The Breast and Cervical Cancer Prevention and Treatment Act program in Georgia creates a quicker pathway for low-income, uninsured women with breast cancer to access services and receive more treatment than women enrolled in traditional Medicaid eligibility groups.
Abstract
Purpose:
To investigate breast cancer treatment of patients enrolled under traditional Medicaid categories versus those in the Breast and Cervical Cancer Prevention and Treatment Act (BCCPTA) in Georgia.
Methods:
Georgia Comprehensive Cancer Registry linked to Medicaid enrollment files were used to identify 2,048 enrollees with a primary cancer of the breast, of whom 1,046 were enrolled in BCCPTA, 674 were disabled, and 328 were in other Medicaid eligibility groups. Logistic regressions were used to estimate factors associated with the odds of receiving lumpectomy, mastectomy, or other surgery in addition to any drug regimen (hormonal or chemotherapy) and radiation.
Results:
Women in BCCPTA were more likely to receive any treatment (odds ratio [OR] = 4.71; 95% CI, 2.48 to 8.96), any drug regimen (OR = 3.58; 95% CI, 2.32 to 5.51), any radiation (OR = 1.61; 95% CI, 1.15to 2.24), and any definitive surgery (OR = 2.52; 95% CI, 1.74 to 3.66) than the “other” eligibility group after controlling for covariates. There were no significant differences by eligibility group in the receipt of a lumpectomy versus a mastectomy. However, women in BCCPTA were more likely to receive more adjuvant follow-up after a mastectomy.
Conclusion:
The BCCPTA program in Georgia appears to create a quicker pathway for low-income, previously uninsured women with breast cancer to access services and, in turn, receive more treatment than women enrolled in the other, more traditional Medicaid eligibility groups. Yet the overall rate of adjuvant therapy, whether radiation, hormonal, or chemotherapy, appears to fall short of national criteria. This deserves attention in Georgia and, most likely, Medicaid programs in other states as well.
Introduction
Breast cancer is the most common site of a new cancer and is second only to lung cancer as a leading cause of cancer deaths among women. Because most risk factors for breast cancer are not easily modified early enough in life,1–4 breast cancer control has focused on early detection and effective treatment.5 However, lack of insurance poses a barrier to age-appropriate screening,6–8 and low-income women often enter Medicaid at a later stage of their cancer.9–11Historically, Medicaid covered patients with cancer only if they were already enrolled under traditional eligibility categories (largely low-income women and children; pregnant women; and the elderly, blind, and disabled). However, the Breast and Cervical Cancer Prevention and Treatment Act (BCCPTA) of 2000 allowed states to cover women diagnosed with breast cancer, cervical cancer, or precancerous cervical conditions at diagnosis. Because eligibility for BCCPTA relates to the financial criteria for the National Breast and Cervical Cancer Early Detection Program (NBCCEDP)—generally up to 250% of the federal poverty level (FPL) versus less than 100% FPL for other Medicaid eligibility groups in most states—BCCPTA provided Medicaid to relatively higher income cancer patients. Georgia's BCCPTA program (eligibility < 200% FPL), called the Women's Health Medicaid Program, allowed non-NBCCEDP providers to screen for cancer and Medicaid eligibility, used a more streamlined and less burdensome process for determining eligibility (self-reported income), and included presumptive eligibility. This likely meant greater access for women, as providers were more willing to initiate treatment given the certainty of payment. Finally, because women enrolled in BCCPTA needed physician certification of active treatment for continued eligibility, they were more connected to the medical system.
Earlier work in Georgia showed that participation in BCCPTA shortened the time between diagnosis and Medicaid enrollment by 7 to 8 months12 and that, once in Medicaid, women were far less likely to disenroll13 after joining BCCPTA. Thus, women in BCCPTA might access care earlier, receive more services, and/or receive more clinically appropriate care. Whether or not these differences are associated with women in BCCPTA exhibiting a different treatment pattern than other women receiving Medicaid is the focus of this study. We asked the following questions:
Among women with a diagnosis of breast cancer, do those enrolled under BCCPTA differ from other women enrolled in Medicaid?
Are women in BCCPTA more or less likely to receive treatment, after controlling for other factors?
Do the groups differ in terms of specific treatments such as lumpectomy versus mastectomy, and adjuvant therapies?
We identified two groups of relatively younger women (age < 65) with breast cancer insured largely by Medicaid who were comparable to those eligible through BCCPTA. The “disabled” group included patients enrolled under Medicaid's disability eligibility; these women generally had income below 74% FPL in Georgia and had to have doctor certification that they were unable to work for at least 1 year. The “other” eligibility category included those enrolled in Medicaid because they had dependent children and had very low income (< 50% FPL) or were pregnant and had income similar to the BCCPTA eligibility level. We hypothesized that treatment patterns would differ among women with breast cancer who received Medicaid and were in BCCPTA compared with these other eligibility groups, as each represented somewhat different populations in terms of health status and income.
