Abstract
Study Objectives:
This study examined changes in sleep parameters between the laboratory and the home setting before and after laboratory monitoring in depressed insomniacs undergoing treatment.
Methods:
This study was a post hoc analysis of a double-blind, randomized, placebo-controlled clinical trial performed with 60 depressed, insomniac outpatients. Patients underwent actigraphic monitoring along with sleep diaries over a continuous 2-week period. After one week of baseline monitoring, subjects spent one night in the laboratory with concurrent actigraphic and PSG monitoring with sleep diaries. Actigraphic monitoring and sleep diaries were continued for another week at home, along with initiation of open-label fluoxetine (FLX).
Results:
Actigraphically recorded laboratory sleep during the night in the laboratory was found to be improved relative to actigraphically recorded sleep at home, with less wake time and greater sleep time and sleep efficiency occurring in the laboratory. In contrast, sleep diaries indicated a slight worsening of sleep in the laboratory compared to home, with significantly more awakenings in the laboratory compared to the week at home before and after the laboratory night.
Conclusions:
The differences between objective and subjective sleep measurements seen in depressed insomniacs may be influenced by the monitoring setting and measurement modality.
Clinical Trial Information:
ClinicalTrials.gov Identifier: NCT00247624
Citation:
McCall C; McCall WV. Objective vs. subjective measurements of sleep in depressed insomniacs: first night effect or reverse first night effect? J Clin Sleep Med 2012;8(1):59-65.
Keywords: First night effect, reverse first night effect, actigraphy, sleep diaries, insomnia, depression
Many individuals experience worse sleep during their first night in a sleep laboratory than on successive nights. This “first night effect” (FNE) is typically characterized by increased sleep onset latency, increased REM latency, a lower percentage of REM stage sleep, and lower sleep efficiency as measured by polysomnography (PSG). While many studies have observed the FNE phenomenon in normal subjects and in those with depression or insomnia,1–3 others have observed a reduced or absent FNE.4–6 It has been suggested that factors such as the location of monitoring or pleasantness of the laboratory environment may attenuate this effect, or that individual variability may cancel out differences for entire samples.5,7–10
Many studies observing a FNE have also noted a “paradoxical” or “reverse” first night effect (RFNE) in some of their subjects.11–14 The RFNE is characterized by the observation of decreased sleep onset latency, decreased REM latency, a higher percentage of REM, and greater sleep efficiency in the first night at the laboratory relative to successive nights, as measured by PSG.13 For insomniacs who demonstrate this phenomenon, several explanations have been proposed. Hauri et al. suggested that maladaptive reinforcements are formed between the insomniac's sleeping problems and their normal sleep environment.13,15 This conditioned arousal reaction to their normal sleep environment does not generalize to the novel sleep environment of the laboratory, where they fall asleep more easily. Alternatively, de la Pena proposed that some insomniacs require a higher level of waking sensory stimulation to induce sleep, and the greater sensory stimulation of the novel laboratory environment improves their sleep.16
BRIEF SUMMARY
Current Knowledge/Study Rationale: Actigraphy and subjective sleep reports are used to measure sleep continuity, but few studies have compared these methods in both home and laboratory environments. This study analyzed differences between sleep continuity in the laboratory and sleep at home before and after laboratory monitoring using actigraphy, PSG, and subjective sleep reports, in depressed insomniacs undergoing treatment.
Study Impact: This study found that the presence of a first night effect versus a reverse first night effect depended upon the measurement modality used. Measurement of sleep using actigraphy and sleep diaries produced contradictory patterns of sleep continuity between home and laboratory settings in this sample of depressed insomniacs.
Studies investigating sleep laboratory adaptation effects have typically focused on comparing the first night of sleep in the laboratory with successive nights in the laboratory. In the explanations for the RFNE postulated by Hauri et al. and de la Pena, one assumption is that sleep during the first laboratory night is not only better than sleep during successive nights in the laboratory, but also better than sleep at home; however, this assumption has not been tested. Studies on the FNE comparing home sleep monitoring with laboratory sleep monitoring have typically compared the first night at home to successive nights at home.5,17–20 None to our knowledge have directly compared sleep in the laboratory with sleep at home before and after laboratory monitoring.
Also, studies reporting a RFNE in insomniacs have largely excluded patients with significant psychiatric illnesses such as depression; however, it has been shown that insomnia and depression are frequently comorbid conditions, with over 90% of depressed patients suffering from sleep complaints.21,22 Some studies on first night adaptation effects in depressed patients have observed a reduced or absent FNE2,9,23; however, it is unknown whether patients suffering from both insomnia and depression exhibit similar laboratory adaptation effects as those suffering from only one of these disorders.
