Abstract
Rationale: Acute lung dysfunction of noninfectious etiology, known as idiopathic pneumonia syndrome (IPS), is a severe complication following hematopoietic stem cell transplantation (HSCT). Several mouse models have been recently developed to determine the underlying causes of IPS. A cohesive interpretation of experimental data and their relationship to the findings of clinical research studies in humans is needed to better understand the basis for current and future clinical trials for the prevention/treatment of IPS.
Objectives: Our goal was to perform a comprehensive review of the preclinical (i.e., murine models) and clinical research on IPS.
Methods: An ATS committee performed PubMed and OVID searches for published, peer-reviewed articles using the keywords “idiopathic pneumonia syndrome” or “lung injury” or “pulmonary complications” AND “bone marrow transplant” or “hematopoietic stem cell transplant.” No specific inclusion or exclusion criteria were determined a priori for this review.
Measurements and Main Results: Experimental models that reproduce the various patterns of lung injury observed after HSCT have identified that both soluble and cellular inflammatory mediators contribute to the inflammation engendered during the development of IPS. To date, 10 preclinical murine models of the IPS spectrum have been established using various donor and host strain combinations used to study graft-versus-host disease (GVHD). This, as well as the demonstrated T cell dependency of IPS development in these models, supports the concept that the lung is a target of immune-mediated attack after HSCT. The most developed therapeutic strategy for IPS involves blocking TNF signaling with etanercept, which is currently being evaluated in clinical trials.
Conclusions: IPS remains a frequently fatal complication that limits the broader use of allogeneic HSCT as a successful treatment modality. Faced with the clinical syndrome of IPS, one can categorize the disease entity with the appropriate tools, although cases of unclassifiable IPS will remain. Significant research efforts have resulted in a paradigm shift away from identifying noninfectious lung injury after HSCT solely as an idiopathic clinical syndrome and toward understanding IPS as a process involving aspects of both the adaptive and the innate immune response. Importantly, new laboratory insights are currently being translated to the clinic and will likely prove important to the development of future strategies to prevent or treat this serious disorder.
Keywords: hematopoietic stem cell transplant, pulmonary complications, lung injury, mouse models
CONTENTS
Introduction
Methods
Results
Idiopathic Pneumonia Syndrome and the clinical spectrum of lung injury after HSCT
The Pathogenesis of IPS after Allogeneic HSCT Mouse Models of Noninfectious Lung Injury after HSCT
The Role of Soluble Mediators in the Development of IPS
TNF-α and TNF Receptors
LPS
Gut-Lung-Liver Axis of Inflammation
Oxidative Stress and Pulmonary Dysfunction during IPS
Depletion of Pulmonary Surfactant during IPS
The Role of Cellular Effectors in the Development of IPS
Donor-derived T Cell Effectors
Role of Host APCs
Donor Accessory Cells
Mechanisms of Leukocyte Recruitment to the Lung during the Development of IPS
Chemokine Receptor–Ligand Interactions during IPS
CC Chemokine Family Members
CXC Chemokine Family Members
Adhesion Molecules and IPS
Targets of Inflammation and Injury during the Development of IPS
Preclinical Interventions for IPS
The Diagnostic and Therapeutic Approach to Patients with IPS
Conclusions and Future Directions
INTRODUCTION
Acute pulmonary dysfunction is a frequently fatal complication following hematopoietic stem cell transplantation (HSCT). Previously, idiopathic pneumonia syndrome (IPS) was defined to describe a subset of these patients who have signs and symptoms of pneumonia, and evidence for widespread alveolar injury in the absence of lower respiratory tract infection (1). Over the years, this definition has been adopted into clinical practice and has been useful in guiding medical management in afflicted patients. However, outcomes of patients diagnosed with IPS remain unacceptable; mortality rates are high, and time to death following diagnosis is short. An improved understanding of the clinical spectrum of disease and the mechanisms responsible for IPS is therefore essential for physician-scientists to make progress in the treatment and prevention of this disorder. Our goal was to perform a comprehensive review of the preclinical (i.e., murine models) and clinical research on IPS.
METHODS
An ATS committee, including representation from the adult and pediatric communities (national and international), was formed by invitation based on expertise in the care of patients with IPS after HSCT and/or expertise in preclinical models of IPS. Committee members performed PubMed and OVID searches for published, peer-reviewed articles using the keywords “idiopathic pneumonia syndrome” or “lung injury” or “pulmonary complications” AND “bone marrow transplant” or “hematopoietic stem cell transplant.” Each committee member was assigned to sections as outlined in this statement and asked to review the literature for the sections assigned. No specific inclusion or exclusion criteria were determined a priori for this review. All committee members reviewed and edited all drafts and the final version of this research statement, and reached consensus for its accuracy and relevance as a review of the current state of the science of IPS research.
RESULTS
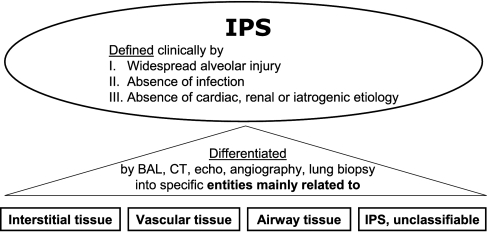
The definition of IPS has been updated by the committee authoring this statement and is now shown in Table 1. The ATS defines IPS as an idiopathic syndrome of pneumopathy after HSCT, with evidence of widespread alveolar injury and in which infectious etiologies and cardiac dysfunction, acute renal failure, or iatrogenic fluid overload have been excluded. Moreover, IPS has been further classified into specific entities (Figure 1 and Table 2) based in large part on the primary anatomical sites of inflammation and dysfunction. The intent is to better characterize the clinical spectrum of disease and to more carefully match subtypes of IPS with specific preclinical models that have been developed to mimic and ultimately understand them. These animal models have proved valuable in defining specific contributors to the pathogenesis of inflammation and evaluating promising new therapeutic modalities. For example, results using novel approaches to use reduced intensity conditioning regimens or to neutralize tumor necrosis factor-α have been encouraging, and these approaches were developed primarily from insights gained from preclinical studies. The purpose of this statement is to provide a brief overview of the spectrum of noninfectious lung injury that occurs after HSCT, to relate these various forms of lung inflammation to the definition of IPS, and to provide a comprehensive review of data generated using experimental animal models that have defined our current understanding of mechanisms of this frequently fatal disorder.
TABLE 1.
DEFINITION OF IDIOPATHIC PNEUMONIA SYNDROME
| I: Evidence of widespread alveolar injury: |
|---|
| a. Multilobar infiltrates on routine chest radiographs or computed tomography |
| b. Symptoms and signs of pneumonia (cough, dyspnea, tachypnea, rales) |
| c. Evidence of abnormal pulmonary physiology |
| 1. Increased alveolar to arterial oxygen difference |
| 2. New or increased restrictive pulmonary function test abnormality |
| II: Absence of active lower respiratory tract infection based upon: |
| a. Bronchoalveolar lavage negative for significant bacterial pathogens including acid-fast bacilli, Nocardia, and Legionella species |
| b. Bronchoalveolar lavage negative for pathogenic nonbacterial microorganisms: |
| 1. Routine culture for viruses and fungi |
| 2. Shell vial culture for CMV and respiratory RSV |
| 3. Cytology for CMV inclusions, fungi, and Pneumocystis jirovecii (carinii) |
| 4. Direct fluorescence staining with antibodies against CMV, RSV, HSV, VZV, influenza virus, parainfluenza virus, adenovirus, and other organisms |
| c. Other organisms/tests to also consider: |
| 1. Polymerase chain reaction for human metapneumovirus, rhinovirus, coronavirus, and HHV6 |
| 2. Polymerase chain reaction for Chlamydia, Mycoplasma, and Aspergillus species |
| 3. Serum galactomannan ELISA for Aspergillus species |
| d. Transbronchial biopsy if condition of the patient permits |
| III: Absence of cardiac dysfunction, acute renal failure, or iatrogenic fluid overload as etiology for pulmonary dysfunction |
Definition of abbreviations: CMV = cytomegalovirus; HSV = herpes simplex virus; RSV = respiratory syncytial virus; VZV = varicella zoster virus.
Table updated from Reference 1.
Figure 1.
Overview of idiopathic pneumonia syndrome (IPS).
TABLE 2.
CATEGORIZATION OF THE CLINICAL SPECTRUM OF LUNG INJURY FOLLOWING HEMATOPOIETIC STEM CELL TRANSPLANTATION
| Clinical Spectrum of Disease as Categorized by Presumed Site of Primary Tissue Injury | ||
|---|---|---|
| Pulmonary Parenchyma | Vascular Endothelium | Airway Epithelium |
| Acute interstitial pneumonitis (AIP)* | Peri-engraftment respiratory distress syndrome (PERDS)* | Cryptogenic organizing pneumonia (COP)/Bronchiolitis obliterans organizing pneumonia (BOOP)* |
| Acute respiratory distress syndrome (ARDS)* | Noncardiogenic capillary leak syndrome (CLS)* | Bronchiolitis obliterans syndrome (BOS)* |
| BCNU pneumonitis | Diffuse alveolar hemorrhage (DAH)* | |
| Radiation pneumonitis | Pulmonary veno-occlusive disease (PVOD) | |
| Delayed pulmonary toxicity syndrome (DPTS)* | Transfusion-related acute lung Injury (TRALI) | |
| Post-transplant lymphoproliferative disease (PTLD) | Pulmonary cytolytic thrombi (PCT) | |
| Eosinophilic pneumonia (EP) | Pulmonary arterial hypertension (PAH) | |
| Pulmonary alveolar proteinosis (PAP) | Pulmonary thromboembolus (PTE) | |
Conditions routinely included under the classification of idiopathic pneumonia syndrome (IPS).
IDIOPATHIC PNEUMONIA SYNDROME AND THE CLINICAL SPECTRUM OF LUNG INJURY AFTER HSCT
In 1993, a National Institutes of Health workshop proposed a broad definition of IPS that included widespread alveolar injury in the absence of active lower respiratory tract infection or cardiogenic causes after HSCT (1). As shown in Table 1, the diagnostic criteria of IPS have been updated and consider newly described pathogens (2), as determined on bronchoalveolar lavage (BAL) or lung biopsy (1, 3). In a practical sense, the diagnosis of IPS is based on exclusion of infectious organisms, cardiac dysfunction, acute renal failure, or iatrogenic fluid overload in the setting of pulmonary dysfunction after HSCT. IPS encompasses a spectrum of clinical presentations, in both children and adults, and is thought to result from a variety of lung insults, including toxic effects of HSCT conditioning regimens, immunologic cell-mediated injury, inflammatory cytokines, and occult pulmonary infections (1, 4–11).
Historically, the incidence of IPS in the first 120 days after allogeneic HSCT with myeloablative conditioning is 3 to 15% (12–19). Recently, the cumulative incidence of IPS after allogeneic HSCT was significantly lower following nonmyeloablative conditioning than conventional, full-intensity preparative regimens (2.2 versus 8.4%; P = 0.003) (15). Once established, however, pulmonary toxicity was severe in each group and resulted in respiratory failure in the majority of patients (15). These findings suggest that the intensity of HSCT conditioning plays an important role in the development of IPS, and are consistent with data generated from mouse models showing that the lung is sensitive to the combined effects of radiation and alloreactive T cells (7, 20).
The median time of onset for IPS was initially reported to be 6 to 7 weeks (range, 14–90 d) following allogeneic HSCT, but more recent studies have indicated an earlier median time of onset at 19 days (range, 4–106 d) (1, 14, 15). Mortality rates in allogeneic HSCT recipients range from 60 to 80% overall, and are greater than 95% for patients requiring mechanical ventilation (3, 12–15, 21, 22). While IPS has been reported following autologous HSCT (22–29), the incidence is lower, the median time to onset is generally longer (63 d; range, 7–336 d), and the two entities differ sharply with respect to overall outcome. Inflammation in the autologous setting responds promptly to corticosteroids and is associated with a favorable prognosis, whereas IPS occurring in an allogeneic environment responds poorly to standard therapy and commonly results in rapid respiratory failure and death in the majority of patients.
Risk factors for IPS after allogeneic HSCT include full-intensity conditioning with total body irradiation (TBI), acute graft-versus-host disease (GVHD), older recipient age, and an underlying diagnosis of acute leukemia or myelodysplastic syndrome (MDS) (3, 12, 13, 15, 21, 22, 30). GVHD is a major cause of morbidity and mortality following HSCT and is caused by donor cell reactivity against host tissues, due to major and minor human leukocyte antigen (HLA) disparities, and primarily affects the gut, liver, and skin. While one report showed that a single, very low dose, nonmyeloablative conditioning regimen was associated with a reduced incidence of IPS (15), it remains to be fully determined what impact the frequent use of other more robust but reduced-intensity regimens will have on IPS. In a recent meta-analysis, investigators found that lung irradiation dose, cyclophosphamide dose, and the addition of busulfan were significantly associated with the development of interstitial pneumonitis and that lung shielding reduced the incidence of HSCT-related IPS (30). Although earlier studies identified methotrexate (MTX) as a risk factor for the development of interstitial pneumonia, these studies did not distinguish inflammation from infectious and noninfectious causes. More recent studies have reported that MTX is not a risk factor for IPS (3, 21, 22, 26, 31–34). Risk factors for IPS following autologous HSCT include older patient age, severe oral mucositis, TBI-based or bischloroethylnitrosourea (BCNU)-containing conditioning regimens, chest irradiation within 2 weeks of transplant, female sex, and an underlying diagnosis of solid tumor (24, 25, 27). To date, the impact that stem cell source (bone marrow versus peripheral blood versus umbilical cord blood) has on the development of IPS has not been fully evaluated. Thus, how the expanded use of peripheral blood and umbilical cord blood from unrelated donors will affect the incidence and severity of disease remains to be elucidated. Despite the identification and attempts to reduce risk factors, the overall mortality due to IPS remains unacceptably high (35, 36).
As initially defined by Clark and colleagues, the clinical spectrum of IPS encompasses several descriptive forms of pulmonary dysfunction (1, 37, 38). In one small subset of patients with IPS, acute pulmonary hemorrhage or hemorrhagic alveolitis occurs. Diffuse alveolar hemorrhage (DAH) generally develops in the immediate post-HSCT period and is characterized by progressive shortness of breath, cough, and hypoxemia with or without fever. DAH has been reported in 5 to 12% of HSCT patients with a median time to onset of 19 days (range, 5–34 d) in allogeneic recipients and 12 days (range, 0–40 d) in autologous patients (24, 39–44). A disproportionately higher incidence of DAH post-HSCT has been reported in patients with mucopolysaccharidosis (45). The diagnosis of DAH is based on progressively bloodier return of BAL fluid, but frank hemoptysis is rare (24). The presence of greater than 20% hemosiderin-laden macrophages in the BAL fluid may give an indication of previous pulmonary hemorrhage. However, this finding has a very low specificity for DAH; BAL fluid hemosiderin-laden macrophages are also common in patients with low platelet counts or pulmonary hypertension (46). Mortality from DAH ranges from 60 to 100% despite treatment with high-dose corticosteroids (2 mg/kg/d to 1 g/m2/d), with death usually the result of multiorgan failure within 3 weeks of diagnosis (40, 41, 47). Majhail and colleagues recently compared patients with DAH and infection-associated alveolar hemorrhage who presented with similar clinical and radiographic findings in the setting of progressively bloodier BAL fluid following allogeneic HSCT. Alveolar hemorrhage from infectious and noninfectious causes were found to be related but distinct entities with extremely poor outcomes following therapy with conventional agents, including steroids (43).
