Abstract
The pathogenesis of coma in severe Plasmodium falciparum malaria remains poorly understood. Obstruction of the brain microvasculature because of sequestration of parasitized red blood cells (pRBCs) represents one mechanism that could contribute to coma in cerebral malaria. Quantitative postmortem microscopy of brain sections from Vietnamese adults dying of malaria confirmed that sequestration in the cerebral microvasculature was significantly higher in patients with cerebral malaria (CM; n = 21) than in patients with non-CM (n = 23). Sequestration of pRBCs and CM was also significantly associated with increased microvascular congestion by infected and uninfected erythrocytes. Clinicopathological correlation showed that sequestration and congestion were significantly associated with deeper levels of premortem coma and shorter time to death. Microvascular congestion and sequestration were highly correlated as microscopic findings but were independent predictors of a clinical diagnosis of CM. Increased microvascular congestion accompanies coma in CM, associated with parasite sequestration in the cerebral microvasculature.
Cerebral malaria (CM) is a common clinical presentation and cause of death in adults with severe malaria in many parts of the world. CM causes a diffuse encephalopathy associated with reduced levels of consciousness or coma, often accompanied by seizures [1]. Focal neurological deficits are relatively unusual. CM represents the lethal end of a disease spectrum, because the majority of Plasmodium falciparum malaria cases involve either asymptomatic parasitemia or mild clinical disease. The clinical presentation of malaria depends on both host and parasite factors, including age and immunity. In areas where malaria is highly endemic, young children are most at risk of severe disease. Migrant workers, nonimmune travelers, or persons growing up in areas of low seasonal transmission remain vulnerable to severe malaria throughout life. CM causes 15%–20% mortality despite treatment [2] and long-term neurological sequelae or developmental/cognitive impairment in as many as 1 in 4 child survivors [3, 4]. This results in a continued major worldwide burden of mortality and morbidity, despite improved malaria control measures, such as insecticide-treated bed nets and introduction of early diagnosis and treatment with artemesinin combination therapies [5, 6]. The need to understand the pathophysiology of CM is therefore important in the search for adjuvant neuroprotective treatments for coma.
Pathological studies of brain tissue samples from patients dying of severe malaria have reported a range of findings, the most common of which is the sequestration of parasitized red blood cells (pRBCs) in cerebral microvessels in human adults [7–10] and children [11]. Erythrocytes infected with the later trophozoite and schizont stages of the parasite disappear from the peripheral circulation and preferentially localize in microvessels through specific molecular adhesion between parasite-encoded molecules, such as PfEMP-1, on the pRBC surface, and host ligands, such as CD36 or ICAM-1, on vascular endothelium [12, 13]. Sequestration is a pathological hallmark of human malaria, in contrast with the predominantly inflammatory picture seen in the brain microvasculature in murine models of experimental malaria, which lack cyto-adherent pRBC sequestration [14, 15].
Sequestration occurs to a variable degree throughout the microvasculature in different vital organs, and previous quantitative light and electron microscopic studies have confirmed a quantitative association between sequestration and coma, with higher levels of sequestration seen in the cerebral microvasculature in patients dying of CM [7–11]. In the brain, sequestration causes functional and structural changes to cerebral endothelial cells [16, 17], leading to activation and changes in blood-brain barrier permeability and contributing to secondary neuropathological events, including cerebral edema and axonal injury [18].
Cytoadherence to vascular endothelial cells is one of several different adhesion phenotypes demonstrated by P falciparum–infected pRBCs, which also bind in rosettes to uninfected red blood cells (uRBCs), host leukocytes, or other pRBCs in platelet-mediated clumps [19]. The formation of aggregates of cells in vitro has been linked with disease severity and may predispose to microvascular obstruction and interference with blood flow [20]. Studies have shown that the rheology of uRBCs in malaria is abnormal [21] and that ring-stage parasites, which lack cytoadherence phenotype, also preferentially sequester in the brain [9]. We were therefore interested in examining microvascular obstruction in the cerebral microvasculature to determine whether there was a relationship with sequestration or coma.
The role of vascular obstruction in causing coma and death, compared with other pathological processes, such as metabolic changes, inflammation, or edema, remains a subject of controversy. Imaging studies suggest that adult patients with CM show mildly swollen brains [22], although without frank brain stem herniation in the majority of cases. The relative contributions of extravascular edema and intravascular congestion, caused by sequestration or congestion, are unclear. We performed quantitative morphometric and immunohistochemical analysis of postmortem brain specimens from Vietnamese adults with fatal severe malaria, with and without CM, to examine the relationship between parasite sequestration, intravascular congestion, and coma.