The aim of this study was to analyze differences between sleep continuity variables in the laboratory and sleep at home before and after laboratory monitoring using actigraphic monitoring and sleep diaries, in subjects undergoing a 9-week clinical trial of simultaneous treatment of major depressive episode (MDE) with open-label fluoxetine (FLX) and insomnia with eszopiclone (ESZ) or placebo. Actigraphy was used as an objective sleep measurement method because of its utility as a noninvasive, cost-effective method of capturing sleep intervals over the entire 10-week course of the study. Although the use of actigraphy is controversial in some populations, including insomniacs,24–26 it has been indicated by the American Academy of Sleep Medicine as a valid method to characterize sleep patterns in insomnia.27 In previous analyses of this sample, actigraphy was found to provide a close approximation to polysomnography (PSG) in measurements of sleep onset latency (SOL), wake time after sleep onset (WASO), total sleep time (TST), and sleep efficiency (SE).28 Although actigraphy and subjective reports provide a far less invasive way to test differences between familiar and novel sleep environments, few studies have used actigraphy or subjective reports in testing the FNE, and those studies did not report a FNE with these methods when used at home.18,29 This study thus also examines the utility of actigraphy and subjective reports in capturing changes between home and laboratory environments.
METHODS
This is a post hoc analysis of a study whose primary endpoint was quality of life; full details of the research protocol are described elsewhere.30 Briefly, 60 patients diagnosed with both insomnia and MDE underwent a week of continuous baseline data collection with actigraphy and sleep diaries, followed by one night in a sleep laboratory with actigraphy, sleep diary, and polysomnographic data collection. This was followed by a second week of continuous data collection with actigraphy and sleep diaries. During this second week of the study, subjects also began the clinical treatment component of the study with the initiation of open-label FLX monotherapy, starting at 20 mg each morning.
Subjects
Sixty participants aged 19-64 years participated in the study. At baseline, participants either (a) reported sleep latency > 30 min and sleep efficiency < 85% at least 4 nights per week, or (b) met Research Diagnostic Criteria (RDC) for insomnia ≥ 4 nights per week.31 Phone screening confirmed a body mass index (BMI) ≤ 35, absence of habitual snoring or daytime sleepiness, absence of significant restless leg symptoms, and absence of substance abuse or medical illnesses likely to interfere with sleep.32
A confirmation diagnosis of unipolar MDE per Structural Clinical Interview for DSM-IV (SCID) was made during the first face-to-face visit after > 7 days of abstinence from all psychotropic medications (4 weeks for FLX).33 After baseline monitoring with PSG, participants with significant sleep apnea (apnea-hypopnea index [AHI] > 15) or clinically significant periodic limb movement (PLM) disorder (PLM-arousal index [PLMAI] of > 15) were excluded.34
Measurements of Sleep
The measurements of sleep continuity variables for this study included self-reported sleep diaries and actigraphy. PSG data were also obtained during laboratory monitoring nights.
Actigraphy
Participants continuously wore a Mini Mitter actigraphy monitor (AW64 Actiwatch) on their non-dominant wrist for the duration of the study. The AW64 output was scored using the proprietary Actiware Software, version 5.0. The medium sensitivity setting at 30-sec epochs was used for actigraphic data. The first week of the study collected baseline data prior to beginning treatment. The laboratory monitoring period occurred at the end of the first week, and was timed to begin at each participant's median bedtime as defined from prospective diary collection of the week prior. Actigraphic data for the second week was collected at the end of that week. Weekly averages of sleep variables obtained from week 1 and week 2 were computed from actigraphy software algorithms.
Sleep Diaries
Participants were instructed to keep prospective, daily sleep diary entries of their bedtime, sleep latency, number of awakenings, WASO, TST, and rising time for the duration of the study. Sleep diaries were collected at the end of each week and at the end of the laboratory monitoring night between week 1 and week 2. Participants also completed the Insomnia Severity Index (ISI) at baseline. Higher scores on the ISI represent greater degrees of insomnia.35
Sleep parameters obtained from actigraphic recordings and sleep diaries included SOL, number of awakenings, WASO, and TST. Actigraphic recordings additionally obtained sleep efficiency (SE) and rest period data.