Peri-engraftment respiratory distress syndrome (PERDS) represents another clinical subset of IPS. By definition, PERDS occurs within 5 days of engraftment and can account for 33% of IPS cases after allogeneic HSCT (48). Although the clinical presentation of PERDS after conventional allogeneic HSCT is similar to that observed in autologous patients or allogeneic patients who receive reduced-intensity conditioning regimens or T cell–depleted stem cell grafts, lung dysfunction is more responsive to corticosteroids and is associated with a better prognosis in the these patients (49–51). Delayed pulmonary toxicity syndrome (DPTS) has also traditionally fallen within the definition of IPS (52). The incidence of DPTS is 29 to 64% in autologous patients receiving chemotherapy with regimens that contain BCNU, cyclophosphamide, and cisplatin. The median time to onset of DPTS is 45 days (range, 21–149 d), and treatment with corticosteroids (1 mg/kg/d) leads to resolution in up to 92% of cases (52–54). High-dose inhaled corticosteroid prophylaxis may also be beneficial in these patients (55). The implication that DPTS is directly related to a specific HSCT conditioning regimen suggests that this entity should technically not be categorized as IPS per se.
Potential etiologies for IPS include direct toxic effects of HSCT conditioning regimens, occult pulmonary infections, and the release of inflammatory cytokines that have been implicated in other forms of pulmonary injury. As noted above, the dose intensity of HSCT conditioning may affect the frequency of IPS, and specific combinations of chemotherapeutic agents can predispose to pulmonary dysfunction in subsets of HSCT recipients. Historically, approximately one half of all pneumonias seen after HSCT have been secondary to infection, but the judicious use of broad-spectrum antimicrobial agents has tipped the balance to noninfectious causes. Together, broad-spectrum prophylaxis and the development and application of advanced diagnostic techniques, including immunohistochemical staining, indirect fluorescent antibody testing, and polymerase chain reaction (PCR), have significantly reduced the incidence of pneumonitis from CMV and Pneumocystis jirovecii and enhanced the utility of bronchoalveolar lavage to rule out the presence of other opportunistic pathogens. This is of particular importance given the impact that making the correct diagnosis has on the approach to subsequent medical therapy; as discussed later, management of IPS (enhanced immunosuppression) and infection (antimicrobial therapy) are quite divergent.
The association between IPS and severe GVHD reported in several large series (1, 3, 14, 15, 21, 22) suggests that immunologic factors may also be operative in the development of lung injury. Acute GVHD often precedes IPS, suggesting a possible causal relationship between the two disorders (3, 33, 56, 57). Although IPS can also occur when signs and symptoms of GVHD are limited or absent, the consistent association between lung injury and GVHD in experimental models also supports such an etiology (1, 3, 6, 7, 15, 20–22, 58).
Despite the aforementioned clinical association, the lung has not been traditionally recognized as a classic GVHD target organ, and the specific role of alloreactive donor T lymphocytes in the pathogenesis of IPS remains a topic of considerable debate. Epithelial apoptosis is usually attributed to T cell–mediated injury and is considered pathognomonic for acute GVHD. Although identified in the lungs of some patients with IPS (56, 59), epithelial apoptosis has not been consistently observed in allogeneic HSCT recipients with pulmonary dysfunction. A histologic spectrum of pulmonary GVHD has recently been described that ranges from diffuse alveolar injury early after HSCT to cicatrical bronchiolitis obliterans, a late and irreversible form of lung injury (59). Bronchitis/bronchiolitis with interstitial pneumonitis was the most common finding and included a lymphocytic infiltration around bronchial structures along with a mononuclear inflammation in the perivascular zones and alveolar septa. Bronchiolitis obliterans syndrome (BOS), also called obliterative bronchiolitis (OB), and bronchiolitis obliterans organizing pneumonia (BOOP), now called cryptogenic organizing pneumonia (COP [60]), are two late-onset noninfectious lung complications that are associated with allo-HSCT and the presence of GVHD (61–65). Incidence of BOS varies from approximately 2% to almost 30%, and time of onset ranges from 2.5 months to almost 10 years after HSCT, but most commonly between 7 and 15 months from time of transplant (63, 65, 66). The incidence of COP (BOOP) is much lower, with a median onset of 3 months after transplant (36, 67). Generally, COP/BOOP can be treated with corticosteroids in the majority of cases while BOS is resistant to this treatment (reviewed in Reference 66).
While histopathology is generally considered the “gold standard” to the diagnostic approach of a clinical disorder, the diagnosis of IPS is rarely made or confirmed by tissue biopsy given the significant mortality risks of open lung biopsy in the early transplant period. The lack of a pathognomonic histologic pattern for IPS and the heterogeneity of pulmonary pathology after allogeneic HSCT are complicated further by the nonspecific lung injury changes that may occur after mechanical ventilation and by the limited quality and quantity of transbronchial lung biopsy specimens obtained from HSCT recipients.
Unfortunately, categorizing patients with IPS based upon descriptive clinical terminology is equally unrewarding. With the help of new and improved diagnostic techniques in immunohistochemistry, radiology, and microbiology, we proposed to categorize the disease entities falling under the umbrella of IPS by the primary anatomic site of cellular damage, including the interstitial tissue, vascular endothelium, or airway epithelium (Table 3). While some cases may remain unclassifiable, this approach may focus future lines of investigation on specific pathways of tissue injury and thereby foster the development of therapeutic interventions that can be tailored to distinct subtypes of disease. To this end and to better define the pathogenesis of IPS, several murine models have been developed and used extensively to investigate immunologic mechanisms of injury that contribute to damage to the vascular endothelium as well as alveolar and bronchiolar epithelium. In this context, a wealth of experimental data has markedly improved our understanding of the pathophysiologic processes that regulate IPS, and has revealed that both cellular effectors and a variety of soluble effectors contribute to the inflammation engendered during disease progression.
TABLE 3.
CLINICAL CATEGORIZATION, HISTOLOGY PATTERN, RADIOGRAPHIC FEATURES, AND MURINE MODEL SYSTEMS OF LUNG INJURY FOLLOWING HEMATOPOIETIC STEM CELL TRANSPLANTATION
| Clinical Categorization (Ref. no.) | Symptoms | Onset | Histologic Pattern | Radiographic Features | Murine HSCT Model? (Ref. no.) |
|---|---|---|---|---|---|
| Lung parenchyma (interstitium and alveolar) | |||||
| AIP (59) | Fever, cough, dyspnea, hypoxemia | Acute onset, 2 to 6 mo after HSCT,typically < 120 d | Diffuse alveolar damage | Bilateral interstitial infiltrates | Yes (6–7, 20, 68, 70, 165, 172) |
| ARDS (59) | Fever, cough, dyspnea, hypoxemia | Acute onset, 2 to 6 mo after HSCT | Diffuse alveolar damage | Bilateral interstitial infiltrates | Yes (6–7, 68, 70) |
| Radiation (210), BCNU pneumonitis (211-212) | Dyspnea, nonproductive cough, rales, pleural friction rub, low-grade fever, restrictive PFTs, impaired DlCO | Subacute, 2 to 4 mo after therapy | Acute fibrinous exudate with chronic fibrosis and sclerosis; often initially subtle pneumonitis with fibrosis in biopsy | Bilateral interstitial infiltrates, initial diffuse infiltrates around hilus | No |
| DPTS (52–54) | Fever, dry cough, dyspnea | Late, months to years after auto HSCT for breast cancer | Hyperplastic alveolar type II cells, septal thickening, interstitial fibrosis, small vessel endothelial injury | Bilateral interstitial infiltrates, ground glass opacities, abnormalities can initially be absent | Yes (71) |
| PTLD (213–214) | Dyspnea, hypoxemia; diffuse lymphadenopathy, may also have more systemic symptoms (viral-like illness to fulminant sepsis) | 1–5 mo, rarely years following allogeneic HSCT with T cell depletion | Lymphoid proliferation in association with EBV | Diffuse infiltrates preferentially located in the basal and subpleural areas | No |
| EP (215–217) | Cough, progressive dyspnea, BAL eosinophilia (> 5%) | Late, 3 mo to years after HSCT | Alveolar and bronchiolar tissue eosinophilia, peripheral blood eosinophilia is also possible | Diffuse infiltrates, often of transient localization, subpleural localization | No |
| PAP (218–219) | Dyspnea, hypoxemia | Early (days) to late (1–2 yr) | BAL milky, extracellular, eosinophilic PAS+ material; alveolar filling with PAS+ material, normal BAL fluid possible | Bilateral patchy alveolar infiltrates; crazy paving pattern | No BMT model; GM-CSFR−/− mice (220) |
| Vascular endothelium | |||||
| PERDS (50) | Fever, dyspnea, cough, hypoxemia, skin rash, weight gain, edema | Acute, within 5–7 d of neutrophil engraftment | Diffuse alveolar damage (but biopsy contraindicated due to thrombocytopenia), neutrophilic inflammation in BAL | Bilateral interstitial infiltrates, pleural effusions; intensity from mild to noncardiogenic pulmonary edema to ARDS | No |
| DAH (40) | Cough, progressive dyspnea, rarely hemoptysis, progressively bloodier BAL fractions | Acute, 1–3 mo after HSCT | Diffuse alveolar damage with intra-alveolar red blood cells and hemosiderin-laden macrophages | Diffuse infiltrates, central appearance initially noted | Yes (6) |
| PVOD (221–223) | Dyspnea | 2–6 mo after HSCT | Fibrous intima proliferation of pulmonary venules, arteriolar involvement also possible | Infiltrates, cardiomegaly, pulmonary hypertension | No |
| TRALI (224) | Acute dyspnea, PaO2/FiO2 ratio < 300 mm Hg, hypotension, fever, chills, leukopenia | Within 6 h of blood product transfusion | Granulocyte aggregation in pulmonary vasculature | Bilateral infiltrates | No |
| PCT (225) | Fever, cough, chest pain, progressive respiratory distress. Observed in pediatric HSCT recipients. | 2–3 mo after HSCT | Open lung biopsy necessary for diagnosis showing thrombi consisting of basophilic, amorphous material which may extend through the vascular wall into adjacent tissue: hemorrhagic infarcts | CXR: normal in 25%, nodules, interstitial markings, atelectasis also seen. CT scan: multiple peripheral pulmonary nodules. | No |
| PAH (226–229) | Asymptomatic/insidious onset of dyspnea | Typically within the first 6 mo after HSCT | Intimal hyperplasia in small pulmonary arteries and arterioles, veins and venules remain unaffected | Pronounced pulmonary artery on CXR or CT | No |
| PTE (218, 230) | Acute onset of dyspnea, chest pain, hypoxemia | Associated with central venous catheter and hypercoagulability | Fibrin-containing thrombus in vein | Embolus detected by thoracic CT or lung angiography | No |
| Airway epithelium | |||||
| BOOP (36, 66) | Fever, dry cough, dyspnea; spirometry: restrictive findings, reduced FVC | 2–12 mo after transplant | Organizing pneumonia (OP), intraluminal organizing fibrosis in distal airspaces with mild interstitial inflammation | Patchy airspace disease, ground glass appearance, nodular opacities | No |
| BOS (66) | Cough, dyspnea, wheezing, lack of fever; spirometry: diminished FEV1 | 3–24 mo after transplant | Bronchiolitis obliterans (BO), cellular and constrictive bronchiolitis | CXR: hyperinflation. Otherwise routinely normal.CT: (early) mosaic attenuation, air trapping; (late) septal lines, centrilobular nodules, bronchial dilatation. | Yes (69) |
Definition of abbreviations: AIP = acute interstitial pneumonitis; ARDS = acute respiratory distress syndrome; BCNU = bischloroethylnitrosourea; BOOP = bronchiolitis obliterans organizing pneumonia; BOS = bronchiolitis obliterans syndrome; DAH = diffuse alveolar hemorrhage; DPTS = delayed pulmonary toxicity syndrome; EP = eosinophilic pneumonia; PAH = pulmonary arterial hypertension; PAP = pulmonary alveolar proteinosis; PCT = pulmonary cytolytic thrombi; PERDS = peri-engraftment respiratory distress syndrome; PTLD = post-transplant lymphoproliferative disease; PVOD = pulmonary veno-occlusive disease; TRALI = transfusion-related acute lung injury.
THE PATHOGENESIS OF IPS AFTER ALLOGENEIC HSCT
Mouse Models of Noninfectious Lung Injury after HSCT
Several murine models of lung injury after HSCT have been established using a variety of strain combinations and conditioning regimens (Table 4). They include a lethally irradiated major histocompatibility complex (MHC)-matched, multiple minor antigen mismatch (6, 20, 68, 172); a complete MHC mismatch model with and without cyclophosphamide (7, 69), lethally irradiated, haploidentical, parent into F1 model (20, 146, 147, 165); a MHC class I mismatch (68, 70); a MHC class II mismatch (68); and a syngeneic HSCT model using high-dose chemotherapy (71).
TABLE 4.
MURINE HEMATOPOIETIC STEM CELL TRANSPLANTATION MODELS OF IDIOPATHIC PNEUMONIA SYNDROME
| Setting | Donor Cells | Recipient Conditioning | Histologic Pattern | Reference No. |
|---|---|---|---|---|
| Complete MHC mismatch (Class I, Class II and minors)C57BL/6 into B10.BR | 20 × 106 BM (T cell depleted) and 15 × 106 spleen cells from B6 donors | 7.5 Gy TBI Day −1 ± Cy (120 mg/kg) Days −3 and −2 | Periluminal infiltrates, diffuse alveolar damage, foamy alveolar macs | (7) |
| Complete MHC mismatch (Class I, Class II and minors)C57BL/6 into B10.BR B10.BR into C57BL/6 C57BL/6 into FVB/N BALB/C into C57BL/6 | 10 × 106 BM (T cell depleted) and 1 × 106 spleen cells from BMT donors | 7.5 Gy TBI Day −1 + Cy (120 mg/kg) Days −3 and −2 | Obliterative bronchiolitis, fibrosis, periluminal infiltration, patchy diffuse alveolar damage | (69) |
| Class I mismatch C57BL/6 into B6.C-H2bm1/By | 5 × 106 splenocytes from B6 donors | 6.75 Gy TBI | Perivascular infiltration, mild alveolar damage | (68) |
| Class I mismatch C57BL/6 into B6.C-H2bm1/By | 5 × 106 BM and 2 × 106 splenic T cells from B6 donors | 11 Gy TBI (split) | Periluminal infiltration, diffuse alveolar damage | (70) |
| Class II mismatch C57BL/6 into B6.C-H2bm12/KhEg | 5 × 106 splenocytes from B6 donors | 6.75 Gy TBI | Perivascular and peribronchiolar cuffing, diffuse alveolar damage | (68) |
| Semi-allogeneic, Haploidentical: Parent into F1C57BL/6 into B6D2F1 hybrid (C57BL/6 × DBA) | 5 × 106BM and 2–10 × 106 splenic T cells from B6 donors | 11–13 Gy TBI (split dose), 9 Gy of TBI | Periluminal infiltration, interstitial pneumonitis, increased cellularity in BAL | (20, 146, 165) |
| Semi-allogeneic, Haploidentical: Parent into F1 BALB/c into CB6F1 hybrid (C57BL/6 × BALB/c) | 10 × 106Rag2−/− BM and 5 × 106 splenocytes or 2 × 106 sorted splenic T cells from Tbet−/− or IFNγ−/− BALB/c donors (or IFNγR−/− recipients) | 13 Gy TBI (split dose) | Perivascular and peribronchiolar cuffing, parenchymal pneumonitis | (147) |
| Minor MHC mismatch B10.BR into CBA | 5 × 106BM and 1 × 105 splenic T cells from B10.BR donors | 11 Gy TBI (split dose) | Mononuclear infiltration, diffuse alveolar damage. DAH with LPS challenge | (6) |
| Minor MHC mismatch LP/J into C57BL/6 | 5 × 106BM and 2 × 106 splenic T cells from LP/J donors | 13 Gy TBI (split dose) | Periluminal infiltration, interstitial infiltration | (172) |
| Syngeneic B6C3F1 hybrid (C57BL/6 × C3H) into B6C3F1 hybrid (C57BL/6 × C3H) | 20 × 106 BM cells from B6C3F1 donors | Cy (105 mg/kg) + cisplatin (3.59 mg/kg) Days −5, −4, and −3 + BCNU (42.9 mg/kg) Day −2 | Large, foamy alveolar macrophages, septal capillary congestion, reactive proliferation of bronchiolar epithelial cells, minimal edema | (71) |
Definition of abbreviations: BCNU = bischloroethylnitrosourea; BM = bone marrow; Cy = cyclophosphamide; DAH = diffuse alveolar hemorrhage; LPS = lipopolysaccharide; MHC = major histocompatibility complex.
