Abstract
Background:
The “Diagnosis and Treatment of Sleep Apnea in Cerebrovascular Disease” (GoToSleep) study is evaluating a strategy to improve the diagnosis and treatment of sleep apnea among veterans with stroke or transient ischemic attack (TIA) who also have hypertension. Specifically, the GoToSleep study was designed to overcome some of the barriers that exist within the Veterans Health Administration (VHA) to the timely diagnosis and treatment of sleep apnea by using ambulatory home-based polysomnography and auto-titrating continuous positive airway pressure (CPAP) to reduce the reliance on laboratory-based sleep studies.
Methods:
The GoToSleep study is a prospective, multi-site, randomized, controlled strategy trial among an expected 318 veterans with cerebrovascular disease and hypertension who are assigned to an intervention group or a control group. Patients in the intervention group receive unattended polysomnography at baseline, and those with sleep apnea receive auto-titrating CPAP therapy for up to one year. Patients in the control group receive usual care and unattended polysomnography at the end of the study to identify the rate of undiagnosed sleep apnea. The primary objectives of the GoToSleep study are to determine whether a diagnostic and therapeutic intervention strategy among veterans with cerebrovascular disease and hypertension improves: (1) detection of sleep apnea; (2) appropriate treatment for sleep apnea; and (3) control of hypertension. Twenty-four-hour blood pressure assessments are made at baseline and at the end of the one-year study period for both groups. Antihypertensive medications and their doses are recorded at the time of the 24-hour blood pressure measurements.
Discussion:
This manuscript provides the rationale for 4 key components of the design of the GoToSleep trial: the inclusion of patients with cerebrovascular disease and hypertension without the use of a measure of daytime sleepiness as an eligibility criterion; the use of portable polysomnography and auto-titrating CPAP in patients' homes rather than using sleep laboratory polysomnography with fixed pressure CPAP; the analytic approach to evaluating change in blood pressure in the context of change in antihypertensive medications; and the use of a usual care control group.
Citation:
Bravata DM; Ferguson J; Miech EJ; Agarwal R; McClain V; Austin C; Struve F; Foresman B; Li X; Wang Z; Williams LS; Dallas MI; Couch CD; Sico J; Fragoso C; Matthias MS; Chumbler N; Myers J; Burrus N; Dube A; French DD; Schmid AA; Concato J; Yaggi HK. Diagnosis and treatment of sleep apnea in patients' homes: the rationale and methods of the “GoToSleep” randomized-controlled trial. J Clin Sleep Med 2012;8(1):27–35.
Keywords: Sleep apnea, brain ischemia, hypertension
BRIEF SUMMARY
Current Knowledge/Study Rationale: This manuscript describes the rationale and methods of the GoToSleep study which seeks to evaluate a strategy of diagnosing and treating sleep apnea among veterans with cerebrovascular disease and hypertension. The intervention strategy was designed to reduce the reliance on laboratory-based sleep studies and to enhance treatment adherence by conducting diagnostic and therapeutic activities within patients' homes.
Study Impact: This manuscript describes four key elements of the GoToSleep study design that are either unique to this study or rarely employed in clinical trials: the inclusion of patients with cerebrovascular disease and hypertension without use of a measure of daytime sleepiness as an eligibility criterion; the use of portable polysomnography and auto-titrating CPAP in patients' homes rather than using sleep laboratory polysomnography with fixed pressure CPAP; the analytic approach to evaluating change in blood pressure in the context of change in antihypertensive medications; and the use of a usual care control group.
Sleep apnea is common, affecting as many as one-fifth of the adult population,1 and found in approximately two-thirds of patients who have had a stroke or transient ischemic attack (TIA).2–10 Sleep apnea has been independently associated with a variety of adverse metabolic and disease states and outcomes including hypertension, diabetes, stroke and TIA, incident coronary events and cardiovascular mortality, and all-cause mortality.1,4,11–13 Unfortunately, estimates suggest that as many as 70% to 80% of patients with sleep apnea are neither diagnosed nor treated.14 Even among patients with stroke and TIA, who are at particularly high risk of recurrent vascular events related to sleep apnea, screening for sleep apnea is uncommon. The barriers to diagnosing and treating sleep apnea consist of a variety of patient, provider, and system issues, including case-identification, provider awareness, and access to laboratory-based testing.14
The “Diagnosis and Treatment of Sleep Apnea in Cerebrovascular Disease” (GoToSleep) study is evaluating a strategy to improve the diagnosis and treatment of sleep apnea among veterans with stroke or TIA who also have hypertension. Specifically, the GoToSleep study was designed to overcome some of the barriers that exist within the Veterans Health Administration (VHA) to the timely diagnosis and treatment of sleep apnea by using home-based ambulatory polysomnography and auto-titrating continuous positive airway pressure (CPAP) to reduce the reliance on laboratory-based sleep studies.