PSG
At the end of their first week, participants completed one night of 8-h PSG in the sleep laboratory for baseline data collection. At this time participants were free of psychotropic medications for > 2 weeks. PSG was started at their median bedtime as defined from the prospective diary collection. The PSG montage included 4 EEG channels (C3-A2, C4-A1, C3-O1, and C4-O2), left and right EOG, chin EMG, right and left anterior tibialis EMG, EKG, nasal thermistor, oral thermistor, respiratory effort, and pulse oximetry. The PSG data were digitally collected on a VIASYS SomnoStar system. Scoring was completed according to standard criteria, and was blind to participant identity.36
Analysis
Post hoc analyses of the differences between sleep continuity variables at home and sleep in the lab were performed using SAS JMP (version 8.0) data analysis software. Basic analytic approaches included two-tailed paired t-tests to calculate whether the difference between home and laboratory sleep parameters were different from “0.” For all analyses, a two-tailed p-value < 0.05 was considered statistically significant.
Using this approach, we compared the mean values of sleep parameters during the first week of the study to the values obtained during the first night of laboratory testing, which occurred at the end of the first week. We also compared the laboratory values of that night with the mean values of the week afterwards. The rest period as recorded by actigraphy was also compared between home and laboratory environments, as differences in the rest period may potentially impact differences in the total amount of sleep and sleep efficiency seen between sleeping environments. Bonferroni corrections were applied to address the problem of multiple comparisons, with p < 0.005 required to achieve significance.
RESULTS
Patient Demographics and Baseline Symptoms
The overall randomized sample was middle-aged, two-thirds women, and 23% minorities. The baseline severity of insomnia as reflected by the Insomnia Severity Index (ISI) was in the moderate range, while depression severity as reflected by the Hamilton Rating Scale for Depression (HRSD) was in the moderate to severe range.37 Most participants experienced MDE of the melancholic subtype (Table 1).
Table 1.
Baseline demographics and measures of depression and insomnia (N = 60)
| Characteristic | Total sample |
|---|---|
| Gender | |
| Male | 20 (33.3) |
| Female | 40 (66.7) |
| Age | 41.5 ± 12.5 |
| Race | |
| Caucasian | 46 (76.7) |
| African American | 12 (20) |
| Other | 2 (3.3) |
| Marital status | |
| Married/Living with Someone | 23 (38.3) |
| Separated/Divorced/Widowed | 22 (36.7) |
| Never married | 15 (25) |
| Years of education | |
| < High school | 5 (8.3) |
| High school diploma/GED | 22 (36.7) |
| Associate degree | 9 (15) |
| Bachelor's degree | 16 (26.7) |
| Master's/Doctorate Degree | 8 (13.3) |
| Body Mass Index (BMI) | 27.8 ± 4.9 |
| Mini Mental State Exam (MMSE)40 | 29.4 ± 0.9 |
| Age at first lifetime major depressive episode | 29.9 ± 13.1 |
| Number of prior lifetime major depressive episodes | |
| 0 Episodes | 2 (3.3) |
| 1 Episodes | 15 (25) |
| 2 Episodes | 18 (30) |
| 3 Episodes | 11 (18.3) |
| 4 Episodes | 7 (11.7) |
| 5 Episodes | 7 (11.7) |
| Duration of present episode of major depression (weeks) | 169.6 ± 310.1 |
| Duration of insomnia complaint (weeks) | 165.5 ± 322.7 |
| SCID: MDE Specifier | |
| None | 17 (28.3) |
| Melancholic | 39 (65) |
| Atypical | 4 (6.7) |
| Dysthymic Disorder (No) | 60 (100) |
| Presence of any lifetime dependence or abuse (Yes) | 22 (36.7) |
| Presence of any anxiety disorder | 32 (53.3) |
| Index | |
| 24-item Hamilton Rating Scale for Depression (HRSD)37 | 27.1 ± 3.9 |
| 21-item HRSD (No Insomnia Items) | 22.4 ± 3.8 |
| Sum of HRSD Insomnia Items | 4.7 ± 1.2 |
| ISI Total Score | 20.7 ± 4.0 |
N (%) or mean ± standard deviation.
Actigraphy
In the analysis which compared week 1 mean values with laboratory night 1 values (following week 1), actigraphic recordings for laboratory night 1 demonstrated improved sleep for all parameters, except the number of awakenings, compared to the mean values of week 1 (p < 0.005), including a significantly shorter SOL, shorter WASO, and longer TST. There was no significant difference between the duration of the rest period between week 1 and laboratory night 1 (Table 2).
