Incomplete or inadequate bowel cleansing continues to hamper successful colonoscopy, and frustrate physicians and patients. Although several bowel preparations initially demonstrated promising results, side effects and factors affecting compliance led to their diminishing and, in some cases, complete withdrawal from use. Only a small number of studies examining the use of newer regimens and agents have been reported; although safe and well tolerated, however, their efficacy remains unclear. This study investigated cleansing efficacy, patient tolerability and the safety of different regimens and combinations of cleansing agents in colonoscopies performed in the morning versus the afternoon.
Keywords: Bowel preparation, Colonoscopy
Abstract
BACKGROUND:
The ideal bowel cleansing regimen for colonoscopy has yet to be determined.
OBJECTIVE:
To compare the cleansing efficacy, and patient tolerability and safety of four bowel preparation regimens.
METHODS:
A total of 834 patients undergoing outpatient colonoscopy were randomly assigned to one of four regimens: 4 L polyethylene glycol (PEG); 2 L PEG + 20 mg bisacodyl; 90 mL of sodium phosphate (NaP); or two sachets of a commercially available bowel cleansing solution (PSMC) + 300 mL of magnesium citrate (M). The primary outcome measure was cleansing efficacy, which was scored by blinded endoscopists using the Ottawa Bowel Preparation Scale. Secondary outcome measures were bowel preparation quality according to time of colonoscopy, and patient tolerability and safety.
RESULTS:
The mean total cleansing score was significantly worse in the NaP group compared with the other three groups (P<0.0001). The mean cleansing scores were worse in patients who underwent morning versus afternoon colonoscopy, a finding that was consistent in all four groups. PSMC + M was the best tolerated regimen. No clinically significant mean changes in creatinine or electrolyte levels were identified, although a significantly higher proportion of patients in the NaP group developed hypokelemia (P<0.0001).
CONCLUSIONS:
2 L PEG + 20 mg bisacodyl, or PSMC + M was as efficacious as 4 L PEG and superior to NaP for bowel cleansing. A short interval between the completion of bowel preparation and the start of colonoscopy (ie, ‘runway time’), irrespective of bowel preparation regimen, appeared to be a more important predictor of bowel cleanliness than the cathartic agents used.
Abstract
HISTORIQUE :
Le régime de nettoyage intestinal idéal en vue de la coloscopie n’a pas encore été déterminé.
OBJECTIF :
Comparer l’efficacité du nettoyage, la tolérabilité des patients et l’innocuité de quatre régimes de préparation intestinale.
MÉTHODOLOGIE :
Au total, 834 patients qui ont subi une coloscopie en consultations externes ont été répartis au hasard entre l’un des quatre régimes suivants : 4 L de polyéthylène glycol (PEG), 2 L de PEG + 20 mg de bisacodyl, 90 mL de phosphate de sodium (NaP) ou deux sachets d’une solution de nettoyage intestinal (PSMC) offerte sur le marché + 300 mL de citrate de magnésium (M). La mesure d’issue primaire était l’efficacité du nettoyage, notée en aveugle par des endoscopistes au moyen de l’échelle de préparation intestinale d’Ottawa. Les mesures d’issue secondaire étaient la qualité de la préparation intestinale d’après le moment de la coloscopie, la tolérabilité des patients et l’innocuité.
RÉSULTATS :
L’indice de nettoyage total moyen était beaucoup moins bon dans le groupe de NaP que dans les trois autres groupes (P<0,0001). Les indices de nettoyage moyens étaient pires chez les patients qui subissaient la coloscopie le matin plutôt que l’après-midi, une observation uniforme dans les quatre groupes. Le régime le mieux toléré était le PSMC + M. Les chercheurs n’ont remarqué aucun changement moyen significatif sur le plan clinique des taux de créatinine ou d’électrolytes, même si une proportion considérablement plus élevée de patients du groupe de NaP a présenté une hypokaliémie (P<0,0001).
CONCLUSIONS :
2 L de PEG + 20 mg de bisacodyl ou le PSMC + M étaient aussi efficaces que 4 L de PEG et supérieurs au NaP pour le nettoyage intestinal. Un court intervalle entre la fin de la préparation intestinale et le début de la coloscopie (c’est-à-dire le « temps d’activité »), quelle que soit la préparation intestinale utilisée, semblait être un prédicteur plus important de la propreté intestinale que le type d’agents cathartiques utilisés.
Adequate bowel preparation is essential for successful colonoscopy. Unfortunately, despite advances in technology and pharmacology, inadequate bowel cleansing outcomes continue to frustrate physicians and patients. Suboptimal bowel preparation not only prolongs procedure time and reduces completion rate, but also increases the likelihood of lesions being missed, especially those in the right colon (1).
For years, polyethylene glycol (PEG) was regarded as the ‘gold standard’. However, the large volume of the PEG preparation (4 L), its salty taste and sulphur smell frequently reduce patient acceptance and compliance, thus resulting in suboptimal bowel preparation (2,3). Given its low volume and good efficacy, sodium phosphate (NaP) was popular among clinicians and patients in the past (4–6); however, NaP use can lead to significant fluid and electrolyte shifts, and acute phosphate nephropathy (7–11).