METHODS
Clinical Definitions and Specimen Collection
Cortical brain samples were obtained from patients who died of severe P. falciparum malaria in a large double-blind comparative trial of parenteral artemether vs quinine for the treatment of severe malaria conducted at the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam, during 1991–1996 [23]. Autopsy was performed as quickly as possible (median time to autopsy, 7 hours); the brain was removed whole and preserved in 10% buffered formalin before a formal brain cut within 6 weeks. Samples taken from the cortex, including white and grey matter in the same block, were embedded and processed using standard histological techniques to formalin-fixed, paraffin-embedded blocks. Patients with CM (n = 21) in this study were defined clinically according to the World Health Organization criteria [1], rather than histologically. This means that patients had a Glasgow Coma Score (GCS) <11 (at admission or defined by neurological examination every 4 hours during the course of disease before death), with associated peripheral parasitemia with P. falciparum proven on microscopy of serial blood smears, and exclusion of other causes of unconsciousness, such as status epilepticus, hypoglycemia, or other central nervous system infections. Patients were defined as not having CM (n = 23) when the GCS was not <11 at any stage before death. Some of this group survived for days after admission and had cleared parasitemia from the peripheral blood smear (and on subsequent histological examination from the cerebral microvasculature) at the time of death but died of other complications of disease, such as renal failure or respiratory disease. None of the non-CM patients had coma at admission without peripheral parasitemia; during subsequent postmortem examination, none of the patients in this study had meningitis or other causes of death that may have simulated severe malaria premortem.
Nonmalarial control samples (n = 10) were from patients dying of non-neurological causes and were collected postmortem at the John Radcliffe Hospital, Oxford, United Kingdom, during 1995–2000. Eight control subjects were age-matched to the patients with malaria, and 2 control subjects were older patients, although there was no statistically significant difference in the mean age between the control and malaria groups (controls: median age, 37 years; interquartile range [IQR], 30–56 years). Brain samples were collected using autopsy protocols as described elsewhere [10, 24]. Use of postmortem samples from both the malaria autopsy study in Vietnam and control subjects in Oxford was with informed consent of the relatives of the deceased, and all protocols for use of the tissue samples were approved by local and institutional ethical review boards. Clinical details of the malaria and control cases are shown in Table 1.
Table 1.
Clinical Details of Patients With Malaria
| Patient | Age, y | Sex | Admission Parasite Level, Parasites/μL | Admission GCS | WHO Diagnosis | Other Clinical Complications |
| 1 | 24 | M | 67 824 | 4 | CM | J |
| 2 | 50 | F | 8290 | 14 | NCM | Sh, J, ARF |
| 3 | 30 | M | 529 278 | 3 | CM | J, Sh, PEd, HyperP |
| 4 | 51 | F | 69 206 | 5 | CM | An, Hyp, ARF |
| 5 | 26 | M | 3140 | 11 | NCM | An |
| 6 | 27 | M | 1 044 866 | 15 | NCM | Sh, Hyp, ARF, An, PEd, HyperP |
| 7 | 46 | M | 212 264 | 3 | CM | An, J |
| 8 | 43 | M | 105 504 | 5 | CM | Sh, PEd, HyperP |
| 9 | 34 | M | 805 912 | 15 | NCM | ARF, AN, Ped |
| 10 | 44 | M | 221 558 | 7 | CM | ARF, J, PEd |
| 11 | 40 | F | 4522 | 14 | NCM | Hyp, ARF, An, J, PEd |
| 12 | 33 | M | 1 450 680 | 15 | NCM | ARF, An, J, HyperP |
| 13 | 44 | F | 15 072 | 7 | CM | Sh, An |
| 14 | 28 | M | 546 360 | 9 | CM | Sh, ARF, HyperP |
| 15 | 33 | M | 121 330 | 14 | NCM | Sh, ARF, An, J |
| 16 | 28 | M | 619 208 | 11 | NCM | ARF, An, J, HyperP |
| 17 | 27 | M | 55 264 | 15 | NCM | An |
| 18 | 47 | M | 1 161 298 | 14 | NCM | Sh, ARF, HyperP |
| 19 | 28 | M | 45 342 | 8 | CM | Sh, Hyp, ARF, An, J |
| 20 | 34 | M | 150 720 | 8 | CM | Sh, Hyp, ARF, An, J |
| 21 | 22 | F | 802 584 | 12 | NCM | Sh, Hyp, ARF, HyperP |
| 22 | 35 | M | 119 320 | 7 | CM | Sh, Hyp, ARF, J, PEd |
| 23 | 24 | M | 1 026 152 | 14 | NCM | Sh,J, PEd, HyperP |
