Abstract
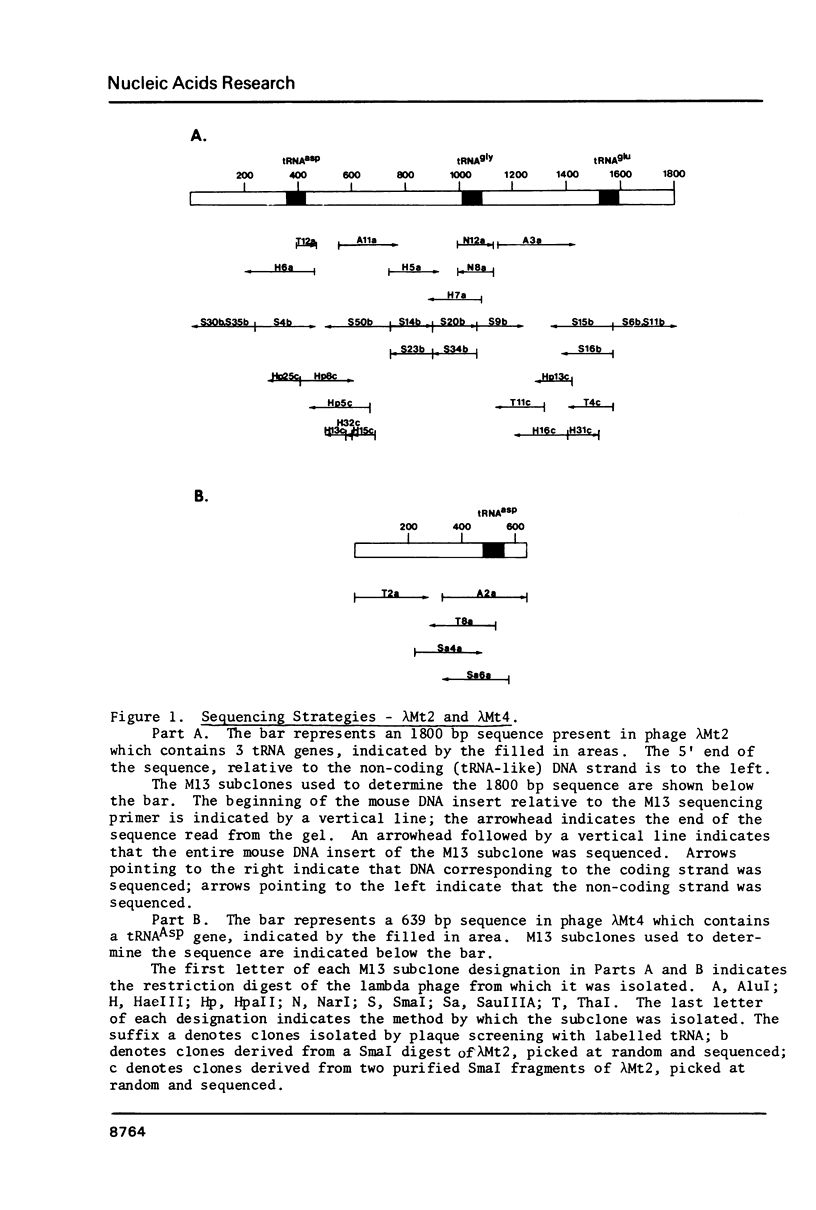
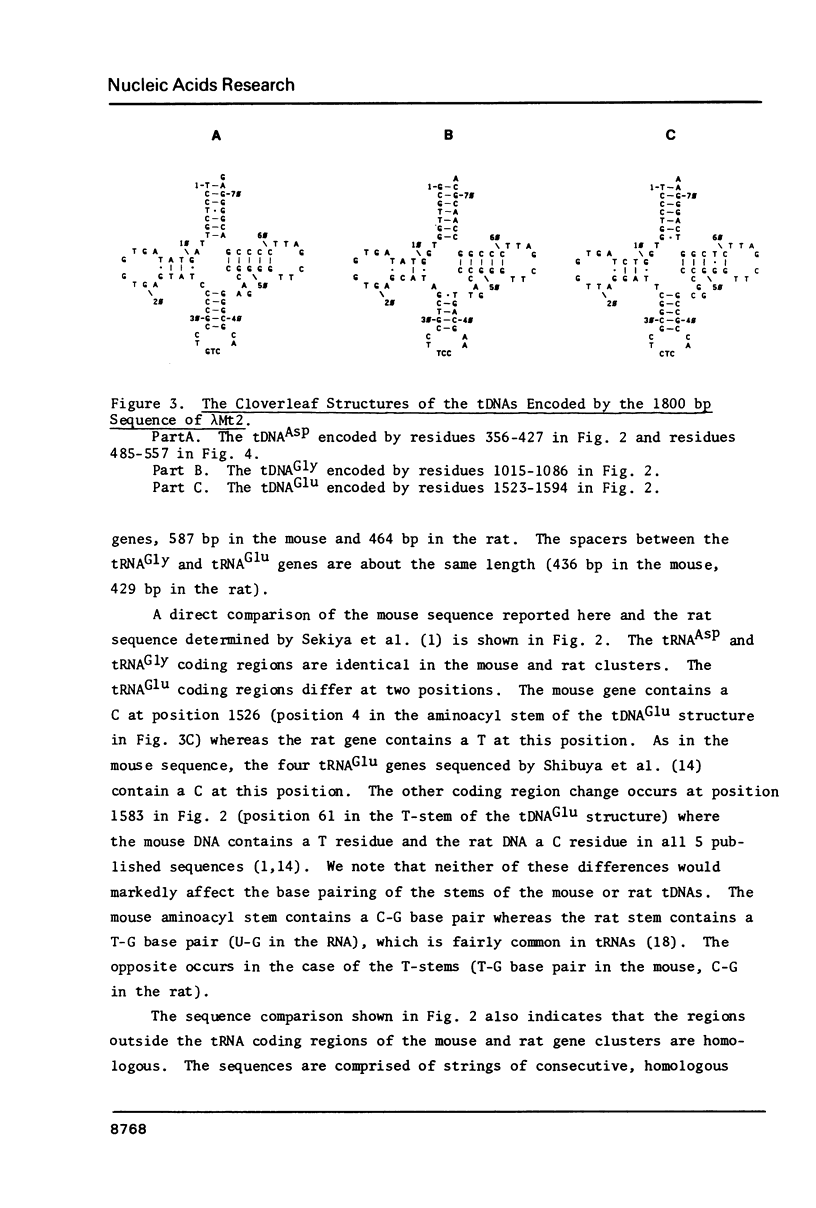
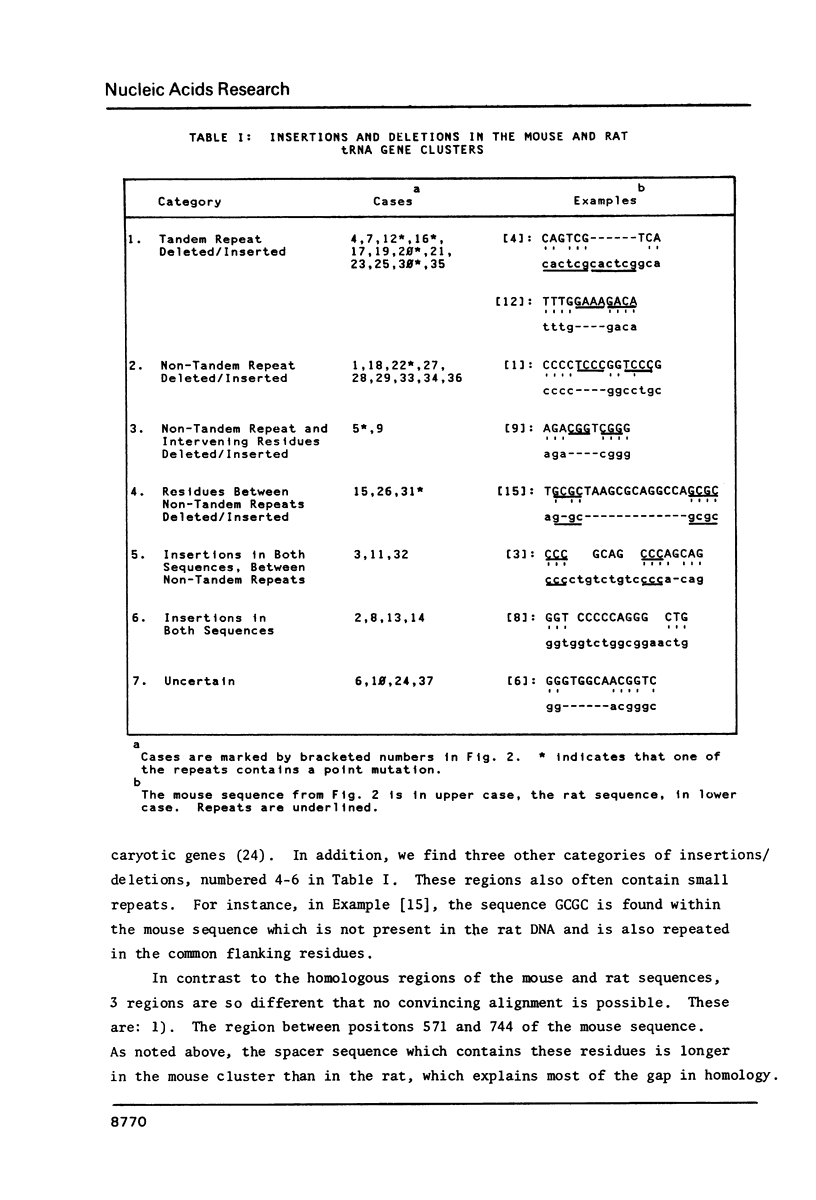
We have sequenced mouse tRNA genes from two recombinant lambda phage. An 1800 bp sequence from one phage contains 3 tRNA genes, potentially encoding tRNAAsp, tRNAGly, and tRNAGlu, separated by spacer sequences of 587 bp and 436 bp, respectively. The mouse tRNA gene cluster is homologous to a rat sequence (Sekiya et al., 1981, Nucleic Acids Res. 9, 2239-2250). The mouse and rat tRNAAsp and tRNAGly coding regions are identical. The tRNAGlu coding regions differ at two positions. The flanking sequences contain 3 non-homologous areas: a c. 100 bp insertion in the first mouse spacer, short tandemly repeated sequences in the second spacers and unrelated sequences at the 3' ends of the clusters. In contrast, most of the flanking regions are homologous, consisting of strings of consecutive, identical residues (5-17 bp) separated by single base differences and short insertions/deletions. The latter are often associated with short repeats. The homology of the flanking regions is c. 75%, similar to other murine genes. The second lambda clone contains a solitary mouse tRNAAsp gene. The coding region is identical to that of the clustered tRNAAsp gene. The 5' flanking regions of the two genes contain homologous areas (10-25 bp) separated by unrelated sequences. Overall, the flanking regions of the two mouse tRNAAsp genes are less homologous than those of the mouse and rat clusters.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison W. R., Astell C. R., Delaney A. D., Gillam I. C., Hayashi S., Miller R. C., Rajput B., Smith M., Taylor D. M., Tener G. M. The structures of genes hybridizing with tRNA4Val from Drosophila melanogaster. J Biol Chem. 1982 Jan 25;257(2):670–673. [PubMed] [Google Scholar]