Table 2.
Actigraphic differences between home and laboratory settings
| Week 1 (Home) to Laboratory Night 1 (End of Week 1) (N = 57) | ||||||
|---|---|---|---|---|---|---|
| Sleep Measure | Week 1 Mean ± SD (min) | Lab Night 1 ± SD (min) | Difference ± SE (min) | t-Ratio | DF | p-value |
| Sleep onset latency | 45.2 ± 34.9 | 23.8 ± 27.5 | −21.4 ± 5.4 | −4.0 | 56 | < 0.001* |
| Number of wake bouts | 34.1 ± 10.9 | 29.4 ± 11.8 | −4.6 ± 1.7 | −2.7 | 56 | 0.009 |
| Wake time after sleep onset | 76.4 ± 42.1 | 59.8 ± 39.3 | −16.6 ± 5.2 | −3.2 | 56 | 0.002* |
| Total sleep time | 370.4 ± 59.6 | 402.2 ± 41.2 | 31.9 ± 8.1 | 4.0 | 56 | < 0.001* |
| Sleep efficiency | 75.8 ± 8.7 | 83.1 ± 9.6 | 7.3 ± 1.3 | 5.5 | 56 | < 0.001* |
| Laboratory Night 1 (End of Week 1) to Week 2 (Home) (N = 58) | ||||||
| Sleep Measure | Lab Night 1 ± SD (min) | Week 2 Mean ± SD (min) | Difference ± SE (min) | t-Ratio | DF | p-value |
| Sleep onset latency | 23.5 ± 27.4 | 36.4 ± 31.7 | 12.9 ± 4.1 | 3.2 | 57 | 0.003* |
| Number of wake bouts | 29.5 ± 11.7 | 32.0 ± 10.0 | 2.5 ± 1.5 | 1.7 | 57 | 0.1 |
| Wake time after sleep onset | 59.9 ± 39.1 | 74.2 ± 34.3 | 14.2 ± 5.1 | 2.8 | 57 | 0.007 |
| Total sleep time | 402.3 ± 41.3 | 371.6 ± 53.2 | −30.7 ± 7.0 | −4.4 | 57 | < 0.001* |
| Sleep efficiency | 83.1 ± 9.6 | 77.5 ± 9.0 | −5.6 ± 1.2 | −4.5 | 57 | < 0.001* |
With Bonferroni corrections, < 0.005 was considered significant.
Mean actigraphic recordings for week 2 (following laboratory night 1) demonstrated significantly longer mean SOL and shorter TST compared to laboratory night 1 (p < 0.005). The number of awakenings was also higher for week 2 than laboratory night 1, but this difference was not significant. As before, there was no significant difference between the duration of the rest period between laboratory night 1 and week 2 (Table 2).
Sleep Diaries
In contrast with actigraphy, sleep diaries recorded slightly worse sleep in the laboratory compared to home (Table 3). For the analysis between week 1 and laboratory night 1, sleep diaries recorded significantly more awakenings in the laboratory relative to the week prior (p < 0.005). Differences for SOL, WASO, and TST recorded by sleep diaries were not significant. The mean number of awakenings was also significantly lower during week 2 compared to laboratory night 1 (p < 0.005). SOL, WASO, and TST showed nonsignificant improvements during week 2 relative to laboratory night 1.
Table 3.
Sleep diary differences between home and laboratory settings
| Week 1 (Home) to Laboratory Night 1 (End of Week 1) (N = 58) | ||||||
|---|---|---|---|---|---|---|
| Sleep Measure | Week 1 Mean ± SD (min) | Lab Night 1 ± SD (min) | Difference ± SE (min) | t-Ratio | DF | p-value |
| Sleep onset latency | 70.5 ± 40.9 | 62.8 ± 57.7 | −7.6 ± 7.2 | −1.1 | 57 | 0.3 |
| Number of awakenings | 2.1 ± 1.3 | 3.5 ± 2.7 | 1.4 ± 0.3 | 4.2 | 57 | < 0.001* |
| Wake time after sleep onset | 62.6 ± 55.7 | 82.6 ± 82.5 | 20.0 ± 9.5 | 2.1 | 57 | 0.04 |
| Total sleep time (N = 57) | 356.1 ± 66.6 | 336.6 ± 104.8 | −19.5 ± 13.9 | −1.4 | 56 | 0.2 |
| Laboratory Night 1 (End of Week 1) to Week 2 (Home) (N = 58) | ||||||
| Sleep Measure | Lab Night 1 ± SD (min) | Week 2 Mean ± SD (min) | Difference ± SE (min) | t-Ratio | DF | p-value |
| Sleep onset latency | 62.8 ± 57.7 | 56.6 ± 40.6 | −6.3 ± 6.3 | −1.0 | 57 | 0.3 |
| Number of awakenings | 3.5 ± 2.7 | 1.9 ± 1.4 | −1.6 ± 0.3 | −5.1 | 57 | < 0.001* |
| Wake time after sleep onset | 82.6 ± 82.5 | 65.0 ± 63.5 | −17.6 ± 9.8 | −1.8 | 57 | 0.08 |
| Total sleep time (N = 57) | 336.6 ± 104.8 | 371.4 ± 74.7 | 34.8 ± 13.5 | 2.6 | 56 | 0.01 |
With Bonferroni corrections, < 0.005 was considered significant.