The objectives of this manuscript are to describe the methodological approach being employed in the GoToSleep study. Specifically, this manuscript provides the rationale for 4 key components of the design of the GoToSleep trial: the inclusion of patients with cerebrovascular disease and hypertension without use of a measure of daytime sleepiness as an eligibility criterion; the use of portable polysomnography and auto-titrating CPAP in patients' homes rather than using sleep laboratory polysomnography with fixed pressure CPAP; the analytic approach to evaluating change in blood pressure in the context of change in antihypertensive medications; and the use of a usual care control group.
DESIGN AND METHODS
Overall Trial Design
The GoToSleep study is a prospective, multi-site, randomized, controlled strategy trial (NCT00984308). The primary objectives of the GoToSleep study are to determine whether a diagnostic and therapeutic intervention strategy among veterans with cerebrovascular disease and hypertension improves: (1) the detection of sleep apnea; (2) appropriate treatment for obstructive sleep apnea; and (3) control of hypertension.
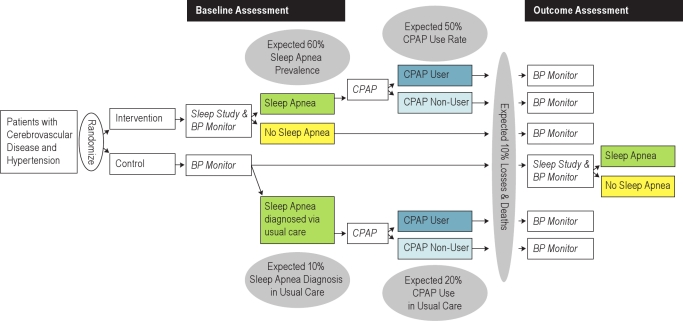
The intervention consists of a strategy intended to improve the diagnosis and long-term management of sleep apnea among veterans with cerebrovascular disease and hypertension. The GoToSleep study is randomly assigning 318 veterans with cerebrovascular disease and hypertension to an intervention group or a usual care control group in a 1:1 randomization scheme (Figure 1). The randomization is stratified by medical center (Connecticut versus Indiana), baseline blood pressure (mean systolic blood pressure from the 24-h ambulatory blood pressure monitor < 135 mm Hg versus ≥ 135 mm Hg for patients without diabetes, and < 125 mm Hg versus systolic ≥ 125 mm Hg for patients with diabetes), and risk of sleep apnea from the Berlin Questionnaire15 (“high risk” versus “low risk”). Patients in the intervention group receive unattended polysomnography, usually in their home, at baseline; those with sleep apnea receive auto-titrating CPAP therapy for up to one year. Patients in the usual care control group receive unattended polysomnography at the end of the study to identify undiagnosed sleep apnea. Twenty-four hour blood pressure assessments are made at baseline and at the end of the one-year study period for both groups. Antihypertensive medications are recorded with their doses at the time of the 24-h blood pressure measurements. Primary analyses will be conducted as intention-to-treat analyses.
Figure 1.
GoToSleep trial design
Study Administration
The GoToSleep project is being conducted at 2 VHA medical centers (West Haven, CT and Indianapolis, IN). Progress is evaluated via weekly video-conference research team meetings. Institutional review board (IRB) approvals have been received at both participating centers.
Inclusion and Exclusion Criteria
Patients are included if they have a history of ischemic stroke or TIA and either a diagnosis of hypertension or blood pressure ≥ 140/≥ 90 mm Hg.16 This study was designed prior to the 2009 revision of the American Heart Association/American Stroke Association definition of TIA and employs the following criteria: an ischemic stroke is a persistent focal neurological deficit of presumed ischemic origin lasting > 24 h,17 and a TIA is a focal neurological deficit of presumed ischemic origin lasting < 24 h.18 Brain imaging is not required for the diagnosis of stroke or TIA. We include the full spectrum of stroke severity, but given that we require patients to provide their own informed consent, patients with severe language impairments are unlikely to be eligible. Patients are recruited ≥ 30 days after their most recent stroke and at any point after their most recent TIA. No limits on the time from the last cerebrovascular event were imposed; we therefore anticipate that the GoToSleep cohort will include a spectrum of patients including those with a relatively recent event and those who are years after their last cerebrovascular event. The exclusion criteria and their justifications are provided in Table 1.
Table 1.
Exclusion criteria
| Exclusion Criterion | Rationale |
|---|---|
| Known sleep apnea | Such patients should already be receiving CPAP therapy |
| Suspected sleep disorder other than sleep apnea (e.g., narcolepsy) | Such patients should be referred for a formal polysomnography in a sleep laboratory |
| Life expectancy is less than 6 months | Care may be directed toward maximizing comfort and such patients may not be able to comply with the research protocol |
| Patients unable to use either a nasal or face mask (e.g., facial trauma) | CPAP* could not be administered |
| Patients who do not speak English | The intervention strategy involves forming a relationship between the patient and the staff who are performing auto-titrating CPAP administration and a language barrier would reduce the likelihood that such a relationship could be formed |
| Inability to provide informed consent (e.g., no proxy consents will be accepted) | This intervention requires active participation from the patient |
CPAP refers to continuous positive airway pressure.