- Bogenhagen D. F., Brown D. D. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981 Apr;24(1):261–270. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Breiner A. V., Brandt C. R., Milcarek C., Sweet R. W., Warszawski D., Ziv E., Schechter I. Recent duplication and germ-line diversification of rat immunoglobulin kappa chain gene joining segments. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5993–5997. doi: 10.1073/pnas.79.19.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Financsek I., Mizumoto K., Muramatsu M. Nucleotide sequence of the transcription initiation region of a rat ribosomal RNA gene. Gene. 1982 May;18(2):115–122. doi: 10.1016/0378-1119(82)90109-3. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Harding J. D. Isolation and nucleotide sequence of a mouse histidine tRNA gene. Nucleic Acids Res. 1982 Aug 25;10(16):4891–4900. doi: 10.1093/nar/10.16.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H., Harding J. D. Using iodinated single-stranded M13 probes to facilitate rapid DNA sequence analysis--nucleotide sequence of a mouse lysine tRNA gene. Nucleic Acids Res. 1983 Apr 11;11(7):2053–2064. doi: 10.1093/nar/11.7.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosbach H. A., Silberklang M., McCarthy B. J. Evolution of a D. melanogaster glutamate tRNA gene cluster. Cell. 1980 Aug;21(1):169–178. doi: 10.1016/0092-8674(80)90124-5. [DOI] [PubMed] [Google Scholar]
- Hovemann B., Sharp S., Yamada H., Söll D. Analysis of a drosophila tRNA gene cluster. Cell. 1980 Apr;19(4):889–895. doi: 10.1016/0092-8674(80)90080-x. [DOI] [PubMed] [Google Scholar]