The model using a MHC class I and class II mismatched donor/recipient strain combination best reproduces acute, early-onset IPS. In this model, pulmonary toxicity is caused by the influx of host monocytes and donor T cells into the lungs of lethally irradiated mice within the first 2 weeks of HSCT (7). Intensifying the pre-HSCT conditioning with cyclophosphamide accelerates the development of IPS consistent with human data. From a classification standpoint (see Figure 1), lung injury is characterized by injured type II alveolar (ATII) epithelial cells, injured endothelial cells, and increased frequencies of cytotoxic T lymphocytes and cells expressing B7 family costimulatory ligands in the alveolar and interstitial space (7, 72). Lung dysfunction in this acute model reflects this interstitial and alveolar damage and presents as reduced specific compliance, decreased total lung capacity and increased wet and dry lung weights. BAL fluid of mice with IPS contained elevated levels of soluble mediators including cytokines as well as nitrites, lactate dehydrogenase, and total protein indicative of lung inflammation and pulmonary endothelial damage and leak (73). Similar findings of decreased compliance and oxidative stress were seen in the early-onset, syngeneic IPS model of DPTS in which mice are conditioned with high-dose chemotherapy alone (71).
Murine systems wherein donor and host differ at multiple minor HC antigens or at isolated MHC class I or class II loci more closely reflect conditions that are typically seen during human HSCT and specifically model IPS that develops during the first 3 to 6 weeks after transplant and is characterized by leukocyte infiltration into the lung as observed on lung biopsy (59). Regardless of strain combination, two primary, reproducible abnormalities are apparent in these experimental models: a dense mononuclear cell infiltrate around both pulmonary vessels and bronchioles and an acute pneumonitis involving the interstitium and alveolar spaces representing each of the three anatomic categories outlined in Figure 1. When measured, alterations in pulmonary function are associated with lung histopathology demonstrating that the observed lung inflammation is physiologically relevant (6, 7, 75). The alveolar infiltrate is composed of macrophages, lymphocytes, epithelial cells, and scattered polymorphonuclear cells within a fibrin matrix, whereas the periluminal (around vascular and bronchial structures) infiltrates are composed primarily of activated donor T cells. Moreover, significant pulmonary endothelial apoptosis and dysfunction has been identified in this context; endothelial injury coincides with the onset of pulmonary pathology, is associated with elevations in BAL fluid TNF-α levels, and the extent of injury directly correlates with the severity of lung inflammation as IPS progresses (76). Despite the presence of endothelial damage, diffuse alveolar hemorrhage is not regularly seen in preclinical IPS models. The only exception to this is when mice are challenged with lipopolysaccharide (LPS). In this scenario, hemorrhage is only observed in mice with advanced GVHD and is associated with large increases in BAL fluid levels of neutrophils, TNF-α, and LPS (6, 11).
Collectively, a significant body of experimental data suggests that mouse models of IPS reproduce many of the histologic and functional changes observed during human disease. To this end, several investigators have used these models to uncover pathways of inflammation that may be operative during human disease in an attempt to identify opportunities to more effectively treat or prevent this complication.
THE ROLE OF SOLUBLE MEDIATORS IN THE DEVELOPMENT OF IPS
TNF-α and TNF Receptors
The mixed inflammatory alveolar infiltrates found in mice with IPS are associated with increased TNF-α in both lung tissue and BAL fluid (6, 11, 20, 77). A causal role for TNF-α in the development of IPS has been established by neutralizing this cytokine in experimental HSCT models (11, 77). Administration of a soluble, dimeric, TNF-binding protein (rhTNFR:Fc; Amgen Corp., Thousand Oaks, CA) from Week 4 to Week 6 after HSCT reduced the progression of lung injury during this time period (11). The use of mutant mice deficient in TNF-α, has shown that lung injury after allogeneic HSCT is dependent upon donor, rather than host-derived TNF-α and that cytokine production from both donor accessory cells (macrophage/monocytes) and T cells significantly contributes to this toxicity (78).
TNF-α is likely to contribute to the development of IPS through both direct and indirect mechanisms. TNF-α increases MHC complex expression, modulates leukocyte migration, facilitates cell-mediated cytotoxicity, and is itself cytotoxic. Recently it has been shown that TNF-α contributes to pulmonary vascular endothelial cell apoptosis that accompanies lung inflammation during the development of IPS (76). Moreover, donor-derived TNF-α serves as both a facilitator and an effector of lung injury. TNF-α secreted by donor T cells regulates the chemokine milieu in the lung within the first 2 weeks after HSCT, which directly contributes to the subsequent recruitment of monocytes/macrophages as lung injury progresses (78).
The actions of TNF-α are mediated by two receptors: a 55- to 60-kD type I receptor (TNFRI; p55/60; CD120a) and a 75- to 80-kD type II receptor (TNFRII; p75/80; CD120b) (79–81). These two receptors are co-expressed in almost every cell in the body. In contrast to the constitutive expression of TNFRI, the expression of TNFRII is strongly modulated by various cytokines and other inflammatory stimuli like LPS (82–84). TNFRI is generally regarded as the dominant receptor in TNF-α biology; it is the high-affinity receptor for soluble TNF-α (sTNF-α), and mediates many of the proinflammatory effects of this cytokine (85–88). While both sTNF-α and memTNF-α (transmembrane form of TNF-α) are able to activate TNFRI, TNFRII can only be fully activated by memTNF-α (89). TNFRII contributes to the cytocidal effects of TNF-α by its own signaling, and also by regulating the access of this cytokine to TNFRI thereby enhancing TNFRI signaling (90–93).
A role for TNFRI in the induction of GVHD, early after allo-HSCT, has been previously described (94). sTNF-α production is directly related to toxicity from pre-transplant conditioning and to the proinflammatory response that accompanies donor T cell activation and expansion early after allo-HSCT. It was demonstrated that absence of TNFRI in allo-HSCT recipients resulted in improved early post-HSCT survival that was associated with decreased pulmonary edema and improved lung compliance on Day 7 after HSCT (95). However, cellular infiltration into the lung, and BAL fluid levels of proinflammatory cytokines and chemokines were actually higher in BAL fluid from TNFRI−/− HSCT recipients compared with TNFRI+/+ controls. These findings were consistent with those reported when IPS was induced across minor H antigens or isolated MHC class I differences wherein interactions between TNFRII and TNF-α contributed to the leukocyte infiltration and pulmonary inflammation (96). In these studies, the reduction in lung injury observed in TNFRII−/− allo-HSCT recipients directly mirrored the more favorable outcome observed when TNF-α−/− mice were used as HSCT donors.
LPS
Increases in neutrophils and TNF-α in the lungs of allogeneic HSCT recipient without evidence of infection, suggest that host flora-derived LPS might play an important role in IPS pathophysiology. LPS is involved in initiating the innate immune response and is a potent enhancer of inflammatory cytokine release. In non-HSCT experimental models, intratracheal administration of LPS elicits a severe, acute inflammatory response in the lungs of animals (97–99). Recent work has also demonstrated that LPS is an important effector molecule in the development of acute GVHD; translocation of LPS across a gut mucosa damaged early in the post-transplant period provides access to the systemic circulation, where it stimulates leukocytes to release inflammatory mediators that subsequently contribute to GVHD target organ damage and dysfunction (74, 100–105). LPS levels are elevated in the BAL fluid of mice with IPS, and LPS stimulates the release of inflammatory cytokines that directly contribute to lung damage: intravenous LPS injection 6 weeks after allogeneic HSCT significantly amplifies lung injury (6). This amplification is only seen in mice with advanced GVHD and is associated with large increases in BAL fluid levels of TNF-α and LPS and the development of alveolar hemorrhage (6, 11).
Gut-Lung-Liver Axis of Inflammation
Collectively, these data demonstrate that the inflammatory mediators TNF-α and LPS both contribute to experimental IPS. Moreover, they support the hypothesis of a “gut-liver-lung” axis of inflammation in IPS pathophysiology. Any process that ultimately results in large amounts of endotoxin and/or TNF-α in the pulmonary circulation may contribute to the development of lung injury in this setting. This hypothesis is consistent with the observation that serum and BAL fluid TNF-α levels are increased in patients with IPS (106, 107). A clinical linkage of hepatic dysfunction to lung injury after HSCT is also suggested by associations between veno-occlusive disease (VOD) and IPS and between hepatic failure and death from IPS (3, 14). Furthermore, evidence for cytokine activation and LPS amplification observed in the BAL fluid of patients with acute respiratory distress syndrome (ARDS) (108) has been demonstrated in patients with IPS after allogeneic HSCT; increased pulmonary vascular permeability and increases in BAL fluid levels of IL-1, IL-12, IL-6, TNF-α, LPS-binding protein (LBP), and soluble CD14 were also observed in these patients (8).
The incomplete protection provided by abrogating the effects of TNF-α after HSCT by various strategies is consistent with reports from many groups (58, 74, 77, 100, 109–111), and suggests that other inflammatory and cellular mechanisms such as the Fas-FasL pathway that mediate acute GVHD may also contribute to the development of IPS (109, 112–114). For example, IL-1β, TGF-β, and nitrating species including nitric oxide and peroxynitrite have also been implicated in the development of IPS, particularly when cyclophosphamide is included in the conditioning regimen (7, 73, 115).
Oxidative Stress and Pulmonary Dysfunction during IPS
Proinflammatory events after HSCT are frequently accompanied by enhanced production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with a concomitant increase in oxidative and nitrosative stress (116–118). Exposure to radiochemotherapy further increases oxidant stress by depletion of antioxidant defenses, including reduced glutathione, the major antioxidant in the epithelial lining fluid of the lung (119, 120). These observations have been confirmed in murine IPS models after allogeneic and autologous HSCT (71, 121). HSCT is also associated with a state of iron excess in which free iron becomes available to catalyze the conversion of ROS intermediates to highly toxic free radicals such as hydroxyl radical (122). The lung is particularly sensitive because of its oxygen-rich environment. Exhaled nitric oxide is also increased in the lower respiratory tract after HSCT (123). Potential sources of nitric oxide are chemotherapy-induced inflammation of lung epithelium and lung-infiltrating inflammatory cells (118). When simultaneously generated in large amounts, nitric oxide rapidly reacts with superoxide to form peroxynitrite, a potent oxidant and nitrating species (73).
Oxidant/antioxidant imbalance may directly cause cellular injury, and may trigger multiple inflammatory signal transduction pathways (124). Direct evidence that oxidative stress plays a distinct role in the development of IPS injury in humans is lacking. However, experimental models indicate that recipient mice given high-dose chemotherapy and treated with N-acetylcysteine exhibit substantially less lung injury compared with mice receiving high-dose chemotherapy without N-acetylcysteine (71). The most likely mechanism for the protective effects of N-acetylcysteine is the replenishment and/or preservation of glutathione redox potential, although direct scavenging of reactive species by N-acetylcysteine may also contribute. Furthermore, overexpression of extracellular superoxide dismutase (EC-SOD), the major extracellular antioxidant enzyme in the lung, confers protection against radiation-induced acute lung injury (125, 126). Together, these data suggest that amelioration of oxidative stress may reduce the development of severe IPS injury; however, human studies using lung-targeted antioxidant approaches are lacking, and questions concerning the potential of these antioxidants to affect the efficacy of the chemotherapy need to be addressed.
Depletion of Pulmonary Surfactant during IPS
Pulmonary surfactant, produced by alveolar type II cells, is composed of a complex mixture of lipids and at least four surfactant proteins (SPs), named in the order of discovery. The presence of surfactant at the blood–gas interface is essential for the survival of air-breathing mammals. By reducing surface tension, surfactant decreases the work of breathing, allows alveoli to remain open at end expiration, and keeps alveoli dry. In addition, surfactant and particularly SP-A and SP-D have a major role in host defense and in regulating immune responses in the lung (127–131). Surfactant dysfunction caused by decreased synthesis in alveolar type II cells, inhibition of function by extravasated serum proteins, or degradation by lipases and oxidants (132, 133) likely contributes to the IPS clinical picture of hypoxemia, progressive dyspnea, and pulmonary edema (134, 135).
There are a limited number of experimental and human studies evaluating the role of surfactant during lung dysfunction following HSCT. In a model of severe IPS following lethal irradiation, mice lacking SP-A or SP-D exhibited exaggerated allogeneic T cell–dependent inflammation that was associated with enhanced markers of lung injury (136). Furthermore, transtracheal instillation of human SP-A attenuated the manifestations of IPS in mice (137). In humans, pre-transplant serum levels of SP-D were identified as a risk factor for the development of IPS and bronchiolitis obliterans after allogeneic HSCT (138). The investigators speculate that certain polymorphisms in the human SP-D gene cause subnormal levels of SP-D in the alveoli and serum (139). Low levels of SP-D may predispose these individuals to enhanced inflammatory responses after exposure to conditioning regimens and allogeneic immune responses that contribute to the development of IPS. Other investigators have shown that serum level of SP-D is a valuable biomarker in acute lung injury that can predict outcome (140). Similarly, serum SP-D may also be a useful test in following the clinical course of IPS, but the extent of this relationship first needs to be established in clinical trials. Further studies are required to determine the specific role of surfactant proteins during IPS injury.
Despite the overwhelming success in premature infants, results of surfactant replacement trials during acute lung injury have been variable (141, 142). Phase III studies are underway to determine the efficacy of novel surfactant preparations that closely resemble natural surfactant. If these investigations reveal beneficial effects on mortality, further trials of optimal surfactant preparation, dosing, and timing of administration need to be performed.
THE ROLE OF CELLULAR EFFECTORS IN THE DEVELOPMENT OF IPS
Donor-derived T Cell Effectors
The role of alloreactive, donor T cells in the pathogenesis of IPS has been a topic of considerable debate, particularly since the lung has not been considered a classic target organ of acute GVHD. The importance of lymphocytes to lung injury after experimental HSCT has, however, been shown by several groups (5, 7, 143, 144). Donor T cells are critical to the early proinflammatory events associated with lung injury that develops within the first week of HSCT across MHC antigens, whereas in minor H antigen mismatch systems, donor lymphocytes continue to respond to host antigens and contribute to physiologically significant lung injury at later time points (7, 144). Furthermore, the infusion of donor T cell clones that recognize allelic differences in cell surface expression of the CD45 molecule into nonirradiated recipients results in a rapidly progressive pulmonary vasculitis within 3 days of their injection (5, 145).
The origin and functional capacity of T cells infiltrating the lung have been examined by using differences in the T cell Vβ repertoire between donor and recipient (144). Flow cytometric analysis demonstrated that the TCRαβ+ T cells found in the lung 6 weeks after allogeneic HSCT were of donor origin. When these donor T cells were recultured with irradiated host antigen-presenting cells (APCs), they proliferated vigorously and produced significant amounts of IFN-γ. Using a CD8-driven model of IPS wherein donor and host differ by class I MHC antigens only, it was shown that donor-derived T cells expressing IFN-γ and CXCR3 were present in the lungs of allo-HSCT recipients as early as Day 7. The influx of these cells correlated with elevations in protein levels of both CXCL9 and CXCL10, two of the principal ligands for CXCR3 (see below), and heralded the onset of significant pulmonary inflammation (70). While robust infiltration occurs reproducibly in these models when IFN-γ receptor–ligand interactions are completely intact, a recent study paradoxically showed that pulmonary inflammation is accelerated when IFN-γ signaling is absent and that this regulatory effect was entirely mediated by pulmonary parenchyma (146). These results were later independently confirmed and extended to show that IFN-γ negatively regulates the expansion of Th17+CD4+ T cells in the lungs during IPS (147). Furthermore, it has recently been demonstrated that in vitro differentiated Th17 cells mediate severe pulmonary pathology in a mouse model of GVHD (148). The role of Th17 cells in tissue inflammation is currently an area of intense study (149).