All Patients
Baseline and Follow-up Assessments
Patient interviews and medical record reviews are conducted at baseline and at the end of study by trained research staff to obtain information about demographics, comorbidities, medication use, and symptoms. At these time points, patients are also examined to measure heart rate, neck circumference, height, and weight (using portable digital scale) for the calculation of body mass index (BMI). Daytime sleepiness is assessed using the Epworth Sleepiness Scale (ESS).19,20 The ESS is sensitive to change with appropriate treatment for sleep apnea.21,22 Functional status is measured with the Modified Rankin Scale which is sensitive to the full range of functional status from mild to severe impairment.23,24 Overall health status is assessed with the 12-item Short Form Health Survey (SF-12).25,26 The Berlin Questionnaire asks about snoring and daytime sleepiness and is used to classify patients according to high versus low risk for sleep apnea.15 All of the questionnaires are completed independent of the study staff and are scored centrally by a blinded member of the team. Neurological functioning is measured with the National Institutes of Health Stroke Scale (NIHSS).27,28
Blood Pressure Measurements
Blood pressure is measured for both control and intervention patients. Blood pressure measurements are taken at baseline and at the end of the study by trained research staff both manually and with a 24-h ambulatory monitor. Sitting blood pressure measurements are taken twice with a sphygmomanometer and stethoscope, using the right arm when possible. Arm and position are noted, and when possible, the same arm and position are used for each measurement for an individual patient. Orthostatic measurements are also recorded. A Doppler ultrasound is used to obtain ankle blood pressure measurements to calculate an ankle-brachial index.
Ambulatory 24-h blood pressure measurements are taken using the Dynapulse 5000A ambulatory blood pressure monitor (Pulsemetric Inc. San Diego, CA). Blood pressure measurements are taken every 30 min for a 24-h period. We use both a sleep diary and actigraphy (Actiwatch, Philips Respironics; placed on a non-paretic arm) to categorize blood pressure data as either sleeping or waking.29 Our primary approach for identifying periods of sleep is based on actigraphy data; we use sleep diary data for patients with incomplete actigraphy data. Nocturnal blood pressure dipping patterns will be assessed by comparing mean systolic blood pressure during sleep with mean systolic pressure during wake.30
Antihypertensive medications are not manipulated as part of the study protocol. All antihypertensive medications are recorded for each patient at baseline and at the end of the study. The Defined Daily Dose (DDD) of all antihypertensive medications is calculated using the World Health Organization (WHO) DDD methodology which allows for comparison across antihypertensive regimens.31 The DDD is defined as “the average maintenance dose per day for a drug used for its main indication in adults.”31 For example, the DDD for lisinopril is 10 mg per day and for hydrochlorothiazide is 25 mg per day. Therefore, if a patient is taking lisinopril 5 mg per day and hydrochlorothiazide 25 mg per day, their total DDD is 1.5 (0.5 for the lisinopril plus 1.0 for the hydrochlorothiazide).
A consolidated measure that includes both blood pressure and antihypertensive medication dose is needed to evaluate the potential blood pressure lowering effect of CPAP across the wide variety of antihypertensive medication regimens employed in routine clinical practice. In particular, an approach that includes both blood pressure and antihypertensive medications is needed in a long-term study of patients with hypertension who are receiving primary care, given that such patients are likely to have their antihypertensive medications adjusted over the course of the study period. For example, if CPAP therapy were to maintain “stable” blood pressure among patients in the intervention group, whereas patients in the control group were concurrently having their antihypertensive treatment up-titrated, then a blood pressure lowering impact of CPAP therapy would be masked if medication adjustments were not included in the analysis. The calculation of this consolidated measure that is used in the GoToSleep study is based on a meta-analysis of 354 randomized controlled trials of antihypertensive medications including the 5 most commonly used categories of antihypertensives: thiazides, β-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, and calcium channel blockers.32 This report found that all drug categories produced similar reductions in blood pressure, but the greatest reductions were observed among patients with the highest pretreatment blood pressures.32 The blood pressure lowering effect was also found to be additive across the various antihypertensive medication classes. The addition of each new antihypertensive medication at half the standard dose reduced systolic blood pressure by an average of 6.7 mm Hg.32 Given that the average reduction in blood pressure was 20% lower when medications were used at half the standard dose compared with full standard dose regimens,32 the addition of each full dose medication (1 DDD) is expected to reduce systolic blood pressure by 8.0 mm Hg. For each patient in the GoToSleep study, the medication-adjusted mean systolic blood pressure will be calculated from the mean systolic blood pressure measurement from the 24-h ambulatory monitor and the patient's DDD as follows: medication-adjusted SBP = [mean systolic blood pressure in mm Hg] + [patient's DDD × (8.0 mm Hg)].32 The medication-adjusted SBP is reported in mm Hg and provides a method for comparing observed blood pressures across a variety of antihypertensive medication regimens. For example, if a patient has a mean systolic blood pressure of 140 mm Hg and a DDD of 0.5 then the medication-adjusted SBP would be 144 mm Hg (140 mm Hg + [0.5 × 8 mm Hg]).