Figures 1-4 plot the mean weekly home values as well as laboratory values for actigraphy and sleep diaries over the course of the study.
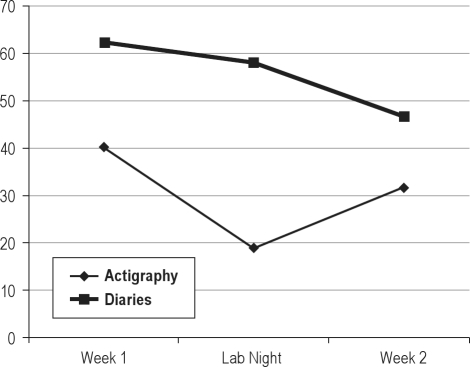
Figure 1.
Changes in sleep onset latency over course of study
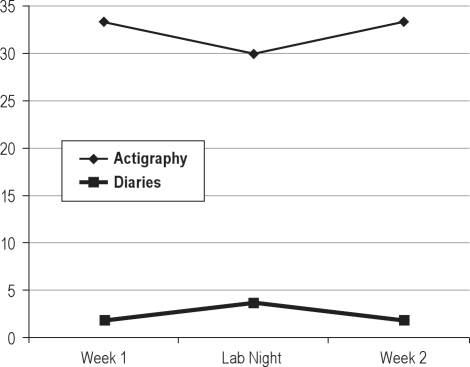
Figure 2.
Changes in number of awakenings (diaries) and wake bouts (actigraphy) over course of study
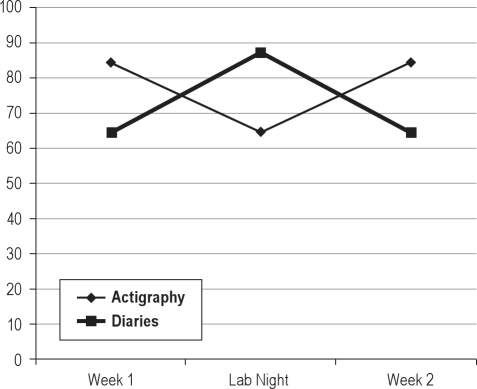
Figure 3.
Changes in wake time after sleep onset over course of study
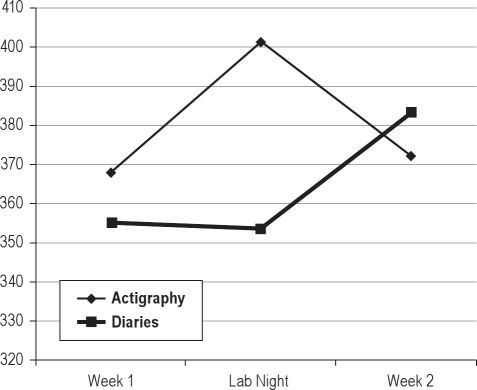
Figure 4.
Changes in total sleep time over course of study
Polysomnography
Table 4 provides data for polysomnography measurements, as well as actigraphy and sleep diaries, for all subjects during the laboratory monitoring session.
Table 4.