Role of Host APCs
While experimental data suggest that donor-derived T cell effectors can home to the lungs early after HSCT and contribute to physiologically significant tissue injury, the precise cytolytic targets of these cells and the process through which they are activated by host antigens remain unresolved. Each process is likely to be complex and to involve interactions with both pulmonary parenchymal cells and APCs (150, 151).
Likewise, the cells responsible for activating T cell effectors that are ultimately recruited to the lung and the specific location of these cellular activating events also remain enigmatic. Donor T cells contributing to IPS may initially encounter host APCs residing in more distant secondary lymphoid tissues and are subsequently specifically recruited to the lung following up-regulation of chemotactic receptors, including CXCR3. It is also possible that donor T cells are activated in the pulmonary microenvironment. The pulmonary dendritic cell (DC) is significantly more efficient than the alveolar macrophage (AM) in antigen presentation and functions as the dominant APC in the lung (152–154). Pulmonary DCs located in the pulmonary interstitium and the bronchial epithelium and submucosa are the sole source of MHC class II expression within the epithelial lining of the airway during steady-state conditions (155, 156). The antigen-presenting capacity of pulmonary DCs is regulated by AMs, which exist in close proximity to DCs in both the airway and lung parenchyma; the addition of AMs to in vitro DC cultures enhances this regulatory effect, whereas depletion of AMs in vivo enhances the APC function of freshly isolated DCs (156). Pulmonary DCs play a critical role in the initiation and regulation of immune responses in the lung, and recent data suggest that they are important to both acute and chronic rejection of lung allografts (155, 157, 158). It is possible that radioresistant host DCs persist longer in the lung than in other organs and allow for sustained presentation of host antigens in that organ (159). Activated donor T cells that can cause progressive lung injury might therefore remain within the pulmonary microvascular circulation because persistent host DCs serve as a continuing site of alloantigen presentation.
Donor Accessory Cells
The role of donor accessory cell populations (monocytes, macrophages, neutrophils) in the pathophysiology of IPS has been examined using HSCT donors that differ in their response to LPS by virtue of a genetic mutation in the Toll-like receptor 4 (TLR 4) gene (160) or the absence of CD14, a key cell surface receptor for the LPS–LBP complex (161). In each case, a significant decrease in lung injury was observed in recipients of LPS resistant donor cells compared with wild-type, LPS-sensitive donor cells. The results obtained using CD14-deficient donors are consistent with the report that monocytes recruited to an inflamed lung up-regulate CD14 expression and show enhanced sensitivity to LPS stimulation (162), and with the clinical evidence for cytokine activation and components of the LPS-activating system including LBP and soluble CD14 present in the BAL fluid of HSCT patients with IPS (8).
Polymorphonuclear cells are a major component of the BAL fluid of animals with IPS (6). In some mouse IPS models, the BAL fluid neutrophilia is prominent between Weeks 4 and 6 after HSCT and is associated with increases in BAL fluid levels of TNF-α and LPS (6, 11). Neutralization of TNF-α with rhTNFR:Fc during this time interval prevents the influx of neutrophils and reduces the progression of lung injury and dysfunction (11). Administration of rhTNFR:Fc following LPS challenge completely abrogates the recruitment of neutrophils into the lungs and prevents further damage (including hemorrhage), underscoring the relationship between neutrophils, TNF-α, and LPS in this setting (11). Neutrophil products such as elastase, myeloperoxidase, metalloproteinases, and oxidants are abundant in the BAL fluid of patients with ARDS and are believed to contribute to the endothelial and epithelial damage that occurs in this setting. Neutrophils are likely to play a role in patients with IPS as well; their appearance in the bloodstream is often temporally associated with the onset of lung inflammation (14).
MECHANISMS OF LEUKOCYTE RECRUITMENT TO THE LUNG DURING THE DEVELOPMENT OF IPS
Chemokine Receptor–Ligand Interactions during IPS
Cellular effectors play a significant role in the development of IPS, but the molecular mechanisms by which leukocytes traffic to the lung and cause damage have yet to be fully elucidated. The persistent elicitation of leukocytes into the lung after allogeneic HSCT requires an intercellular communication between infiltrating leukocytes, endothelium, parenchymal cells, and components of the extracellular matrix. The chemokines, by virtue of their specific cell surface receptor expression, can selectively mediate the local recruitment/activation of distinct leukocytes allowing for migration across the endothelium and beyond the vascular compartment along established chemotactic gradients.
The chemokine superfamily is divided into four subfamilies (C, CC, CXC, and CX3C) based on the presence of conserved cysteine residues at the NH2-terminus (163, 164). CXC chemokines have been further subdivided on the basis of the presence or absence of the sequence glutamic acid–leucine–arginine (ELR) near the NH2-terminal. ELR+ CXC chemokines are neutrophil chemoattractants with angiogenic properties. Interferon-inducible ELR- CXC chemokines are chemoattractants of lymphocytes with angiostatic properties. CC chemokines predominantly recruit mononuclear cells. The C and CX3C subfamily predominantly attract lymphocytes. All chemokine action is mediated through seven-transmembrane–spanning G protein–coupled receptors (163, 164).
CC Chemokine Family Members
CCL2 (MCP-1) is a CC chemokine and its role in IPS was evaluated using the lethally irradiated haploidentical (B6→B6D2F1) murine model. Investigators found increased expression of CCL2 in lungs undergoing IPS that paralleled the recruitment of leukocytes and cellular expression of CCR2 (165). Importantly, when CCR2−/− donor cells were used there was a reduction in lung infiltrating mononuclear cells resulting in the attenuation of IPS (165). Conversely, in a more aggressive lethally irradiated, MHC disparate IPS model (B10.BR→C57BL/6), the use of CCL2−/− or CCR2−/− as recipients resulted in a similar degree of host monocyte and donor T cell recruitment and IPS compared with wild type recipients (166). Collectively, these studies demonstrate that CCR2/CCL2 biology is more important in models where IPS evolves over the time course of two to four weeks and reflects the sequential recruitment of CCR2 expressing donor cells as compared with more aggressive forms of IPS where host APCs and other chemokines acting in parallel/series may play more important roles.
CCL3 (MIP-1α) is a potent chemoattractant of mononuclear cells expressing CCR1 and CCR5. Using the lethally irradiated, MHC disparate (C57BL/6 → B10.BR) murine model of IPS described above, investigators found elevated lung levels of CCL3 (167). Surprisingly, when donor CCL3−/− T cells were used in this model there was an increased sequestration of T cells into the lung resulting in an increase in IPS severity (168). Mechanistically, the recipients of the CCL3−/− donor T cells were found to have an increased type I cytokine profile, demonstrating that chemokines have a role in modulating type I and II immune responses during IPS. Similar to CCL3, CCL5 (RANTES) also recruits mononuclear cells expressing CCR1 and CCR5. However, when CCL5−/− donor cells were used in a lethally irradiated haploidentical (B6 → B6D2F1) murine model of IPS there was an attenuation of IPS (169). Collectively, these studies demonstrate the importance of specific CC chemokines in mediating the inflammatory response engendered in the lung during the pathogenesis of IPS.
CXC Chemokine Family Members
Using the lethally irradiated haploidentical murine model of IPS, investigators also found augmented lung expression of CXCL10 (IP-10) and CXCL11 (I-TAC) that paralleled mononuclear cell recruitment during IPS (170). When other investigators used an irradiated murine MHC class I mismatched model of IPS, they too found elevated levels of CXCL9 (MIG) and CXCL10 that correlated with the recruitment of CXCR3-expressing CD8+ T cells to the lung by Day 7 after HSCT (70). In vivo neutralization of CXCL9 or CXCL10, or HSCT using CXCR3−/− donor leukocytes led to a reduction of lung infiltrating CD8+ T cells and attenuated IPS demonstrating the importance of CXCR3/ligand biology in this model of IPS.
Adhesion molecules and IPS
In lethally irradiated allogeneic mouse models using ICAM−/− recipients, an aspect of the pathogenesis of IPS was found to be distinct from the development of GVHD in other traditional target organs (171, 172). The influx of T cells, macrophages, and neutrophils into the lungs and the production of inflammatory cytokines were dramatically decreased in ICAM−/− allogeneic HSCT recipients compared with wild-type controls. In contrast, systemic levels of these cytokines were unaffected, and GVHD-induced lesions in the liver and colon were at least as severe as those of the wild-type recipients. Furthermore, GVHD-mediated mortality was accelerated in ICAM-1−/− recipients at doses of allogeneic spleen cells that were otherwise not uniformally lethal, implying that ICAM-1 plays a critical role in the generation of IPS and may be a discerning element that segregates IPS from GVHD injury.
Attempts to block adhesion molecule interactions in humans undergoing HSCT have shown mixed results. In one series, patients with leukocyte adhesion deficiency (LAD) given allogeneic HSCT combined with anti-leukocyte function-associated antigen (LFA) and anti-CD2 treatment, achieved reasonable engraftment, but a majority of the patients developed GVHD (173). In a phase II study for high-risk ALL, graft failure and GVHD was prevented in patients given anti–LFA-1 and anti-CD2 (174). However, these patients tended to have long-lasting immunodeficiency and infectious complications.
TARGETS OF INFLAMMATION AND INJURY DURING THE DEVELOPMENT OF IPS
Pulmonary endothelial and epithelial cells can express MHC Class I, MHC Class II, and minor histocompatibility (H) antigens, and the expression of these molecules on vascular endothelium is enhanced by TNF-α and IFN-γ (175). It is conceivable, therefore, that pulmonary parenchymal cells can serve as targets for direct cell-mediated damage, but definitive proof for this has yet to be shown.
Allogeneic-mediated endothelial damage has been demonstrated by transfer of allogeneic lymphocytes to immune-deficient mice (176, 177). Endothelial cell (EC) injury has been observed after allogeneic HSCT and has been implicated as a direct contributor to the development of several complications, including GVHD, VOD, and thrombotic microangiopathy (178). Clinical and experimental IPS is associated with evidence for EC injury and leak as demonstrated by pulmonary edema, enhanced total protein levels in BAL fluid, and increased wet-to-dry lung weight ratios. Moreover, leukocyte infiltration during IPS is accompanied by significant apoptosis of the pulmonary vascular endothelium (76). EC apoptosis coincides with the onset of pulmonary pathology, is associated with elevations in BAL fluid TNF-α levels, and is accompanied by evidence for EC activation as measured by enhanced mRNA expression of adhesion molecules (76). The administration of a soluble TNF-α–binding protein (rhTNFR:Fc) from Week 4 to Week 6 after allogeneic HSCT significantly reduces EC apoptosis and lung histopathology observed in mice (76).
By contrast, epithelial apoptosis, generally ascribed to T cell–mediated injury and considered pathognomonic for acute GVHD in other target tissues, has not been consistently observed in allogeneic HSCT recipients with lung injury. The unique aspects of epithelial anatomy in the lung may help explain this discrepancy. Since there is no stratification or layering of pulmonary epithelial cells as in the skin or intestine, identification of epithelial cell apoptosis by histologic criteria can be more challenging. Experimental studies have, however, provided evidence for epithelial injury during IPS. Panoskaltsis-Mortari and coworkers demonstrated that IPS was associated with injured alveolar type II cells and increased frequencies of cytotoxic T lymphocytes in a model of early-onset IPS (7). The same group later showed that keratinocyte growth factor, a mediator of epithelial cell proliferation and a growth factor for type II pneumocytes, diminished IPS injury by dampening the immune response to chemoradiotherapy and by accelerating repair of the damaged tissue, specifically alveolar type II epithelial cells (72). It was further shown in a murine model of obliterative bronchiolitis after HSCT that bronchiolar epithelial cells expressed MHC class II and were surrounded by cells expressing granzyme B prior to the occlusion of the airways (69).
PRECLINICAL INTERVENTIONS FOR IPS
Keratinocyte growth factor (KGF), or FGF7, is a mediator of epithelial cell proliferation and mesenchymal–epithelial interactions, and a growth factor for type II pneumocytes (179–181). KGF is protective against chemotherapy- and radiation-induced injury in various rodent models (182). In mouse HSCT models, in vivo administration of exogenous KGF, completed prior to conditioning, ameliorates GVHD (183, 184). In investigations targeting pulmonary injury, KGF was protective in lethal models of radiation-, hyperoxia-, acid-, and bleomycin-induced lung injury in rats (185–187), possibly by facilitating repair of DNA damage in alveolar epithelial cells (188, 189). Furthermore, KGF induces increased lung surfactant levels (190, 191), potentiates alveolar fluid clearance by increasing Na,K-ATPase activity (192), decreases hyperoxia-induced apoptosis of alveolar type II cells, and may detoxify reactive oxygen species generated by injured cells (reviewed in Reference 193). In the acute, early-onset IPS mouse model, KGF diminished IPS injury by dampening the immune response to chemoradiotherapy and by accelerating repair of the damaged tissue, specifically alveolar type II (ATII) cells (72). Furthermore, an association between the ability of rHuKGF to up-regulate the expression of SP-A and to limit IPS injury in recipients of allogeneic HSCT was observed (194–196). To establish a causal relationship, rHuKGF was administered subcutaneously, for 3 consecutive days before irradiation, to SP-A–sufficient (SP-A+/+) and SP-A–deficient (SP-A−/−) mice given inflammation-inducing allogeneic T cells at the time of HSCT. rHuKGF failed to suppress the high levels of TNF-α, IFN-γ, and nitric oxide contained in BAL fluid collected on Day 7 after HSCT from SP-A−/− mice compared with wild-type, SP-A+/+ mice.
KGF-treated mice also had significantly lower levels of serum TNF-α than non–KGF-treated counterparts. This finding was consistent in both the complete MHC mismatched and the parent into F1 mouse HSCT models (184, 197). The association of lower TNF-α and LPS levels with less severe manifestations of GVHD and lung injury post-HSCT is consistent with the hypothesis that TNF-α and LPS contribute to IPS injury.
THE DIAGNOSTIC AND THERAPEUTIC APPROACH TO PATIENTS WITH IPS
The approach to HSCT patients with acute pulmonary dysfunction is complex and requires a variety of diagnostic tests and possible consultation with experts in the fields of pulmonology, cardiology, nephrology, radiology, and critical care medicine. Because symptoms of respiratory distress can progress rapidly once established, the timely coordination of care is essential to optimizing outcomes. In addition, there are several diagnostic challenges to address in this setting as both pulmonary and nonpulmonary causes of respiratory compromise are possible. Determination of the severity of respiratory dysfunction, including an assessment of the need for supplemental oxygen support, overall fluid balance, renal function, and cardiac output, should be followed by radiographic imaging. In general, an initial chest X-ray or CT scan will identify the presence of lobar, multilobar, or diffuse pulmonary infiltrates. While such findings may impact the decision-making process, they are nondiagnostic in and of themselves.
In the absence of obvious left-sided heart failure or iatrogenic fluid overload, bronchoscopy with BAL should be strongly considered when multilobar or diffuse infiltrates are present. BAL fluid samples should be sent for a variety of diagnostic tests to determine the presence of community-acquired, hospital-acquired, and otherwise opportunistic infections. While accepted at many large transplant centers, the need to complete a BAL in HSCT recipients with significant respiratory compromise remains a matter of debate, particularly in those patients who are critically ill. Recently, Yanik and colleagues reported on the results of 444 bronchoscopy procedures completed on 300 (20% of all patients) who received HSCT at the University of Michigan from 2001 to 2007 (198). Procedural complications were noted in only 3.6% of cases, including hypoxemia (1.8%), bleeding (1.7%), and hypotension (0.2%). Importantly, only 2% of patients that were breathing spontaneously before bronchoscopy required endotracheal intubation and mechanical ventilation within the initial 48 hours after the procedure. While only 13% of BAL specimens collected in the first 30 days of HSCT were positive for infection, this number increased to 33% between Days 31 and 100. Hence, although the majority of HSCT patients requiring BAL within the first 100 days may have IPS, a significant number of individuals will have evidence for infection. To this end, BAL data resulted in changes in medical management in approximately 60% of cases, and modifications in antimicrobial therapy in just under half. Of note, only one recipient of allogeneic HSCT had evidence for Pneumocystis, underscoring the effect antimicrobial prophylaxis has on the scope of subsequent infectious complications. Since medical management of IPS (immunosuppression) and infection (antimicrobial therapy) are rather divergent, making the appropriate diagnosis has significant merit.
