Abstract
Study Objectives:
Although the prevalence of obstructive sleep apnea/hypopnea syndrome (OSAHS) is high in patients with acute coronary syndromes (ACS), there is little knowledge about the persistence of OSAHS in ACS patients after the acute event. We aimed to assess the prevalence and time course of OSAHS in patients with ACS during and after the acute cardiac event.
Methods:
Fifty-two patients with first-ever ACS, underwent attended overnight polysomnography (PSG) in our sleep center on the third day after the acute event. In patients with an apnea hypopnea index (AHI) > 10/h, we performed a follow up PSG 1 and 6 months later.
Results:
Twenty-eight patients (54%) had an AHI > 10/h. There was a significant decrease in AHI 1 month after the acute event (13.9 vs. 19.7, p = 0.001), confirming the diagnosis of OSAHS in 22 of 28 patients (79%). At 6-month follow-up, the AHI had decreased further (7.5 vs. 19.7, p < 0.05), and at that time only 6 of the 28 patients (21%) were diagnosed as having OSAHS. Twelve of the 16 current smokers stopped smoking after the acute event.
Conclusions:
We have demonstrated a high prevalence of OSAHS in ACS patients, which did not persist 6 months later, indicating that, to some degree, OSAHS may be transient and related with the acute phase of the underlying disease or the reduction in the deleterious smoking habit.
Citation:
Schiza SE; Simantirakis E; Bouloukaki I; Mermigkis C; Kallergis EM; Chrysostomakis S; Arfanakis D; Tzanakis N; Vardas P; Siafakas NM. Sleep disordered breathing in patients with acute coronary syndromes. J Clin Sleep Med 2012;8(1):21-26.
Keywords: Acute coronary syndromes, obstructive sleep apnea hypopnea syndrome, polysomnography
The high prevalence and clinical significance of sleep disordered breathing, (SDB) as well as its effects on a range of cardiovascular conditions have attracted growing interest. Obstructive sleep apnea/hypopnea syndrome (OSAHS) is strongly associated with the incidence and consequences of hypertension,1,2 arrhythmia,3,4 heart failure,5–7 and stroke.8,9 Treatment of OSAHS with CPAP has positive results on its well-known cardiovascular consequences.10
In addition, OSAHS has been found to be a factor involved in endothelial dysfunction,11 inflammation,12 and sympathetic activation, which may promote ischemic events. Although an increased incidence of CAD has been found in patients with OSAHS,13,14 and even mild to moderate OSAHS has been associated with a worse prognosis in patients with existing CAD,15,16 the characterization of OSAHS in the acute myocardial ischemia setting is less clear.17–25 Based on the results of recent studies19,20,25 it is difficult to determine whether OSAHS contributes to the occurrence of ACS, or whether OSAHS is a consequence of the acute phase in ACS patients that may improve after medical stabilisation. We therefore conducted a longitudinal study to assess the prevalence of OSAHS in a homogeneous group of patients with ACS, who had no other medical or known sleep disorder, to examine whether OSAHS persists over time and to elucidate any possible connection with the severity of the underlying disease.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Several studies have shown a high prevalence of Obstructive sleep apnea/hypopnea syndrome (OSAHS) in patients with acute coronary syndromes (ACS) in the acute setting. The aim of this study was to assess the prevalence and time course of OSAHS in patients with ACS, during and after the acute cardiac event.
Study Impact: The issue on the ideal timing of investigation for OSAHS after an acute cardiovascular event remains unresolved. Based on our results, attempting to diagnose OSAHS early in patients with ACS should be done with caution, because initial polysomnography (PSG) findings in the acute phase of ACS are not necessarily indicative of an ongoing problem.