Methods
Data Sources
We linked the Georgia Cancer Comprehensive Registry (GCCR) and Georgia Medicaid enrollment/claims data for analysis. The GCCR is a population-based cancer registry that collects all cancer cases diagnosed in Georgia since 1999. Medicaid enrollment files offer monthly records of all linked cancer beneficiaries. Medicaid claims files contain patients' diagnosis and procedure fields coded by the International Classification of Disease, ninth Revision, Clinical Modification (ICD-9-CM) and Current Procedural Terminology (CPT) schema. We used county data from the Area Resource File, a publicly available data file from the Health Resources and Services Administration. We also used data from the Commission on Cancer (CoC) and the Consolidated Analysis Center to reflect county characteristics that might affect utilization patterns of Medicaid enrollees. The CoC (of the American College of Surgeons) gives recognition to facilities committed to providing the best in diagnosis and treatment of cancers only after a rigorous evaluation process. The Consolidated Analysis Center is a 50-year-old firm specializing in data and information system technology.
Study Sample
To derive our study sample, we included the 2,543 women between the ages of 19 and 64 years who were in the GCCR with a primary cancer site of the breast between January 1, 2002 and December 31, 2004. We excluded those who enrolled in Medicaid > 6 months after their diagnosis (n = 153) because they may have enrolled as a result of other medical needs. We also excluded those with more than one primary cancer site (n = 274). To ensure that all women could be monitored for at least 2 years, we excluded those older than 63 years, as we do not observe Medicare claims (n = 38). We also excluded women in BCCPTA who were not continuously enrolled in the first 2 months (n = 30) because, as a result of presumptive eligibility, women could enroll in Medicaid immediately after diagnosis without verification of eligibility for payment purposes. Their quick disenrollment indicates they were not actually BCCPTA eligible. To identify Medicaid eligibility groups, we placed women into (A) BCCPTA, (B) disabled, or (C) “other” groups on the basis of the most frequently observed eligibility category in the 6 months after enrollment. A hierarchy was used if we had a tie (3 months each) to give priority to BCCPTA, followed by disabled. The final sample included 2,048 women, of whom 1,046 were in BCCPTA, 674 were disabled, and 328 were “other.”
Study Variables
Using CPT, ICD-9-CM, Healthcare Common Procedure Coding System, and state-specific procedure codes, we identified any medical claim related to breast cancer within 2 years of Medicaid enrollment in the following categories: (A) lumpectomy, (B) mastectomy, (C) any drug regimen (hormonal or chemotherapy), and (D) radiation). (A full list of drug names, National Drug Code numbers, and treatment procedure codes used in this analysis are available in the Data Supplement.)
We further analyzed only those patients with a lumpectomy or mastectomy code and identified the last date of service for either of these surgeries to flag these patients as having undergone definitive surgery. We then analyzed whether they received (A) lumpectomy versus mastectomy, (B) lumpectomy with or without radiation, or (C) mastectomy with or without adjuvant therapy. The first date of service after the definitive surgery was defined as adjuvant therapy. To ensure that patient's cancer stage corresponded to their treatment options, we excluded patients with an in situ diagnosis from our analysis of the receipt of any drug regimen and adjuvant therapy after definitive surgery, because these patients are often not candidates for these treatments. Moreover, we excluded patients with distant metastases from analysis of any type of surgery because they are often treated only with systemic therapy.
Given that the differences in demographics across the three groups could affect treatment patterns, we controlled for age at Medicaid enrollment, race/ethnicity, stage of disease, and enrollment status. To adjust for a noncancer illness that might affect patients' treatment options, we adopted Romano's modification of the comorbidity index originally developed by Charlson,14–16 using medical claims up to 1 year after enrollment. We also flagged women who enrolled before versus after their cancer diagnosis and those continuously enrolled over the 24 months of observation. Finally, we included county data on the percentage of households with annual income less than $15K; whether there was a hospital with oncology services and/or a CoC-approved approval certification; and the number of obstetrician/gynecologists per 1,000 women, to reflect provider capacity in the area. The sources of variables and detailed coding information are available in Appendix Table A1 (online only) and the Data Supplement, respectively.
Statistical Analyses
All analyses were conducted using Stata version 9.2 (Stata, College Station, TX). All statistical tests were two-sided.
Results
Women with breast cancer who were enrolled under BCCPTA were less likely to be under age 44 than women in the “other” eligibility group (largely women receiving welfare or pregnant women) and more likely to be in this younger age group than disabled women (Appendix Table A2, online only). Women in BCCPTA were more likely to be white and far less likely to have comorbidities complicating their cancer diagnosis. Approximately 50% of women in all three groups had either in situ or local stage disease, but far fewer women in BCCPTA, compared with disabled women, were categorized as having distant metastases. Women in BCCPTA remained enrolled slightly longer than the other groups but were not different with respect to urban versus rural residency or whether they lived in a county with at least one CoC-approved hospital. They were, however, more likely than disabled women to live in a county that has a hospital with oncology services.
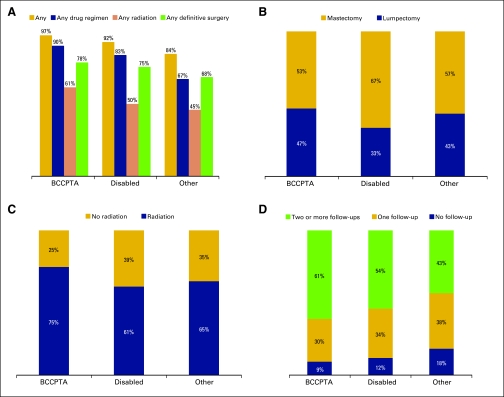
The data in Figure 1A show that women in BCCPTA were more likely than other eligible women to receive any treatment, drug regimen, and radiation, as well as to undergo definitive surgery. Women with an earlier stage of cancer were more likely to have a mastectomy than a lumpectomy as their definitive surgery, regardless of eligibility group, although women in BCCPTA had the lowest percentage of mastectomies (53%; Figure 1B). Regarding adjuvant therapy (Figures 1C and 1D) after definitive surgery, women in BCCPTA were far more likely to receive radiation after a lumpectomy (75% v 61%–65% of other eligibility groups), as well as follow-up treatment after a mastectomy.