| 24 | 54 | M | 3517 | 11 | NCM | Sh, ARF, An, J |
| 25 | 56 | F | 21 101 | 13 | NCM | Sh, ARF, J, PEd |
| 26 | 36 | M | 193 424 | 8 | CM | Sh, Hyp, ARF, J, HyperP |
| 27 | 22 | M | 112 538 | 7 | CM | Sh, ARF, J, PEd |
| 28 | 22 | M | 47 728 | 14 | NCM | Sh, ARF, An, J |
| 29 | 32 | M | 241 152 | 15 | NCM | Sh, ARF, An, J |
| 30 | 30 | M | 412 847 | 7 | CM | Sh, ARF, An, J |
| 31 | 50 | F | 76 365 | 15 | NCM | Sh, ARF, An, J |
| 32 | 36 | F | 300 | 6 | CM | Sh, Hyp, ARF, An, J |
| 33 | 22 | M | 768 421 | 11 | NCM | Sh, ARF, An, J, HyperP |
| 34 | 25 | M | 480 | 15 | NCM | Sh, Hyp, ARF, An, J |
| 35 | 25 | M | 335 980 | 15 | NCM | Hyp, ARF, An, J |
| 36 | 43 | F | 567 712 | 15 | NCM | Sh, Hyp, ARF, An, J, PEd, HyperP |
| 37 | 69 | M | 92 442 | 8 | CM | Sh, Hyp, ARF, An, J |
| 38 | 63 | M | 120 576 | 10 | CM | Sh, Hyp, An, PEd |
| 39 | 22 | M | 297 421 | 7 | CM | Sh, Hyp, J, PEd |
| 40 | 63 | M | 136 653 | 11 | NCM | Sh, ARF, An, J, PEd |
| 41 | 32 | M | 11 304 | 15 | NCM | Sh, ARF, J |
| 42 | 45 | F | 89 553 | 10 | CM | Sh, J |
| 43 | 30 | M | 21 980 | 7 | CM | An, J |
| 44 | 52 | F | 7536 | 8 | CM | Sh, Hyp, An |
| Control Patients | Age (y) | Sex | Neurological History | Additional Clinical History |
| 1 | 30 | M | None | Hemorrhagic pericarditis, splenomegaly, myelodysplasia |
| 2 | 56 | F | Stupor due to depression/schizophrenia | Aspiration; dehydration; bronchopneumonia |
| 3 | 35 | M | None | Extensive severe burns of the skin; smoke inhalation |
| 4 | 35 | M | Epilepsy; previous encephalitis | PEd |
| 5 | 27 | M | Unconscious, right-sided focal | PEd; anorexia nervosa; Hyp |
| 6 | 23 | F | None | Sickle cell trait; severe hemorrhage |
| 7 | 45 | M | None | Splenomegaly |
| 8 | 39 | F | None | Chronic renal failure; gastric bleed |
| 9 | 95 | F | None | Hepatocellular carcinoma |
| 10 | 86 | M | None | Acute upper GI bleeding and ischemic heart disease |
Abbreviations: An, anemia; ARF, acute renal failure; CM, cerebral malaria; GCS, Glasgow Coma Score; GI, gastrointestinal; Hyp, hypoglycemia; HyperP, hyperparasitemia; J, jaundice; NCM, non–cerebral malaria; PEd, pulmonary edema; Sh, shock; WHO, World Health Organization.
Immunohistochemistry
Immunohistochemistry was performed on cortical brain sections (from the frontal, parietal, or occipital lobes) with use of antibodies to glycophorin A as a marker of red blood cells (RBCs; clone JC159) and CD31 (clone JC70A) as a constitutive vascular marker (both gifts from Jackie Cordell, OxFABs, NDCLS, University of Oxford). Paraffin sections were dewaxed and rehydrated and then underwent microwave (for glycophorin) or trypsin antigen retrieval (for CD31), according to standard protocols, as described elsewhere [25, 26]. The primary antibodies were incubated for 45 minutes on the section, the slides were washed in Tris buffered saline 3 times for 5 minutes, and incubation was done with biotinylated goat antimouse immunoglobulin for 30 minutes, followed by Streptavidin ABComplex alkaline phosphatase. The chromogen used was the new fuchsin substrate system (all kits and substrates obtained from DAKO). The reaction was observed under a microscope and quenched by washing with Tris buffered saline. Slides were mounted in Aquamount (BDH) and counterstained with hematoxylin. Negative controls comprised sections immunostained using the aforementioned methods, except for omission of the primary antibody.
Microscopy and Quantitation of Vessels by Image Analysis
Images were captured under ×250 magnification with use of QImaging software with a Zeiss microscope and analyzed using Adobe Photoshop software. CD31 and glycophorin were used as markers for endothelial cells and the erythrocyte membrane, respectively, on slides of cortical brain. The number of vessels stained with CD31 was quantitated with a graticule to provide a measure of vessels per unit area of cortex (per square millimeter). This was done in total for the section and using separate counts for grey and white matter areas. The individual counts of vessels from 6 separate fields from each case were averaged to produce a vessel density per square millimeter for each case. This single figure was correlated with clinical, biochemical, and histopathological data.