MATERIALS AND METHODS
Patients
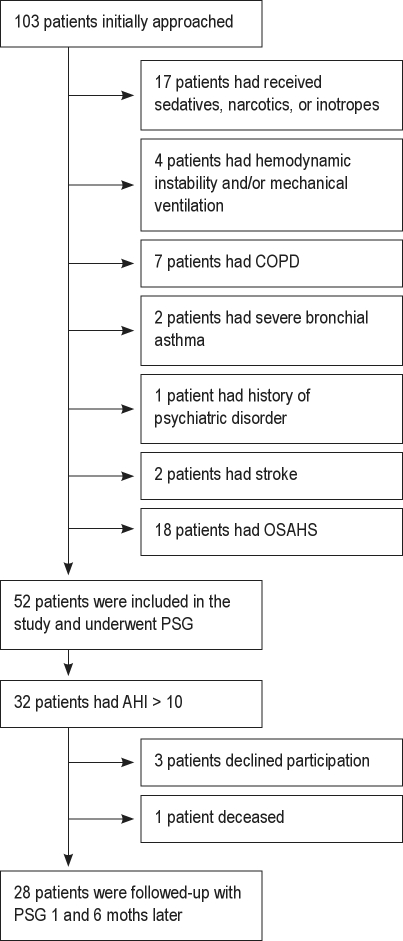
Fifty-two of 103 consecutive patients (40 men, 12 women) admitted to the CCU with a first-ever ACS, and with preserved left ventricular function (LVEF > 40%), satisfied the inclusion criteria and were invited to participate voluntarily in this study. The purpose of the study was explained to patients and relatives by a study author within the first 2 days after admission. ACS included first ever-acute myocardial infarction (AMI), acute EKG changes (i.e., ST-segment elevation > 1 mm in 2 contiguous leads, new Q waves, ST-segment depression, or T wave inversion), along with positive cardiac enzymes and unstable angina. Excluded were patients who were confused; patients who had received sedatives or narcotics within the previous 48 h or inotropes on the day of the sleep study; patients who had suffered cardiac arrest; patients with chronic obstructive pulmonary disease, severe bronchial asthma, a history of psychiatric disorders, stroke or sepsis, hemodynamic instability, or a need for mechanical ventilation and cardiac assist, or ongoing supplementary oxygen therapy at the time of the sleep study; and those with a known history of diagnosed OSAHS, restless legs syndrome, or periodic leg movement disorder.
Study Design
Overnight attended polysomnography (PSG) was carried out in our sleep disorders unit, away from the CCU environment but in the same hospital, on the third day after the acute event. Safety and feasibility of PSG for acutely ill patients in the sleep disorders center has been described previously.26 A sleep history was taken from each patient and his/her relatives, and the Epworth Sleepiness Scale (ESS) was used to evaluate daytime sleepiness. Left ventricular ejection fraction (LVEF) was estimated by echocardiography using the biplane Simpson method before every sleep study. Patients with an apnea/hypopnea index (AHI) > 10/h were followed up with PSGs 1 and 6 months later.
Patients while in the CCU environment were kept in rooms with windows, and the light was dimmed every night at 22:00 to avoid circadian rhythm disturbances; they were encouraged to follow a normal sleep schedule and to avoid napping. Moreover, they were continuously video-recorded in order to rule out any abnormalities in their sleep schedule that might influence the first PSG, performed 3 days after the acute event. On the day of the sleep study, the PSG procedure was explained to the patients and a short visit to our sleep disorders center was arranged.
The study was approved by the hospital's ethics committee, and each participant gave written informed consent.
Polysomnography
Overnight attended polysomnography (Alice 5, Respironics) was performed in our sleep disorders center, located in the same hospital as the CCU. Patients underwent 3 nights of full diagnostic PSG, one at each time point, according to our standard techniques, with monitoring of the electroencephalogram (EEG) using frontal, central, and occipital leads; electroculogram (EOG); electromyogram (EMG); flow (by oronasal thermistor and nasal air pressure transducer); thoracic and abdominal respiratory effort (inductance plethysmography); oximetry; and body position. Snoring was recorded by a microphone placed on the anterior neck. A single modified EKG lead II was used for baseline cardiac monitoring (additional leads I, II, aVL, aVR, and aVF were used as backup for further evaluation of features suggestive of cardiac ischemia observed in the main EKG lead). An infrared camera synchronized to the PSG was used, and the number and timing of interactions between nurse and patient were documented.