Other bowel cleansing regimens that have been examined include sodium picosulphate plus magnesium oxide-containing preparations (PSMC, Pico-Salax [Ferring Pharmaceuticals Inc, Canada]) and low-volume PEG, alone or in combination with an adjunct such as bisacodyl (B) and magnesium citrate (M). Although these regimens are reported to be safe and well-tolerated by patients (12), their cleansing efficacy remains uncertain because, to date, only a small number of studies have been published (13–15). One recent randomized controlled trial (16) reported that PSMC + B was superior to either PSMC alone or NaP, specifically in the right colon.
A meta-analysis published in 2006 (6) examined 18 studies comparing PEG (n=1629) to NaP (n=1855), three studies comparing PEG (n=104) with PSMC (n=112) and three studies comparing NaP (n=369) with PSMC (n=381). It concluded that NaP was more effective than either high-volume PEG or PSMC. It also concluded that PEG and PSMC were similar in efficacy. However, a 2007 systematic review of 24 trials comparing PEG (n=2107) with NaP (n=1984) (12) concluded that there was no significant difference in efficacy between these two regimens (OR 1.0 [95% CI 0.67 to 1.5]). There are little data available on how low-volume PEG or PSMC compare with NaP (17,18).
Furthermore, factors other than cathartic agents themselves have been shown to influence bowel preparation quality. The interval at which the cleansing agents are taken has been shown to affect this outcome (19–21). For example, two doses of NaP taken 12 h or 24 h apart result in better bowel preparations than two doses taken 6 h apart (19). Data have shown better quality bowel preparation to follow from morning (AM), rather than afternoon (PM), colonoscopies (20,22,23). This association appeared to be inversely related to the duration of the interval between the completion of bowel preparation and the start of colonoscopy (also referrred to as ‘runway time’), as described by Siddiqui et al (24) and others (25,26). The optimal ‘runway time’ appeared to be 4 h to 8 h.
The objectives of the present study were to compare both AM and PM colonoscopies for the following outcomes: cleansing efficacy; patient tolerability; and the safety of 4 L PEG, 2L PEG + B, NaP and PSMC + M.
METHODS
Protocol
The present prospective, randomized, endoscopist-blinded study was conducted at the University of Alberta Hospital (Edmonton, Alberta). The study was approved by the Health Research Ethics Board of the University of Alberta and registered with clinicaltrials.gov (NCT00831064). Informed consent was obtained from each patient before enrollment. Ambulatory gastroenterology clinic patients between 18 and 75 years of age who underwent elective outpatient colonoscopy were recruited for participation. Patients with the following conditions were excluded: renal insufficiency or abnormal creatinine clearance (glomerular filtration rate <59 mL/L); congestive heart failure; recent acute coronary syndrome or unstable angina; liver cirrhosis or ascites; chronic furosemide therapy; previous colorectal resection; and known or suspected bowel obstruction, megacolon or ileus.
Assignment
Participants were randomly assigned to receive one of four bowel cleansing regimens before colonoscopy: group 1 received 4 L PEG (4L PEG); group 2 received 2 L PEG + 20 mg of B (2 L PEG+B); group 3 received two doses of 45 mL NaP 24 h apart; and group 4 received PSMC + 300 mL of M (PSMC+M). In January 2009, NaP was voluntarily withdrawn by the manufacturers in Canada; therefore, allocation to this arm was terminated. Patients who had previously been randomly assigned to this arm were randomly re-assigned to one of the other groups.
Participants were instructed to ingest a clear fluid diet on the day before colonoscopy; they were also given explicit instruction to hydrate liberally with water or a clear electrolyte replacement solution until 2 h before the procedure. Written cleansing procedure instruction sheets were given to all participants.
Participants who were scheduled to undergo colonoscopy between 08:00 and 12:00 (AM colonoscopy) and between 12:30 and 17:00 (PM colonoscopy) took their preparations according to the schedules in Table 1. The schedules were designed in such a way so that they were convenient for participants to follow, and the interval between the completion of bowel preparation and the start of colonoscopy were as similar as possible for the AM and PM groups.
TABLE 1.
Bowel cleansing schedules, according to group, for morning (AM) and afternoon (PM) colonoscopy
| Group 1 (AM) 4L PEG |
4 L PEG over 4 h starting at 18:00 on the day before colonoscopy |
| Group 2 (AM) 2L PEG+B |
20 mg B at 16:00 + 2 L PEG over 2 h starting at 20:00 on the day before colonoscopy |
| Group 3 (AM) NaP |
45 mL NaP at 20:00 two days before colonoscopy + 45 mL NaP at 20:00 on the day before colonoscopy |
| Group 4 (AM) PSMC+M |
One sachet of PSMC at 08:00 and at 14:00 + 300 mL M at 20:00 on the day before colonoscopy |
| Group1 (PM) 4L PEG |
2L PEG at 20:00 on the day before colonoscopy + 2L PEG at 06:00 on the day of colonoscopy |
| Group 2 (PM) 2L PEG+B |
20 mg B at 20:00 on the day before colonoscopy + 2L PEG at 06:00 on the day of colonoscopy |
| Group 3 (PM) NaP |
45 mL NaP at 06:00 on the day before colonoscopy + 45 mL NaP at 06:00 on the day of colonoscopy |
| Group 4 (PM) PSMC+M |
One sachet of PSMC at 18:00 and at 22:00 on the day before colonoscopy + 300 mL M at 06:00 on the day of colonoscopy |
B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
Masking
Eight experienced endoscopists participated in the present study. Randomization of the bowel preparation allocation was conducted using blocks of eight, and stratified according to AM and PM colonoscopy using a computer-generated table of random numbers. The concealment of allocation was maintained through the use of consecutively numbered, sealed, opaque envelopes.