Laboratory night measurements for all subjects (mean ± SD, minutes)
| Sleep Measure | Actigraphy (N = 59) | PSG (N = 58) | Sleep Diaries (N = 58) |
|---|---|---|---|
| Sleep onset latency | 23.3 ± 27.2 | 27.0 ± 36.0 | 62.8 ± 57.7 |
| Latency to persistent sleep | N/A | 35.1 ± 36.5 | N/A |
| Wake time after sleep onset | 59.7 ± 38.8 | 59.8 ± 51.5 | 82.6 ± 82.5 |
| Total sleep time | 402.5 ± 41.0 | 389.4 ± 63.1 | 336.6 ± 104.8 |
| Sleep efficiency | 83.2 ± 9.6 | 81.1 ± 13.1 | N/A |
DISCUSSION
The results of this study found that, overall, actigraphic sleep measurements demonstrated significantly increased sleep time and decreased wake time in the laboratory relative to the home environment; all nonsignificant differences occurred in the same direction of a greater quantity of actigraphic sleep in the lab relative to home. In contrast, sleep diary variables showed significantly increased number of awakenings in the laboratory relative to home; nonsignificant differences occurred in the same direction of a lower quantity of self-reported sleep obtained in the laboratory relative to home, with the exception of sleep latency.
The actigraphic results support the hypothesis that some insomniacs may sleep better in the laboratory setting than at home.13,16 While previous studies have supported this hypothesis only by demonstrating worsening of sleep from the first night to successive nights, this study directly compared actigraphic sleep at home versus the lab, and found that actigraphic sleep was better in the laboratory than at home. Unlike Hauri's finding that insomniacs demonstrating a RFNE were less depressed than those demonstrating a FNE, the current study observed a RFNE with insomniacs diagnosed with depression. However, the authors noted in their study that patients diagnosed with mood disorders other than dysthymia were typically excluded by their selection process.13
As Hauri et al. suggested, the RFNE effect may be due to maladaptive associations of insomnia, formed in the normal sleeping environment of the insomnia patient, that are then disrupted by the novel sleeping environment of the lab.13 Alternatively, the increased sensory stimulation of the novel sleep environment and monitoring equipment may provide better sleep in those insomniacs whose poor sleep may be caused by “information underload” in their normal sleep environment.16 A third possible explanation is related to the nature of the sleep laboratory, as it has been suggested that the quality of the sleep environment and staff interactions may have an effect on sleep in a laboratory setting.7,8 The laboratory environment used for this study was underground and sound attenuated, with little ambient light and simple bedroom furnishings; it is possible that this setting facilitated better sleep than the patients' normal sleep environment.
In contrast to the actigraphic data, sleep diaries indicated no significant changes between self-reported sleep in the laboratory setting except an increased number of awakenings in the laboratory setting. Actigraphic differences between home and laboratory were much greater than the differences for sleep diaries, showing a strong reverse first night effect measured by actigraphy, and a weak first night effect measured by sleep diaries.
There are several possible explanations for these discrepancies. Differences between objective and subjective measurements of sleep are commonly reported in insomniacs. In studies using PSG, patients with insomnia are noted for underestimating their total amount of sleep and overstating sleep latency, compared to PSG.3,38 Insomniacs often report a degree of sleep disturbance not reflected in PSG sleep, and may report improvements also not reflected in PSG sleep.39 A previous study by Saletu et al. also found that FNE observed with PSG was not reflected in subjectively experienced sleep quality.18 Therefore, it might be concluded that these depressed insomniacs underestimated their self-reported sleep in the laboratory relative to actigraphy.
Another possibility is that participants accurately portrayed their self-reported sleep as being slightly worse in the laboratory, while actigraphy falsely recorded better sleep in the laboratory. This might occur if the presence of PSG monitoring equipment caused participants to lie awake but still, thus leading to incorrect recording of sleep continuity by actigraphy. This is supported by the fact that the differences between home and laboratory measurements were much greater with actigraphy than with the sleep diaries. To address the validity of actigraphy versus PSG and sleep diaries in the laboratory, previous analyses of this sample have been performed to compare these methods.28 In those analyses, it was found that sleep diary measurements were significantly different from PSG in variables of SOL, WASO, and TST, whereas actigraphy and PSG were not significantly different for those variables. Because it was found that actigraphic sleep provided a closer approximation to PSG sleep than did sleep diaries during the laboratory night in this sample, it may be concluded that the lack of agreement between actigraphy and sleep diaries may be due more to subjective misperception of sleep duration in this sample of depressed insomniacs, rather than actigraphy falsely recording sleep continuity when subjects are lying awake but still.