Polysomnographic recordings were manually interpreted in 30-sec intervals, in accordance with the guidelines of Rechtschaffen and Kales and the new AASM guidelines27,28; the scorer was always the same, blinded to the patients' clinical condition and the PSG findings from the first assessment. The definitions of apneas and hypopneas were based on the new AASM standard criteria.28 Hypopneas were scored with the “recommended” AASM hypopnea rule that requires a ≥ 30% drop in the nasal pressure transducer signal and a desaturation ≥ 4% compared to the prior event baseline. The same PSG protocol and criteria were used on each of the 3 nights.
Statistical Analysis
Summary descriptive statistics are reported as mean ± SD (standard deviation) or frequency (%), as appropriate. The Kolmogorov-Smirnov test was used to confirm normality. Differences between consecutive values (BMI, sleep architecture parameters, respiratory and oxygenation indices) were assessed by a one-way analysis of variance (ANOVA) test with the Bonferroni correction. A p-value < 0.05 was considered to indicate statistical significance. The statistical package SPSS 16 (Chicago, IL, USA) was used for the entire analysis.
RESULTS
Of 103 consecutive patients, 51 were excluded based on the above-mentioned exclusion criteria (Figure 1). Table 1 shows the patients' demographics and clinical characteristics. Admission diagnoses were AMI in 47 patients (91%) and unstable angina (UA) in 5 patients (9%). All patients received the same scheme of potentially sleep-affecting medications, of which the administration remained unchanged during the study period. In detail, the vast majority of participants, in accordance with international guidelines, received β-blockers (77%), angiotensin converting enzyme inhibitors (90%), or angiotensin-1 inhibitors (10%), while all of them received aspirin and/or clopidogrel and were continued on these medications upon discharge.
Figure 1.
Flowchart of participation
Table 1.
Patients' demographics and clinical characteristics
| Characteristics | Values |
|---|---|
| Number | 52 |
| Age, y | 55.8 ± 13 |
| Sex | |
| Male | 40 |
| Female | 12 |
| Hypertension | 19 (37%) |
| Diabetes mellitus | 8 (28%) |
| Hypercholesterolemia | 16 (31%) |
| BMI, kg/m2 | 28.4 ± 3.9 |
| ESS score | 5.9 ± 1.3 |
| LVEF | 47 ± 9.8% |
Values are given as mean ± SD, or No (%).
ESS, Epworth Sleepiness Scale; LVEF, left ventricular ejection fraction.
Coronary angiography was performed before discharge and in any case 3 days after the first PSG. No correlation was found between the apnea-hypopnea index and the number of coronary arteries involved or the site of myocardial ischemia or infarction. No ischemic changes were documented on EKG during the sleep study, and none of the participants reported chest pain during PSG monitoring. The LVEF was slightly but not significantly increased during the follow-up period.
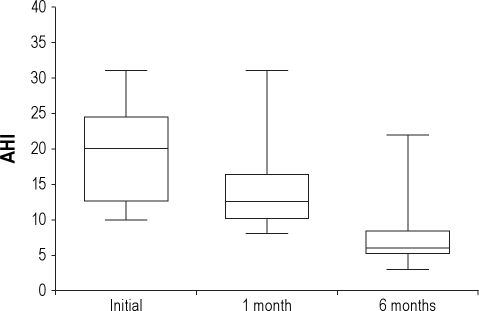
OSAHS (AHI > 10/h) was identified in 32 of 52 patients, giving a prevalence of OSAHS of 62%. Of those 32 patients, 3 patients declined to participate further and one died. The remaining 28 patients were followed up with PSG 1 and 6 months later. Sixteen were current smokers, 6 were former smokers, and 6 were nonsmokers. Data collected from patients who underwent all 3 PSG studies are shown in Table 2. We found that the BMI and ESS did not change significantly between the 3 assessments. As depicted in Figure 2, there was a significant decrease in AHI 1 month after the acute event (13.9 ± 5.9 vs. 19.7 ± 6.7, p = 0.001), confirming the diagnosis of OSAHS in 22 of 28 patients (79%), and giving a prevalence of OSAHS of 42% (22 out of 52 patients). The AHI decreased further 6 months later compared with the initial PSG (7.5 ± 4.6 vs. 19.7 ± 6.7, p < 0.05), confirming the diagnosis of OSAHS in only 6 of 28 patients (21%), and giving the final prevalence of OSAHS of 12% (6 out of 52 patients; Figure 2). In addition, 12 of the 16 current smokers stopped smoking after the acute event. Specifically, 2 of the quitters were in the group of 6 patients with the final diagnosis of OSAHS; the remaining 10 were in the group of 22 with no confirmed OSAHS diagnosis at 6 months. Central events represented less than 5% of scored respiratory events during the initial PSG. The mean percentage of time spent in the supine position did not change significantly between the 3 studies, and none of the patients met formal criteria for a diagnosis of positional OSAHS during our study.29,30
Table 2.