Protocol for Intervention Patients without Sleep Apnea and Control Patients
Intervention patients without sleep apnea and control patients receive an in-person visit at baseline, a telephone call at every other month, and an in-person visit at the end of the study.
Intervention Patients
Intervention Protocol: Full Unattended Sleep Study to Diagnose Sleep Apnea
Unattended polysomnography has been used with excellent reliability in other large scale trials and cohort studies.1,33–35 For the GoToSleep study, full unattended polysomnography is conducted using the portable Safiro device (Compumedics) providing data on: electroencephalogram (EEG), electrooculogram, chin electromyogram, thoracic and abdominal displacement, airflow, finger pulse oximeter, electrocardiogram, body position, leg movement, and audio recording of participant's snoring. Trained research staff members travel to the patient to apply the sleep monitor in the location that is most convenient for the patient (usually the patient's home). Research staff member travel to the same location the following morning to remove the sleep monitor. The polysomnographic data are read centrally and scored according to standard criteria.36
Polysomnographic data are used to identify respiratory events (apneas and hypopneas) while the patient is asleep. The sensor used to detect absence of airflow (for the identification of an apnea) is an oral nasal thermal sensor. The sensor used for the detection of airflow (for the identification of an hypopnea) is a nasal pressure transducer. An apnea-hypopnea index (AHI) is calculated as the number of respiratory events per hour of sleep.36–38 Patients with an AHI ≥ 5 events/h are diagnosed as having sleep apnea.37 Respiratory events made in the presence of thoracic or abdominal effort are scored as obstructive, whereas respiratory events made in the absence of thoracic and abdominal effort are scored as central events. If ≥ 50% of respiratory events are obstructive, patients are classified as having obstructive sleep apnea. If ≥ 50% of respiratory events are central, patients are classified as having central sleep apnea. Patients are classified has having Cheyne-Stokes respiration if the predominant breathing pattern (> 50%) of respiratory events takes the form of a waxing and waning ventilatory effort, and airflow with arousals typically occurring at the peak of the hyperpnea phase. If such a pattern is detected on the ambulatory sleep study, patients are continued in the study. However, such patients are not initiated on auto-titrating CPAP, but rather are referred through the local clinical sleep programs for initiation on adaptive servo-ventilation (ASV).
Auto-Titrating Continuous Positive Airway Pressure
Patients in the intervention group with obstructive sleep apnea are offered auto-titrating CPAP (System One, Respironics) consistent with the American College of Chest Physicians (ACCP) treatment guidelines to treat patients with documented cardiovascular disease including hypertension and stroke.37 The auto-titrating device being used in this study is able to distinguish central from obstructive respiratory events and respond accordingly.37,39,40
With the use of data card technology, the auto-titrating CPAP machine records pressure, residual AHI, air leak, and compliance data. We download data from the CPAP machines at least once during the first week, weekly during the first month, at the sixth month, and then at the final (close-out) visit. We assess treatment efficacy in 3 ways: (1) by examining and addressing any existing leak; (2) evaluating the residual AHI (targeted AHI < 5); and (3) evaluating oxygenation among patients with hypoxia on initial polysomnography. Oxygen saturation is measured concomitantly with the auto-titrating CPAP via one night of overnight oximetry performed during the first month of CPAP use for patients with good mask fit who have evidence of oxygen desaturations by polysomnography (< 88% for > 5 min during polysomnography). Patients with persistent desaturations despite auto-titrating CPAP are provided supplemental nocturnal oxygen via the CPAP machine.
Auto-titrating CPAP is administered to sleeping patients via a mask. Patients in the intervention group with sleep apnea are originally fit with their CPAP masks by members of the sleep medicine service in accordance with usual practice at the participating VA sites. Our study staff provides ongoing support to patients to ensure that patients receive the mask that is most comfortable and effective, with a goal unintentional leak of < 10 liters/minute.
Our approach to improving CPAP adherence consists of early intensive education and support, followed by regular and ongoing contact with patients. The education and support program is based on a patient narrative conceptual model.41 Based on this conceptual framework, for patients with sleep apnea, during the in-home visit where the staff members give the patients the results of the baseline polysomnography, staff members provide patients with information to about sleep apnea and CPAP and then ask patients to describe their understanding of sleep apnea, how sleep apnea may be related to their overall health status, and their reasons for trying to use CPAP.
During this session, staff answers questions that the patient or family members may have about CPAP. During the first month after delivery of the CPAP machine, the patient is visited each of the first 2 days and then weekly for the first month; thereafter patients receive monthly telephone calls. An in-home visit is scheduled after 6 months of CPAP use and at the end of the study to download the CPAP adherence data. In-person visits are also scheduled as needed upon patient request (e.g., for mask refitting). During the in-home visits, staff review with patients data from the CPAP machine (e.g., number of nights of use, number of hours of use, leak). Patients are instructed to call staff to answer questions about CPAP or the study at any point during the study period.