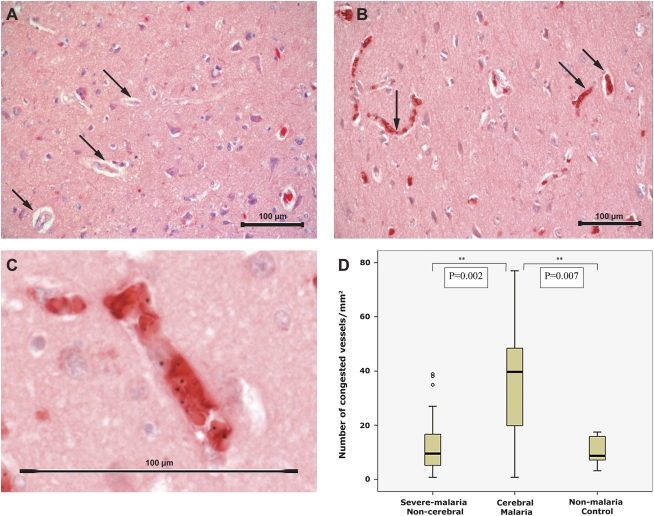
To assess microvascular congestion, the blood vessels examined were defined as small- to medium-sized vessels (2–10 erythrocytes in diameter, representing small arterioles and venules) that contained RBCs on glycophorin immunostaining (Figure 1). Congestion was defined by the presence in a vessel lumen of erythrocytes (both uninfected and infected) staining positively with glycophorin. Congestion was therefore a measure of the number of small vessels containing RBCs in a section.
Figure 1.
A–C, Images of cortical brain sections from patients with malaria that were stained for glycophorin A. A, Low-power image showing noncongested vessels. Examples of vessels without erythrocytes indicated with arrows (magnification ×250; scale bar, 100 μm; counterstained with hematoxylin). B, Low-power image showing vessels congested with parasitized and nonparasitized erythrocytes (arrows; magnification ×250; scale bar 100 μm; counterstained with hematoxylin). C, High-power image of a congested vessel showing both infected and uninfected red blood cells in a congested vessel (scale bar, 100 μm). D, Box plots showing a comparison of the number of congested vessels per square millimeter in non–cerebral malaria, cerebral malaria, and control cases. Boxes show median values with interquartile ranges and limiting values.
Sequestration was defined morphologically as the presence of pRBCs in a vessel. Both uRBCs and pRBCs stained for glycophorin; thus, the presence of malaria pigment in RBCs or the presence of a visible parasite nucleus was used to differentiate between pRBCs and uRBCs. The number of vessels showing either congestion or sequestration was quantitated and calculated as a percentage of the total vessel count (ratio of congested or sequestered vessels to total vessels on CD31 staining).
Statistical Analysis
All analyses were performed using SPSS, version 17, or Stata, version 11 (StataCorp). Comparisons were made across the 3 groups: CM, non-CM, and controls. Differences between groups were assessed using the Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables. Variables were log-transformed to a normal distribution when possible, but analysis of variance was used for normally distributed continuous variables only if the variances were similar for all groups. To further elucidate the relationship between exposure and outcome, post hoc pairwise comparisons (ie, CM vs non-CM, CM vs controls, and non-CM vs controls) were made only when the initial comparison showed a significant association or when data were available for only the 2 severe malaria groups. Student t test or Mann–Whitney U test was used to assess pairwise comparisons, as appropriate. Correlations for continuous variables among patients with malaria were tested using Spearman rank correlation coefficient.
To quantify the risk associated with clinical and neuropathological features that were significant predictors of cerebral malaria, logistic regression modeling was used. Cerebral malaria was the dependent variable, and all quantitative measures of neuropathology that were significant in the univariate analysis at P < .05 were included as covariates. The best predictor or combination of predictors was determined using the likelihood ratio test and by checking the area under the receiver-operating characteristic (ROC) curves. Goodness-of-fit for the models was confirmed using the Homer-Lemeshow χ2 test.
No adjustment was made for multiple comparisons; however, for P values > .001, exact numbers are reported so that a Bonferroni-Dunn correction can be calculated (ie, α = .05/k, where k = number of tests).
RESULTS
Patients with CM showed a higher degree of congestion, as measured by the number of congested blood vessels per unit area of the cerebral cortex (median density, 39.7 blood vessels/mm2; IQR, 19.8–48.4 blood vessels/mm2), compared with non-CM (9.52 blood vessels/mm2; IQR, 4.76–20.6 blood vessels/mm2; P = .002) and nonmalarial controls (8.73 blood vessels/mm2; IQR, 7.14–15.9 blood vessels/mm2; P =.007) (Figure 1D). There was no statistically significant difference in congestion between controls and patients with non-CM (P = .78), implying that the differences in observed vascularity and microvascular congestion were not simply attributable to postmortem leaching of RBCs from patent vessels.