Current standard treatment strategies for IPS include supportive care measures in conjunction with broad-spectrum antimicrobial agents and intravenous corticosteroids. Unfortunately, responses to standard therapy are limited, and the mortality of patients diagnosed with IPS remains unacceptably high. Response to corticosteroid therapy has shown mixed but limited efficacy. On the one hand, high-dose corticosteroid therapy (> 4 mg/kg/d of methylprednisolone equivalent) has not been shown to improve outcome when compared with lower doses of corticosteroids (≤ 2 mg/kg/d) in patients diagnosed with IPS (15). On the other hand, in the presence of the DAH form of IPS, high-dose steroids may have increased efficacy with improved mortality (91% versus 67%) (199) and the addition of aminocaproic acid may further improve outcomes (41). However, the overall response to steroids for patients presenting with DAH remains disappointing (41, 47). Advances in supportive care, including the early institution of continuous veno-venous hemofiltration (200), may help to improve survival in some patients, but prospective studies addressing the treatment of IPS, including the specific use of steroids, are lacking in the literature.
Insights generated from preclinical models of IPS suggest that neutralization of TNF-α may be a useful therapeutic strategy for IPS. In a recent translational research effort, etanercept was given subcutaneously at a dose of 0.4 mg/kg twice weekly for a maximum of eight doses in combination with systemic steroids and empiric antimicrobial therapy to a total of 15 patients with IPS (198). All patients had evidence for systemic inflammation and pulmonary vascular dysfunction prior to enrollment on study. Therapy was well tolerated overall. Ten of the 15 patients were able to completely withdraw from supplemental oxygen support within 28 days of therapy, and survival at Day 28 and Day 56 (from the first etanercept dose) was significantly improved. Based on these encouraging results, larger phase II (pediatric) and phase III (adult) trials are ongoing within the Bone Marrow Transplant Clinical Trials Network (phase III) and the Children's Oncology Group (phase II).
Consistent with animal studies, not all patients with IPS responded to etanercept therapy. Categorizing patients with IPS based upon the presumed anatomic site of primary injury and exploiting mechanistic insights gained in the laboratory, as described above, may avail the use of other promising, non–cross-reactive agents for the treatment of IPS. It is conceivable that approaches to maintain EC integrity may be effective at preventing or treating IPS. The administration of molecules that function as survival factors for ECs has been successful in preventing endothelial damage and mortality from septic shock and radiation injury (201, 202). Specifically, KGFs have been shown to be efficacious in reducing epithelial damage and the severity of acute GVHD in mice and humans (183, 184, 203) and experimental IPS after allogeneic HSCT (72), as well as protecting pulmonary endothelium from oxygen-induced injury. Similarly, future studies examining the role of surfactant replacement therapy, perhaps in combination with etanercept, might prove useful in overcoming the effects of epithelial injury and dysfunction on ventilation and oxygenation.
Finally, the role for specific chemokine receptor:ligand interactions in the development of experimental IPS suggests that these proteins may also be operative in the clinical setting as well, particularly if disease onset is later and inflammation by cellular, rather than soluble, effectors predominates. Since IPS develops and progresses to respiratory failure despite significant systemic immunosuppression, it is possible that novel strategies directed toward inhibiting pathways of leukocyte recruitment to the lung may serve as future adjuncts to standard therapy intended to prevent or treat this serious complication.
CONCLUSIONS AND FUTURE DIRECTIONS
IPS remains a frequent and severe complication of allogeneic HSCT, and despite significant advances in critical care medicine, mortality rates remain unacceptably high with standard therapy. Although such lung injury occasionally occurs after autologous transplants, the allogeneic setting significantly exacerbates toxicity and increases the likelihood of treatment failure. Recent efforts in pre-HSCT prognostic risk assessments for developing IPS, including evaluation of pulmonary function (FEV1 and DlCO), BAL fluid inflammatory mediator levels, and genetic analyses (single-nucleotide polymorphisms), show promise for identifying those patients at highest risk of developing IPS (45, 204–207).
In addition, the use of established preclinical models of IPS has resulted in a more robust understanding of the mechanisms that contribute to inflammation during the development of IPS. To this end, the findings of this statement support a shift away from the current paradigm, in which noninfectious lung injury after HSCT is viewed simply as an idiopathic clinical syndrome and toward a process in which the lung is the target of a complex cytotoxic and immune-mediated attack. Soluble inflammatory mediators like TNF-α, LPS, and reactive oxygen species are significant, albeit not exclusive, contributors to IPS, and cells of both lymphoid and myeloid origin play a direct role in lung injury that occurs in this setting. Donor-derived cellular effectors may also be recruited to the lung by using specific receptor–ligand interactions that allow them to traverse through an inflamed pulmonary vascular endothelium and into the parenchyma, where they contribute to epithelial damage and dysfunction. Donor bone marrow–derived cells, epithelial progenitors, and other stem cells with multipotent differentiative capacity may also be recruited to the lungs after HSCT. The roles of such cells in repairing the injured lung after transplant is currently the subject of much debate and anticipation (reviewed in References 208–209). It is hoped that mechanistic insights from experimental models will form the basis for translational clinical research protocols with the goal of treating or preventing IPS after HSCT. As our understanding of pathways involved with the development of IPS improves, determining which patient will respond best to the new agents available in the therapeutic armamentarium will be critical to improving outcomes for HSCT patients.
This official Research Statement was prepared by an ad hoc subcommittee of the Assembly on Respiratory Cell and Molecular Biology.
Members of the subcommittee:
Angela Panoskaltsis-Mortari, Ph.D. (Chair)
Matthias Griese, M.D.
David K. Madtes, M.D.
John A. Belperio, M.D.
Imad Y. Haddad, M.D.
Rodney J. Folz, M.D., Ph.D.
Kenneth R. Cooke, M.D.
Acknowledgments
The authors thank Drs. Joan Clark and David Ingbar for helpful comments and suggestions. They also thank Dr. JoAnne Young for helpful comments in revising the definition of IPS in Table 1 of this manuscript.
This official Research Statement of the American Thoracic Society (ATS) was approved by the ATS Board of Directors, September 2010
Author Disclosure: A.P-M. reported a grant from the NIH paid to her institution in support of the work reported in this manuscript, and that Amgen provided KGF for experimental studies cited within the manuscript. M.G. reported that he received no payments or services from a third party for the work submitted. He had paid consultant relationships with Novartis and Vertex, and his institution received grants from DFG and BMBF for activities outside of the submitted work. D.K.M., J.A.B., I.Y.H., and R.J.F. reported that they received no payments or services from a third party for the work submitted, and had no relevant financial activities outside the submitted work. K.R.C. reported that he received no payments or services from a third party for the work submitted. He received a grant from Athersys and support from Amgen (study drug and central pharmacy support) for activities outside of the submitted work. Note: disclosures made consistent with International Committee of Medical Journal Editors recommendations.
References
- 1.Clark JGHJ, Hertz MI, Parkman R, Jensen L, Peavy HH. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis 1993;147:1601–1606. [DOI] [PubMed] [Google Scholar]
- 2.Englund JA, Boeckh M, Kuypers J, Nichols WG, Hackman RC, Morrow RA, Fredricks DN, Corey L. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006;144:344–349. [DOI] [PubMed] [Google Scholar]
- 3.Crawford SW, Hackman RC. Clinical course of idiopathic pneumonia after bone marrow transplantation. Am Rev Respir Dis 1993;147:1393–1400. [DOI] [PubMed] [Google Scholar]
- 4.Shankar G, Cohen DA. Idiopathic pneumonia syndrome after bone marrow transplantation: the role of pre-transplant radiation conditioning and local cytokine dysregulation in promoting lung inflammation and fibrosis. Int J Exp Pathol 2001;82:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JG, Madtes DK, Hackman RC, Chen W, Cheever MA, Martin PJ. Lung injury induced by alloreactive Th1 cells is characterized by host-derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol 1998;161:1913–1920. [PubMed] [Google Scholar]
- 6.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood 1996;88:3230–3239. [PubMed] [Google Scholar]
- 7.Panoskaltsis-Mortari A, Taylor PA, Yaeger TM, Wangensteen OD, Bitterman PB, Ingbar DH, Vallera DA, Blazar BR. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogeneic recipients are due to donor T cell infusion and potentiated by cyclophosphamide. J Clin Invest 1997;100:1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark JG, Madtes DK, Martin TR, Hackman RC, Farrand AL, Crawford SW. Idiopathic pneumonia after bone marrow transplantation: cytokine activation and lipopolysaccharide amplification in the bronchoalveolar compartment. Crit Care Med 1999;27:1800–1806. [DOI] [PubMed] [Google Scholar]
- 9.Shankar G, Scott Bryson J, Darrell Jennings C, Kaplan AM, Cohen DA. Idiopathic pneumonia syndrome after allogeneic bone marrow transplantation in mice: role of pretransplant radiation conditioning. Am J Respir Cell Mol Biol 1999;20:1116–1124. [DOI] [PubMed] [Google Scholar]
- 10.Della Volpe A, Ferreri AJ, Annaloro C, Mangili P, Rosso A, Calandrino R, Villa E, Lambertenghi-Deliliers G, Fiorino C. Lethal pulmonary complications significantly correlate with individually assessed mean lung dose in patients with hematologic malignancies treated with total body irradiation. Int J Radiat Oncol Biol Phys 2002;52:483–488. [DOI] [PubMed] [Google Scholar]
- 11.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, Brewer JP, Ferrara JL. Tumor necrosis factor-alpha neutralization reduces lung injury after experimental allogeneic bone marrow transplantation. Transplantation 2000;70:272–279. [DOI] [PubMed] [Google Scholar]
- 12.Wingard JR, Mellits ED, Sostrin MB, Chen DY, Burns WH, Santos GW, Vriesendorp HM, Beschorner WE, Saral R. Interstitial pneumonitis after allogeneic bone marrow transplantation: nine-year experience at a single institution. Medicine (Baltimore) 1988;67:175–186. [DOI] [PubMed] [Google Scholar]
- 13.Meyers JD, Flournoy N, Thomas ED. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years' experience. Rev Infect Dis 1982;4:1119–1132. [DOI] [PubMed] [Google Scholar]
- 14.Yanik G, Hellerstedt B, Custer J, Hutchinson R, Kwon D, Ferrara JL, Uberti J, Cooke KR. Etanercept (enbrel) administration for idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2002;8:395–400. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, Maloney DG, Deeg HJ, Martin PJ, Storb RF, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003;102:2777–2785. [DOI] [PubMed] [Google Scholar]
- 16.Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and outcome of idiopathic pneumonia syndrome in pediatric stem cell transplant recipients. Bone Marrow Transplant 2006;38:285–289. [DOI] [PubMed] [Google Scholar]
- 17.Huisman C, van der Straaten HM, Canninga-van Dijk MR, Fijnheer R, Verdonck LF. Pulmonary complications after T-cell-depleted allogeneic stem cell transplantation: Low incidence and strong association with acute graft-versus-host disease. Bone Marrow Transplant 2006;38:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patriarca F, Skert C, Bonifazi F, Sperotto A, Fili C, Stanzani M, Zaja F, Cerno M, Geromin A, Bandini G, et al. Effect on survival of the development of late-onset non-infectious pulmonary complications after stem cell transplantation. Haematologica 2006;91:1268–1272. [PubMed] [Google Scholar]
- 19.Griese M, Rampf U, Hofmann D, Fuhrer M, Reinhardt D, Bender-Gotze C. Pulmonary complications after bone marrow transplantation in children: twenty-four years of experience in a single pediatric center. Pediatr Pulmonol 2000;30:393–401. [DOI] [PubMed] [Google Scholar]
- 20.Shankar G, Bryson JS, Jennings CD, Morris PE, Cohen DA. Idiopathic pneumonia syndrome in mice after allogeneic bone marrow transplantation. Am J Respir Cell Mol Biol 1998;18:235–242. [DOI] [PubMed] [Google Scholar]
- 21.Weiner RS, Bortin MM, Gale RP, Gluckman E, Kay HE, Kolb HJ, Hartz AJ, Rimm AA. Interstitial pneumonitis after bone marrow transplantation: assessment of risk factors. Ann Intern Med 1986;104:168–175. [DOI] [PubMed] [Google Scholar]
- 22.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: changing spectrum of lung injury after marrow transplantation. Transplantation 1997;63:1079–1086. [DOI] [PubMed] [Google Scholar]
- 23.Pecego R, Hill R, Appelbaum FR, Amos D, Buckner CD, Fefer A, Thomas ED. Interstitial pneumonitis following autologous bone marrow transplantation. Transplantation 1986;42:515–517. [DOI] [PubMed] [Google Scholar]
- 24.Robbins RA, Linder J, Stahl MG, Thompson AB III, Haire W, Kessinger A, Armitage JO, Arneson M, Woods G, Vaughan WP, et al. Diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Am J Med 1989;87:511–518. [DOI] [PubMed] [Google Scholar]
- 25.Carlson K, Backlund L, Smedmyr B, Oberg G, Simonsson B. Pulmonary function and complications subsequent to autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:805–811. [PubMed] [Google Scholar]
- 26.Granena A, Carreras E, Rozman C, Salgado C, Sierra J, Algara M, Rovira M, Valls A. Interstitial pneumonitis after BMT: 15 years experience in a single institution. Bone Marrow Transplant 1993;11:453–458. [PubMed] [Google Scholar]
- 27.Rubio C, Hill ME, Milan S, O'Brien ME, Cunningham D. Idiopathic pneumonia syndrome after high-dose chemotherapy for relapsed Hodgkin's disease. Br J Cancer 1997;75:1044–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CS, Boeckh M, Seidel K, Clark JG, Kansu E, Madtes DK, Wagner JL, Witherspoon RP, Anasetti C, Appelbaum FR, et al. Incidence, risk factors, and mortality from pneumonia developing late after hematopoietic stem cell transplantation. Bone Marrow Transplant 2003;32:515–522. [DOI] [PubMed] [Google Scholar]
- 29.Bilgrami SF, Metersky ML, McNally D, Naqvi BH, Kapur D, Raible D, Bona RD, Edwards RL, Feingold JM, Clive JM, et al. Idiopathic pneumonia syndrome following myeloablative chemotherapy and autologous transplantation. Ann Pharmacother 2001;35:196–201. [DOI] [PubMed] [Google Scholar]
- 30.Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys 2005;63:876–884. [DOI] [PubMed] [Google Scholar]
- 31.Weiner RS, Horowitz MM, Gale RP, Dicke KA, van Bekkum DW, Masaoka T, Ramsay NK, Rimm AA, Rozman C, Bortin MM. Risk factors for interstitial pneumonia following bone marrow transplantation for severe aplastic anaemia. Br J Haematol 1989;71:535–543. [DOI] [PubMed] [Google Scholar]
- 32.Lazarus HM, Coccia PF, Herzig RH, Graham-Pole J, Gross S, Strandjord S, Gordon E, Cheung NK, Warkentin PI, Spitzer TR, et al. Incidence of acute graft-versus-host disease with and without methotrexate prophylaxis in allogeneic bone marrow transplant patients. Blood 1984;64:215–220. [PubMed] [Google Scholar]
