leishmania guyanensis | 5 (20.0) | 11 (31.4) | |
| Leishmania braziliensis | 1 (4.0) | 0 | |
| Total | 25 (43.1) | 35 (60.3) |
Mann–Whitney test.
χ2test.
Before enrollment.
Fisher’s exact test.
Parasites were isolated and identified in 51.7% of participants (60/116) from samples obtained at the baseline visit. The majority (71.6%) were L. panamensis, followed by L. guyanensis (26.6%). Only L. panamensis was isolated from 32 of 54 patients enrolled in Tumaco, and only L. guyanensis was isolated from 16 of 50 patients enrolled in Chaparral. L. braziliensis was isolated from 1 patient in Cali; all others (11/12) were L. panamensis.
Primary Outcome
Therapeutic failures were diagnosed in 23 patients (Table 2): 16 of 56 in the meglumine antimoniate group and 7 of 55 in the miltefosine group. By ITT analysis, the failure rate was 17.2% (98% CI, 5.7%–28.7%) for miltefosine and 31% (98% CI, 16.9%–45.2%) for meglumine antimoniate. The difference in outcome between the 2 treatment groups was 13.8% (98% CI, −4.5% to 32%). Results of PP analysis were similar (Table 2), with a 15.9% (98% CI, −1.7% to 33.4%) lower failure rate for miltefosine. In both analyses, the lower limit of the confidence interval of the difference was well above −15% (the predetermined delta), establishing noninferiority of miltefosine.
Table 2.
Response to Treatment According to Study Group
| Variable | Meglumine Antimoniate (n = 58) | Miltefosine (n = 58) |
| Primary outcome | ||
| Definitive cure at week 26 | 40 | 48 |
| Treatment failure | 16 | 7 |
| Unavailable for follow-up | 1 | 2 |
| Did not complete treatment | 1 | 1 |
| Failure (98% CI), %a | ||
| Per-protocol population | 28.6 (14.5–42.6) | 12.7 (2.3–23.2) |
| Intention-to-treat population | 31 (16.9–45.2) | 17.2 (5.7–28.8) |
Abbreviation: CI, confidence interval.
The difference in median failure rates between treatment groups was 15.9 (98% CI, −1.7 to 33.4) for the per-protocol population and 13.8 (−4.5 to 32.0) for the intention-to-treat population.
Early treatment failure was diagnosed in 1 patient receiving meglumine antimoniate at the end of treatment (Table 3). Ten additional therapeutic failures in the meglumine antimoniate group and 4 therapeutic failures in the miltefosine group were diagnosed at or before the week 13 evaluation. All other therapeutic failures—5 in the meglumine antimoniate group and 3 in the miltefosine group—were diagnosed at the week 26 evaluation (Table 3).
Table 3.
Response to Treatment Over Period of Follow-up
| Subjects, No. (%) |
||
| Follow-up Period and Outcome | Meglumine Antimoniate (n = 58) | Miltefosine (n = 58) |
| End of treatmenta | ||
| Apparent cure | 17 (29.8) | 27 (47.4) |
| Improvement | 39 (68.4) | 30 (52.6) |
| Early failure | 1 (1.8) | 0 (0) |
| Withdrawal without completion of treatment | 1 (1.8) | 1 (1.8) |
| Total | 58 | 58 |
| Week 13 | ||
| Initial therapeutic response | 41 (70.6) | 53 (91.3) |
| Therapeutic failure at week 13 | 10 (17.2) | 4 (6.9) |
| Unavailable for follow-up | 1 (1.8) | 0 |
| Early failure | 1 (1.8) | 0 |
| Did not attend week 13 visit | 4 (6.9) | 0 |
| Withdrawal without completion of treatment | 1 (1.8) | 1 (1.8) |
| Total | 58 | 58 |
| Week 26 | ||
| Final therapeutic response | 40 (68.9) | 48 (82.8) |
| Therapeutic failure at week 26 | 5 (8.6) | 3 (5.2) |
| Early failure | 1 (1.8) | 0 |
| Failure at week 13 | 10 (17.2) | 4 (6.9) |
| Unavailable for follow-up | 1 (1.8) | 2 (3.4) |
| Withdrawal without completion of treatment | 1 (1.8) | 1 (1.8) |
| Total | 58 | 58 |
| Case closure | ||
| Cure | 40 (69.0) | 48 (82.8) |
| Failure | 18 (31.0) | 10 (17.2) |
| Total | 58 | 58 |
The treatment period was 20 days for meglumine antimoniate and 28 days for miltefosine.