- Indik Z. K., Tartof K. D. Glutamate tRNA genes are adjacent to 5S RNA genes in Drosophila and reveal a conserved upstream sequence (the ACT-TA box). Nucleic Acids Res. 1982 Jul 24;10(14):4159–4172. doi: 10.1093/nar/10.14.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran P., Forget B. G., Weissman S. M. Short interspersed repetitive DNA elements in eucaryotes: transposable DNA elements generated by reverse transcription of RNA pol III transcripts? Cell. 1981 Oct;26(2 Pt 2):141–142. doi: 10.1016/0092-8674(81)90296-8. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Kafatos F. C. Accepted mutations in a gene family: evolutionary diversification of duplicated DNA. J Mol Evol. 1982;19(1):87–103. doi: 10.1007/BF02100227. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Shindo-Okada N., Ando N., Watanabe S., Nishimura S. Nucleotide sequences of two aspartic acid tRNAs from rat liver and rat ascites hepatoma. J Biol Chem. 1981 Sep 10;256(17):9059–9062. [PubMed] [Google Scholar]
- Munz P., Amstutz H., Kohli J., Leupold U. Recombination between dispersed serine tRNA genes in Schizosaccharomyces pombe. Nature. 1982 Nov 18;300(5889):225–231. doi: 10.1038/300225a0. [DOI] [PubMed] [Google Scholar]
- Reilly J. G., Ogden R., Rossi J. J. Isolation of a mouse pseudo tRNA gene encoding CCA--a possible example of reverse flow of genetic information. Nature. 1982 Nov 18;300(5889):287–289. doi: 10.1038/300287a0. [DOI] [PubMed] [Google Scholar]
- Robinson R. R., Davidson N. Analysis of a drosophila tRNA gene cluster: two tRNALeu genes contain intervening sequences. Cell. 1981 Jan;23(1):251–259. doi: 10.1016/0092-8674(81)90289-0. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Cooke H., Buckland R. Nucleotide sequence of a segment of human DNA containing the three tRNA genes. Nucleic Acids Res. 1982 Nov 25;10(22):7313–7322. doi: 10.1093/nar/10.22.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Santos T., Zasloff M. Comparative analysis of human chromosomal segments bearing nonallelic dispersed tRNAimet genes. Cell. 1981 Mar;23(3):699–709. doi: 10.1016/0092-8674(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Kuchino Y., Nishimura S. Mammalian tRNA genes: nucleotide sequence of rat genes for tRNAAsp, tRNAGly and tRNAGlu. Nucleic Acids Res. 1981 May 25;9(10):2239–2250. doi: 10.1093/nar/9.