Comparison Group (Control) Patients
Control patients receive usual care as directed by their physicians, including care of their vascular risk factors such as hypertension and referral for usual care sleep apnea testing. Many veterans receive care both from VHA providers and from non-VHA providers. The research staff members inquire about all care that patients receive during the study period (e.g., medications provided by the VHA as well as medications obtained at private pharmacies). Control group patients who did not receive polysomnography as part of their usual care (expected to be the overwhelming majority of control group patients) will receive an unattended polysomnography at the end of the study. Polysomnographic data obtained as part of this study on control group patients are communicated with their primary care providers and the facility's sleep medicine service. We defer to the primary care provider regarding the long-term management of control group patients with sleep apnea, given that this diagnosis is made at the end of the study period.
OUTCOMES
Sleep Apnea Diagnosis Rate
The proportion of intervention and control patients with a diagnosis of sleep apnea will be calculated from the unattended polysomnography performed in the intervention group and from any polysomnography that was performed as part of usual care among control patients. We will use polysomnography data obtained as part of the study protocol at the end of the study period in the control group to determine the prevalence of undiagnosed sleep apnea among control patients.
Obstructive Sleep Apnea Treatment Rate
The proportion of intervention and control patients with obstructive sleep apnea who are receiving treatment will be calculated. Treatment of sleep apnea is defined as use of CPAP ≥ 4 h/night for ≥ 70% of nights42 or use of alternative (non-CPAP) therapies (e.g., positional therapy, oral appliance therapy, or upper airway surgical intervention). Within the VHA, the use of alternative therapies is rare; it is therefore expected that the treatment rate will be based primarily upon the use of CPAP.
Hypertension
The primary outcome measure for hypertension is change in mean 24-h systolic blood pressure from baseline to the end of the study. Ambulatory blood pressure monitoring has been shown to perform better than the use of single blood pressure assessments with a sphygmomanometer for the purpose of cardiovascular risk stratification, and it can be used to describe sleep versus wake blood pressure patterns.29,43 Our secondary hypertension outcomes include: (a) the change in medication-adjusted SBP from baseline to the end of the study; (b) the change in DDD from baseline to the end of the study; (c) the proportion of patients with a nocturnal decline (“dipping”) in systolic blood pressure of ≥ 10%30; and (d) the proportion of patients with either a decrease in mean systolic blood pressure of 5 mm Hg or any increase in blood pressure medication.
Cost Analysis
A cost analysis is planned as a secondary analysis for the GoToSleep trial. We are prospectively collecting all non-VHA direct healthcare costs (e.g., hospitalizations, outpatient visits, durable medical equipment) and overall and specific intervention costs. The prospective data are supplemented with administrative data from the VHA Decision Support System (DSS) that provides patient-level data for all VHA and fee-basis healthcare costs (e.g., hospitalizations, outpatient care, medications) by medical facility (adjusted for regional variation). Unlike Medicare, Medicaid and other prospective payment systems that are charge- or payment-based, the VHA Decision Support System captures actual costs of healthcare provided. For intervention costs, the research staff members perform micro-costing by recording the time (and mileage) they spend with study patients and record details about the activities and care they are engaged in with the patient. In this way, the overall intervention costs can be compared with potential economic savings or benefits of the intervention (reduced health care utilization and costs) to determine the overall net present value of the program (cost-benefit analysis).
Sample Size
The GoToSleep trial was designed to have ≥ 80% power to detect the following pre-specified differences in the 3 primary aims: 60% of intervention patients compared with 10% of control patients will be diagnosed with sleep apnea; 50% of intervention patients diagnosed with sleep apnea as part of the study protocol, compared with 15% of control patients diagnosed with sleep apnea as part of usual care, will use CPAP therapy; and the baseline mean systolic blood pressure in both the intervention and usual care groups will be 135 mm Hg with a standard deviation of 20 mm Hg, but the 1-year mean systolic blood pressure in the intervention group will be 125 mm Hg with a standard deviation of 20 mm Hg, whereas in the control group it will be 135 mm Hg with a standard deviation of 20 mm Hg. The sample size estimates further assumed an estimated 10% loss to follow-up in both the intervention and usual care arms.
DISCUSSION
Our purpose was to describe the methodological approach being employed in the GoToSleep study and to provide the rationale for the components of the design that are either unique to this study or rarely employed in clinical trials: the inclusion of patients with cerebrovascular disease and hypertension without use of a measure of daytime sleepiness as an eligibility criterion; the use of portable polysomnography and auto-titrating CPAP in patients' homes rather than using sleep laboratory polysomnography with fixed pressure CPAP; the analytic approach to evaluating change in blood pressure in the context of change in antihypertensive medications; and the use of a usual care control group.
Methodological Rationale
Rationale for Inclusion Criteria Based on History of Cerebrovascular Disease Rather Than Daytime Sleepiness Symptoms
Patients with cerebrovascular disease have a high prevalence of sleep apnea. Most studies in this area have reported that 60% to 70% of patients with stroke or TIA have sleep apnea.2,3,4,5,6,7,8,9–10,13,44 Therefore, in the GoToSleep study, patients with cerebrovascular disease were included on the basis of their high pre-test probability of having sleep apnea. No additional clinical characteristic (e.g., daytime sleepiness) were used as part of the inclusion criteria because we sought to include both patients who are symptomatic and patients who are asymptomatic but who nonetheless experience the physiologic consequences of their sleep related respiratory events.