Analysis
The main hypothesis of the present study was that one of the four commonly used bowel cleansing preparations was superior with respect to cleansing efficacy. The primary outcome measure was the quality of bowel preparation, which was assessed using the total Ottawa Bowel Preparation Scale (OBPS). Each endoscopist measured the quality of bowel preparation using the OBPS, which has been shown to be a valid and reliable tool for assessing bowel cleansing (27). It uses ratings from 0 to 4 (0 = excellent to 4 = inadequate) for assessment of the right, middle and rectosigmoid colon, and includes a separate score for overall fluid (0 to 2). These four individual scores are added, and the total score of between 0 and 14 provides a global assessment of bowel preparation quality. An excellent bowel preparation is judged to have a total score of 0 to 1, while a good bowel preparation has a score of 2 to 4 (27). To ensure that similar criteria were used to rate bowel preparation quality, an experienced clinician independently trained all endoscopists in advance of the present trial.
The secondary outcomes assessed were cleanliness score for individual OBPS components (right, middle, and rectosigomid colon and fluid level); differences within bowel preparation groups according to colonoscopy timing; patient tolerance of preparations, assessed by a questionnaire that included questions about symptom severity (nausea, vomiting, chest pain, and abdominal cramps or distension); ability to complete bowel preparation; number of hours slept the night before colonoscopy and willingness to repeat the same bowel preparation; and safety of bowel preparation, as assessed by changes in serum electrolyte (sodium, potassium, calcium and phosphate) and creatinine levels before and after the bowel preparation, as well as the number of patients with ischemic colitis – a recently reported rare complication of B (28,29). Patient tolerance was assessed by categorical outcomes on symptoms and ability to complete the preparation (mild to moderate, or severe to intolerable), willingness to repeat the same preparation (yes or no), and by 7-point Likert scales on other outcomes.
Sample size calculation and data analyses
A 2-point difference between the mean scores for bowel preparation on the OBPS was considered to be the minimal clinically significant difference. A sample size of 732 was estimated to be necessary to yield 80% power at a two-sided alpha of 0.05 to detect this difference. To allow for a 15% dropout rate and incomplete colonoscopy, the final sample size was calculated to be 840.
The mean total OBPS scores and individual OBPS components were analyzed as a continuous variable using one-way ANOVA tests with correction for multiple comparisons. Differences in bowel preparation according to time of colonoscopy (AM or PM) and effects of sex and age on preparation quality were assessed post hoc. A separate post hoc analysis was also performed using χ2 analysis to determine the proportion of patients in each group with total OBPS scores of greater than 5.
Tolerability was analyzed using the Mantel-Haenszel test. All other variables were described as mean ± SD, and compared using one-way ANOVA, or described as counts and percentages. Regression analyses were used to determine possible interactions among OBPS, age and sex.
RESULTS
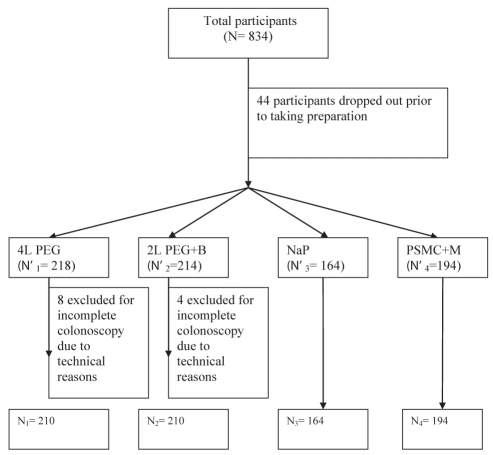
A total of 834 patients were enrolled in the present study between September 2007 and July 2009 (Figure 1). There were no significant differences among the study groups in baseline characteristics (Table 2). Fifty-six patients were excluded from the primary analyses: 44 withdrew before undergoing the assigned cleansing regimens, and 12 due to technical difficulties preventing the completion of the OBPS. Four patients underwent incomplete colonoscopy due to poor bowel preparation (group 1: n=1, and group 2: n=3) and were assigned the maximum OBPS score of 14. One hundred and fifteen patients were excluded from tolerability analyses due to missing questionnaire data, while 329 patients were excluded from safety analyses due to missing postcolonoscopy electrolyte and creatinine data. The missing data were equally divided among the four groups.
Figure 1).
Consolidated Standards of Reporting Trials study flow chart. B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
TABLE 2.