Clinical and polysomnography (PSG) parameters of 28 patients who showed an AHI > 10/h at first assessment, measured during the acute stage, and 1 and 6 months later
| First PSG | Second PSG | Third PSG | |
|---|---|---|---|
| N = 28 | N = 28 | N = 28 | |
| BMI | 28.5 ± 4.9 | 28.1 ± 4.6 | 27.9 ± 4.6 |
| TIB (min) | 366 ± 41.8 | 393.3 ± 60.1 | 383.2. ± 77.2 |
| TST (min) | 233.2 ± 42.5 | 301.8 ± 24.5* | 341.3 ± 23.7† |
| Arousal index | 26.9 ± 10.9 | 12.9 ± 3.8* | 4.3 ± 3.5† |
| Sleep efficiency (%) | 62.3 ± 9.6 | 75.9 ± 7.9* | 83.8 ± 5.6† |
| AHI (total) | 19.7 ± 6.9 | 13.9 ± 5.9* | 7.5 ± 4.6† |
| REM AHI | 21.9 ± 11.7 | 14.2 ± 6.7* | 8.1 ± 5.3† |
| CAHI | 0.9 ± 0.7 | 0.7 ± 0.4 | 0.7 ± 0.3 |
| OAHI | 18.8 ± 6.6 | 13.3 ± 5.9* | 6.9 ± 4.7† |
| ODI (≥ 3%) | 24.2 ± 12.9 | 13.3 ± 6.0* | 8.3 ± 4.0† |
| Mean SaO2 | 93.5 ± 1.1 | 95.1 ± 1.1* | 95.4 ± 0.8† |
| Min SaO2 | 84.5 ± 3.7 | 88.3 ± 3.2* | 89.7 ± 1.9† |
| Time lying supine (% TST) | 47.7 ± 7 | 46.9 ± 4.9 | 45.7 ± 6.2 |
| AHI supine | 23 ± 7.4 | 16.8 ± 6.8* | 8.8 ± 5.1† |
| AHI non-supine | 17 ± 6.8 | 11.5 ± 5.2* | 6.4 ± 4.3† |
| WASO (min) | 130.1 ± 28.2 | 77.1 ± 17.7* | 16.9 ± 6.1† |
| SWS (%) | 5.5 ± 2.1 | 10.7 ± 2.1* | 13.7 ± 3.2† |
| REM (%) | 2.4 ± 2.9 | 11 ± 1.3* | 13.6 ± 3.7† |
Data are presented as mean ± SD.
TIB, time in bed; TST, total sleep time; WASO, wake duration after sleep onset; REM(%), rapid eye movement percentage (%) of TST; SWS (%), slow wave sleep percentage (%) of TST; AHI, apnea hypopnea index; CAHI, central apnea-hypopnea index; OAHI, obstructive apnea-hypopnea index; ODI, oxygen desaturation index; SaO2, oxygen saturation.
p < 0.05 between second and first PSG,
p < 0.05 between third and first PSG.
Figure 2.
Box plots showing total apnea-hypopnea index (AHI) values.
Middle horizontal line inside box indicates median. Bottom and top of the box are 25th and 75th percentiles, and the error bars outside the box represent maximum and minimum values, respectively.