It is expected that control group patients diagnosed with sleep apnea as part of their usual care are likely to be more symptomatic than intervention patients diagnosed with sleep apnea as part of the study, because patients in the usual care group with excessive daytime sleepiness may be more likely to receive polysomnography than control patients without symptoms. Despite conventional wisdom to the contrary, however, symptoms correlate poorly with sleep apnea severity.45 Yet symptomatic patients may be more likely to adhere to CPAP treatment. Therefore, the control patients with sleep apnea who are diagnosed as part of usual care may be more likely to use CPAP than patients diagnosed with sleep apnea in the intervention group. As part of the secondary analyses, we will examine the treatment rate among asymptomatic patients (ESS < 10) and among mild sleep apnea patients (AHI 5-15 events/h).
Rationale for Using Portable Unattended Polysomnography and Auto-titrating CPAP Rather than In-Laboratory Polysomnography and Fixed Pressure CPAP
Polysomnography performed in a sleep laboratory by a sleep technician is the gold standard for diagnosing sleep apnea, but inadequate sleep laboratory infrastructure to accommodate the US population is a major barrier to its use. The Institute of Medicine in its report “Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem” articulated the problem as follows: “…the current diagnostic and therapeutic capacity is not sufficient for the present demand, let alone the predicted increase in demand arising from the proposed public education campaign. Thus, additional technology development is required.”14 Unattended polysomnography is an alternative to polysomnography in a sleep laboratory. Several studies have reported on the agreement between unattended polysomnography and formal polysomnography with sensitivities of 75% to 100% and specificities of 87% to 100%.46 Alternatives to full polysomnography may be acceptable when patients cannot be assessed with polysomnography in a sleep laboratory.42 We designed the GoToSleep study to use portable technology so that sleep apnea could be diagnosed and treated in the patients' homes. Only in rare circumstances do we anticipate that patients will require additional sleep testing—either repeat portable testing or referral to in-laboratory polysomnography for more complex sleep disordered breathing (e.g., Cheyne-Stokes central sleep apnea).
The traditional approach to the treatment of sleep apnea involves the use of fixed-pressure CPAP. This approach requires a two-step process. First, a sleep study is performed to diagnose sleep apnea. Next, CPAP titration is performed by a sleep therapist who increases the fixed-pressure CPAP until the apneas and hypopneas are eliminated. Hence, the use of fixed-pressure CPAP requires that the patient have a sleep study performed in the presence of a sleep technician. Auto-titrating CPAP machines eliminate respiratory disturbances by varying the pressure delivered depending on the patients' respiratory efforts. We use auto-titrating CPAP in the GoToSleep study because it is as effective as fixed-pressure CPAP39,40 and because it does not require an in-laboratory sleep study for titration.
Rationale for Approach to Evaluating Blood Pressure in Context of Antihypertensive Medications
Although some clinical trials have used a measure of blood pressure as a clinical endpoint without evaluation of antihypertensive medication use, other trials have used a consolidated measure of antihypertensive management that includes both blood pressure and antihypertensive medication use (e.g., achievement of a goal blood pressure or any decrement in antihypertensive medication).47 Given that the patients in the GoToSleep study are all cared for by VA primary care providers, and given the emphasis in VA primary care on the management of blood pressure, we anticipate that patients in the GoToSleep study are likely to have their blood pressure measured and managed (e.g., antihypertensive medications adjusted) intensively over the course of the one-year study period.
Our primary blood pressure outcome is change in 24-hour mean systolic blood pressure, with a power calculation based on this outcome measure. We have pre-specified secondary blood pressure outcomes as described above. The medication-adjusted SBP measure will be used to evaluate the effect of the intervention strategy on hypertension control in the context of antihypertensive medications. The advantage of the medication-adjusted SBP outcome is that it includes both blood pressure and antihypertensive medications in a consolidated continuous measure where the output is in mm Hg, a unit that is readily interpretable by clinicians. Moreover, given the known relationship between impaired nocturnal blood pressure dipping and sleep apnea, we will also examine the change in the proportion of patients with a normal nocturnal decline in systolic blood pressure of at least 10% between the two groups.
Rationale for Using a Usual Care Control Group
There are a number of challenges regarding designing long-term randomized controlled trials using CPAP, including pragmatic issues (such as strategies to increase long-term CPAP adherence) as well as conceptual issues (such as safety/ethical considerations among control patients diagnosed with sleep apnea not receiving treatment). For example, one approach would be to use sham-CPAP as the control comparison.48 Significant safety considerations (e.g., drowsiness related motor vehicle accidents, recurrent vascular events, depression/suicide) arise, however, if sham positive pressure were to be used over a one-year intervention period among patients with known sleep apnea. In addition, the use of a sham-CPAP control group might reduce participant enrollment (if participants are unwilling to be randomized to a sham-CPAP group) or overall CPAP adherence (if participants are uncertain about the benefits of the CPAP that they are using).