Baseline characteristics according to preparation group*
|
Preparation group |
P | ||||
|---|---|---|---|---|---|
| 4 L PEG | 2 L PEG+B | NaP | PSMC+M | ||
| n | 218 | 214 | 164 | 194 | – |
| Age, years | 49.8 | 50.8 | 49.6 | 48.4 | 0.368 |
| Male, % | 41.7 | 35.2 | 39.8 | 40.7 | 0.551 |
| Morning colonoscopy, % | 63.8 | 63.6 | 61.0 | 62.4 | 0.946 |
Morning and afternoon colonoscopy groups combined. B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
Cleansing efficacy
Colonoscopy cleansing data were obtained for 778 patients. The mean total score was significantly higher in the NaP group than in the other three groups (Table 3), although the mean differences between the groups did not reach 2, which was predetermined to be the minimal clinically significant difference. The NaP group was the only group with a total mean OBPS score >4, which represented a less than ‘good’ bowel preparation (24). In addition, the NaP group also had the highest proportion of patients (29%) with total scores >5 compared with the other groups: 4 L PEG (19%), 2 L PEG+B (17%) and PSMC (15%) (P=0.004). The mean total scores were significantly higher for the AM compared with the PM colonoscopies, a finding that was consistent across all four groups (Table 3), even after adjusting for sex and age (P=0.65).
TABLE 3.
Mean bowel Ottawa Bowel Preparation Scale cleansing scores according to colonoscopy preparation group
|
Morning and afternoon colonoscopy groups combined | |||||
|---|---|---|---|---|---|
|
Preparation group |
P* | ||||
| 4L PEG (n=210) | 2L PEG+B (n=210) | NaP (n=164) | PSMC+M (n=194) | ||
| Right colon | 1.35 | 1.10 | 2.04 | 1.37 | <0.0001† |
| Middle colon | 0.89 | 0.82 | 1.24 | 0.88 | 0.001‡ |
| Rectosigmoid colon | 0.96 | 0.86 | 1.13 | 0.71 | 0.007§ |
| Overall fluid | 0.62 | 0.66 | 0.46 | 0.54 | 0.002¶ |
| Total score (95% CI) | 3.72 (3.29– 4.14) | 3.36 (2.99–3.73) | 4.83 (4.35–5.32) | 3.46 (3.08–3.84) | <0.0001** |
Corrected for multiple comparisons;
Significant difference between NaP and 4 L PEG, between NaP and 2 L PEG+B, and between NaP and PSMC;
Significant difference between NaP and 4L PEG, between NaP and 2 L PEG+B, and between NaP and PSMC.
Significant difference between NaP and PSMC;
Significant difference between NaP and 2 L PEG+B;
Significant difference between NaP and 4L PEG, between NaP and 2 L PEG+B, and between NaP and PSMC. B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
The right colon was consistently more difficult to clean in all groups, especially when the colonoscopy was performed in the morning (Table 3). Interestingly, differences in total OBPS scores and scores for various colonic segments between groups were small and not statistically significant when colonoscopies were performed in the PM (Table 3).
Patient tolerance of preparations
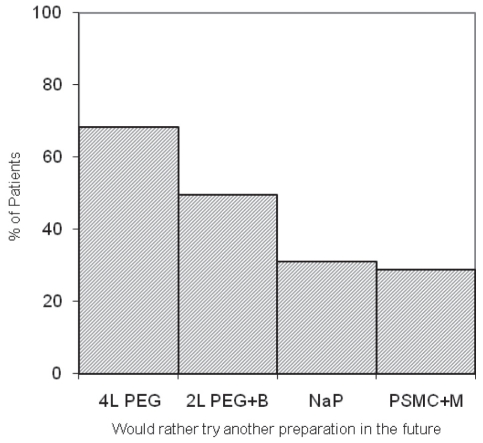
There were no significant group differences for reported symptoms of nausea, vomiting, abdominal cramping, bloating or chest pain (Table 4). Significantly fewer patients reported mild or moderate dizziness after taking 2 L PEG+B compared with the other groups (Table 4). Patients in the NaP group slept longer the night before colonoscopy (6.81 h) than did those in the other three groups (5.73 h, 5.13 h and 5.21 h, respectively [P<0.0001]). More patients assigned to 4 L PEG and 2L PEG+B found it difficult to complete the preparations than did those assigned to NaP or PSMC+M (P<0.0001). More patients assigned to PEG-based regimens preferred a different regimen in the future (Figure 2) than those assigned to take NaP or PSMC+M. A significantly higher proportion of patients assigned to PSMC+M found the taste of the cleansing agent to be more tolerable than in the other three groups (P<0.0001). The mean overall tolerability score was the lowest for the 4 L PEG group (4.19) compared with that of the other three groups (4.60, 4.75 and 4.66, respectively [P<0.011]).
TABLE 4.