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Nishizawa R., Matsuda K., Taya Y., Nishimura S. A rat tRNA gene cluster containing the genes for tRNAPro and tRNALys. Analysis of nucleotide sequences of the genes and the surrounding regions. Nucleic Acids Res. 1982 Oct 25;10(20):6411–6419. doi: 10.1093/nar/10.20.6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., DeFranco D., Silberklang M., Hosbach H. A., Schmidt T., Kubli E., Gergen J. P., Wensink P. C., Söll D. The initiator tRNA genes of Drosophila melanogaster: evidence for a tRNA pseudogene. Nucleic Acids Res. 1981 Nov 25;9(22):5867–5882. doi: 10.1093/nar/9.22.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K., Noguchi S., Nishimura S., Sekiya T. Characterization of a rat tRNA gene cluster containing the genes for tRNAAsp, tRNAGly and tRNAGlu, and pseudogenes. Nucleic Acids Res. 1982 Jul 24;10(14):4441–4448. doi: 10.1093/nar/10.14.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vakharia V. N., Singhal R. P. The structure of aspartate transfer RNA from rabbit liver. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1072–1081. doi: 10.1016/0006-291x(82)91079-8. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Denison R. A., Bernstein L. B., Weiner A. M., Manser T., Gesteland R. F. Direct repeats flank three small nuclear RNA pseudogenes in the human genome. Cell. 1981 Oct;26(1 Pt 1):11–17. doi: 10.1016/0092-8674(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Woo S. L. A sensitive and rapid method for recombinant phage screening. Methods Enzymol. 1979;68:389–395. doi: 10.1016/0076-6879(79)68028-x. [DOI] [PubMed] [Google Scholar]