Figure 1.
Receipt of treatment for women with breast cancer enrolled in Medicaid in Georgia by eligibility groups. (A) Percentage of receipt of any treatment (P < .001), any drug regimen (P < .001), any radiation (P < .001), and any definitive surgery (P = .002) among Medicaid eligibility groups. (B) Distribution of definitive surgery (P < .001). (C) Distribution of receipt of adjuvant radiation for patients who underwent lumpectomy (P = .017). (D) Distribution of receipt of adjuvant follow-up among patients who underwent mastectomy (P = .013). χ2 tests are used for analysis of treatment patterns among the three Medicaid eligibility groups. BCCPTA, Breast and Cervical Cancer Prevention and Treatment Act.
We show multivariate analyses beginning in Table 1; the reference category for both the BCCPTA and disabled is the “other” eligibility group. After controlling for other factors, women in BCCPTA were almost five times more likely (odds ratio[OR] = 4.71; 95% CI, 2.48 to 8.96) than “other” Medicaid-enrolled women to receive any treatment, more than three times as likely to receive any drug regimen, and almost 60% more likely to receive any radiation. They were approximately three times more likely to receive any definitive surgery (OR = 2.52; 95% CI, 1.74 to 3.66) during our 2-year observation period.
Table 1.
Multivariate Analysis, Receipt of Treatment Among Women With Breast Cancer Enrolled in Medicaid in Georgia
| Independent Variables | Any Treatment (n = 1,911) |
Any Drug Regimen (n = 1,680) |
Any Radiation (n = 1,680) |
Any Definitive Surgery (n = 1,772) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Medicaid eligibility group | ||||||||||||
| BCCPTA | 4.71 | 2.48 to −8.96 | < .001 | 3.58 | 2.32 to −5.51 | < .001 | 1.61 | 1.15 to −2.24 | .005 | 2.52 | 1.74 to 3.66 | < .001 |
| Disabled | 2.02 | 1.08 to −3.79 | .029 | 2.29 | 1.51 to −3.47 | < .001 | 0.97 | 0.69 to −1.34 | .834 | 1.02 | 0.68 to 1.52 | .920 |
| Others | Ref. | Ref. | Ref. | Ref. | ||||||||
| Previously enrolled | 2.41 | 1.28 to −4.56 | .007 | 1.07 | 0.72 to −1.59 | .726 | 1.14 | 0.86 to −1.53 | .355 | 7.66 | 5.06 to 11.59 | < .001 |
| Enrolled > 24 months | 1.42 | 0.88 to −2.31 | .153 | 1.47 | 1.08 to −1.99 | .013 | 1.07 | 0.87 to −1.33 | .500 | 1.46 | 1.14 to 1.87 | < .001 |
| Age at Medicaid enrollment, years | ||||||||||||
| 19-44 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 45-54 | 0.84 | 0.46 to −1.54 | .577 | 0.73 | 0.49 to −1.08 | .111 | 0.83 | 0.64 to −1.08 | .164 | 0.95 | 0.70 to 1.29 | .720 |
| 55-63 | 0.63 | 0.34 to −1.18 | .149 | 0.56 | 0.38 to −0.84 | .005 | 0.79 | 0.60 to −1.03 | .085 | 0.76 | 0.55 to 1.05 | .090 |
| Race/ethnicity | ||||||||||||
| White | Ref. | Ref. | Ref. | Ref. | ||||||||
| Black | 0.75 | 0.45 to −1.25 | .277 | 0.96 | 0.70 to −1.33 | .819 | 1.13 | 0.91 to −1.42 | .268 | 0.99 | 0.76 to 1.28 | .920 |
| Other | 1.32 | 0.37 to −4.72 | .664 | 2.43 | 0.99 to −5.95 | .052 | 1.57 | 0.93 to −2.63 | .089 | 1.74 | 0.96 to 3.14 | .060 |
| Stage at diagnosis | ||||||||||||
| In situ | Ref. | — | — | — | — | — | — | Ref. | ||||
| Local | 1.18 | 0.58 to −2.42 | .650 | Ref. | Ref. | 1.02 | 0.69 to 1.51 | .900 | ||||
| Regional | 2.26 | 1.03 to −4.93 | .041 | 0.94 | 0.57 to −1.55 | .811 | 0.65 | 0.44 to −0.95 | .028 | 1.04 | 0.70 to 1.54 | .830 |
| Distant metastases | 0.51 | 0.21 to −1.21 | .125 | 2.38 | 1.42 to −4.00 | .001 | 1.58 | 1.08 to −2.33 | .019 | — | — | — |
| Comorbidity index | ||||||||||||
| 0 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 1 | 3.33 | 1.61 to −6.89 | .001 | 2.09 | 1.41 to −3.09 | < .001 | 1.04 | 0.81 to −1.32 | .778 | 1.17 | 0.88 to 1.56 | .290 |
| ≥ 2 | 1.47 | 0.76 to −2.83 | .254 | 1.30 | 0.86 to −1.97 | .216 | 0.85 | 0.64 to −1.14 | .284 | 1.45 | 1.00 to 2.10 | .040 |
| Resident county | ||||||||||||
| Central city, large metropolitan area | Ref. | Ref. | Ref. | Ref. | ||||||||
| Fringe county, large metropolitan area | 1.17 | 0.61 to −2.23 | .635 | 0.83 | 0.55 to −1.25 | .362 | 1.15 | 0.86 to −1.53 | .342 | 1.23 | 0.88 to 1.72 | .220 |
| Small metropolitan area | 0.72 | 0.35 to −1.47 | .365 | 0.98 | 0.60 to −1.59 | .919 | 0.68 | 0.50 to −0.94 | .019 | 0.55 | 0.38 to 0.81 | < .001 |
| Completely rural | 2.19 | 0.27 to −17.76 | .464 | 1.93 | 0.56 to −6.72 | .301 | 0.90 | 0.50 to −1.64 | .735 | 1.07 | 0.48 to 2.41 | .860 |