Regimen tolerability: Reported patient symptoms for morning and afternoon colonoscopy groups combined
| Symptom |
4L PEG (n=184) |
2L PEG + B (n=179) |
NaP (n=153) |
PSMC + M (n=178) |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mild-moderate | Severe | Mild-moderate | Severe | Mild-moderate | Severe | Mild-moderate | Severe | ||
| Nausea | 86 (46.9) | 8 (4.5) | 68 (37.9) | 10 (5.6) | 67 (43.9) | 6 (4.1) | 74 (41.6) | 4 (2.2) | 0.444 |
| Vomiting | 20 (10.6) | 4 (2.2) | 15 (8.5) | 5 (2.8) | 8 (5.4) | 1 (0.7) | 16 (9.0) | 3 (1.7) | 0.515 |
| Abdominal cramps | 77 (41.9) | 6 (3.4) | 67 (37.3) | 9 (5.1) | 62 (40.5) | 5 (3.4) | 67 (37.6) | 3 (1.7) | 0.634 |
| Bloating | 95 (51.4) | 11 (6.1) | 77 (42.9) | 6 (3.4) | 58 (37.8) | 8 (5.4) | 66 (37.1) | 7 (3.9) | 0.051 |
| Chest pain | 6 (3.4) | 1 (0.6) | 6 (3.4) | 0 (0) | 6 (4.1) | 0 (0) | 5 (2.8) | 1 (0.6) | 0.901 |
| Dizziness | 40 (21.8) | 1 (0.6) | 18 (10.2) | 3 (1.7) | 40 (26.4) | 0 (0) | 50 (28.2) | 3 (1.7) | 0.001* |
Data presented as n (%) unless otherwise indicated.
Corrected for multiple comparisons. Significant difference between 4 L PEG and 2L PEG+B, between 2 L PEG + B, between 2L PEG + B and PSMC + M. B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
Figure 2).
Percentage of patients who would rather try a different regimen in the future according to group (P<0.0001). M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Phamaceuticals Inc, Canada)
Patient safety
There were no clinically significant changes in mean electrolyte or creatinine levels in any of the study groups (Table 5). However, a significantly higher proportion of patients (10.6%) with potassium levels of lower than 3 mmol/L was observed in the NaP group than in the other three groups. Six per cent of patients in the NaP group developed hyper-phosphatemia (phosphate >2.0 mmol/L), a finding that was not seen in any other group. Hypocalcemia (calcium <2.2 mmol/L) was frequent in the NaP group (25%), although it was also seen in the 4L PEG group (18%). After taking the cathartic agent, 21 patients experienced a rise in creatinine levels of 20 μmol/L or more compared with baseline (4L PEG, n=4; 2L PEG+B, n=6; NaP, n=2 and PSMC+M, n=9), although none of these patients’ creatinine levels actually reached an abnormal value. No cases of ischemic colitis were identified.
TABLE 5.
Mean changes (Δ) from baseline in serum electrolyte and creatinine levels
| Parameter | Colonoscopy preparation group | P | |||
|---|---|---|---|---|---|
| 4L PEG (n=117) | 2L PEG + B (n=135) | NaP (n=99) | PSMC + M (n=129) | ||
| ΔNa, mmol/L | 1.87 | 2.51 | 2.05 | 2.48 | 0.011 |
| Serum Na | 1 (0.6) | 0 (0) | 0 (0) | 1 (0.6) | 0.332 |
| >145 mmol/L*, n (%) | |||||
| ΔK, mmol/L | 0.74 | 0.65 | 0.80 | 0.57 | <0.0001 |
| Serum K | 1 (0.6) | 0 (0) | 11 (11) | 1 (0.6) | <0.0001 |
| <3.0 mmol/L*, n (%) | |||||
| ΔCl, mmol/L | 2.85 | 3.14 | 3.77 | 3.41 | 0.61 |
| ΔCa, mmol/L | 0.075 | 0.089 | 0.081 | 0.084 | 0.259 |
| Serum Ca | 21 (18) | 9 (7) | 25 (25) | 7 (5) | <0.0001 |
| <2.2 mmol/L*, n (%) | |||||
| ΔPO4, mmol/L | 0.17 | 0.16 | 0.36 | 0.18 | <0.001 |
| Serum PO4 | 0 (0) | 0 (0) | 6 (6) | 0 (0) | <0.0001 |
| >2.0 mmol/L*, n (%) | |||||
| ΔCreatinine, μmol/L | 9.04 | 9.32 | 7.52 | 9.96 | 0.074 |
| Rise in creatinine | 2 (2) | 4 (3) | 1 (1) | 6 (5) | 0.0681 |
| ≥20 μmol/L*, n (%) | |||||
Abnormal laboratory value. B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
DISCUSSION
The results of the present study demonstrate that NaP, although favoured by patients and clinicians in the past, is inferior to 4 L PEG, 2 L PEG + B and PSMC + M in cleansing efficacy, although the difference did not reach the predetermined minimal clinically significant score of 2. Previous studies using the OBPS have also found the score differences between PEG and NaP and between PSMC and NaP to be less than 2 and, therefore, one could argue that the statistically significant differences observed in bowel preparation quality following different cleansing regimens may not be clinically significant (16,19). In the present study, the NaP group also had a significantly higher proportion of patients with less than ideal bowel preparations compared with the other three groups. Given the added risks of electrolyte disturbance and acute phosphate nephropathy, NaP should not be used as a cleansing agent, especially because more effective alternatives are available. In fact, the NaP manufacturer has voluntarily withdrawn this product from the market in the United States and in Canada.