This study was limited by a number of factors. One general limitation was that there was no systematic measurement of daytime sleeping that could have affected nighttime measurements of sleep at home. Also, the study was conducted with thermistor technology without a nasal pressure transducer, so it is possible the extent of sleep apnea in this sample was underestimated. Given the high proportion of comorbid anxiety in this sample, it is possible that anxiety may also have influenced some of the effects noted in this study. However, trait anxiety and bedtime state anxiety have each been more closely associated with FNE rather than RFNE.13,14 In our analyses, the number of direct comparisons between variables introduced the possibility of falsely significant differences; we attempted to reduce this possibility with Bonferroni-corrected significance levels < 0.005.
Measurement-specific limitations may also have affected the results of this study. Choice of actigraphy product and accompanying proprietary algorithm, sensitivity level, use of non-dominant versus dominant wrist, and the analysis of the first night in the sleep lab may have affected our actigraphic findings. In regards to sleep diaries, it is possible that changes in medication, alcohol use, or tobacco use may have affected self-reporting. PSG monitoring, particularly that of REM sleep, may have been affected by the washout of prior antidepressant medications used before the initiation of the study. Lastly, although we speculate that PSG monitoring equipment may have influenced participants to lie still in the laboratory relative to home (thus increasing actigraphically measured sleep), we did not conduct PSG monitoring in the home environment. Future research to isolate changes in actigraphic measurements based on the environment should conduct simultaneous PSG and actigraphic monitoring in both environments.
In summary, the present study found that the first night in the laboratory resulted in better actigraphic sleep for this sample of depressed insomniacs, but slightly worse self-reported sleep as measured by sleep diaries. These results emphasize the importance of using multiple methods for measuring sleep continuity in populations of patients such as those with depression and insomnia, particularly in the assessment and diagnosis of sleep problems. It may be that the discrepant results between objective and subjective methods frequently seen in insomniacs are influenced by the laboratory setting. While comparisons between actigraphy and the gold standard of PSG have found that actigraphy data more closely mirrored PSG data than did sleep diaries, this study revealed significant differences between the laboratory and home for actigraphic sleep that were not reflected in sleep diaries, calling into question whether actigraphy is appropriate for all monitoring environments. It may be that actigraphy has better application as a monitoring tool in the home than in the laboratory, especially when PSG monitoring equipment may influence body movement and thus affect actigraphic sleep recording. It must also be noted that although this sample as a whole demonstrated a strong RFNE by actigraphic measurements, a small number of individual subjects may have demonstrated variable results that included FNE or no adaptation effects at all. Further research is needed in comparing sleep in the laboratory with sleep at home using actigraphy and sleep diaries to determine if this phenomenon is truly a “reverse” first night effect.
DISCLOSURE STATEMENT
This study was partially funded and medications provided by Sepracor; in addition, funding and material support was provided by Mini Mitter. Dr. W. Vaughn McCall was on the Speaker Bureau for Sepracor and Merck and has received research support from Sealy, Sepracor, Mini Mitter, Corcept, and Sanofi. He is an advisor to Merck. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was performed at Wake Forest University Health Sciences. This study was funded by NIH grants: MH70821 and M01-RR07122.
REFERENCES
- 1.Agnew H, Webb W, Williams R. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 2.Mendels J, Hawkins DR. Sleep laboratory adaptation in normal subjects and depressed patients (“first night effect”) Electroencephalogr Clin Neurophysiol. 1967;22:556–8. doi: 10.1016/0013-4694(67)90063-6. [DOI] [PubMed] [Google Scholar]
- 3.Hauri P. Persistent psychophysiologic (learned) insomnia. Sleep. 1986;9:38–53. doi: 10.1093/sleep/9.1.38. [DOI] [PubMed] [Google Scholar]
- 4.Kupfer DJ, Weiss BL, Detre TP, et al. First night effect revisited: a clinical note. J Nerv Ment Dis. 1974;159:205–9. doi: 10.1097/00005053-197409000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Coates TJ, George JM, Killen JD, et al. First night effects in good sleepers and sleep-maintenance insomniacs when recorded at home. Sleep. 1981;4:293–8. doi: 10.1093/sleep/4.3.293. [DOI] [PubMed] [Google Scholar]