| % Household income < $15K | 1.04 | 1.00 to −1.08 | .034 | 1.01 | 0.99 to −1.04 | .274 | 1.01 | 1.00 to −1.03 | .069 | 1.05 | 1.03 to 1.07 | < .001 |
| With at least one oncology service hospital | 0.96 | 0.49 to −1.88 | .907 | 0.87 | 0.55 to −1.39 | .563 | 1.00 | 0.73 to −1.38 | .977 | 1.22 | 0.85 to 1.76 | .280 |
| With at least one CoC approval hospital | 1.57 | 0.73 to −3.37 | .244 | 0.81 | 0.49 to −1.33 | .400 | 0.98 | 0.69 to −1.39 | .916 | 0.77 | 0.51 to 1.16 | .210 |
| Ob/Gyn per 1,000 women | 0.69 | 0.14 to −3.27 | .635 | 0.99 | 0.37 to −2.65 | .984 | 0.60 | 0.30 to −1.21 | .152 | 1.03 | 0.45 to 2.39 | .930 |
NOTE. Bold type indicates statistical significance. Dashes indicate data not reported.
Abbreviations: BCCPTA, Breast and Cervical Cancer Prevention and Treatment Act; CoC, Commission on Cancer; Ob/Gyn, obstetrician/gynecologist; OR, odds ratio; Ref., reference.
Of the other covariates considered, women enrolled in Medicaid before diagnosis were more likely than those who enrolled at or after diagnosis to have received any surgery. Those continuously enrolled in Medicaid over the 2-year period were also more likely than their counterparts to receive a definitive surgery or a drug regimen. Later stage was associated with higher odds of receiving any drug regimen and any radiation, whereas greater comorbidities were associated with a higher likelihood of any treatment, any drug regimen, and definitive surgery.
The receipt of a lumpectomy versus a mastectomy involves both a clinical and personal decision; this decision also affects the type of adjuvant therapy recommended and/or received. As the results in Table 2 show, there were no significant differences by eligibility group in the receipt of lumpectomy versus mastectomy. However, women in BCCPTA were more likely to receive more follow-up after mastectomy; their odds of receiving two or more follow-up therapies were almost three times higher (OR = 2.70; 95% CI, 1.20 to 6.10) than those of women in the “other” eligibility group. Other factors affecting these odds include race/ethnicity, disease stage, and the health resources available in the county.
Table 2.
Multiple Analysis, Definitive Surgery and Receipt of Adjuvant Treatment Among Women With Breast Cancer Enrolleed in Medicaid in Georgia
| Variable | Lumpectomy Versus Mastectomy (n = 1,387) | Lumpectomy With Versus Without Radiation (n = 479) | Mastectomy and Follow-Up (n = 723) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One Versus None |
Two or More Versus None |
|||||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Medicaid eligibility group | ||||||||||||
| BCCPTA | 1.42 | 0.96 to −2.10 | .080 | 1.56 | 0.77 to −3.17 | .218 | 1.81 | 0.78 to −4.19 | .167 | 2.70 | 1.20 to −6.10 | .017 |
| Disabled | 0.76 | 0.52 to −1.10 | .140 | 0.78 | 0.39 to −1.58 | .495 | 1.14 | 0.53 to −2.43 | .742 | 1.53 | 0.73 to −3.24 | .264 |
| Other | Ref. | Ref. | Ref. | Ref. | ||||||||
| Previously enrolled | 1.35 | 0.96 to −1.90 | .081 | 1.33 | 0.69 to −2.56 | .400 | 1.29 | 0.63 to −2.63 | .491 | 0.99 | 0.50 to −1.98 | .984 |
| Enrolled > 24 months | 0.93 | 0.74 to −1.18 | .549 | 0.55 | 0.35 to −0.87 | .010 | 1.18 | 0.70 to −2.01 | .535 | 1.45 | 0.87 to −2.41 | .157 |
| Age at Medicaid enrollment, years | ||||||||||||
| 19-44 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 45-54 | 1.11 | 0.83 to −1.48 | .477 | 1.59 | 0.94 to −2.67 | .081 | 1.01 | 0.52 to −1.96 | .977 | 0.67 | 0.35 to −1.25 | .207 |
| 55-63 | 1.10 | 0.81 to −1.49 | .535 | 1.84 | 1.06 to −3.21 | .032 | 1.33 | 0.65 to −2.74 | .435 | 0.86 | 0.43 to −1.71 | .673 |
| Race/ethnicity | ||||||||||||
| White | Ref. | Ref. | Ref. | |||||||||
| Black | 0.86 | 0.67 to −1.10 | .239 | 0.99 | 0.64 to −1.55 | .981 | 2.09 | 1.17 to −3.73 | .013 | 2.04 | 1.17 to −3.56 | .012 |
| Other | 1.48 | 0.88 to −2.51 | .142 | 12.38 | 1.61 to −95.29 | .016 | 2.62 | 0.50 to −13.83 | .256 | 3.93 | 0.83 to −18.61 | .084 |
| Stage at diagnosis | ||||||||||||
| In situ | Ref. | — | — | — | — | — | — | — | — | — | ||
| Local | 0.67 | 0.47 to −0.94 | .021 | Ref. | Ref. | Ref. | ||||||
| Regional | 0.36 | 0.25 to −0.51 | < .001 | 1.07 | 0.70 to −1.63 | .759 | 1.48 | 0.86 to −2.53 | .157 | 0.33 | 0.19 to −0.54 | < .001 |