The bowel preparation schedules were designed so that the intervals between the completion of bowel preparation and the start of colonoscopy (ie, ‘runway time’) were as similar and convenient as possible for the AM and PM procedures in all groups. For practical reasons, the start time of 20:00 (18:00 for 4 L PEG) was chosen for commencing the preparation the night before the procedure for AM colonoscopies, and 06:00 for the PM procedures. The consequence of this bowel preparation schedule was shorter intervals for the PM colonoscopies (4 h to 8 h) compared with AM colonoscopies (10 h to 14 h). This is the likely explanation for the better overall bowel preparation quality of the PM colonoscopies, irrespective of the purging regimens. Furthermore, the observed group differences in bowel preparation quality in AM colonoscopies (ie, NaP was the worst group among the four regimens) was not present in the PM colonoscopies, suggesting that ‘runway time’ may be a more important factor than the cathartic agents used in determining bowel cleanliness. There are now emerging data suggesting that same-day preparation may improve the quality of bowel cleansing for afternoon colonoscopy (30,31), again reflecting the fact that shorter runway time is critical for a good bowel preparation.
Several studies have shown that split-dose preparations are more efficacious than single-dose regimens (32–35). In fact, the most current colon cancer screening guidelines from the American College of Gastroenterology (36) recommended split-dose regimens. However, split-dosing in a regimen requires patients scheduled for AM colonoscopy to rise in the early hours of the day to finish the final cathartic dose, likely decreasing compliance, especially if patients are not sufficiently educated about the importance of a good bowel preparation. Interestingly, Gupta et al (37) have shown that prior-day dosing disrupts sleep and travel to the endoscopy units to the same degree as split dosing. Experts believe that most patients would comply with split-dosing schedules by getting up early – just as they would for other important reasons (38) – if they were given the right information. The reason better bowel preparations may be more effective with a split-dose regimen may be explained by a shorter runway time.
Patient factors may also have an impact on the quality of bowel preparation. For example, obesity (body mass index >30 kg/m2) and chronic constipation have been shown to be independent predictors of inadequate bowel preparation (39,40).
The combination of PSMC + M was rated the best-tolerated regimen from the perspective of taste, ease of completion and a low frequency of symptoms with ingestion, compared with PEG-based regimens or NaP. PSMC + M also appeared to be safe, with very few associated cases of hypokelemia or hypocalcemia, although our sample size was not large enough to specifically address safety concerns. A PSMC-based regimen could be a good low-volume bowel preparation alternative.
CONCLUSION.
The present study demonstrated equal cleansing efficacies for 4 L PEG, 2 L PEG + B and PSMC + M. PSMC + M appeared to be the best-tolerated regimen, and it was as safe as PEG-based regimens. A short runway time may be the single most important factor determining the quality of bowel preparation and should be taken into consideration in the design of bowel preparation protocols.
Morning colonoscopy
|
Preparation group |
P* | ||||
|---|---|---|---|---|---|
| 4L PEG (n=134) | 2L PEG + B (n=136) | NaP (n=100) | PSMC + M (n=121) | ||
| Right colon | 1.49 | 1.17 | 2.38 | 1.58 | <0.0001† |
| Middle colon | 1.00 | 0.87 | 1.37 | 1.02 | 0.002‡ |
| Rectosigmoid colon | 1.09 | 0.94 | 1.30 | 0.80 | 0.004§ |
| Overall fluid | 0.56 | 0.56 | 0.33 | 0.46 | 0.12 |
| Total score (95%CI) | 4.14 (3.63–4.64) | 3.51 (3.06–3.96) | 5.37 (4.80–5.94) | 3.84 (3.35–4.34) | <0.0001¶ |
Corrected for multiple comparisons;
Significant difference between NaP and 4L PEG, between NaP and 2L PEG+B, and between NaP and PSMC;
Significant difference between NaP and 4L PEG, between NaP and 2L PEG + B, and between NaP and PSMC;
Significant difference between NaP and 4L PEG, between NaP and 2L PEG + B, and between NaP and PSMC.