Rather than a double-blind sham-CPAP control design, the GoToSleep study is a randomized diagnosis and treatment strategy trial. Such a study design serves to ask an important clinical question in the context of usual care (where patients are not prohibited from seeking care for sleep disordered breathing): What additional benefit is conferred by a universal approach to diagnosing and treating sleep apnea? We assert that “usual care” provides a safe means of evaluating the effectiveness of the intervention without incurring undue risks to patients. Patients who are in the control group may receive polysomnography during the study as part of their usual care, and they receive an unattended polysomnography at the end of the study period. The polysomnographic and other medical information (e.g., blood pressure data) are provided to the patients and to their primary care providers. We do not, however, automatically refer patients in the control group who are diagnosed with sleep apnea for treatment with CPAP as part of the study. Rather, we communicate with the primary care providers and offer to assist with establishing treatment depending on the primary care providers' preference.
Summary
As a study designed to address the question of whether a strategy of diagnosing and treating sleep apnea among patients with cerebrovascular disease and hypertension can improve rates of sleep apnea diagnosis, sleep apnea treatment, and blood pressure control, the GoToSleep trial has several strengths. First, the GoToSleep study has a relatively long intervention period of up to one year. Much of the literature has focused on short intervention periods (days or weeks), with very few studies examining long-term CPAP use. Second, the GoToSleep study will provide new information about the use of in-home management for both the diagnosis and treatment of sleep apnea. Several studies have examined the use of in-home or portable polysomnography for use in diagnosing sleep apnea, but few data exist on the use of in-home support to improve CPAP adherence.46 Although sleep medicine clinics may be effective in promoting CPAP adherence in general, our anecdotal experience suggests that the older population with cerebrovascular disease is often unable or unwilling to visit the sleep clinic because of difficulties with transportation or competing medical demands. These data will be especially relevant to hospital administrators who might reallocate some sleep medicine resources to the home setting. Third, the GoToSleep study has been designed to provide sufficient sample size to evaluate the issue of whether CPAP can improve hypertension control among patients with sleep apnea. Several studies have evaluated the relationship between CPAP and blood pressure, but the GoToSleep study has a randomized controlled trial design; a sample size large enough to provide sufficient power; and includes measurement of a variety of blood pressure related outcomes.49–53
The primary limitation of the GoToSleep trial is its focus on the veteran population, which may limit generalizability to women. Also, although the sample size has been designed to provide adequate power for the primary analyses, sample size constraints will not allow for subgroup analyses based either on cerebrovascular event type (stroke or TIA) or time from event (recent or distant).
CONCLUSION
The GoToSleep trial is expected to complete follow-up in 2011. Current guidelines include sleep apnea as a modifiable risk factor for stroke, but the current standard of care does not include routine assessment for sleep apnea among patients with stroke or TIA.54,55 We believe that the GoToSleep trial will provide needed information to clinicians caring for patients with cerebrovascular disease and hypertension and for policy makers deciding about how to allocate limited sleep medicine resources.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This project is supported by the Department of Veterans Affairs Health Services Research and Development Service (VA HSR&D; IIR-06-233-2).
REFERENCES
- 1.Young T, Peppard P, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Wessendorf T, Teschler H, Wang Y-M, Konietzko N, Thilmann A. Sleep-disordered breathing among patients with first-ever stroke. J Neurol. 2000;247:41–7. doi: 10.1007/pl00007787. [DOI] [PubMed] [Google Scholar]
- 3.Turkington P, Bamford J, Wanklyn P, Elliott M. Prevalence and predictors of upper airway obstruction in the first 24 hours after acute stroke. Stroke. 2002;33:2037–42. doi: 10.1161/01.str.0000023576.94311.27. [DOI] [PubMed] [Google Scholar]
- 4.Harbison J, Ford G, James O, Gibson G. Sleep-disordered breathing following acute stroke. Q J Med. 2002;95:741–7. doi: 10.1093/qjmed/95.11.741. [DOI] [PubMed] [Google Scholar]
- 5.Iranzo A, Santamaria J, Berenguer J, Sanchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;58:911–6. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 6.Dyken M, Somers V, Yamada T, Ren Z, Zimmerman M. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–7. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 7.Bassetti C, Aldrich M. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–23. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 8.Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–80. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg O, Franklin K, Bucht G, Gustafson Y. Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. J Am Geriatr Soc. 2001;49:391–7. doi: 10.1046/j.1532-5415.2001.49081.x. [DOI] [PubMed] [Google Scholar]
- 10.Kapen S, Goldberg J, Wynter J. The incidence and severity of obstructive sleep apnea in ischemic cerebrovascular disease. Neurology. 1991;41:125. [Google Scholar]
- 11.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi H. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122:1122–7. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. New Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 13.Mohsenin V, Valor R. Sleep apnea in patients with hemispheric stroke. Arch Phys Med Rehabil. 1995;76:71–6. doi: 10.1016/s0003-9993(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 14.Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academy of Sciences; 2006. [PubMed] [Google Scholar]
- 15.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Matchar DB. Clinical assessment of stroke. JAMA. 1994;271:1114–20. [PubMed] [Google Scholar]
- 18.Johnston S. Transient ischemic attack. New Engl J Med. 2002;347:1687–92. doi: 10.1056/NEJMcp020891. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. Reliability and factor analysis of the Epworth sleepiness scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 21.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial.[comment] Lancet. 2002;359:204–10. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 22.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 23.Rankin J. Cerebral vascular accidents in patients over the age of 60: prognosis. Scott Med J. 1957;2:200–15. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 24.Bonita R, Beaglehole R. Modification of Rankin Scale: Recovery of motor function after stroke. Stroke. 1988;19:1497–500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart. 2004;90:523–7. doi: 10.1136/hrt.2003.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupelian V, Wei JT, O'Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381–7. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 27.Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–31. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein L, Bertels C, Davis J. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660–2. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 29.Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension. 2000;35:844–51. doi: 10.1161/01.hyp.35.3.844. [DOI] [PubMed] [Google Scholar]
- 30.Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study.[see comment] J Hypertens. 2002;20:2183–9. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Norwegian Institute of Public Health. http://www.whocc.no/atcddd/. 2006 2005-10-19.