- 33.Bortin MM, Kay HE, Gale RP, Rimm AA. Factors associated with interstitial pneumonitis after bone-marrow transplantation for acute leukaemia. Lancet 1982;1:437–439. [DOI] [PubMed] [Google Scholar]
- 34.Crawford SW, Longton G, Storb R. Acute graft-versus-host disease and the risks for idiopathic pneumonia after marrow transplantation for severe aplastic anemia. Bone Marrow Transplant 1993;12:225–231. [PubMed] [Google Scholar]
- 35.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;28:425–434. [DOI] [PubMed] [Google Scholar]
- 36.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood 2003;102:3822–3828. [DOI] [PubMed] [Google Scholar]
- 37.Afessa B, Peters SG. Noninfectious pneumonitis after blood and marrow transplant. Curr Opin Oncol 2008;20:227–233. [DOI] [PubMed] [Google Scholar]
- 38.Watkins TR, Chien JW, Crawford SW. Graft versus host-associated pulmonary disease and other idiopathic pulmonary complications after hematopoietic stem cell transplant. Semin Respir Crit Care Med 2005;26:482–489. [DOI] [PubMed] [Google Scholar]
- 39.Raptis A, Mavroudis D, Suffredini A, Molldrem J, Rhee FV, Childs R, Phang S, Barrett A. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in allogeneic bone marrow stem cell transplant recipients. Bone Marrow Transplant 1999;24:879–883. [DOI] [PubMed] [Google Scholar]
- 40.Afessa B, Tefferi A, Litzow MR, Krowka MJ, Wylam ME, Peters SG. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 2002;166:641–645. [DOI] [PubMed] [Google Scholar]
- 41.Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant 2006;12:949–953. [DOI] [PubMed] [Google Scholar]
- 42.Heggen J, West C, Olson E, Olson T, Teague G, Fortenberry J, Yeager AM. Diffuse alveolar hemorrhage in pediatric hematopoietic cell transplant patients. Pediatrics 2002;109:965–971. [DOI] [PubMed] [Google Scholar]
- 43.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant 2006;12:1038–1046. [DOI] [PubMed] [Google Scholar]
- 44.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Alveolar hemorrhage following allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Bone Marrow Transplant 2006;38:765–768. [DOI] [PubMed] [Google Scholar]
- 45.Kharbanda S, Panoskaltsis-Mortari A, Haddad IY, Blazar BR, Orchard PJ, Cornfield DN, Grewal SS, Peters C, Regelmann WE, Milla CE, et al. Inflammatory cytokines and the development of pulmonary complications after allogeneic hematopoietic cell transplantation in patients with inherited metabolic storage disorders. Biol Blood Marrow Transplant 2006;12:430–437. [DOI] [PubMed] [Google Scholar]
- 46.Salih ZN, Akhter A, Akhter J. Specificity and sensitivity of hemosiderin-laden macrophages in routine bronchoalveolar lavage in children. Arch Pathol Lab Med 2006;130:1684–1686. [DOI] [PubMed] [Google Scholar]
- 47.Lewis ID, DeFor T, Weisdorf DJ. Increasing incidence of diffuse alveolar hemorrhage following allogeneic bone marrow transplantation: cryptic etiology and uncertain therapy. Bone Marrow Transplant 2000;26:539–543. [DOI] [PubMed] [Google Scholar]
- 48.Gorak E, Geller N, Srinivasan R, Espinoza-Delgado I, Donohue T, Barrett AJ, Suffredini A, Childs R. Engraftment syndrome after nonmyeloablative allogeneic hematopoietic stem cell transplantation: incidence and effects on survival. Biol Blood Marrow Transplant 2005;11:542–550. [DOI] [PubMed] [Google Scholar]
- 49.Cahill RA, Spitzer TR, Mazumder A. Marrow engraftment and clinical manifestations of capillary leak syndrome. Bone Marrow Transplant 1996;18:177–184. [PubMed] [Google Scholar]
- 50.Capizzi SA, Kumar S, Huneke NE, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Gastineau DA, Prakash UB, Tefferi A. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant 2001;27:1299–1303. [DOI] [PubMed] [Google Scholar]
- 51.Nurnberger W, Willers R, Burdach S, Gobel U. Risk factors for capillary leakage syndrome after bone marrow transplantation. Ann Hematol 1997;74:221–224. [DOI] [PubMed] [Google Scholar]
- 52.Wilczynski SW, Erasmus JJ, Petros WP, Vredenburgh JJ, Folz RJ. Delayed pulmonary toxicity syndrome following high-dose chemotherapy and bone marrow transplantation for breast cancer. Am J Respir Crit Care Med 1998;157:565–573. [DOI] [PubMed] [Google Scholar]
- 53.Cao TM, Negrin RS, Stockerl-Goldstein KE, Johnston LJ, Shizuru JA, Taylor TL, Rizk NW, Wong RM, Blume KG, Hu WW. Pulmonary toxicity syndrome in breast cancer patients undergoing BCNU-containing high-dose chemotherapy and autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2000;6:387–394. [DOI] [PubMed] [Google Scholar]
- 54.Bhalla KS, Wilczynski SW, Abushamaa AM, Petros WP, McDonald CS, Loftis JS, Chao NJ, Vredenburgh JJ, Folz RJ. Pulmonary toxicity of induction chemotherapy prior to standard or high-dose chemotherapy with autologous hematopoietic support. Am J Respir Crit Care Med 2000;161:17–25. [DOI] [PubMed] [Google Scholar]
- 55.McGaughey DS, Nikcevich DA, Long GD, Vredenburgh JJ, Rizzieri D, Smith CA, Broadwater G, Loftis JS, McDonald C, Morris AK, et al. Inhaled steroids as prophylaxis for delayed pulmonary toxicity syndrome in breast cancer patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Biol Blood Marrow Transplant 2001;7:274–278. [DOI] [PubMed] [Google Scholar]
- 56.Beschorner WE, Saral R, Hutchins GM, Tutschka PJ, Santos GW. Lymphocytic bronchitis associated with graft-versus-host disease in recipients of bone-marrow transplants. N Engl J Med 1978;299:1030–1036. [DOI] [PubMed] [Google Scholar]
- 57.Zhu KE, Hu JY, Zhang T, Chen J, Zhong J, Lu YH. Incidence, risks, and outcome of idiopathic pneumonia syndrome early after allogeneic hematopoietic stem cell transplantation. Eur J Haematol 2008;81:461–466. [DOI] [PubMed] [Google Scholar]
- 58.Piguet PF, Grau GE, Collart MA, Vassalli P, Kapanci Y. Pneumopathies of the graft-versus-host reaction: alveolitis associated with an increased level of tumor necrosis factor mRNA and chronic interstitial pneumonitis. Lab Invest 1989;61:37–45. [PubMed] [Google Scholar]
- 59.Yousem SA. The histological spectrum of pulmonary graft-versus-host disease in bone marrow transplant recipients. Hum Pathol 1995;26:668–675. [DOI] [PubMed] [Google Scholar]
- 60.American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- 61.Holland HK, Wingard JR, Beschorner WE, Saral R, Santos GW. Bronchiolitis obliterans in bone marrow transplantation and its relationship to chronic graft-v-host disease and low serum IgG. Blood 1988;72:621–627. [PubMed] [Google Scholar]
- 62.Chien JW, Martin PJ, Gooley TA, Flowers ME, Heckbert SR, Nichols WG, Clark JG. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:208–214. [DOI] [PubMed] [Google Scholar]
- 63.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biol Blood Marrow Transplant 2003;9:657–666. [DOI] [PubMed] [Google Scholar]
- 64.Santo Tomas LH, Loberiza FR Jr, Klein JP, Layde PM, Lipchik RJ, Rizzo JD, Bredeson CN, Horowitz MM. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest 2005;128:153–161. [DOI] [PubMed] [Google Scholar]
- 65.Sakaida E, Nakaseko C, Harima A, Yokota A, Cho R, Saito Y, Nishimura M. Late-onset noninfectious pulmonary complications after allogeneic stem cell transplantation are significantly associated with chronic graft-versus-host disease and with the graft-versus-leukemia effect. Blood 2003;102:4236–4242. [DOI] [PubMed] [Google Scholar]
- 66.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007;13:749–759. [DOI] [PubMed] [Google Scholar]
- 67.Patriarca F, Skert C, Sperotto A, Damiani D, Cerno M, Geromin A, Zaja F, Stocchi R, Prosdocimo S, Fili C, et al. Incidence, outcome, and risk factors of late-onset noninfectious pulmonary complications after unrelated donor stem cell transplantation. Bone Marrow Transplant 2004;33:751–758. [DOI] [PubMed] [Google Scholar]
- 68.Serody JS, Burkett SE, Panoskaltsis-Mortari A, Ng-Cashin J, McMahon E, Matsushima GK, Lira SA, Cook DN, Blazar BR. T-lymphocyte production of macrophage inflammatory protein-1alpha is critical to the recruitment of CD8 (+) T cells to the liver, lung, and spleen during graft-versus-host disease. Blood 2000;96:2973–2980. [PubMed] [Google Scholar]
- 69.Panoskaltsis-Mortari A, Tram KV, Price AP, Wendt CH, Blazar BR. A new murine model for bronchiolitis obliterans post-bone marrow transplant. Am J Respir Crit Care Med 2007;176:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hildebrandt GC, Corrion LA, Olkiewicz KM, Lu B, Lowler K, Duffner UA, Moore BB, Kuziel WA, Liu C, Cooke KR. Blockade of CXCR3 receptor:Ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol 2004;173:2050–2059. [DOI] [PubMed] [Google Scholar]
- 71.Bhalla KS, Folz RJ. Idiopathic pneumonia syndrome after syngeneic bone marrow transplant in mice. Am J Respir Crit Care Med 2002;166:1579–1589. [DOI] [PubMed] [Google Scholar]
- 72.Panoskaltsis-Mortari A, Ingbar DH, Jung P, Haddad IY, Bitterman PB, Wangensteen OD, Farrell CL, Lacey DL, Blazar BR. KGF pretreatment decreases B7 and granzyme B expression and hastens repair in lungs of mice after allogeneic BMT. Am J Physiol Lung Cell Mol Physiol 2000;278:L988–L999. [DOI] [PubMed] [Google Scholar]
- 73.Haddad IY, Panoskaltsis-Mortari A, Ingbar DH, Yang S, Milla CE, Blazar BR. High levels of peroxynitrite are generated in the lungs of irradiated mice given cyclophosphamide and allogeneic T cells: a potential mechanism of injury after marrow transplantation. Am J Respir Cell Mol Biol 1999;20:1125–1135. [DOI] [PubMed] [Google Scholar]
- 74.Cooke KR, Hill GR, Crawford JM, Bungard D, Brinson YS, Delmonte J Jr, Ferrara JL. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest 1998;102:1882–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miklos S, Mueller G, Chang Y, Schubert TE, Holler E, Hildebrandt GC. Pulmonary function changes in experimental graft-versus-host disease of the lung. Biol Blood Marrow Transplant 2008;14:1004–1016. [DOI] [PubMed] [Google Scholar]
- 76.Gerbitz A, Nickoloff BJ, Olkiewicz K, Willmarth NE, Hildebrandt G, Liu C, Kobzik L, Eissner G, Holler E, Ferrara JL, et al. A role for tumor necrosis factor-alpha-mediated endothelial apoptosis in the development of experimental idiopathic pneumonia syndrome. Transplantation 2004;78:494–502. [DOI] [PubMed] [Google Scholar]
- 77.Piguet PF, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med 1987;166:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hildebrandt GC, Olkiewicz KM, Corrion LA, Chang Y, Clouthier SG, Liu C, Cooke KR. Donor-derived TNF-alpha regulates pulmonary chemokine expression and the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 2004;104:586–593. [DOI] [PubMed] [Google Scholar]
- 79.Brockhaus M, Schoenfeld HJ, Schlaeger EJ, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci USA 1990;87:3127–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loetscher H, Pan YC, Lahm HW, Gentz R, Brockhaus M, Tabuchi H, Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell 1990;61:351–359. [DOI] [PubMed] [Google Scholar]
- 81.Schall TJ, Lewis M, Koller KJ, Lee A, Rice GC, Wong GH, Gatanaga T, Granger GA, Lentz R, Raab H, et al. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell 1990;61:361–370. [DOI] [PubMed] [Google Scholar]
- 82.Kalthoff H, Roeder C, Brockhaus M, Thiele HG, Schmiegel W. Tumor necrosis factor (TNF) up-regulates the expression of p75 but not p55 TNF receptors, and both receptors mediate, independently of each other, up-regulation of transforming growth factor alpha and epidermal growth factor receptor mRNA. J Biol Chem 1993;268:2762–2766. [PubMed] [Google Scholar]
- 83.Winzen R, Wallach D, Kemper O, Resch K, Holtmann H. Selective up-regulation of the 75-kDa tumor necrosis factor (TNF) receptor and its mRNA by TNF and IL-1. J Immunol 1993;150:4346–4353. [PubMed] [Google Scholar]
- 84.Tannenbaum CS, Major JA, Hamilton TA. IFN-gamma and lipopolysaccharide differentially modulate expression of tumor necrosis factor receptor mRNA in murine peritoneal macrophages. J Immunol 1993;151:6833–6839. [PubMed] [Google Scholar]
- 85.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell 1993;74:845–853. [DOI] [PubMed] [Google Scholar]
- 86.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol 1998;160:943–952. [PubMed] [Google Scholar]
- 87.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to l. monocytogenes infection. Cell 1993;73:457–467. [DOI] [PubMed] [Google Scholar]
- 88.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci USA 1998;95:570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 1995;83:793–802. [DOI] [PubMed] [Google Scholar]
- 90.Weiss T, Grell M, Hessabi B, Bourteele S, Muller G, Scheurich P, Wajant H. Enhancement of TNF receptor p60-mediated cytotoxicity by TNF receptor p80: requirement of the TNF receptor-associated factor-2 binding site. J Immunol 1997;158:2398–2404. [PubMed] [Google Scholar]
- 91.Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kD tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem 1993;268:18542–18548. [PubMed] [Google Scholar]
- 92.Bigda J, Beletsky I, Brakebusch C, Varfolomeev Y, Engelmann H, Bigda J, Holtmann H, Wallach D. Dual role of the p75 tumor necrosis factor (TNF) receptor in TNF cytotoxicity. J Exp Med 1994;180:445–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grell M, Zimmermann G, Hulser D, Pfizenmaier K, Scheurich P. TNF receptors TR60 and TR80 can mediate apoptosis via induction of distinct signal pathways. J Immunol 1994;153:1963–1972. [PubMed] [Google Scholar]
- 94.Speiser DE, Bachmann MF, Frick TW, McKall-Faienza K, Griffiths E, Pfeffer K, Mak TW, Ohashi PS. TNF receptor p55 controls early acute graft-versus-host disease. J Immunol 1997;158:5185–5190. [PubMed] [Google Scholar]
- 95.Shukla M, Yang S, Milla C, Panoskaltsis-Mortari A, Blazar BR, Haddad IY. Absence of host tumor necrosis factor receptor 1 attenuates manifestations of idiopathic pneumonia syndrome. Am J Physiol Lung Cell Mol Physiol 2005;288:L942–L949. [DOI] [PubMed] [Google Scholar]
- 96.Hildebrandt GC, Olkiewicz KM, Corrion L, Clouthier SG, Pierce EM, Liu C, Cooke KR. A role for TNF receptor type II in leukocyte infiltration into the lung during experimental idiopathic pneumonia syndrome. Biol Blood Marrow Transplant 2008;14:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith S, Skerrett SJ, Chi EY, Jonas M, Mohler K, Wilson CB. The locus of tumor necrosis factor-alpha action in lung inflammation. Am J Respir Cell Mol Biol 1998;19:881–891. [DOI] [PubMed] [Google Scholar]
- 98.Nelson S, Bagby GJ, Bainton BG, Wilson LA, Thompson JJ, Summer WR. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis 1989;159:189–194. [DOI] [PubMed] [Google Scholar]
- 99.Ulich TR, Watson LR, Yin SM, Guo KZ, Wang P, Thang H, del Castillo J. The intratracheal administration of endotoxin and cytokines: I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am J Pathol 1991;138:1485–1496. [PMC free article] [PubMed] [Google Scholar]
- 100.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997;90:3204–3213. [PubMed] [Google Scholar]
- 101.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med 1992;175:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000;95:2754–2759. [PubMed] [Google Scholar]
- 103.Cooke KR, Gerbitz A, Crawford JM, Teshima T, Hill GR, Tesolin A, Rossignol DP, Ferrara JL. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest 2001;107:1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fegan C, Poynton CH, Whittaker JA. The gut mucosal barrier in bone marrow transplantation. Bone Marrow Transplant 1990;5:373–377. [PubMed] [Google Scholar]
- 105.Jackson SK, Parton J, Barnes RA, Poynton CH, Fegan C. Effect of IgM-enriched intravenous immunoglobulin (pentaglobin) on endotoxaemia and anti-endotoxin antibodies in bone marrow transplantation. Eur J Clin Invest 1993;23:540–545. [DOI] [PubMed] [Google Scholar]
- 106.Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H, Lehmacher W, Ruckdeschel G, Gleixner B, Riedner C, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood 1990;75:1011–1016. [PubMed] [Google Scholar]