| Distant metastases | — | — | — | — | — | — | — | — | — | — | — | — |
| Comorbidity index | ||||||||||||
| 0 | Ref. | Ref. | Ref. | Ref. | ||||||||
| 1 | 0.87 | 0.67 to −1.14 | 0.319 | 1.21 | 0.73 to −2.03 | .457 | 1.02 | 0.54 to −1.93 | .956 | 1.35 | 0.73 to −2.49 | .339 |
| ≥ 2 | 0.78 | 0.57 to −1.09 | 0.143 | 0.83 | 0.46 to −1.50 | .536 | 1.11 | 0.54 to −2.28 | .769 | 1.19 | 0.59 to −2.37 | .630 |
| Resident county | ||||||||||||
| Central city, large metropolitan area | Ref. | Ref. | Ref. | Ref. | ||||||||
| Fringe county, large metropolitan area | 1.12 | 0.82 to −1.53 | 0.466 | 1.60 | 0.91 to −2.82 | .103 | 0.38 | 0.17 to −0.85 | .019 | 0.52 | 0.24 to −1.14 | .103 |
| Small metropolitan area | 0.72 | 0.50 to −1.04 | 0.079 | 1.64 | 0.82 to −3.29 | .162 | 0.59 | 0.25 to −1.36 | .214 | 0.72 | 0.32 to −1.63 | .435 |
| Completely rural | 1.02 | 0.54 to −1.93 | 0.953 | 0.61 | 0.20 to −1.80 | .369 | 0.57 | 0.12 to −2.73 | .486 | 0.57 | 0.13 to −2.57 | .465 |
| % Household income < $15K | 0.98 | 0.96 to −1.00 | 0.029 | 1.01 | 0.98 to −1.05 | .419 | 0.97 | 0.92 to −1.01 | .106 | 0.97 | 0.93 to −1.01 | .156 |
| With at least one oncology service hospital | 1.05 | 0.74 to −1.49 | 0.786 | 0.71 | 0.37 to −1.36 | .303 | 0.33 | 0.15 to −0.75 | .008 | 0.38 | 0.18 to −0.83 | .015 |
| With at least one CoC-approved hospital | 0.67 | 0.45 to −0.99 | 0.044 | 2.13 | 1.05 to −4.33 | .036 | 0.69 | 0.28 to −1.68 | .411 | 0.70 | 0.30 to −1.63 | .408 |
| Ob/Gyn per 1,000 women | 2.55 | 1.20 to −5.44 | 0.015 | 0.35 | 0.08 to −1.56 | .170 | 6.25 | 1.08 to −36.08 | .041 | 3.24 | 0.59 to −17.71 | .175 |
NOTE. Bold type indicates statistical significance. Dashes indicate data not reported.
Abbreviations: BCCPTA, Breast and Cervical Cancer Prevention and Treatment Act; CoC, Commission on Cancer; Ob/Gyn, obstetrician/gynecologist; OR, odds ratio; Ref., reference.
Discussion
Georgia's BCCPTA program, the Women's Health Medicaid Program, appears to create a quicker pathway to Medicaid eligibility12 and, as shown here, provide greater access to services, after controlling for observed patient characteristics. Although care patterns of Medicaid enrollees have been studied in other settings using linked Medicaid and Surveillance, Epidemiology, and End Results registry data, these studies took place before the establishment of BCCPTA.17 Results indicated that Medicaid-insured women were less likely to receive radiation if they had breast-conserving surgery, more likely to receive no surgery, and more likely to have died within the study period than non–Medicaid-insured women. Using linked Medicaid and state registry (North Carolina) data, Kimmick et al18 found that 67% (n = 974) received mastectomy and 43% received adjuvant chemotherapy, and of those women who received breast-conserving surgery, 67% received adjuvant therapy.
The advantage of the present study is our ability to identify cancer treatment through Medicaid claims beyond the period covered by the GCCR and for a post-BCCPTA period. By using longitudinal data on inpatient, outpatient, and drug claims, we were able to capture not only surgical and radiation procedures but also detailed drug regimens. We used the drug claims files along with generic/brand names and National Drug Code numbers to capture the receipt of oral chemotherapy (if not captured through administration codes) as well as hormonal oral agents (selective estrogen receptor modulators, aromatase inhibitors, and other oral hormonal agents) covered by Georgia Medicaid. Our estimated levels of service receipt are comparable to those found in the study of women receiving Medicaid in North Carolina18 but indicate higher rates of chemotherapy receipt and, in particular, any adjuvant therapy after a mastectomy. An important difference is our finding that black women who had undergone mastectomy were more likely to receive adjuvant therapy, whereas Kimmick et al18 found no differences by race. The need for adjuvant therapy is higher among those with triple-negative disease, for which black women are at higher risk.26 Our sample may include more black women with this type of breast cancer, but differences in the two studies are also likely due to our inclusion of women in BCCPTA.