An analysis of sleep stages comparing the initial PSG with the first follow-up PSG and the second follow-up PSG revealed difficulties in initiating and maintaining sleep, significantly shorter total sleep time (233.2 ± 42.5 min vs. 301.8 ± 24.5 min vs. 341.3 ± 23.7 min, p < 0.05) and poorer sleep efficiency, significantly greater arousal index (26.9 ± 10.9 vs. 12.9 ± 3.8 vs. 4.3 ± 3.5, p < 0.05), and significantly less slow wave sleep (expressed as a percentage of total sleep time: 5.5 ± 2.1 vs. 10.7 ± 2.1 vs. 13.7 ± 3.2, p < 0.05), as well as percentage REM sleep (2.4 ± 2.9 vs. 11 ± 1.3 vs. 13.6 ± 3.7, p < 0.05). Sleep architecture was less altered 1 month after the acute event, while 6 months later sleep duration and stages were within normal range. There were no significant differences in light-off/light-on or total time in bed.
No patients were placed on nasal positive pressure therapy during the 6-month follow-up period. We arranged further clinical follow-up in all 6 patients who still had confirmed OSAHS at the last PSG study. Two of them received treatment with continuous positive airway pressure (CPAP), and for the others we recommended that CPAP therapy should be initiated in the future if patient's cardiac status deteriorated, if hypertension became difficult to stabilize, or if daytime sleepiness developed.
DISCUSSION
A few studies have evaluated the time course of SDB in patients with ACS.19,20,25 The present study was performed to investigate the time course of OSAHS in a homogeneous group of patients with ACS and preserved left ventricular function, who were not mechanically ventilated or on oral or intravenous sedation. To try to explain the association between ACS and OSAHS, patient selection was strict enough to exclude other comorbidities (details in the materials and methods section) that may be causative related to OSAHS. Our results indicated a high prevalence of OSAHS (AHI > 10/h) in the acute phase of ACS. However, the AHI was significantly lower in the second PSG with a further reduction near to the upper normal values in the chronic phase namely, 6 months after the acute event. The significant decrease of AHI during consecutive PSGs represents a decrease in scored obstructive respiratory events. Central events were less than 5% of scored respiratory events during the initial PSG.
Previous studies have shown a high prevalence of sleep disordered breathing and oxygen desaturation in patients with ACS.17–25,31 Additionally, a temporal association has been found between nocturnal myocardial ischemia and sleep apnea and hypopnea or desaturation in some patients with nocturnal ST-segment depression.32 The frequency of sleep disordered breathing in our study population at the time of the acute event (AHI > 10, 28 of 52 patients [54%]) and one month later (22 of 52 patients [42%]) is similar to that reported in previous studies.
However previous studies have several limitations, because the exact timing of the investigation in relation to cardiac events was either not stated or variable,18,25 and most of them calculated the AHI with a relatively less accurate monitoring system that did not employ an EEG.19,20,22–24,31 Furthermore, the patient samples were heterogeneous, since conditions that could increase the prevalence of OSAHS—confused patients, patients who had received sedatives or narcotics, and patients with chronic obstructive pulmonary disease, severe bronchial asthma, or stroke—were not excluded.18,19,23,24 Moreover, sleep position and the fact that, as sensors need to be attached, patients are more likely to lay supine in the coronary care unit (CCU) or sleep laboratory setting as opposed to their own home, was not taken into consideration. Therefore, the possibility of transient OSAHS or a false-positive diagnosis cannot be excluded.
There are only a few reports of worsening of OSAHS in the acute phase of ACS.19,21,25,31 Our results from the follow-up investigation, in accordance with previous studies, showed that in a significant number of patients, the findings were transient and could not be repeated. A final diagnosis of OSAHS was confirmed in 6 of 28 patients (or 6 of the 52 [12%] of the original study group) 6 months later, indicating that PSG performed early after the acute event does not have good diagnostic value for determining the severity of OSAHS. However, our findings seem to contradict those previously reported by Bahammam et al., who showed a high prevalence of OSAHS persisting at 6 months after the acute cardiac event.20
The question of the ideal timing of an investigation for OSAHS after an acute cardiovascular event remains unresolved. It is well known that making the diagnosis of OSAHS in patients with cardiovascular disease is important, particularly given that adequate management improves its cardiovascular consequences. It could be argued that the diagnosis should be made as early as possible, when patients are in contact with clinical staff and in a setting where facilities for immediate investigation are more likely to be available. However, based on our results, attempting to diagnose OSAHS early in patients with ACS should be done with caution, because initial PSG findings in the acute phase of ACS are not necessarily indicative of an ongoing problem. In addition, disrupted sleep with alterations in sleep macro- and microarchitecture that is common during acute cardiac events may temporally increase sleep disordered breathing. Therefore, PSG should not be performed early after ACS to reliably establish the diagnosis of OSA, given the high rate of false-positive diagnoses (22 of 28 diagnoses: 79%) and should only very restrictively be performed in patients during ACS because PSG worsens the frail sleep in these already distressed patients. Additionally, performing sleep studies shortly after admission to the CCU may be a waste of resources.