One patient in the meglumine antimoniate group and 3 in the miltefosine group had received treatment with meglumine antimoniate >2 months before enrollment in the study. Treatment failed only in the child randomized to meglumine antimoniate.
Secondary Outcomes and Post Hoc Analyses
Post hoc analyses suggest a possible association between treatment response and age, Leishmania species, and study site (Table 4) However, none of these associations reached statistical significance in Mantel–Haenszel tests of homogeneity. Neither sex nor clinical characteristics of lesions were associated with outcome of treatment.
Table 4.
Frequency of Treatment Failures by Age, Study Site, and Infecting Leishmania Species
| Meglumine Antimoniate Failures |
Miltefosine Failures |
|||||
| Variable | No. | % | No. | % | Pa | M–H Test |
| Age | 0.086 | |||||
| <7 years | 12 (28) | 42.9 | 3 (27) | 11.1 | .008 | |
| ≥7 years | 6 (30) | 20.0 | 7 (31) | 22.6 | .81 | |
| Site | 0.064 | |||||
| Tumaco | 9 (27) | 33.3 | 2 (27) | 7.4 | .02 | |
| Cali | 1 (6) | 16.7 | 0 (6) | 0 | .30 | |
| Chaparral | 8 (25) | 32.0 | 8 (25) | 32.0 | 1.0 | |
| Leishmania species | ND | |||||
| L. panamensis | 7 (19) | 36.8 | 2 (24) | 8.3 | .02 | |
| L. guyanensis | 1 (5) | 20.0 | 5 (11) | 45.5 | .33 | |
| L. braziliensis | 0 (1) | 0 | 0 | 0 | … | |
Abbreviations: M–H test, Mantel–Haenszel test of homogeneity; ND, not done.
χ2 test.
Number parentheses corresponds to total number of children in each group.
In children aged <7 years, therapeutic response to miltefosine was significantly higher than response to meglumine antimoniate (89.9% vs 57.1%; P = .008). The response to meglumine antimoniate was lower in this age group than in children aged ≥7 years (57.1% vs 80%; P = .06), but this difference did not reach statistical significance.
Among patients from whom parasites were isolated at diagnosis, infections caused by L. panamensis presented a higher failure rate with meglumine antimoniate than with miltefosine (36.8% vs 8.3%; P = .02). The low proportion of patients from whom L. guyanensis was achieved precludes conclusions regarding treatment response based only on patients from whom this species was isolated. However, assuming that most, if not all, patients in Chaparral were infected by L. guyanensis, the equal failure rate (8/25) for miltefosine and meglumine antimoniate among participants enrolled at this study site indicates that infections with L. guyanensis were similarly responsive to both drugs.
L. panamensis was cultured from lesion aspirates after treatment in only 3/51 patients evaluated. All were from patients treated with meglumine antimoniate who presented therapeutic failure during follow-up. The low efficiency of culture post-treatment precluded analysis of association of parasitologic response with treatment.
Adverse Events
Adverse events were evaluated in all patients that completed treatment and observed in 91, (48/57 meglumine antimoniate; 43/57 miltefosine). Ninety-five percent of clinical adverse events were grade 1 according to Common Terminology Criteria for Adverse Events classification (mild symptoms that do not interfere with regular activities). Patients receiving meglumine antimoniate more frequently had elevated levels of hepatic enzymes AST (16/57 vs 5/57; P = .01) and ALT (10/57 vs 2/57; P = .01), compared with patients receiving miltefosine (Table 5). Gastrointestinal symptoms were more frequent for patients receiving miltefosine than for those receiving meglumine antimoniate; these included nausea (9/57 vs 2/57; P = .02) and vomiting (15/57 vs 2/57; P < .001). Fever and loss of appetite were more frequent in the meglumine antimoniate group, and abdominal pain was more frequent in the miltefosine group, but neither difference was statistically significant. In no case was treatment with either drug stopped because of intolerance.
Table 5.