Although more than 90% of women in our sample received some treatment, only 70% of women received radiation after a lumpectomy. This compares poorly with the 95.4% of women in the general population of patients with breast cancer who had a strong indication for radiation23 but is similar to the results in the North Carolina Medicaid study completed by Kimmick et al.18 Currently, many breast cancer centers are using a National Quality Forum indicator, which calls for monitoring the rate of radiation therapy after breast-conserving surgery within 1 year of diagnosis for those younger than 70.24 To increase compliance with the National Quality Forum indicators in patients with historically poorer access to care, researchers in the AVON Foundation Comprehensive Breast Center at Grady hired a medical oncology nurse practitioner to coordinate care, trained patient navigators to emphasize the importance of multimodal therapy, and focused the role of the radiation oncology social worker on tracking patients who missed appointments, resulting in an increase from 75.8% to 95.8% compliance.25 To meet today's quality standards, practitioners treating patients with breast cancer throughout Georgia could benefit from such an approach.
Although this is one of the few studies to examine the treatment patterns of women enrolled in BCCPTA, we note several limitations. First, the Georgia data cannot be generalized because each state operates its BCCPTA program differently. Georgia is one of 12 states that chose the most expansive coverage27and potentially, enrolled more uninsured women in need of cancer treatment. This may result in larger variation in breast cancer cases and treatment patterns and may also put more of a burden on the capacity of cancer care providers in a given state.
We identified a group of women with breast cancer who were insured largely by Medicaid and found the characteristics of women in the BCCPTA, disabled, and “other” groups to be significantly different. We controlled for these observed confounders but note that unobserved differences, such as perceptions of body image, that exist across the groups could lead to bias. We did, however, control for factors such as age, which relates to issues of childbearing status,22 and race/ethnicity, which relates to unmeasured aspects of clinical diagnosis, as well as the direct measures of relative severity (stage and comorbidities) we controlled for.
We also lacked detail on hormonal status and clinical cancer stage. Because we know that younger women are more prone to hormone receptor–negative breast disease and black women are more prone to triple-negative disease, this could have biased our results if there were differences across the Medicaid eligibility groups on these measures. Moreover, we were unable to create a subset of patients with localized breast radiotherapy, which is usually not appropriate for patients with distant metastases. Instead, we restricted the sample to low-income, Medicaid-insured women younger than 65 and used multiple regression analysis to control for confounders such as age and race that affect hormone receptivity.
Another limitation may be due to unequal disenrollment from Medicaid across eligibility groups, which might bias our analysis. Relative to the other Medicaid eligibility groups, women in BCCPTA were less likely to disenroll from Medicaid because their recertification process was based on whether they were in active treatment. Yet, we still found that approximately 40% of women in BCCPTA did not remain continuously enrolled over a 2-year period. To test whether disenrollment patterns affected the study results, we controlled for variation in enrollment patterns and found that “enrolled over 24 months” increased the odds of receiving any drug regimen, any surgery, and any definitive surgery. We also completed a sensitivity analysis including only those continuously enrolled in Medicaid for more than 2 years. The results are robust.
A key limitation is that we could not track patients' treatments that occurred outside Medicaid coverage or beyond our study period; this may bias our measure of treatments received downward. Finally, it is possible that women in our sample had other cancers that were not readily detected through claims data and not noted in the GCCR. We did restrict our sample, however, to those women for whom the GCCR noted breast cancer as their first cancer diagnosis.
In conclusion, we found that the Georgia BCCPTA provided a new pathway to insurance coverage for low-income, uninsured women with breast cancer and, in turn, significantly more treatment than received by other Medicaid patients. The stronger linkage of women in BCCPTA with their providers and their ability to stay enrolled in Medicaid may mean that they receive not only more services, but also services more in line with clinical guidelines. Still, our finding that the overall rate of adjuvant therapy, whether radiation or hormonal/chemotherapy, appears to fall short of national criteria deserves attention.
Georgia policy makers and providers should be commended for implementing the BCCPTA program in a timely and effective manner and allowing all providers to screen and identify BCCPTA-eligible women. As we wait for the full impact of health care reform under the Patient Protection and Accountability Care Act, such an expansion could provide a much-needed safety net for low-income women with breast cancer. Even with the implementation of the Patient Protection and Accountability Care Act, it is likely that programs such as the patient navigation system set in place at Atlanta's inner-city public hospital will be needed to encourage completion of all modalities of care monitored by the National Quality Forum indicators for low-income women, and especially for racial/ethnic minorities28 within this vulnerable population.
Supplementary Material
Acknowledgment
We thank Laura Schild and Cheryl Raskind-Hood for their able assistance in creating the analytic files for this research.
Supported by Grant No. RSGT-05-004-01-CPHPS from the American Cancer Society (E.K.A., principal investigator).
The opinions reflected herein are those of the authors and do not necessarily reflect those of the funding agency.
Appendix
Table A1.