An explanation for the high prevalence rate in the first study may be the abnormal breathing and negative impaired sleep microarchitecture during sleep from acute cardiovascular pathology, which results in overestimation of the baseline degree of apnea. However, in such cases there is a tendency for central respiratory events to occur, which did not appear in our results since patients with cardiac failure were excluded. Another explanation may be inflammatory changes and sympathetic neural hyperactivity associated with ACS,33 which could lead to destabilization of ventilatory control, affecting upper airway patency as well as chest wall muscles.34 Considering that smoking is a predisposing factor for OSAHS,35 an apparent explanation for the observed decrease of AHI 6 months later is that 12 of 16 current smokers in our study stopped smoking, with most of them included in the group of the 22 with decreased AHI 6 months later. Smoking habit has well-described negative effects in the upper airway via several mechanisms (inflammation, influence of declining nicotine levels in sleep stability, smoking related diseases) and may predispose or increase underlying sleep disordered breathing, especially in vulnerable populations, such as those with coronary disease.36–39 In addition, smoking reduction after an acute cardiac event is usually accompanied by improvement in other behaviors like exercise, alcohol consumption, and alterations in diet that may improve underlying sleep disordered breathing. An alternative explanation is that patients in the first study were more likely to lie supine (or spent more time supine) in the sleep laboratory compared to their own home because of the need for additional instrumentation (e.g., EEG leads) which may also have worsened OSAHS. However, the findings of this study showed that there were no significant changes in time spent in the supine position among the three studies. Furthermore, our group has demonstrated previously that patients with ACS have alterations in sleep architecture, which tend to disappear over time.40 Our study confirms that sleep architecture was clearly worse in the acute post-AMI phase than at follow-up, which suggests that the SDB was worse in the acute phase. Finally, it is worth noting that, according to Feinsilver, an alternative explanation for our findings is that these patients are not really “false positives” but demonstrate true OSAHS, albeit transient, during an acute cardiac illness.41
The present study did have some limitations that deserve comment. First our study had the limitation that it did not include a control group to control for regression to the mean. The absence of control group and the individual's selection based on one measurement followed by repeated measurements is definitely linked with both intra-subject and between-subject variations. The regression to the mean bias could affect particularly, the observed differences between baseline and first time-point measurements (1 month after initial estimation). However, our data of the second time-point (6 months) showed that these differences sustained not only between baseline and 6 months but also between 1 month and 6 months. Thus, we feel pretty confident that our entire conclusion that “to some degree, OSAHS may be transient and related with the acute phase of the underlying disease” is correct. Second, it is possible that gender factors could play a role, as the data presented were mainly based on male subjects, and therefore the results cannot be extrapolated to women. Another limitation of the study was the relatively small number of subjects. Nevertheless, previous studies exploring the time course of OSAHS in patients with ACS recruited similarly small numbers of patients. Taking the above into account, further studies using a larger number of patients will be needed to investigate the exact mechanism.