The difference among patients with CM and other clinical groups remained statistically significant when adjusted for variation in the vessel density between areas of the cortex. Grey matter, which normally has a higher density of vessels than white matter [27, 28], logically showed a higher degree of congested vessels (grey matter to white matter ratio: CM, 1.55 [median, 44.4; IQR, 17.5–68.3 to 28.6; IQR, 12.7–38.1], non-CM, 1 [median, 9.52; IQR, 4.76–30.2 to 9.52; IQR, 3.17–19.1], controls, 1.30 [median, 10.3; IQR, 7.94–14.3 to 7.94; IQR, 3.17–11.1]), although this was only borderline significantly different in cases of cerebral malaria (P = .06).
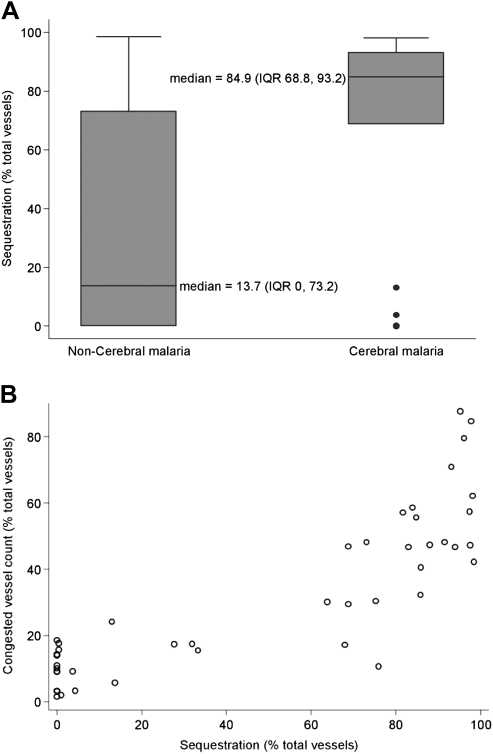
Sequestration of pRBCs was significantly higher in the cortical brain sections of patients with CM than in patients with non-CM (P = .01) (Table 2 and Figure 2A). The percentage of congested microvessels was strongly positively correlated with percentage of vessels showing sequestration of parasitized erythrocytes (Spearman ρ = 0.85; P < .0001) (Figure 2B). Moreover, both sequestration and congestion were inversely associated with time to death after admission to hospital (Spearman ρ = −0.61 and ρ = −0.51, respectively; both P < .001). The level of consciousness, as measured by the GCS, was also inversely correlated with both parameters (Spearman ρ = −0.48; P = .001 for sequestration; Spearman ρ = −0.52; P = .0003 for congestion).
Table 2.
Statistical Comparison of the Clinical Parameters of Cerebral Malaria and Non–Cerebral Malaria Cases
| CM | Non-CM | P Value | |
| Number of patients | 21 | 23 | |
| Age (years) | 36 (22–69) | 32 (22–63) | .31 |
| Drug therapy | A = 11, Q = 10 | A = 7, Q = 16 | .22 |
| Maximum temperature, °C | 39.5 (38.3–40) | 39.0 (38.3–39.8) | .79 |
| Maximum heart rate, beats/min | 120 (120–140) | 120 (116–140) | .95 |
| Time to death, h | 36.0 (13–56) | 44 (18–123.5) | .31 |
| At admission | |||
| Glasgow Coma Score | 7 (3–10) | 14 (11–15) | .0001 |
| Hemotocrit, % | 30 (8–50) | 28 (6–47) | .66 |
| Geometric mean (95% CI) parasitemia/μL | 80 665 (37 403–173 963) | 96 144 (34 836–265 346) | .78 |
| Lactate (plasma), mmol/L | 6.7 (2.2–18.3) | 3.7 (0.4–1.03) | .03 |
| Creatinine (serum), mg/dL | 2.3 (0.7–7.5) | 4.4 (1.03–11) | .02 |
| White cell count × 10−8, cells/mm3 | 12.8 (3.0–31.5) | 11.0 (2.0–36.8) | .30 |
| Platelet count × 10−4, cells/mm3 | 32 (20–194) | 40 (12–120) | .66 |
| Bilirubin, mg/dL | 5.2 (1.0–18.8) | 9.9 (0.6–18.0) | .21 |
| CSF opening pressure, /mm H20 | 17 (10.5–22); n = 16 | 14 (13.5–19); n = 5 | .90 |
| CSF protein | 140 (70–208); n = 18 | 70 (53–130); n = 6 | .04 |
| CSF white cell count | 3 (0–6); n = 17 | 0 (0–2); n = 6 | .04 |
| No. (%) with acute renal failure | 9 (42.9) | 17 (73.9) | .07 |
| No. (%) with jaundice | 14 (66.7) | 18 (78.3) | .50 |
| No. (%) with shock | 8 (34.8) | 6 (28.6) | .75 |
| No. (%) with hypoglycemia | 2 (9.52) | 4 (17.4) | .67 |
| No. (%) with pulmonary edema | 1 (4.76) | 2 (8.70) | 1.0 |
| No. (%) with convulsions | 5 (25.0) | 1 (4.35) | .08 |
| No. (%) with dialysis | 3 (47.8) | 11 (14.3) | .02 |
| Histopathological | |||
| Sequestration, % | 84.9 (68.8–93.2) | 13.7 (0–73.2) | .01 |
| Congestion, vessels/mm3 | 39.7 (19.8–48.1) | 9.52 (4.76–20.6) | .002 |
| Brain mass, g | 1335 (1240–1400) | 1300 (1290–1400) | .95 |
All values are median (interquartile range), unless otherwise specified. Results in bold show significant differences between CM and Non-CM patient groups, P < .05.