- 6.Kader GA, Griffin PT. Reevaluation of the first night effect. Sleep. 1983;6:67–71. doi: 10.1093/sleep/6.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Coble P, McPartland RJ, Silva WJ, et al. Is there a first night effect? (a revisit) Biol Psychiatry. 1969;9:215–9. [PubMed] [Google Scholar]
- 8.Browman CP, Cartwright RD. The first-night effect on sleep and dreams. Biol Psychiatry. 1980;15:809–12. [PubMed] [Google Scholar]
- 9.Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric patients. Sleep. 1995;18:463–9. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 10.Edinger JD, Marsh GR, McCall WV, et al. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14:13–7. [PubMed] [Google Scholar]
- 11.Hartmann E. Adaptation to the sleep laboratory and placebo effect. Psychobiology. 1968;4:389. [Google Scholar]
- 12.Schmidt HS. The differential laboratory adaptation of sleep parameters. Biol Psychiatry. 1971;3:33–45. [PubMed] [Google Scholar]
- 13.Hauri P, Olmstead E. Reverse first night effect in insomnia. Sleep. 1989;12:97–105. doi: 10.1093/sleep/12.2.97. [DOI] [PubMed] [Google Scholar]
- 14.Riedel B, Winfield CF, Lichstein KL. First night effect and reverse night effect in older adults with primary insomnia: does anxiety play a role? Sleep Med. 2001;2:125–33. doi: 10.1016/s1389-9457(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 15.Hauri P. A cluster analysis of insomnia. Sleep. 1983;6:326–38. doi: 10.1093/sleep/6.4.326. [DOI] [PubMed] [Google Scholar]
- 16.de la Pena A. Toward a psychophysiologic conceptualization of insomnia. In: Williams RL, Karacan I, editors. Sleep disorders: diagnosis and treatment. New York: Wiley; 1978. pp. 101–43. [Google Scholar]
- 17.Sharpley AL, Solomon RA, Cowen PJ. Evaluation of first night effect using ambulatory monitoring and automatic sleep stage analysis. Sleep. 1988;11:273–76. doi: 10.1093/sleep/11.3.273. [DOI] [PubMed] [Google Scholar]
- 18.Saletu B, Klosch G, Gruber G, et al. First-night-effects on generalized anxiety disorder (GAD)-based insomnia, laboratory versus home sleep recordings. Sleep. 1996;19:691–7. [PubMed] [Google Scholar]
- 19.Edinger JD, Fins AI, Sullivan RJ, Jr, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119–26. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 20.Edinger JD, Glenn DM, Bastian LA, et al. Sleep in the laboratory and sleep at home II: comparisons of middle-aged insomnia sufferers and normal sleepers. Sleep. 2001;24:761–70. doi: 10.1093/sleep/24.7.761. [DOI] [PubMed] [Google Scholar]
- 21.Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychol Aging. 2000;15:232–40. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]
- 22.Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry. 1999;60(Suppl):28–31. [PubMed] [Google Scholar]
- 23.Rotenberg VS, Hadjez J, Kimhi R, et al. First night effect in depression: new data and a new approach. Biol Psychiatry. 1997;42:267–74. doi: 10.1016/S0006-3223(96)00343-5. [DOI] [PubMed] [Google Scholar]
- 24.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 25.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 26.Verbeek I, Arends J, Declerck G, et al. Wrist actigraphy in comparison with polysomnography and subjective evaluation in insomnia. Sleep-Wake Research in the Netherlands. 1994;5:163–9. [Google Scholar]
- 27.Standards of Practice Committee of the American Sleep Disorders Association. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519–29. [Google Scholar]
- 28.McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2011 Mar 29; doi: 10.1111/j.1365-2869.2011.00917.x. doi: 10.1111/j.1365-2869.2011.00917.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Hilton JJ, Braat EA, van der Velde EA, et al. Ambulatory activity monitoring during sleep: an evaluation of internight and intrasubject variability in healthy persons aged 50-98. Sleep. 1993;16:146–50. doi: 10.1093/sleep/16.2.146. [DOI] [PubMed] [Google Scholar]
- 30.McCall WV, Blocker JN, D'Agostino RB, Jr, et al. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med. 2010;6:322–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 32.Allen R, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology: A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 34.McCall WV, Kimball J, Boggs N, et al. Prevalence and prediction of primary sleep disorders in a clinical trial of depressed patients with insomnia. J Clin Sleep Med. 2009;5:454–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 36.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 37.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCall WV, Edinger JD. Subjective total insomnia: an example of sleep state misperception. Sleep. 1992;15:71–3. [PubMed] [Google Scholar]
- 39.Schneider-Helmert D. Overestimations of hypnotic drug effects by insomniacs - a hypothesis. Psychopharmacology. 1985;87:107–10. doi: 10.1007/BF00431788. [DOI] [PubMed] [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]