Summary Characteristics of Women With Breast Cancer Enrolled in Medicaid in Georgia, by Eligibility Group (full sample)
| Characteristic | Medicaid Eligibility Group |
Total | P | ||
|---|---|---|---|---|---|
| BCCPTA | Disabled | Other | |||
| Sample size | 1,046 | 674 | 328 | 2,048 | |
| Age at Medicaid enrollment, % | < .001 | ||||
| 19-44 | 26.1 | 20.3 | 46.0 | 27.4 | |
| 45-54 | 42.4 | 36.8 | 26.2 | 38.0 | |
| 55-63 | 31.5 | 42.9 | 27.7 | 34.6 | |
| Race/ethnicity, % | < .001 | ||||
| White | 48.9 | 38.6 | 37.8 | 43.7 | |
| Black | 44.7 | 59.1 | 57.9 | 51.6 | |
| Other | 6.4 | 2.4 | 4.3 | 4.7 | |
| Stage at diagnosis, % | < .001 | ||||
| In situ | 10.4 | 12.0 | 13.1 | 11.4 | |
| Local | 42.0 | 32.2 | 43.3 | 39.0 | |
| Regional | 39.2 | 39.3 | 34.5 | 38.5 | |
| Distant metastases | 4.5 | 13.2 | 3.0 | 7.1 | |
| Unstaged | 3.9 | 3.3 | 6.1 | 4.1 | |
| Comorbidity index, % | < .001 | ||||
| 0 | 65.1 | 41.2 | 54.3 | 55.5 | |
| 1 | 24.5 | 26.9 | 22.3 | 24.9 | |
| [gte] 2 | 10.0 | 28.0 | 13.1 | 16.5 | |
| Missing | 0.4 | 3.9 | 10.4 | 3.1 | |
| Previously enrolled, % | 2.6 | 49.3 | 50.3 | 25.6 | < .001 |
| Enrolled > 24 months, % | 60.1 | 52.7 | 38.4 | 54.2 | < .001 |
| Duration of enrollment | < .001 | ||||
| Mean | 21.2 | 19.4 | 18.8 | 20.2 | |
| SD | 4.5 | 6.4 | 5.8 | 5.5 | |
| Residency, % | .002 | ||||
| Central city, large metropolitan area | 31.1 | 33.5 | 29.3 | 31.6 | |
| Fringe county, large metropolitan area | 42.7 | 33.7 | 45.1 | 40.1 | |
| Small metropolitan area | 22.6 | 29.1 | 22.9 | 24.8 | |
| Completely rural | 3.6 | 3.7 | 2.7 | 3.5 | |
| % Household income < $15K | < .001 | ||||
| Mean | 23.1 | 25.0 | 23.3 | 23.7 | |
| SD | 9.9 | 9.2 | 9.8 | 9.7 | |
| With at least oneoncology service hospital, % | 65.3 | 60.4 | 64.9 | 63.6 | .102 |
| With at least one Commission on Cancer–approved hospital, % | 55.0 | 51.9 | 54.9 | 54.0 | .436 |
| Obstetrician/gynecologist per 1,000 women | .939 | ||||
| Mean | 0.27 | 0.27 | 0.27 | 0.27 | |
| SD | 0.2 | 0.2 | 0.2 | 0.2 | |
Abbreviation: BCCPTA, Breast and Cervical Cancer Prevention and Treatment Act; SD, standard deviation.
Table A2.
Sources of Data
| Variable name | Source | Definition |
|---|---|---|
| Age at Medicaid enrollment | Medicaid enrollment file | 19-44, 45-54, 55-63 |
| Race/ethnicity | Georgia Comprehensive Cancer Registry | White, black, other |
| Stage at diagnosis | Georgia Comprehensive Cancer Registry | In situ, local, regional, distant metastases, unstaged |
| Comorbidity index | Medicaid claims | 0, 1, < 2 |
| Previously enrolled | Medicaid enrollment file | Yes, no |
| Enrolled > 24 months | Medicaid enrollment file | Yes, no |
| Duration of enrollment | Medicaid enrollment file | Yes, no |
| Residency | Area Resource File | Central city, large metropolitan area; fringe county, large metropolitan area; small metropolitan area; completely rural |
| % Household income < $15K | Consolidated Analysis Center | Continuous |
| With at least one oncology service hospital | Area Resource File | Yes, no |
| With at least one Commission on Cancer–approved hospital | Commission on Cancer | Yes, no |
| Obstetrician/gynecologist per 1,000 women | Area Resource File | Continuous |
| Type of treatment | Medicaid claims | Any, any drug, any radiation, any definitive surgery |
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: E. Kathleen Adams
Financial support: E. Kathleen Adams
Collection and assembly of data: Li-Nien Chien
Data analysis and interpretation: E. Kathleen Adams, Li-Nien Chien, Sheryl G.A. Gabram-Mendola
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Makuc DM, Freid VM, Parsons PE. Health insurance and cancer screening among women. Advance Data. 1994;254:1–12. [PubMed] [Google Scholar]
- 2.CDC. Health insurance coverate and receipt of preventive health services – United States, 1993. MMWR Morb Mortal Wkly Rep. 1995;44:219–225. [PubMed] [Google Scholar]
- 3.CDC. Self-reported use of mammography and insurance status among women aged or =40 years – United States, 1991-1992 and 1996-1997. MMWR Morb Mortal Wkly Rep. 1998;47:825–830. [PubMed] [Google Scholar]
- 4.O'Malley AS, Mandelblatt J, Gold K, et al. Continuity of care and the use of breast and cervical cancer screening services in a multiethnic community. Arch Int Med. 1997;157:1462–1470. [PubMed] [Google Scholar]
- 5.Pamuk E, Makuc D, Heck K, et al. Health, United States, 1998. Hyattsville, MD: National Center for Health Statistics; 1998. Socioeconomic status and health chartbook. [Google Scholar]
- 6.Fronstin P. The working uninsured: Who they are, how they have changed, and the consequences of being uninsured with presidential candidate proposals outlined. EBRI Issue Brief No. 2000;224:1–23. http://www.ebri.org/publications/ib/index.cfm?fa=ibDisp&content_id=139. [PubMed] [Google Scholar]