Clinical and Laboratory Adverse Events According to Study Group
| Treatment Groups |
|||
| Variable | Meglumine Antimoniate (n = 57)a | Miltefosine (n = 57)a | Pb |
| Adverse events n (%) | |||
| Loss of appetite | 12 (21.1) | 6 (10.5) | .12 |
| Diarrhea | 3 (5.3) | 4 (7.0) | .70 |
| Nausea | 2 (3.5) | 9 (15.8) | .03 |
| Vomiting | 2 (3.5) | 15 (26.3) | <.001 |
| Headache | 7 (12.3) | 4 (7.0) | .34 |
| Abdominal pain | 7 (12.3) | 13 (22.8) | .14 |
| Fever | 14 (24.6) | 8 (14.0) | .16 |
| Any adverse event | 43 (75.4) | .41 | |
| Pancreatic amylasec | |||
| Grade 1 (>ULN ≤1.5 × ULN) | 12 (21.1) | 10 (17.5) | .70 |
| Grade 2 (>1.5 × ULN ≤2.0 × ULN) | 7 (12.3) | 8 (14.0) | .73 |
| Grade 3 (>2.0 × ULN ≤5.0 × ULN) | 18 (31.6) | 18 (31.6) | .90 |
| Grade 4 (>5.0 × ULN) | 3 (5.3) | 0 | .09 |
| Alkaline phosphatasec | |||
| Grade 1 (>ULN ≤2.5 × ULN) | 24 (42.1) | 29 (50.9) | .35d |
| Grade 2 (>2.5 × ULN ≤5.0 × ULN) | 1 (1.8) | 3 (5.3) | .62d |
| Alanine aminotransferasec | |||
| Grade 1 (>ULN ≤2.5 × ULN) | 10 (17.5) | 2 (3.5) | .02 |
| Grade 2 (>2.5 × ULN ≤5.0 × ULN) | 1 (1.8) | 0 | .32 |
| Grade 4 (>20.0 × ULN) | 0 | 1 (1.8) | .31 |
| Aspartate aminotransferasec | |||
| Grade 1 (>ULN ≤2.5 × ULN) | 16 (28.1) | 5 (8.8) | .01 |
| Grade 2 (>2.5 × ULN ≤5.0 × ULN) | 2 (3.5) | 0 | .16 |
| Grade 4 (>20.0 × ULN) | 0 | 1 (1.8) | .31 |
| Hemoglobinc | |||
| Grade 2 (8.0–10 g/dL) | 10 (17.5) | 4 (7.0) | .1 |
| Creatininec | |||
| Grade 1 (>ULN ≤1.5 × ULN) | 5 (8.8) | 13 (22.8) | .03 |
| Grade 2 (>1.5 × ULN ≤3.0 × ULN) | 1 (1.8) | 0 | .32 |
Abbreviation: ULN, upper limit of normal.
One patient per group withdrew within the first days of treatment. These patients were excluded from the analysis of adverse events.
χ2 test.
Classification based on the Common Terminology Criteria for Adverse Events, version 3.0, published 9 August 2006.
Fisher’s exact test.
DISCUSSION
Systemic parenteral treatment with pentavalent antimonial drugs is the current standard of care for cutaneous leishmaniasis in Latin America, where mucosal and chronic disease–causing species of the Viannia subgenus are responsible for the vast majority of cases [29]. Children are particularly vulnerable to transmission within the domestic setting and respond poorly to antimonial treatment [7, 30]. Furthermore, pharmacokinetics of antimony in children results in substantially lower, potentially subtherapeutic, drug exposure compared with adults [8].
This study established the noninferiority of miltefosine to the standard-of-care meglumine antimoniate for the treatment of pediatric cutaneous leishmaniasis in 3 populations and locations where the most prevalent species of the Leishmania (Viannia) subgenus (L. panamensis, L. braziliensis, and L. guyanensis) are actively transmitted. We know of no other randomized efficacy trial of any anti-Leishmania treatment for cutaneous leishmaniasis in children. Although 2 recent studies comparing miltefosine with antimonial drug treatment of cutaneous leishmaniasis caused by L. guyanensis and L. braziliensis have included children, they were not designed to evaluate efficacy in children, who constituted a minority of patients and of whom most were aged >6 years [14, 15].
Clinical and laboratory toxicity were transient and mild for both medications, as reported in prior studies in adults [12, 31, 32]. Daily monitoring detected mild adverse events in 84.2% and 75.4% of children treated with meglumine antimoniate and miltefosine respectively, similar to the proportion and frequency detected by weekly monitoring in Brazilian studies that included children [14, 15]. Importantly, the teratogenicity risk of miltefosine, requiring effective contraception in females of reproductive age, is not an issue in children, and the oral administration of miltefosine obviates the risks and logistical demands of daily intramuscular injections in the rural communities where cutaneous leishmaniasis occurs. These advantages support targeted use of miltefosine for children and the feasibility of home-based treatment, directly observed by a family member, which could assure adherence to treatment and proper usage [33]. Such an approach is encouraged by the 95% adherence to ambulatory miltefosine treatment of visceral leishmaniasis in a phase IV trial in India, in vastly more complex social and medical circumstances, which included treatment together with contraception in females of reproductive age [34].