- 107.Hauber HP, Mikkila A, Erich JM, Kroger N, Meyer A, Schoder V, Zander AR, Pforte A. TNFalpha, interleukin-10 and interleukin-18 expression in cells of the bronchoalveolar lavage in patients with pulmonary complications following bone marrow or peripheral stem cell transplantation: a preliminary study. Bone Marrow Transplant 2002;30:485–490. [DOI] [PubMed] [Google Scholar]
- 108.Martin TR, Rubenfeld GD, Ruzinski JT, Goodman RB, Steinberg KP, Leturcq DJ, Moriarty AM, Raghu G, Baughman RP, Hudson LD. Relationship between soluble CD14, lipopolysaccharide binding protein, and the alveolar inflammatory response in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;155:937–944. [DOI] [PubMed] [Google Scholar]
- 109.Hattori K, Hirano T, Miyajima H, Yamakawa N, Tateno M, Oshimi K, Kayagaki N, Yagita H, Okumura K. Differential effects of anti-Fas ligand and anti-tumor necrosis factor alpha antibodies on acute graft-versus-host disease pathologies. Blood 1998;91:4051–4055. [PubMed] [Google Scholar]
- 110.Vallera DA, Taylor PA, Vannice JL, Panoskaltsis-Mortari A, Blazar BR. Interleukin-1 or tumor necrosis factor-alpha antagonists do not inhibit graft-versus-host disease induced across the major histocompatibility barrier in mice. Transplantation 1995;60:1371–1374. [PubMed] [Google Scholar]
- 111.Clark JG, Mandac JB, Dixon AE, Martin PJ, Hackman RC, Madtes DK. Neutralization of tumor necrosis factor-alpha action delays but does not prevent lung injury induced by alloreactive T helper 1 cells. Transplantation 2000;70:39–43. [PubMed] [Google Scholar]
- 112.Cooke KR, Kobzik L, Teshima T, Lowler K, Cluthier S, Ferrara J. A role for Fas-Fas ligand but not perforin mediated cytolysis in the development of experimental idiopathic pneumonia syndrome. Blood 2000;96:768a.10887147 [Google Scholar]
- 113.Baker MB, Altman NH, Podack ER, Levy RB. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med 1996;183:2645–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braun MY, Lowin B, French L, Acha-Orbea H, Tschopp J. Cytotoxic T cells deficient in both functional Fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med 1996;183:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haddad IY, Ingbar D, Panoskaltsis-Mortari A, Blazar B. Activated alveolar macrophage-derived nitric oxide predicts the development of lung damage after marrow transplantation in mice. Chest 1999;116:37S. [DOI] [PubMed] [Google Scholar]
- 116.Blackwell TS, Christman JW, Hagan T, Price P, Edens T, Morris PE, Wolff SN, Goodman SA, Christman BW. Oxidative stress and NF-kappaB activation: norrelation in patients following allogeneic bone marrow transplantation. Antioxid Redox Signal 2000;2:93–102. [DOI] [PubMed] [Google Scholar]
- 117.Cetin T, Arpaci F, Yilmaz MI, Saglam K, Ozturk B, Komurcu S, Gunay M, Ozet A, Akay C, Kilic S, et al. Oxidative stress in patients undergoing high-dose chemotherapy plus peripheral blood stem cell transplantation. Biol Trace Elem Res 2004;97:237–247. [DOI] [PubMed] [Google Scholar]
- 118.Yang S, Porter VA, Cornfield DN, Milla C, Panoskaltsis-Mortari A, Blazar BR, Haddad IY. Effects of oxidant stress on inflammation and survival of iNOS knockout mice after marrow transplantation. Am J Physiol Lung Cell Mol Physiol 2001;281:L922–L930. [DOI] [PubMed] [Google Scholar]
- 119.Abushamaa AM, Sporn TA, Folz RJ. Oxidative stress and inflammation contribute to lung toxicity after a common breast cancer chemotherapy regimen. Am J Physiol Lung Cell Mol Physiol 2002;283:L336–L345. [DOI] [PubMed] [Google Scholar]
- 120.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–157. [DOI] [PubMed] [Google Scholar]
- 121.Ziegler TR, Panoskaltsus-Mortari A, Gu LH, Jonas CR, Farrell CL, Lacey DL, Jones DP, Blazar BR. Regulation of glutathione redox status in lung and liver by conditioning regimens and keratinocyte growth factor in murine allogeneic bone marrow transplantation. Transplantation 2001;72:1354–1362. [DOI] [PubMed] [Google Scholar]
- 122.Evens AM, Mehta J, Gordon LI. Rust and corrosion in hematopoietic stem cell transplantation: the problem of iron and oxidative stress. Bone Marrow Transplant 2004;34:561–571. [DOI] [PubMed] [Google Scholar]
- 123.Qureshi MA, Girgis RE, Dandapantula HK, Abrams J, Soubani AO. Increased exhaled nitric oxide following autologous peripheral hematopoietic stem-cell transplantation: a potential marker of idiopathic pneumonia syndrome. Chest 2004;125:281–287. [DOI] [PubMed] [Google Scholar]
- 124.Hehner SP, Breitkreutz R, Shubinsky G, Unsoeld H, Schulze-Osthoff K, Schmitz ML, Droge W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J Immunol 2000;165:4319–4328. [DOI] [PubMed] [Google Scholar]
- 125.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys 2003;57:1056–1066. [DOI] [PubMed] [Google Scholar]
- 126.Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, Golson ML, Samulski TS, Dewhirst MW, Vujaskovic Z. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer 2005;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest 2002;109:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Borron PJ, Mostaghel EA, Doyle C, Walsh ES, McHeyzer-Williams MG, Wright JR. Pulmonary surfactant proteins A and D directly suppress CD3+/CD4+ cell function: evidence for two shared mechanisms. J Immunol 2002;169:5844–5850. [DOI] [PubMed] [Google Scholar]
- 129.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003;115:13–23. [DOI] [PubMed] [Google Scholar]
- 130.Epaud R, Ikegami M, Whitsett JA, Jobe AH, Weaver TE, Akinbi HT. Surfactant protein B inhibits endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 2003;28:373–378. [DOI] [PubMed] [Google Scholar]
- 131.Augusto LA, Synguelakis M, Johansson J, Pedron T, Girard R, Chaby R. Interaction of pulmonary surfactant protein C with CD14 and lipopolysaccharide. Infect Immun 2003;71:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kennedy M, Phelps D, Ingenito E. Mechanisms of surfactant dysfunction in early acute lung injury. Exp Lung Res 1997;23:171–189. [DOI] [PubMed] [Google Scholar]
- 133.Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels of nitrate and surfactant protein A nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med 2001;163:166–172. [DOI] [PubMed] [Google Scholar]
- 134.Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J 1999;13:1455–1476. [DOI] [PubMed] [Google Scholar]
- 135.Gunther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 1996;153:176–184. [DOI] [PubMed] [Google Scholar]
- 136.Yang S, Milla C, Panoskaltsis-Mortari A, Ingbar DH, Blazar BR, Haddad IY. Human surfactant protein A suppresses T cell-dependent inflammation and attenuates the manifestations of idiopathic pneumonia syndrome in mice. Am J Respir Cell Mol Biol 2001;24:527–536. [DOI] [PubMed] [Google Scholar]
- 137.Yang S, Milla C, Panoskaltsis-Mortari A, Hawgood S, Blazar BR, Haddad IY. Surfactant protein A decreases lung injury and mortality after murine marrow transplantation. Am J Respir Cell Mol Biol 2002;27:297–305. [DOI] [PubMed] [Google Scholar]
- 138.Nakane T, Nakamae H, Kamoi H, Koh H, Takeoka Y, Sakamoto E, Kanashima H, Nakamae M, Ohta K, Terada Y, Koh KR, Yamane T, Hino M. Prognostic value of serum surfactant protein D level prior to transplant for the development of bronchiolitis obliterans syndrome and idiopathic pneumonia syndrome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2008;42:43–49. [DOI] [PubMed] [Google Scholar]
- 139.Heidinger K, Konig IR, Bohnert A, Kleinsteiber A, Hilgendorff A, Gortner L, Ziegler A, Chakraborty T, Bein G. Polymorphisms in the human surfactant protein-d (SFTPD) gene: strong evidence that serum levels of surfactant protein-D (SP-D) are genetically influenced. Immunogenetics 2005;57:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003;58:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spragg RG. The future of surfactant therapy for patients with acute lung injury: new requirements and new surfactants. Biol Neonate 2002;81:20–24. [DOI] [PubMed] [Google Scholar]
- 142.Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 2005;293:470–476. [DOI] [PubMed] [Google Scholar]
- 143.Watanabe T, Kawamura T, Kawamura H, Haga M, Shirai K, Watanabe H, Eguchi S, Abo T. Intermediate TCR cells in mouse lung: their effector function to induce pneumonitis in mice with autoimmune-like graft-versus-host disease. J Immunol 1997;158:5805–5814. [PubMed] [Google Scholar]
- 144.Cooke KR, Krenger W, Hill G, Martin TR, Kobzik L, Brewer J, Simmons R, Crawford JM, van den Brink MR, Ferrara JL. Host reactive donor T cells are associated with lung injury after experimental allogeneic bone marrow transplantation. Blood 1998;92:2571–2580. [PubMed] [Google Scholar]
- 145.Chen W, Chatta GS, Rubin WD, Clark JG, Hackman RC, Madtes DK, Ligitt DH, Kusunoki Y, Martin PJ, Cheever MA. T cells specific for a polymorphic segment of CD45 induce graft-versus-host disease with predominant pulmonary vasculitis. J Immunol 1998;161:909–918. [PubMed] [Google Scholar]
- 146.Burman AC, Banovic T, Kuns RD, Clouston AD, Stanley AC, Morris ES, Rowe V, Bofinger H, Skoczylas R, Raffelt N, et al. IFNgamma differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood 2007;110:1064–1072. [DOI] [PubMed] [Google Scholar]
- 147.Mauermann N, Burian J, von Garnier C, Dirnhofer S, Germano D, Schuett C, Tamm M, Bingisser R, Eriksson U, Hunziker L. Interferon-gamma regulates idiopathic pneumonia syndrome, a Th17+CD4+ T-cell-mediated graft-versus-host disease. Am J Respir Crit Care Med 2008;178:379–388. [DOI] [PubMed] [Google Scholar]
- 148.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro differentiated Th17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathology. Blood 2008;113:1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Massard G, Tongio MM, Wihlm JM, Morand G. The dendritic cell lineage: a ubiquitous antigen-presenting organization. Ann Thorac Surg 1996;61:252–258. [DOI] [PubMed] [Google Scholar]
- 151.Armstrong LR, Christensen PJ, Paine R III, Chen GH, McDonald RA, Lim TK, Toews GB. Regulation of the immunostimulatory activity of rat pulmonary interstitial dendritic cells by cell-cell interactions and cytokines. Am J Respir Cell Mol Biol 1994;11:682–691. [DOI] [PubMed] [Google Scholar]
- 152.Pollard AM, Lipscomb MF. Characterization of murine lung dendritic cells: similarities to langerhans cells and thymic dendritic cells. J Exp Med 1990;172:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Masten BJ, Yates JL, Pollard Koga AM, Lipscomb MF. Characterization of accessory molecules in murine lung dendritic cell function: roles for CD80, CD86, CD54, and CD40L. Am J Respir Cell Mol Biol 1997;16:335–342. [DOI] [PubMed] [Google Scholar]
- 154.Christensen PJ, Armstrong LR, Fak JJ, Chen GH, McDonald RA, Toews GB, Paine R III. Regulation of rat pulmonary dendritic cell immunostimulatory activity by alveolar epithelial cell-derived granulocyte macrophage colony-stimulating factor. Am J Respir Cell Mol Biol 1995;13:426–433. [DOI] [PubMed] [Google Scholar]
- 155.van Haarst JM, de Wit HJ, Drexhage HA, Hoogsteden HC. Distribution and immunophenotype of mononuclear phagocytes and dendritic cells in the human lung. Am J Respir Cell Mol Biol 1994;10:487–492. [DOI] [PubMed] [Google Scholar]
- 156.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function (s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 1993;177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dupuis M, McDonald DM. Dendritic-cell regulation of lung immunity. Am J Respir Cell Mol Biol 1997;17:284–286. [DOI] [PubMed] [Google Scholar]
- 158.Yousem SA, Ray L, Paradis IL, Dauber JA, Griffith BP. Potential role of dendritic cells in bronchiolitis obliterans in heart-lung transplantation. Ann Thorac Surg 1990;49:424–428. [DOI] [PubMed] [Google Scholar]
- 159.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol 1994;153:256–261. [PubMed] [Google Scholar]
- 160.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, Brewer JP, Ferrara JL. Hyporesponsiveness of donor cells to lipopolysaccharide stimulation reduces the severity of experimental idiopathic pneumonia syndrome: potential role for a gut-lung axis of inflammation. J Immunol 2000;165:6612–6619. [DOI] [PubMed] [Google Scholar]
- 161.Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res 2002;8:441–448. [DOI] [PubMed] [Google Scholar]
- 162.Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, et al. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. Am J Physiol Lung Cell Mol Physiol 2001;280:L58–L68. [DOI] [PubMed] [Google Scholar]
- 163.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med 1998;338:436–445. [DOI] [PubMed] [Google Scholar]
- 164.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International Union of Pharmacology: XXII. Nomenclature for chemokine receptors. Pharmacol Rev 2000;52:145–176. [PubMed] [Google Scholar]
- 165.Hildebrandt GC, Duffner UA, Olkiewicz KM, Corrion LA, Willmarth NE, Williams DL, Clouthier SG, Hogaboam CM, Reddy PR, Moore BB, et al. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 2004;103:2417–2426. [DOI] [PubMed] [Google Scholar]
- 166.Panoskaltsis-Mortari A, Hermanson JR, Taras E, Wangensteen OD, Charo IF, Rollins BJ, Blazar BR. Post-bmt lung injury occurs independently of the expression of CCL2 or its receptor, CCR2, on host cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L284–L292. [DOI] [PubMed] [Google Scholar]
- 167.Panoskaltsis-Mortari A, Strieter RM, Hermanson JR, Fegeding KV, Murphy WJ, Farrell CL, Lacey DL, Blazar BR. Induction of monocyte- and T-cell-attracting chemokines in the lung during the generation of idiopathic pneumonia syndrome following allogeneic murine bone marrow transplantation. Blood 2000;96:834–839. [PubMed] [Google Scholar]
- 168.Panoskaltsis-Mortari A, Hermanson JR, Taras E, Wangensteen OD, Serody JS, Blazar BR. Acceleration of idiopathic pneumonia syndrome (IPS) in the absence of donor MIP-1 alpha (CCL3) after allogeneic BMT in mice. Blood 2003;101:3714–3721. [DOI] [PubMed] [Google Scholar]
- 169.Hildebrandt GC, Olkiewicz KM, Choi S, Corrion LA, Clouthier SG, Liu C, Serody JS, Cooke KR. Donor T-cell production of RANTES significantly contributes to the development of idiopathic pneumonia syndrome after allogeneic stem cell transplantation. Blood 2005;105:2249–2257. [DOI] [PubMed] [Google Scholar]
- 170.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, Kirby SL, Luster AD, McKinnon K, Blazar BR, Serody JS. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol 2004;173:845–854. [DOI] [PubMed] [Google Scholar]
- 171.Panoskaltsis-Mortari A, Hermanson JR, Haddad IY, Wangensteen OD, Blazar BR. Intercellular adhesion molecule-1 (ICAM-1, CD54) deficiency segregates the unique pathophysiological requirements for generating idiopathic pneumonia syndrome (IPS) versus graft-versus-host disease following allogeneic murine bone marrow transplantation. Biol Blood Marrow Transplant 2001;7:368–377. [DOI] [PubMed] [Google Scholar]
- 172.Gerbitz A, Ewing P, Olkiewicz K, Willmarth NE, Williams D, Hildebrandt G, Wilke A, Liu C, Eissner G, Andreesen R, et al. A role for CD54 (intercellular adhesion molecule-1) in leukocyte recruitment to the lung during the development of experimental idiopathic pneumonia syndrome. Transplantation 2005;79:536–542. [DOI] [PubMed] [Google Scholar]
- 173.Thomas C, Le Deist F, Cavazzana-Calvo M, Benkerrou M, Haddad E, Blanche S, Hartmann W, Friedrich W, Fischer A. Results of allogeneic bone marrow transplantation in patients with leukocyte adhesion deficiency. Blood 1995;86:1629–1635. [PubMed] [Google Scholar]
- 174.Cavazzana-Calvo M, Jabado N, Bordigoni P, Michel G, Haddad E, Mechinaud F, Landman-Parker J, Leblanc T, Plouvier E, Baruchel A, et al. In vivo infusion of anti-LFA-1 and anti-CD2 antibodies prevents graft failure after HLA partially incompatible bone marrow transplantation in children with high risk acute lymphoblastic leukaemia. Leuk Lymphoma 1997;28:103–112. [DOI] [PubMed] [Google Scholar]
- 175.Madtes DK, Crawford SW. Lung injuries associated with graft-versus-host reactions. In: Ferrara JLM, Deeg HJ, Burakoff SL, editors. Graft-vs.-host disease, 2nd ed. New York: Marcel Dekker; 1997. pp. 425–446.