Abbreviations: A, Artemether; CI, confidence interval; CM, cerebral malaria; CSF, cerebrospinal fluid; Q, Quinine.
Figure 2.
A, Box plot showing the difference between sequestration in cerebral malaria and non–cerebral malaria cases. This measured the percentage of vessels in cortical brain sections showing sequestration of parasitized red blood cells. Boxes show median values with interquartile ranges and limiting values. B, Dot plot showing the correlation between sequestration and microvascular congestion in individual cases.
The clinical and neuropathological correlates of CM and non-CM were examined (Table 2). There was no difference between the CM and non-CM groups in terms of their age, admission parasitemia, or type of treatment received after admission (either artemether or quinine). Across the malaria groups, drug type was also not associated with a difference in the degree of congestion or sequestration observed microscopically (P = .19 and P = 0.61, respectively). No relationship was found between the degree of sequestration or congestion and hematocrit (P = .08 and P = 0.34, respectively), implying that lower rates of congestion or sequestration cannot be explained simply by the presence of anemia.
A number of clinical features were not associated with CM in this series, including shock, anemia, jaundice, hypoglycemia, pulmonary edema, and the incidence of convulsions before admission. Brain weight at autopsy and cerebrospinal fluid (CSF) opening pressure were also not significantly different between the 2 groups.
However, microvascular congestion, sequestration of pRBCs, admission lactate, CSF protein level, and CSF white blood cell count were significantly associated with cerebral malaria with use of the Mann–Whitney U test (Table 2). When the risk was quantified using logistic regression, CSF protein level (n = 24; odds ratio [OR], 1.03; 95% confidence interval [CI], .99–1.06) and CSF white blood cell count (n = 23; OR, 1.75; 95% CI, .81–3.79) were no longer significantly associated with CM possibly because of small sample size. Coma score was significantly associated because of the clinical definition of the CM group; risk could not be quantified, because all patients with GCS ≥11 were defined as having non-CM and all patients with GCS <11 were considered to have CM.
Acute renal failure, as defined by higher levels of plasma creatinine, were associated with lower risk of CM (OR, 0.71; 95% CI, .52–.97), as was the requirement for treatment by dialysis after admission (OR, 0.18; 95% CI, .04–.79). Higher levels of admission lactate level were borderline significantly associated with higher odds of CM (OR, 1.16; 95% CI, .99–1.36). When assessed using likelihood ratio testing, percentage vessel sequestration was a good independent predictor (OR, 1.02; 95% CI, 1.01–10.4; ROC area, 0.72), but microvascular congestion was the best independent predictor of CM (OR, 1.07; 95% CI, 1.02–1.12; P = .0001; ROC area, 0.77). Multivariate combinations of the aforementioned variables either did not provide a good model fit or did not predict as well as the single covariate models.
DISCUSSION
Sequestration of P falciparum–parasitized erythrocytes in the cerebral microvasculature has been a consistent feature of pathological studies of CM since the seminal studies of Marchiafava et al [29] more than a century ago. Because the extent of sequestration is so marked and the microvascular pathology is so distinctive, observers have proposed this as the central pathogenic process causing coma in CM [2, 7, 30, 31]. Previous studies have quantitated the degree of sequestration in cerebral vessels in patients with CM and shown a significant association between premortem coma and cerebral sequestration, using both electron [7, 8, 10] and light microscopy [11, 22] on brain sections or brain smears [9]. This study confirms the significant association between premortem sequestration of malaria-infected erythrocytes in cerebral microvessels and the coma of CM in adults.
Data derived from murine models of experimental CM, in which histological evidence of extensive parasitized RBC sequestration is lacking, have led some authors to suggest that coma and death result from immunopathological processes independent of sequestration [15, 32]. Other studies have reported accumulation of other host cells, such as leukocytes or platelets, predominantly in pediatric African patients with CM [33]. In Southeast Asian adults, our results confirm that histological evidence of cerebral microvascular sequestration with pRBCs is significantly and quantitatively linked to premortem coma. The only other neuropathological correlates that we previously found to be significantly associated with coma in this group is axonal injury [24].