Although subgroup analyses suggest there might be differential treatment response between species, study sites, and age group, these results are inconclusive because the study was not designed or powered to evaluate these factors. A major motivation of this study was evidence from previous studies supporting lower therapeutic response to antimonial drugs and the potential impact of higher elimination rates for this drug in children. For this reason, randomization was stratified by age as well as study site. Subgroup analyses suggest that miltefosine may be superior to meglumine antimoniate in children aged <7 years and noninferior in older children. Species comparison based on isolation is limited by selection bias because of the wide disparity in efficiency of parasite isolation in the study sites, particularly Chaparral, where isolation was achieved in only 30% of participants (16/50) enrolled at this study site. Nevertheless, comparison by study site, assuming that that all or most cases were caused by L. guyanensis in Chaparral [5] and by L. panamensis in Tumaco, supports noninferiority of miltefosine in Chaparral and Tumaco and the conclusion of noninferiority of miltefosine in the target pediatric population.
Prior trials in adults have shown cutaneous leishmaniasis caused by L. panamensis in Colombia to be variably responsive to miltefosine and meglumine antimoniate [31, 32]. Few studies have assessed the efficacy of miltefosine for the treatment of cutaneous leishmaniasis caused by other species. Despite the poor response of L. braziliensis to miltefosine in Guatemala [11], trials conducted in endemic areas for L. braziliensis and L. guyanensis in Brazil and L. braziliensis in Bolivia demonstrated the efficacy of miltefosine for cutaneous leishmaniasis caused by these species in these high-burden countries [14–16].
Distinguishing strengths of this study include randomization by age group and study site, administration of study medications by directly observed treatment, and masked evaluation. These features and the high adherence (96%) of randomized patients to the assigned treatment and retention throughout the 26-week follow-up substantiate the validity of the primary outcome. Although double blinding was not feasible, the few discrepancies between study site physicians and masked expert evaluation were resolved by a second masked evaluation, and any bias of masked evaluation would have equally affected the treatment groups.
None of the currently recommended drugs for the treatment of cutaneous leishmaniasis are ideal, particularly for children in remote endemic settings. Alternatives are needed, as is the evidence base to guide their use. The efficacy, low-toxicity profile, and advantage of oral administration of miltefosine favor the use of miltefosine for the treatment of pediatric cutaneous leishmaniasis caused by Leishmania of the Viannia subgenus. Regulation of the use of the pediatric formulation of miltefosine and adherence to treatment and pharmacovigilance will be necessary to preserve its utility [33, 35–37].
Notes
Acknowledgments.
We thank Barbara Louise Herwalt for her contributions to the conception of this study. The assistance of physicians Michel Talbert, Victor Manuel Blanco, Johana Parra, Adolfo Álvarez, Juan Pablo Valverde, Rafael Montaña, Luisa Vélez, Johana Ospino, Boris Sánchez, and Wilson Cortez, nurses Mary Luz Hurtado, Bladimir Dueñas, Minelly Yepes, and Ruth Janeth Caicedo in the diagnosis, enrollment, treatment, and clinical management of patients is gratefully acknowledged. We thank Robinson Pacheco, Ricardo Obonaga, Javier Dario Martínez, and Rafael Góngora for assistance in sample procurement, shipping, and analysis; Adriana Cruz for masked evaluation of treatment outcomes in discrepant cases and participation on the Data Safety Management Board, together with Helmer Zapata and Mauricio Palacios; Neal Alexander for support in the interim analysis; and James Becerra, Sandra Becerra, Maria Fernanda Collazos, and Olga Fernandez for data entry and security. The Institute of Health of Nariño, the Regional Hospital San Andres de Tumaco, the Regional Hospital San Juan Bautista, and the Secretaries of Public Health of the Departments of Valle del Cauca and Tolima generously provided logistical support for the conduct of this study. The meglumine antimoniate was provided by the Ministry of Social Protection of Colombia and the pediatric formulation of miltefosine was kindly provided by Aeterna Zentaris.
The study protocol and informed consent/assent were approved by Ethical Review Boards of CIDEIM, and the Centro Dermatológico Federico Lleras, Bogotá, in accordance with the Declaration of Helsinki, Guidelines for Good Clinical Practice, and the Colombian Ministry of Social Protection (Resolution 8430 of 1993 and Resolution 2378 of 2008). Written informed consent and assent were provided by participants or their parents or guardians after explanation of study procedures, benefits, and potential risks.
