Diabetic nephropathy is a major complication of both type 1 and type 2 diabetes. It is one of the leading causes of end-stage renal disease and kidney failure.1 The prevalence of diabetes and diabetic complications, such as diabetic nephropathy that is initially characterized by microalbuminuria and later by glomerular sclerosis, is rapidly increasing. Podocytes play a critical role in preventing proteins from leaking into urine.2 Podocyte injury and loss are believed to contribute to the development of diabetic nephropathy.3
Rapamycin is an immunosuppressive drug that is commonly used in organ transplantation, such as in kidney transplantation. The target of rapamycin (TOR) plays a major role in cell growth and cell size control. mTOR forms two distinct structural and functional complexes, mTORC1 and mTORC2. Rapamycin selectively inhibits mTORC1 but not mTORC2. Increased mTORC1 activity and podocyte hypertrophy have been observed in early stages of diabetic nephropathy. Moreover, rapamycin treatment has been reported to prevent diabetic nephropathy in mouse models. Paradoxically, rapamycin causes proteinuria and glomerular sclerosis in some patients.3,4 Therefore, the exact function of mTOR in diabetic nephropathy development is unknown. Recently, two studies have shed lights on the function of mTOR in podocytes using mouse models.3,4
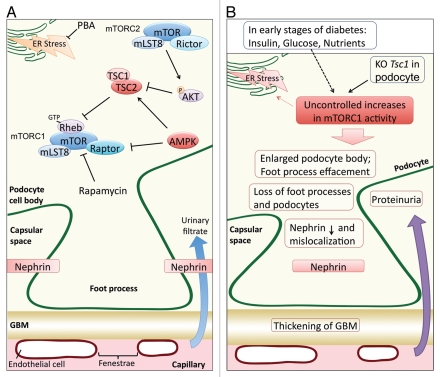
The TSC1/TSC2 complex functions as a GTPase-activating protein toward Rheb, which is a potent direct activator of mTORC1 (Fig. 1A).5 Mutation in either TSC1 or TSC2 causes constitutive activation of mTORC1. Inoki et al. specifically ablated the Tsc1 gene in podocytes. Constitutive activation of mTORC1 in TSC1-knockout podocytes was confirmed by a significant increase in S6 phosphorylation,3 which is a commonly used indicator of mTORC1 activity. Interestingly, uncontrolled mTORC1 activation in the Tsc1-knockout (PcKOTsc1) mice induced proteinuria as early as 2 weeks of age, with massive proteinuria at 4 weeks. The PcKOTsc1 phenotypes resemble many of the pathologies found in diabetic nephropathy, such as effacement of podocyte foot processes, thickening of glomerular basement membrane and podocyte hypertrophy. Constitutive activation of mTORC1 in podocytes also increased mesangial matrix and mesangial cell size. The PcKOTsc1 mice died within the first four months due to kidney failure. Interestingly, rapamycin injection at 2 weeks of age could completely prevent phenotypes associated with the PcKOTsc1, including proteinuria, glomerular sclerosis and loss of podocytes. Moreover, rapamycin treatment at 4 weeks of age could reverse the TSC1 knockout-induced phenotypes as well. Remarkably, the PcKOTsc1 mice with rapamycin treatment appeared to be healthy, although smaller in size, and survived as long as being treated with rapamycin. Ceasing rapamycin treatment rapidly caused kidney failure and death. These results show that sustained high mTORC1 activation in podocytes is responsible for glomerular sclerosis and kidney failure.
Figure 1.
(A) Schematic illustration of mTOR signaling and Nephrin localization in normal podocytes. (B) Pathological mTORC1 activation contributes to podocyte dysfunction.
These authors further investigated the underlying mechanism through which high mTORC1 activity induced podocyte dysfunction. It was found that high mTORC1 activation led to the mislocalization of Nephrin, which is a podocyte cell surface protein that is important for a proper renal filtration barrier. In the TSC1-knockout podocytes, Nephrin was retained in the cytoplasm and, therefore, could not contribute to the formation of the filtration barrier. Moreover, nephrin mislocalization could be recapitulated in transfected cells in a manner dependent on mTORC1 activity. They also showed that reducing endoplasmic reticulum (ER) stress by treatment with chemical chaperone partially prevented podocytes loss, supporting a model that ER stress caused by high mTORC1 activity contributes to podocyte dysfunction.3
Raptor and Rictor are essential subunits in mTORC1 and mTORC2, respectively. Gödel et al. ablated Raptor and/or Rictor in podocytes to investigate the function of mTORC1 and mTORC2. The loss of Raptor in podocytes caused albuminuria, progressive glomerulosclerosis and foot process broadening and effacement. However, the deletion of Raptor at a late stage generated much milder phenotypes. These results indicate that mTORC1 is critical in podocyte development and growth but less important in the developed kidney. In contrast, Rictor knockout, even in early stages, showed little change in phenotype unless under stress conditions (e.g., when injected with bovine serum albumin), indicating a less important role of mTORC2 in podocytes. Moreover, combined knockout of Raptor and Rictor resulted in dramatic renal failure and rapid death. The findings that double knockout produced a stronger phenotype than each single knockout indicate that both mTORC1 and mTORC2 play roles in podocyte development. Together, the two reports suggest that excessive or deficienct mTOR activity restricts podocyte function.
To further support the model that reduced mTORC1 activity may prevent diabetic nephropathy, Inoki et al. generated a Raptor heterozygous in db/db mice, which is commonly used as a model for obesity-induced diabetes. Interestingly, reduction of mTORC1 activity by deleting one copy of Raptor significantly suppressed the proteinuria and mislocalization of Nephrin observed in the db/db mice, further supporting a pathogenic role of increased mTORC1 activity in diabetic nephropathy.
In summary, the two studies have established an important role of mTOR signaling in podocyte development and uncontrolled mTORC1 activation in the development of diabetic nephropathy (Fig. 1b). One may expect an elevated mTORC1 in diabetes because of the high levels of insulin and glucose. These studies also demonstrate an important clinical implication that partial inhibition of mTORC1 by drugs, such as rapamycin, may be a potential therapy for diabetic nephropathy.
Comment on: Inoki K, et al. J Clin Invest. 2011;121:2181–2196. doi: 10.1172/JCI44771. and Gödel M, et al. J Clin Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774.
References
- 1.Rossing P, et al. Kidney Int. 2011;79:28–32. [Google Scholar]
- 2.Pavenstädt H, et al. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 3.Inoki K, et al. J Clin Invest. 2011;121:2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gödel M, et al. J Clin Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoncu R, et al. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]