- 32.Law M, Wald N, Morris J, Jordan R. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonelli de Oliveira A, Martinez D, Vasconcelos L, et al. Diagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoring. Chest. 2009;135:330–6. doi: 10.1378/chest.08-1859. [DOI] [PubMed] [Google Scholar]
- 34.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367–74. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Flemons W. State of home sleep studies. Clin Chest Med. 2003;24:283–95. doi: 10.1016/s0272-5231(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 36.Sleep Disorders Atlas Task Force. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 37.Loube D, Gay P, Strohl K, Pack A, White D, Collop N. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115:863–6. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 38.Meoli A, Casey K, Clark R, et al. Hypopnea in sleep-disordered breathing in adults. Sleep. 2001;24:469–70. [PubMed] [Google Scholar]
- 39.Massie C, McArdle N, Hart R, et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167:20–3. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick M, Alloway C, Wakeford T, MacLean A, Munt P, Day A. Can patients with obstructive sleep apnea titrate their own continuous positive airway pressure? Am J Respir Crit Care Med. 2003;167:716–22. doi: 10.1164/rccm.200204-360OC. [DOI] [PubMed] [Google Scholar]
- 41.Bravata DM, Rastegar A, Horwitz RI. How do women make decisions about hormone replacement therapy. Am J Med. 2002;113:22–9. doi: 10.1016/s0002-9343(02)01148-8. [DOI] [PubMed] [Google Scholar]
- 42.Hirshkowitz M, Littner M, Kuna S, Berry RB, Norris M, Almenoff P. Sleep-related breathing disorders: sourcebook. 2nd ed. Milwaukee, WI: Healthcare Analysis & Information Group (HAIG), VHA; 2003. [Google Scholar]
- 43.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 44.Good D, Henkle J, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–9. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- 45.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 46.Gagnadoux F, Pelletier-Fleury N, Philippe C, Rakotonanahary D, Fleury B. Home unattended vs hospital telemonitored polysomnography in suspected obstructive sleep apnea syndrome: a randomized crossover trial. Chest. 2002;121:753–8. doi: 10.1378/chest.121.3.753. [DOI] [PubMed] [Google Scholar]
- 47.van Jaarsveld B, Krijnen P, Pieterman H, et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med. 2000;342:1007–14. doi: 10.1056/NEJM200004063421403. [DOI] [PubMed] [Google Scholar]
- 48.Kushida C, Nichols D, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2:288–300. [PubMed] [Google Scholar]
- 49.Hla K, Skatrud J, Finn L, Palta M, Young T. The effect of correction of sleep-disordered breathing on BP in untreated hypertension. Chest. 2002;122:1125–32. doi: 10.1378/chest.122.4.1125. [DOI] [PubMed] [Google Scholar]
- 50.Becker H, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 51.Wilcox I, Grunstein R, Hedner J, et al. Effect of nasal continuous positive airway pressure during sleep on 24-hour blood pressure in obstructive sleep apnea. Sleep. 1993;16:539–44. doi: 10.1093/sleep/16.6.539. [DOI] [PubMed] [Google Scholar]
- 52.Mayer J, Becker H, Brandenburg U, Penzel T, Peter J, von Wichert P. Blood pressure and sleep apnea: results of long-term nasal continuous positive airway pressure therapy. Cardiology. 1991;79:84–92. doi: 10.1159/000174864. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, Otsuka K, Guilleminault C. Long-term nasal continuous positive airway pressure administration can normalize hypertension in obstructive sleep apnea patients. Sleep. 1993;16:545–9. doi: 10.1093/sleep/16.6.545. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein L, Adams R, Alberts M, et al. Primary prevention of ischemic stroke. Stroke. 2006;37:1583–633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 55.Sacco R, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–49. [PubMed] [Google Scholar]