Financial support.
This work was supported by the Colombian national Departamento Administrativo de Ciencia, Tecnología e Innovación (COLCIENCIAS) (grant 2229-343-19253). Capacity building for the ethical conduct of clinical trials at the study sites was supported by the National Institute of Allergy and Infectious Diseases International Collaborations in Infectious Disease Research Program (grant 1 U19AIO65866) and Fogarty Global Infectious Diseases Research Training Program (grant D43 TW006589).
Potential conflicts of interest.
M. C. M. joined Sanofi Pasteur in July 2009, a year after the start of this noninferiority trial. All other authors declare no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Campbell-Lendrum D, Dujardin JP, Martinez E, et al. Domestic and peridomestic transmission of American cutaneous leishmaniasis: changing epidemiological patterns present new control opportunities. Mem Inst Oswaldo Cruz. 2001;96:159–62. doi: 10.1590/s0074-02762001000200004. [DOI] [PubMed] [Google Scholar]
- 2.Munoz G, Davies CR. Leishmania panamensis transmission in the domestic environment: the results of a prospective epidemiological survey in Santander, Colombia. Biomedica. 2006;26(Suppl 1):131–44. [PubMed] [Google Scholar]
- 3.Urbanization: an increasing risk factor for leishmaniasis. Wkly Epidemiol Rec. 2002;77:365–70. [PubMed] [Google Scholar]
- 4.Yadon ZE, Rodrigues LC, Davies CR, Quigley MA. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. Am J Trop Med Hyg. 2003;68:519–26. doi: 10.4269/ajtmh.2003.68.519. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Barraquer I, Gongora R, Prager M, et al. Etiologic agent of an epidemic of cutaneous leishmaniasis in Tolima, Colombia. Am J Trop Med Hyg. 2008;78:276–82. [PubMed] [Google Scholar]
- 6.Tuon FF, Amato VS, Graf ME, et al. Treatment of new world cutaneous leishmaniasis—a systematic review with a meta-analysis. Int J Dermatol. 2008;47:109–24. doi: 10.1111/j.1365-4632.2008.03417.x. [DOI] [PubMed] [Google Scholar]
- 7.Palacios R, Osorio LE, Grajalew LF, Ochoa MT. Treatment failure in children in a randomized clinical trial with 10 and 20 days of meglumine antimonate for cutaneous leishmaniasis due to Leishmania viannia species. Am J Trop Med Hyg. 2001;64:187–93. doi: 10.4269/ajtmh.2001.64.187. [DOI] [PubMed] [Google Scholar]
- 8.Cruz A, Rainey PM, Herwaldt BL, et al. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J Infect Dis. 2007;195:602–8. doi: 10.1086/510860. [DOI] [PubMed] [Google Scholar]
- 9.Jha TK, Sundar S, Thakur CP, et al. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 10.Sundar S, Jha TK, Thakur CP, et al. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;347:1739–46. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- 11.Soto J, Arana BA, Toledo J, et al. Miltefosine for New World cutaneous leishmaniasis. Clin Infect Dis. 2004;38:1266–72. doi: 10.1086/383321. [DOI] [PubMed] [Google Scholar]
- 12.van Thiel PP, Leenstra T, Kager PA, et al. Miltefosine treatment of Leishmania major infection: an observational study involving Dutch military personnel returning from northern Afghanistan. Clin Infect Dis. 2010;50:80–3. doi: 10.1086/648726. [DOI] [PubMed] [Google Scholar]
- 13.Mohebali M, Fotouhi A, Hooshmand B, et al. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103:33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Machado PR, Ampuero J, Guimaraes LH, et al. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis. 2010;4:e912. doi: 10.1371/journal.pntd.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrusciak-Talhari A, Dietze R, Chrusciak Talhari C, et al. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am J Trop Med Hyg. 2011;84:255–60. doi: 10.4269/ajtmh.2011.10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto J, Rea J, Balderrama M, et al. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2008;78:210–1. [PubMed] [Google Scholar]
- 17.Yardley V, Croft SL, De Doncker S, et al. The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am J Trop Med Hyg. 2005;73:272–5. [PubMed] [Google Scholar]