Significant difference between NaP and 4L PEG, between NaP and 2L PEG + B, and between NaP and PSMC. B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
Afternoon colonoscopy
|
Preparation group |
P | ||||
|---|---|---|---|---|---|
| 4L PEG (n=76) | 2L PEG + B (n=74) | NaP (n=64) | PSMC + M (n=73) | ||
| Right colon | 0.85 | 0.92 | 1.39 | 0.96 | 0.920 |
| Middle colon | 0.57 | 0.69 | 0.89 | 0.64 | 0.212 |
| Rectosigmoid colon | 0.61 | 0.68 | 0.72 | 0.56 | 0.711 |
| Overall fluid | 0.61 | 0.8 | 0.51 | 0.67 | 0.083 |
| Total score (95%CI) | 2.59 (2.00–3.18) | 3.08 (2.42–3.75) | 3.51 (2.84–4.18) | 2.82 (2.24–3.40) | 0.220 |
B Bisacodyl; M Magnesium citrate; NaP Sodium phosphate; PEG Polyethylene glycol; PSMC Pico-Salax (Ferring Pharmaceuticals Inc, Canada)
Footnotes
CONFLICTS OF INTEREST: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: The European Panel of Appropriateness of Gastrointestinal Endoscopy European Multicenter Study. Gastrointest Endosc. 2005;61:378–84. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 2.Golub RW, Kerner BA, Wise WE, Jr, et al. Colonoscopic bowel preparations – which one? A blinded, prospective, randomized trial. Dis Col Rec. 1995;38:594–9. doi: 10.1007/BF02054117. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JB, Pineda JJ, Barthel JS, King PD. Prospective, randomized trial comparing sodium phosphate solution with polyethylene glycol-electrolyte lavage for colonoscopy preparation. Gastrointest Endosc. 1993;39:631–4. doi: 10.1016/s0016-5107(93)70213-8. [DOI] [PubMed] [Google Scholar]
- 4.Picchio M, Gallinaro L, Ceci F, et al. Comparison of standard polyethylene glycol and two doses of oral sodium phosphate solution in precolonoscopy bowel preparation: A randomized controlled trial. Acta Gastroenterologica Belgica. 2008;71:15–20. [PubMed] [Google Scholar]
- 5.Vanner SJ, MacDonald PH, Paterson WG, Prentice RS, Da Costa LR, Beck IT. A randomized prospective trial comparing oral sodium phosphate with standard polyethylene glycol-based lavage solution (Golytely) in the preparation of patients for colonoscopy. Am J Gastroenterol. 1990;85:422–7. [PubMed] [Google Scholar]
- 6.Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy – a meta-analysis. Col Dis. 2006;8:247–58. doi: 10.1111/j.1463-1318.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 7.Sica DA, Carl D, Zfass AM. Acute phosphate nephropathy – an emerging issue. Am J Gastroenterol. 2007;102:1844–7. doi: 10.1111/j.1572-0241.2007.01047.x. (see comment) [DOI] [PubMed] [Google Scholar]
- 8.Carl DE, Sica DA. Acute phosphate nephropathy following colonoscopy preparation. Am J Med Sci. 2007;334:151–4. doi: 10.1097/MAJ.0b013e318156c529. [DOI] [PubMed] [Google Scholar]
- 9.Markowitz GS, Radhakrishnan J, D’Agati VD. Towards the incidence of acute phosphate nephropathy. J Am Soc Nephrol. 2007;18:3020–2. doi: 10.1681/ASN.2007101073. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz GS, Stokes MB, Radhakrishnan J, D’Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: An under-recognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389–96. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 11.Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349:1006–7. doi: 10.1056/NEJM200309043491020. [DOI] [PubMed] [Google Scholar]
- 12.Belsey J, Epstein O, Heresbach D. Systematic review: Oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373–84. doi: 10.1111/j.1365-2036.2006.03212.x. (see comment) [DOI] [PubMed] [Google Scholar]
- 13.Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: Efficacy, tolerability, and safety – a Canadian Association of Gastroenterology position paper. Can J Gastroenterol. 2006;20:699–710. doi: 10.1155/2006/915368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshioka K, Connolly AB, Ogunbiyi OA, Hasegawa H, Morton DG, Keighley MR. Randomized trial of oral sodium phosphate compared with oral sodium picosulphate (Picolax) for elective colorectal surgery and colonoscopy. Dig Surg. 2000;17:66–70. doi: 10.1159/000018802. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt LM, Williams P, King D, Perera D. Picoprep-3 is a superior colonoscopy preparation to Fleet: A randomized, controlled trial comparing the two bowel preparations. Dis Colon Rectum. 2004;47:238–42. doi: 10.1007/s10350-003-0027-4. [DOI] [PubMed] [Google Scholar]
- 16.Hookey LC, Vanner SJ. Pico-Salax plus two-day bisacodyl is superior to Pico-Salax alone or oral sodium phosphate for colon cleansing before colonoscopy. Am J Gastroenterol. 2009;104:703–9. doi: 10.1038/ajg.2008.167. [DOI] [PubMed] [Google Scholar]
- 17.American Society of Colon and Rectal Surgeons (ASCRS) American Society for Gastrointestinal Endoscopy (ASGE) Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: Prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Surg Endosc. 2006;20:1147–60. doi: 10.1007/s00464-006-0152-y. (see comment) [DOI] [PubMed] [Google Scholar]