In our study, we revealed a significant increase in the number of patent microvessels in the brain after death due to cerebral malaria, because of congestion of vessels by uninfected and infected RBCs. This phenomenon of microvascular congestion showed a significant correlation with sequestration and coma. These results support the hypothesis that cerebral microvascular obstruction is central to the pathogenesis of coma. Extensive sequestration and congestion was associated with a shorter time to death, a lower level of premortem consciousness, and markers of severe disease, such as a high blood lactate concentration, which is an established prognostic marker of poor outcome in severe malaria [34].
One hypothesis to explain microvascular congestion would be backing up behind downstream obstruction in the capillary or postvenular beds, caused by pRBC sequestration. However, although these 2 features were strongly correlated, congestion proved a better predictor of coma, and other factors may contribute to congestion independently of sequestration. These could include reduced deformability of uRBCs during malaria infection or other adhesive phenotypes, such as rosetting and platelet-mediated clumping of pRBCs. Congestion has a number of important consequences, including impaired tissue perfusion that may cause diffuse cerebral ischemia and an increased intracranial blood volume. Global measurement of intravascular blood volume would be important to assess whether histological evidence of microvascular congestion correlates with increased intravascular volume in CM, as opposed to fluid leakage into the brain parenchyma because of vasogenic edema. Our studies of this group indicate that patterns of perivascular or parenchymal edema are not significantly associated with coma in these patients [35].
Congestion represents opening of small vessels that are not usually patent. Blood flow in the cortex is tightly linked to neuronal energy requirements. An inappropriate dilation and packing of multiple small vessels may have a pathological effect on cortical function, through a disconnection between neuronal metabolism and flow. Coma may represent a potentially neuroprotective response of global decreased neuronal function in the face of inadequate blood supply or metabolic competition from developing intravascular parasites. The unique pathology of CM, with sequestration, cerebral microvascular congestion, and obstruction, and the contribution of changes to uninfected and cytoadherent infected erythrocytes is different from other causes of large vessel hypoxic and/or ischemic damage. Whether coma is a neuroprotective response is an important question, because efforts to reverse it with adjuvant therapies could then risk exacerbating injury, causing reflow damage or contributing to long-term neurological sequelae.
Recent work in the Plasmodium coatneyi primate model of CM (in which there is significant cerebral sequestration) using positron-emission tomography to map glucose use revealed diffuse and heterogeneous reduction of metabolism in the cortex during the acute phase of infection [36], which is consistent with an impaired microcirculation. A functioning microcirculation protects organs from the effects of diminished oxygen and metabolite supply, and impairment of microcirculatory function may cause diminished oxygenation despite normal overall oxygen delivery to an organ [37]. Changes in microcirculatory function has been directly visualized in the buccal and rectal vessels in patients with severe malaria, in which the patterns of obstruction and flow differ from sepsis, a widely used model of the contribution of microcirculatory dysfunction to tissue injury [38]. Our results imply that dysregulation of the control of microcirculatory blood supply occurs in the brain during CM.
Brain swelling in CM is a variable finding in radiological and postmortem studies of adults with fatal malaria [21]. With greater occlusion of cerebral blood flow, increased hydrostatic pressure might lead to hemorrhages and cerebral edema. However, preliminary examination of the incidence of edema, fibrinogen leakage, and microscopic hemorrhages in these patients reveal no association with the degree of sequestration or congestion [35]. Systemic, as opposed to local, microcirculatory factors may also contribute to defects in cerebral blood flow during severe malarial illness. Shock is present in only 12% of cases in both adult [39] and pediatric [40] severe malaria but was seen in a disproportionately high number of the fatal cases in this study, reflecting the multisystemic nature of fatal disease in this group. The high incidence of acute renal failure in adults may be important, as shown here by the inverse relationship between creatinine levels and congestion, but requires further investigation.
Our findings emphasize the importance of cerebral microvascular obstruction in contributing to coma during severe malarial in humans. They confirm the association between sequestration of malaria-infected erythrocytes and show, for the first time to our knowledge, a strong association between coma, sequestration, and inappropriate microvascular congestion in human CM.
Notes
Financial support.
This work was supported by the Wellcome Trust of Great Britain; the NIHR Biomedical Research Centre Programme, Oxford; The John Fell Fund of Oxford University; and the Thrasher Research Fund, USA (grant number 02827-2).