- 18.Escobar P, Matu S, Marques C, Croft SL. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 2002;81:151–7. doi: 10.1016/s0001-706x(01)00197-8. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya SK, Jha TK, Sundar S, et al. Efficacy and tolerability of miltefosine for childhood visceral leishmaniasis in India. Clin Infect Dis. 2004;38:217–21. doi: 10.1086/380638. [DOI] [PubMed] [Google Scholar]
- 20.Singh UK, Prasad R, Mishra OP, Jayswal BP. Miltefosine in children with visceral leishmaniasis: a prospective, multicentric, cross-sectional study. Indian J Pediatr. 2006;73:1077–80. doi: 10.1007/BF02763048. [DOI] [PubMed] [Google Scholar]
- 21.Sundar S, Jha TK, Sindermann H, Junge K, Bachmann P, Berman J. Oral miltefosine treatment in children with mild to moderate Indian visceral leishmaniasis. Pediatr Infect Dis J. 2003;22:434–8. doi: 10.1097/01.inf.0000066877.72624.cb. [DOI] [PubMed] [Google Scholar]
- 22.Vega JC, Sanchez BF, Montero LM, et al. Short communication: the cost-effectiveness of cutaneous leishmaniasis patient management during an epidemic in Chaparral, Colombia in 2004. Trop Med Int Health. 2007;12:1540–4. doi: 10.1111/j.1365-3156.2007.01962.x. [DOI] [PubMed] [Google Scholar]
- 23.Saravia NG, Weigle K, Navas C, et al. Heterogeneity, geographic distribution, and pathogenicity of serodemes of Leishmania viannia in Colombia. Am J Trop Med Hyg. 2002;66:738–44. doi: 10.4269/ajtmh.2002.66.738. [DOI] [PubMed] [Google Scholar]
- 24.Corredor A, Kreutzer RD, Tesh RB, et al. Distribution and etiology of leishmaniasis in Colombia. Am J Trop Med Hyg. 1990;42:206–14. doi: 10.4269/ajtmh.1990.42.206. [DOI] [PubMed] [Google Scholar]
- 25.Saravia NG, Segura I, Holguin AF, Santrich C, Valderrama L, Ocampo C. Epidemiologic, genetic, and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am J Trop Med Hyg. 1998;59:86–94. doi: 10.4269/ajtmh.1998.59.86. [DOI] [PubMed] [Google Scholar]
- 26.Instituto Nacional de Salud. Guía para el tratamiento y seguimiento del paciente con leishmaniasis. Convenio 442 de 2008 Versión Final, Colombian Ministry of Social Protection. 2011. [Google Scholar]
- 27.Weigle KA, de Davalos M, Heredia P, Molineros R, Saravia NG, D’Alessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg. 1987;36:489–96. doi: 10.4269/ajtmh.1987.36.489. [DOI] [PubMed] [Google Scholar]
- 28. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. DCTD, NCI, NIH, DHHS, 2006. http://ctep.cancer.gov. [Google Scholar]
- 29.Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- 30.Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J Infect Dis. 1993;168:709–14. doi: 10.1093/infdis/168.3.709. [DOI] [PubMed] [Google Scholar]
- 31.Velez I, Lopez L, Sanchez X, Mestra L, Rojas C, Rodriguez E. Efficacy of miltefosine for the treatment of American cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;83:351–6. doi: 10.4269/ajtmh.2010.10-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soto J, Toledo J, Gutierrez P, et al. Treatment of American cutaneous leishmaniasis with miltefosine, an oral agent. Clin Infect Dis. 2001;33:E57–61. doi: 10.1086/322689. [DOI] [PubMed] [Google Scholar]
- 33.Sundar S, Olliaro PL. Miltefosine in the treatment of leishmaniasis: clinical evidence for informed clinical risk management. Ther Clin Risk Manag. 2007;3:733–40. [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya SK, Sinha PK, Sundar S, et al. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J Infect Dis. 2007;196:591–8. doi: 10.1086/519690. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Victoria FJ, Sanchez-Canete MP, Seifert K, et al. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist Updat. 2006;9:26–39. doi: 10.1016/j.drup.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Berman J, Bryceson AD, Croft S, et al. Miltefosine: issues to be addressed in the future. Trans R Soc Trop Med Hyg. 2006;100(Suppl 1):S41–4. doi: 10.1016/j.trstmh.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Bryceson A. A policy for leishmaniasis with respect to the prevention and control of drug resistance. Trop Med Int Health. 2001;6:928–34. doi: 10.1046/j.1365-3156.2001.00795.x. [DOI] [PubMed] [Google Scholar]