- 18.Poon CM, Lee DW, Mak SK, et al. Two liters of polyethylene glycol-electrolyte lavage solution versus sodium phosphate as bowel cleansing regimen for colonoscopy: A prospective randomized controlled trial. Endoscopy. 2002;34:560–3. doi: 10.1055/s-2002-33207. [DOI] [PubMed] [Google Scholar]
- 19.Rostom A, Jolicoeur E, Dube C, et al. A randomized prospective trial comparing different regimens of oral sodium phosphate and polyethylene glycol-based lavage solution in the preparation of patients for colonoscopy. Gastrointest Endosc. 2006;64:544–52. doi: 10.1016/j.gie.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, et al. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: A randomized study. World J Gastroenterol. 2006;12:6161–6. doi: 10.3748/wjg.v12.i38.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frommer D. Cleansing ability and tolerance of three bowel preparations for colonoscopy. Dis Colon Rectum. 1997;40:100–4. doi: 10.1007/BF02055690. [DOI] [PubMed] [Google Scholar]
- 22.Sanaka MR, Shah N, Mullen KD, Ferguson DR, Thomas C, McCullough AJ. Afternoon colonoscopies have higher failure rates than morning colonoscopies. Am J Gastroenterol. 2006;101:2726–30. doi: 10.1111/j.1572-0241.2006.00887.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanaka MR, Deepinder F, Thota PN, Lopez R, Burke CA. Adenomas are detected more often in morning than in afternoon colonoscopy. Am J Gastroenterol. 2009;104:1659–64. doi: 10.1038/ajg.2009.249. (quiz 1665). [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69(3 Suppl):700–6. doi: 10.1016/j.gie.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 25.Kossi J, Krekela I, Patrikainen H, Vuorinen T, Luostarinen M, Laato M. The cleansing result of oral sodium phosphate is inversely correlated with time between the last administration and colonoscopy. Tech Coloproctol. 2007;11:51–4. doi: 10.1007/s10151-007-0325-2. [DOI] [PubMed] [Google Scholar]
- 26.Aisenberg J. Bowel preparation for colonoscopy: Shortening the “runway time”. Gastrointest Endosc. 2009;69(3 Suppl):707–9. doi: 10.1016/j.gie.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 27.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482–6. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 28.Lopez Morra HA, Fine SN, Dickstein G. Colonic ischemia with laxative use in young adults. Am J Gastroenterol. 2005;100:2134–6. doi: 10.1111/j.1572-0241.2005.50395_8.x. [DOI] [PubMed] [Google Scholar]
- 29.Baudet JS, Castro V, Redondo I. Recurrent ischemic colitis induced by colonoscopy bowel lavage. Am J Gastroenterol. 2010;105:700–1. doi: 10.1038/ajg.2009.637. [DOI] [PubMed] [Google Scholar]
- 30.Gurudu SR, Ratuapli S, Heigh R, DiBaise J, Leighton J, Crowell M. Quality of bowel cleansing for afternoon colonoscopy is influenced by time of administration. Am J Gastroenterol. 2010;105:2318–22. doi: 10.1038/ajg.2010.235. [DOI] [PubMed] [Google Scholar]
- 31.Varughese S, Kumar AR, George A, Castro FJ. Morning-only one-gallon polyethylene glycol improves bowel cleansing for afternoon colonoscopies: A randomized endoscopist-blinded prospective study. Am J Gastroenterol. 2010;105:2368–74. doi: 10.1038/ajg.2010.271. [DOI] [PubMed] [Google Scholar]
- 32.Aoun E, Abdul-Baki H, Azar C, et al. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest Endosc. 2005;62:213–8. doi: 10.1016/s0016-5107(05)00371-8. [DOI] [PubMed] [Google Scholar]
- 33.Church JM. Effectiveness of polyethylene glycol antegrade gut lavage bowel preparation for colonoscopy – timing is the key! Dis Colon Rectum. 1998;41:1223–5. doi: 10.1007/BF02258217. [DOI] [PubMed] [Google Scholar]
- 34.El Sayed AM, Kanafani ZA, Mourad FH, et al. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc. 2003;58:36–40. doi: 10.1067/mge.2003.318. [DOI] [PubMed] [Google Scholar]
- 35.Park JS, Sohn CI, Hwang SJ, et al. Quality and effect of single dose versus split dose of polyethylene glycol bowel preparation for early-morning colonoscopy. Endoscopy. 2007;39:616–9. doi: 10.1055/s-2007-966434. [DOI] [PubMed] [Google Scholar]
- 36.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 37.Gupta T, Mandot A, Desai D, Abraham P, Joshi A, Shah S. Comparison of two schedules (previous evening versus same morning) of bowel preparation for colonoscopy. Endoscopy. 2007;39:706–9. doi: 10.1055/s-2007-966375. [DOI] [PubMed] [Google Scholar]
- 38.Lin OS, Schembre DB. Are split bowel preparation regimens practical for morning colonoscopies? Implications of the new American College of Gastroenterology colon cancer screening guidelines for real-world clinical practice. Am J Gastroenterol. 2009;104:2627–8. doi: 10.1038/ajg.2009.415. (author reply 2628–9). [DOI] [PubMed] [Google Scholar]
- 39.Borg BB, Gupta NK, Zuckerman GR, Banerjee B, Gyawali CP. Impact of obesity on bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2009;7:670–5. doi: 10.1016/j.cgh.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Li X, Ge Z. Comparative study on two colonic bowel preparations for patients with chronic constipation. Scand J Gastroenterol. 2009;44:375–9. doi: 10.1080/00365520802538211. [DOI] [PubMed] [Google Scholar]