- 176.Janin A, Deschaumes C, Daneshpouy M, Estaquier J, Micic-Polianski J, Rajagopalan-Levasseur P, Akarid K, Mounier N, Gluckman E, Socie G, et al. CD95 engagement induces disseminated endothelial cell apoptosis in vivo: immunopathologic implications. Blood 2002;99:2940–2947. [DOI] [PubMed] [Google Scholar]
- 177.Ratajczak P, Desveaux A, Socie G, Janin A. Alveolar hemorrhage and acute graft-versus-host disease. Biol Blood Marrow Transplant 2007;13:1244–1245. [DOI] [PubMed] [Google Scholar]
- 178.Cooke KR, Jannin A, Ho V. The contribution of endothelial activation and injury to end-organ toxicity following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2008;14:23–32. [DOI] [PubMed] [Google Scholar]
- 179.Finch PW, Cunha GR, Rubin JS, Wong J, Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn 1995;203:223–240. [DOI] [PubMed] [Google Scholar]
- 180.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest 1993;92:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ulich TR, Yi ES, Longmuir K, Yin S, Biltz R, Morris CF, Housley RM, Pierce GF. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest 1994;93:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, Yin S, Hill DC, Wiemann B, Starnes CO, et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res 1998;58:933–939. [PubMed] [Google Scholar]
- 183.Panoskaltsis-Mortari A, Lacey DL, Vallera DA, Blazar BR. Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood 1998;92:3960–3967. [PubMed] [Google Scholar]
- 184.Krijanovski OI, Hill GR, Cooke KR, Teshima T, Crawford JM, Brinson YS, Ferrara JL. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood 1999;94:825–831. [PubMed] [Google Scholar]
- 185.Guo J, Yi ES, Havill AM, Sarosi I, Whitcomb L, Yin S, Middleton SC, Piguet P, Ulich TR. Intravenous keratinocyte growth factor protects against experimental pulmonary injury. Am J Physiol 1998;275:L800–L805. [DOI] [PubMed] [Google Scholar]
- 186.Yi ES, Williams ST, Lee H, Malicki DM, Chin EM, Yin S, Tarpley J, Ulich TR. Keratinocyte growth factor ameliorates radiation- and bleomycin-induced lung injury and mortality. Am J Pathol 1996;149:1963–1970. [PMC free article] [PubMed] [Google Scholar]
- 187.Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol 1998;186:90–98. [DOI] [PubMed] [Google Scholar]
- 188.Takeoka M, Ward WF, Pollack H, Kamp DW, Panos RJ. KGF facilitates repair of radiation-induced DNA damage in alveolar epithelial cells. Am J Physiol 1997;272:L1174–L1180. [DOI] [PubMed] [Google Scholar]
- 189.Wu KI, Pollack N, Panos RJ, Sporn PH, Kamp DW. Keratinocyte growth factor promotes alveolar epithelial cell DNA repair after H2O2 exposure. Am J Physiol 1998;275:L780–L787. [DOI] [PubMed] [Google Scholar]
- 190.Sugahara K, Rubin JS, Mason RJ, Aronsen EL, Shannon JM. Keratinocyte growth factor increases mRNAs for SP-A and SP-B in adult rat alveolar type II cells in culture. Am J Physiol 1995;269:L344–L350. [DOI] [PubMed] [Google Scholar]
- 191.Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L1146–L1158. [DOI] [PubMed] [Google Scholar]
- 192.Borok Z, Danto SI, Dimen LL, Zhang XL, Lubman RL. Na (+)-K (+)-ATPase expression in alveolar epithelial cells: upregulation of active ion transport by KGF. Am J Physiol 1998;274:L149–L158. [DOI] [PubMed] [Google Scholar]
- 193.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev 1998;9:153–165. [DOI] [PubMed] [Google Scholar]
- 194.Haddad IY, Milla C, Yang S, Panoskaltsis-Mortari A, Hawgood S, Lacey DL, Blazar BR. Surfactant protein A is a required mediator of keratinocyte growth factor after experimental marrow transplantation. Am J Physiol Lung Cell Mol Physiol 2003;285:L602–L610. [DOI] [PubMed] [Google Scholar]
- 195.Haddad IY, Panoskaltsis-Mortari A, Ingbar DH, Resnik ER, Yang S, Farrell CL, Lacey DL, Cornfield DN, Blazar BR. Interactions of keratinocyte growth factor with a nitrating species after marrow transplantation in mice. Am J Physiol 1999;277:L391–L400. [DOI] [PubMed] [Google Scholar]
- 196.Yang S, Panoskaltsis-Mortari A, Ingbar DH, Matalon S, Zhu S, Resnik ER, Farrell CL, Lacey DL, Blazar BR, Haddad IY. Cyclophosphamide prevents systemic keratinocyte growth factor-induced up-regulation of surfactant protein A after allogeneic transplant in mice. Am J Respir Crit Care Med 2000;162:1884–1890. [DOI] [PubMed] [Google Scholar]
- 197.Panoskaltsis-Mortari A, Taylor PA, Rubin JS, Uren A, Welniak LA, Murphy WJ, Farrell CL, Lacey DL, Blazar BR. Keratinocyte growth factor facilitates alloengraftment and ameliorates graft-versus-host disease in mice by a mechanism independent of repair of conditioning-induced tissue injury. Blood 2000;96:4350–4356. [PubMed] [Google Scholar]
- 198.Yanik GA, Ho VT, Levine JE, White ES, Braun T, Antin JH, Whitfield J, Custer J, Jones D, Ferrara JL, et al. The impact of soluble tumor necrosis factor receptor etanercept on the treatment of idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Blood 2008;112:3073–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Metcalf JP, Rennard SI, Reed EC, Haire WD, Sisson JH, Walter T, Robbins RA. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center bone marrow transplant group. Am J Med 1994;96:327–334. [DOI] [PubMed] [Google Scholar]
- 200.DiCarlo JV, Alexander SR, Agarwal R, Schiffman JD. Continuous veno-venous hemofiltration may improve survival from acute respiratory distress syndrome after bone marrow transplantation or chemotherapy. J Pediatr Hematol Oncol 2003;25:801–805. [DOI] [PubMed] [Google Scholar]
- 201.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards CK III, Schuchman EH, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med 1997;186:1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001;293:293–297. [DOI] [PubMed] [Google Scholar]
- 203.Clouthier SG, Cooke KR, Teshima T, Lowler KP, Liu C, Connolly K, Ferrara JL. Repifermin (keratinocyte growth factor-2) reduces the severity of graft-versus-host disease while preserving a graft-versus-leukemia effect. Biol Blood Marrow Transplant 2003;9:592–603. [DOI] [PubMed] [Google Scholar]
- 204.Ho VT, Weller E, Lee SJ, Alyea EP, Antin JH, Soiffer RJ. Prognostic factors for early severe pulmonary complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2001;7:223–229. [DOI] [PubMed] [Google Scholar]
- 205.Savani BN, Montero A, Srinivasan R, Singh A, Shenoy A, Mielke S, Rezvani K, Karimpour S, Childs R, Barrett AJ. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biol Blood Marrow Transplant 2006;12:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chien JW, Zhao LP, Hansen JA, Fan WH, Parimon T, Clark JG. Genetic variation in bactericidal/permeability-increasing protein influences the risk of developing rapid airflow decline after hematopoietic cell transplantation. Blood 2006;107:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hildebrandt GC, Granell M, Urbano-Ispizua A, Wolff D, Hertenstein B, Greinix HT, Brenmoehl J, Schulz C, Dickinson AM, Hahn J, et al. Recipient NOD2/CARD15 variants: a novel independent risk factor for the development of bronchiolitis obliterans after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2008;14:67–74. [DOI] [PubMed] [Google Scholar]
- 208.Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation workshop. Proc Am Thorac Soc 2006;3:193–207. [DOI] [PubMed] [Google Scholar]
- 209.Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc 2008;5:637–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Movsas B, Raffin TA, Epstein AH, Link CJ Jr. Pulmonary radiation injury. Chest 1997;111:1061–1076. [DOI] [PubMed] [Google Scholar]
- 211.Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics 2000;20:1245–1259. [DOI] [PubMed] [Google Scholar]
- 212.Litam JP, Dail DH, Spitzer G, Vellekoop L, Verma DS, Zander AR, Dicke KA. Early pulmonary toxicity after administration of high-dose bcnu. Cancer Treat Rep 1981;65:39–44. [PubMed] [Google Scholar]
- 213.Kunitomi A, Arima N, Ishikawa T. Epstein-Barr virus-associated post-transplant lymphoproliferative disorders presented as interstitial pneumonia; successful recovery with rituximab. Haematologica 2007;92:e49–e52. [DOI] [PubMed] [Google Scholar]
- 214.Hou HA, Yao M, Tang JL, Chen YK, Ko BS, Huang SY, Tien HF, Chang HH, Lu MY, Lin TT, Lin KH, Hsiao CH, Lin CW, Chen YC. Poor outcome in post transplant lymphoproliferative disorder with pulmonary involvement after allogeneic hematopoietic SCT: 13 years' experience in a single institute. Bone Marrow Transplant 2008;43:315–321. [DOI] [PubMed] [Google Scholar]
- 215.Akhtari M, Langston AA, Waller EK, Gal AA. Eosinophilic pulmonary syndrome as a manifestation of GVHD following hematopoietic stem cell transplantation in three patients. Bone Marrow Transplant 2008;43:155–158. [DOI] [PubMed] [Google Scholar]
- 216.Gross TG, Hoge FJ, Jackson JD, Sammut PH, Warkentin PI. Fatal eosinophilic disease following autologous bone marrow transplantation. Bone Marrow Transplant 1994;14:333–337. [PubMed] [Google Scholar]
- 217.Richard C, Calavia J, Loyola I, Baro J, Cuadrado MA, Gomez-Casares MT, Zurbano F, Ondiviela R, Zubizarreta A. (Chronic eosinophilic pneumonia in a patient treated with allogenic bone marrow transplantation). Med Clin (Barc) 1994;102:462–464. [PubMed] [Google Scholar]
- 218.Sharma S, Nadrous HF, Peters SG, Tefferi A, Litzow MR, Aubry MC, Afessa B. Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest 2005;128:1385–1392. [DOI] [PubMed] [Google Scholar]
- 219.Tomonari A, Shirafuji N, Iseki T, Ooi J, Nagayama H, Masunaga A, Tojo A, Tani K, Asano S. Acquired pulmonary alveolar proteinosis after umbilical cord blood transplantation for acute myeloid leukemia. Am J Hematol 2002;70:154–157. [DOI] [PubMed] [Google Scholar]
- 220.Nishinakamura R, Nakayama N, Hirabayashi Y, Inoue T, Aud D, McNeil T, Azuma S, Yoshida S, Toyoda Y, Arai K, et al. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity 1995;2:211–222. [DOI] [PubMed] [Google Scholar]
- 221.Troussard X, Bernaudin JF, Cordonnier C, Fleury J, Payen D, Briere J, Vernant JP. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax 1984;39:956–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hackman RC, Madtes DK, Petersen FB, Clark JG. Pulmonary venoocclusive disease following bone marrow transplantation. Transplantation 1989;47:989–992. [DOI] [PubMed] [Google Scholar]
- 223.Williams LM, Fussell S, Veith RW, Nelson S, Mason CM. Pulmonary veno-occlusive disease in an adult following bone marrow transplantation: case report and review of the literature. Chest 1996;109:1388–1391. [DOI] [PubMed] [Google Scholar]
- 224.Ganguly S, Carrum G, Nizzi F, Heslop HE, Popat U. Transfusion-related acute lung injury (TRALI) following allogeneic stem cell transplant for acute myeloid leukemia. Am J Hematol 2004;75:48–51. [DOI] [PubMed] [Google Scholar]
- 225.Gulbahce HE, Pambuccian SE, Jessurun J, Woodard P, Steiner ME, Manivel JC, Hite S, Ramsay NK, Baker KS. Pulmonary nodular lesions in bone marrow transplant recipients: impact of histologic diagnosis on patient management and prognosis. Am J Clin Pathol 2004;121:205–210. [DOI] [PubMed] [Google Scholar]
- 226.Steward CG, Pellier I, Mahajan A, Ashworth MT, Stuart AG, Fasth A, Lang D, Fischer A, Friedrich W, Schulz AS. Severe pulmonary hypertension: a frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br J Haematol 2004;124:63–71. [DOI] [PubMed] [Google Scholar]
- 227.Grigg A, Buchanan M, Whitford H. Late-onset pulmonary arterial hypertension in association with graft-versus-host disease after allogeneic stem-cell transplantation. Am J Hematol 2005;80:38–42. [DOI] [PubMed] [Google Scholar]
- 228.Limsuwan A, Pakakasama S, Rochanawutanon M, Hong-eng S. Pulmonary arterial hypertension after childhood cancer therapy and bone marrow transplantation. Cardiology 2006;105:188–194. [DOI] [PubMed] [Google Scholar]
- 229.Shankar S, Choi JK, Dermody TS, Head DR, Bunin N, Iannone R. Pulmonary hypertension complicating bone marrow transplantation for idiopathic myelofibrosis. J Pediatr Hematol Oncol 2004;26:393–397. [DOI] [PubMed] [Google Scholar]
- 230.Uderzo C, Marraro G, Riva A, Bonanomi E, Vaj PL, Marchi PF, Locasciulli A, Masera G. Pulmonary thromboembolism in leukaemic children undergoing bone marrow transplantation. Bone Marrow Transplant 1993;11:201–203. [PubMed] [Google Scholar]