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 2.Newton CR, Taylor TE, Whitten RO. Pathophysiology of fatal falciparum malaria in African children. Am J Trop Med Hyg. 1998;58:673–83. doi: 10.4269/ajtmh.1998.58.673. [DOI] [PubMed] [Google Scholar]
- 3.Carter JA, Mung'ala-Odera V, Neville BG, et al. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476–81. doi: 10.1136/jnnp.2004.043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John CC, Bangirana P, Byarugaba J, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9. doi: 10.1542/peds.2007-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2) doi: 10.1002/14651858.CD000363.pub2. CD000363. [DOI] [PubMed] [Google Scholar]
- 6.White NJ. The role of anti-malarial drugs in eliminating malaria. Malar J. 2008;7(Suppl 1):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 8.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–75. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 9.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–403. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pongponratn E, Turner GD, Day NP, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–59. [PubMed] [Google Scholar]
- 11.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–5. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–80. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Chakravorty SJ, Craig A. The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur J Cell Biol. 2005;84:15–27. doi: 10.1016/j.ejcb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. The murine cerebral malaria phenomenon. Trends Parasitol. 2010;26:11–15. doi: 10.1016/j.pt.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza JB, Hafalla JC, Riley EM, Couper KN. Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology. 2010;137:755–72. doi: 10.1017/S0031182009991715. [DOI] [PubMed] [Google Scholar]
- 16.Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood. 2009;114:4243–52. doi: 10.1182/blood-2009-06-226415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jambou R, Combes V, Jambou MJ, et al. Plasmodium falciparum adhesion on human brain microvascular endothelial cells involves transmigration-like cup formation and induces opening of intercellular junctions. PLoS Pathog. 2010;6:e1001021. doi: 10.1371/journal.ppat.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol. 2006;36:555–68. doi: 10.1016/j.ijpara.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogerson SJ, Grau GE, Hunt NH. The microcirculation in severe malaria. Microcirculation. 2004;11:559–76. doi: 10.1080/10739680490503311. [DOI] [PubMed] [Google Scholar]
- 21.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–17. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Looareesuwan S, Wilairatana P, Krishna S, et al. Magnetic resonance imaging of the brain in patients with cerebral malaria. Clin Infect Dis. 1995;21:300–9. doi: 10.1093/clinids/21.2.300. [DOI] [PubMed] [Google Scholar]
- 23.Tran TH, Day NP, Nguyen HP, et al. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 24.Medana IM, Day NP, Hien TT, et al. Axonal injury in cerebral malaria. Am J Pathol. 2002;160:655–66. doi: 10.1016/S0002-9440(10)64885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parums DV, Cordell JL, Micklem K, et al. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990;43:752–7. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erber WN, McLachlan J, Cordell JL, et al. A new monoclonal antibody (JC159) that detects glycophorin A for the diagnosis of erythroleukaemia. Hematol Rev. 1991;5:113–20. [Google Scholar]
- 27.Lu M, Zhang ZG, Chopp M. Analysis of cerebral microvascular architecture—application to cortical and subcortical vessels in rat brain. J Neurosci Methods. 2004;138:81–7. doi: 10.1016/j.jneumeth.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Lauwers F, Cassot F, Lauwers-Cances V, Puwanarajah P, Duvernoy H. Morphometry of the human cerebral cortex microcirculation: general characteristics and space-related profiles. Neuroimage. 2008;39:936–48. doi: 10.1016/j.neuroimage.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Marchiafava E, Bignami A. Malaria and the parasites of malaria fevers. Vol 150. London: The New Sydenham Society; 1894. “On summer-autumnal malaria fever.”; pp. 1–234. [Google Scholar]
- 30.Newton CR, Hien TT, White N. Cerebral malaria. J Neurol Neurosurg Psychiatry. 2000;69:433–41. doi: 10.1136/jnnp.69.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milner DA., Jr Rethinking cerebral malaria pathology. Curr Opin Infect Dis. 2010;23:456–63. doi: 10.1097/QCO.0b013e32833c3dbe. [DOI] [PubMed] [Google Scholar]
- 32.Clark IA, Cowden WB. The pathophysiology of falciparum malaria. Pharmacol Ther. 2003;99:221–60. doi: 10.1016/s0163-7258(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 33.Grau GE, Mackenzie CD, Carr RA, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–6. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- 34.Day NP, Phu NH, Mai NT, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit Care Med. 2000;28:1833–40. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 35.Medana IM, Day NP, Sachanonta N, et al. Coma in fatal adult human malaria is not caused by cerebral oedema. Malaria J. 2011;10:267. doi: 10.1186/1475-2875-10-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai S, Sugiyama M. Imaging analysis of the brain in a primate model of cerebral malaria. Acta Trop. 2010;114:152–6. doi: 10.1016/j.actatropica.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Ellis CG, Jagger J, Sharpe M. The microcirculation as a functional system. Crit Care. 2005;9(Suppl 4):S3–8. doi: 10.1186/cc3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 39.Dondorp A, Nosten F, Stepniewska K, Day N, White N South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 40.Dondorp AM, Fanello CI, Hendriksen IC, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376:1647–57. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]