- 7.Freeman JD, Kadiyala S, Bell JF, et al. The causal effect of health insurance on utilization and outcomes in adults: A systematic review of US studies. Med Care. 2008;46:1023–1032. doi: 10.1097/MLR.0b013e318185c913. doi:1010.1097/MLR.1020b1013e318185c318913. [DOI] [PubMed] [Google Scholar]
- 8.Sambamoorthi U, McAlpine DD. Racial, ethnic, socioeconomic, and access disparities in the use of preventive services among women. Prev Med. 2003;37:475–484. doi: 10.1016/s0091-7435(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 9.Chen AY, Schrag NM, Halpern MT, et al. The impact of health insurance status on stage at diagnosis of oropharyngeal cancer. Cancer. 2007;110:395–402. doi: 10.1002/cncr.22788. [DOI] [PubMed] [Google Scholar]
- 10.Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: A retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen AY, Schrag NM, Halpern M, et al. Health insurance and stage at diagnosis of laryngeal cancer: Does insurance type predict stage at diagnosis? Arch Otolaryngol Head Neck Surg. 2007;133:784–790. doi: 10.1001/archotol.133.8.784. [DOI] [PubMed] [Google Scholar]
- 12.Adams EK, Chien LN, Florence CS, et al. The Breast and Cervial Cancer Prevention and Treatment Act in Georgia effects on time to Medicaid enrollment. Cancer. 2009;115:1300–1309. doi: 10.1002/cncr.24124. [DOI] [PubMed] [Google Scholar]
- 13.Chien LN, Adams EK. The effect of Breast and Cervical Cancer Prevention and Treatment Act on Medicaid disenrollment. Women's Health Issues. 2010;20:266–271. doi: 10.1016/j.whi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Romano PS, Roos LL, Jollis JG. Presentation adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: An overview. Med Care. 2002;40(suppl):IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 17.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 18.Kimmick G, Camacho F, Foley KL, et al. Racial differences in patterns of care among Medicaid-enrolled patients with breast cancer. J Oncol Pract. 2006;2:205–213. doi: 10.1200/jop.2006.2.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lantz PM, Soliman S. An evaluation of a Medicaid expansion for cancer care: The Breast and Cervical Cancer Prevention and Treatment Act of 2000. Women's Health Issues. 2009;19:221–231. doi: 10.1016/j.whi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Ogilvie GS, Shaw EA, Lusk SP, et al. Access to colposcopy services for high-risk Canadian women: Can we do better? Can J Public Health. 2004;95:346–351. doi: 10.1007/BF03405143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobb R, Allen JD, Emmons KM, et al. Timely care after an abnormal mammogram among low-income women in a public breast cancer screening program. Arch Intern Med. 2010;170:521–528. doi: 10.1001/archinternmed.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien L-N, Adams EK, Flowers CL. Treating cervical cancer: Breast and Cervical Cancer Prevention and Treatment Act patients. Am J Obstet Gynecol. 2011;204:533.e1–533.e8. doi: 10.1016/j.ajog.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 23.Jagsi R, Abrahamse P, Morrow M, et al. Patterns and correlates of adjuvant radiotherapy receipt after lumpectomy and after mastectomy for breast cancer. J Clin Oncol. 2010;28:2396–2403. doi: 10.1200/JCO.2009.26.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyengar R, Lund MJ, Lamson P, et al. Using National Quality Forum breast cancer indicators to measure quality of care for patients in an AVON comprehensive breast center. Breast J. 2010;16:240–244. doi: 10.1111/j.1524-4741.2010.00909.x. [DOI] [PubMed] [Google Scholar]
- 25.Rizzo M, Bumpers H, Okoli J, et al. Improving on National Quality indicators of breast cancer care in a large public hospital as a means to decrease disparities for African American (AA) women. Ann Surg. 2011;18:34–39. doi: 10.1245/s10434-010-1204-z. [DOI] [PubMed] [Google Scholar]
- 26.Ray M, Polite BN. Triple-negative breast cancers: A view from 10,000 Feet. Cancer J. 2010;16:17–22. doi: 10.1097/PPO.0b013e3181d3eef5. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Breast and cervical cancer prevention and treatment activity map. 2010. [Accessed 1/12, 2010]. http://www.cms.hhs.gov/MedicaidSpecialCovCond/Downloads/BREASTandCERVICALCANCERPREVENTIONandTREATMENTACTIVITYMAP.pdf.
- 28.Gabram SG, Lund MJ, Gardner J, et al. Effects of an outreach and internal navigation program on breast cancer diagnosis in an urban cancer center with a large African-American population. Cancer. 2008;113:602–607. doi: 10.1002/cncr.23568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.