In conclusion, our data show a high prevalence of obstructive sleep apnea-hypopnea syndrome in the acute myocardial ischemia setting. In the majority of cases, this did not persist and AHI was significantly lower 6 months later, indicating that OSAHS may be transient. This suggests that the pathophysiological consequences of ACS (increased sympathetic activity and inflammatory changes) may be the main factors causing the high rate of OSAHS diagnosis during the acute phase. Therefore, sleep studies should not be performed early after ACS to reliably establish the diagnosis of OSAHS. However, further studies should explore the above-mentioned changes associated with the underlying cardiac disease and their contribution to the alteration in OSAHS. In addition, further research is needed concerning the role of short-term (one to six months) CPAP therapy in ACS patients with polysomnographic features who exhibit OSAHS around the acute event. This approach might improve patient outcomes (morbidity and mortality) during the period in which we observed a gradual decrease in the apnea-hypopnea index.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 3.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 4.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 5.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 6.Alchanatis M, Tourkohoriti G, Kosmas EN, et al. Evidence for left ventricular dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2002;20:1239–45. doi: 10.1183/09031936.02.00278002. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–31. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 9.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37:967–72. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- 10.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3:333–42. doi: 10.1016/S1474-4422(04)00766-5. [DOI] [PubMed] [Google Scholar]
- 11.Devulapally K, Pongonis R, Jr, Khayat R. OSA: the new cardiovascular disease: part II: Overview of cardiovascular diseases associated with obstructive sleep apnea. Heart Fail Rev. 2009;14:155–64. doi: 10.1007/s10741-008-9101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 13.Barcelo A, Miralles C, Barbé F, Vila M, Pons S, Agustí AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J. 2000;16:644–7. doi: 10.1034/j.1399-3003.2000.16d13.x. [DOI] [PubMed] [Google Scholar]
- 14.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 15.Peker Y, Hedner J, Kraiczi H, Löth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–6. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 16.Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–3. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Yoshikawa T, Sakamoto Y, Tanaka K, Inoue T, Ogawa R. Sleep apnea in patients with acute myocardial infarction. Crit Care Med. 1991;19:938–41. doi: 10.1097/00003246-199107000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 19.Skinner MA, Choudhury MS, Homan SD, Cowan JO, Wilkins GT, Taylor DR. Accuracy of monitoring for sleep-related breathing disorders in the coronary care unit. Chest. 2005;127:66–71. doi: 10.1378/chest.127.1.66. [DOI] [PubMed] [Google Scholar]
- 20.BaHammam A, Al-Mobeireek A, Al-Nozha M, Al-Tahan A, Binsaeed A. Behaviour and time-course of sleep disordered breathing in patients with acute coronary syndromes. Int J Clin Pract. 2005;59:874–80. doi: 10.1111/j.1742-1241.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 21.Moruzzi P, Sarzi-Braga S, Rossi M, Contini M. Sleep apnoea in ischaemic heart disease: differences between acute and chronic coronary syndromes. Heart. 1999;82:343–47. doi: 10.1136/hrt.82.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marin JM, Carrizo SJ, Kogan I. Obstructive sleep apnea and acute myocardial infarction: clinical implications of the association. Sleep. 1998;21:809–15. [PubMed] [Google Scholar]
- 23.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–95. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 24.Mehra R, Principe-Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med. 2006;7:521–8. doi: 10.1016/j.sleep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto K, Ohara A. Temporal worsening of sleep-disordered breathing in the acute phase of myocardial infarction. Circ J. 2006;70:1553–6. doi: 10.1253/circj.70.1553. [DOI] [PubMed] [Google Scholar]
- 26.BaHammam A, Syed S, Al-Mughairy A. Sleep-related breathing disorders in obese patients presenting with acute respiratory failure. Respir Med. 2005;99:718–25. doi: 10.1016/j.rmed.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. US Department of Health, Education, and Welfare Public Health Service—NIH/NIND; 1968. [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–4. doi: 10.1093/sleep/7.2.110. [DOI] [PubMed] [Google Scholar]
- 30.The American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: Recommendations for syndrome definitions and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 31.Hayashi M, Fujimoto K, Urushibata K, Uchikawa S, Imamura H, Kubo K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest. 2003;124:936–41. doi: 10.1378/chest.124.3.936. [DOI] [PubMed] [Google Scholar]
- 32.Mooe, T, Franklin KA, Wiklund U, Rabben T, Holmström K. Sleep-disordered breathing and myocardial ischemia in patients with coronary artery disease. Chest. 2000;117:1597–602. doi: 10.1378/chest.117.6.1597. [DOI] [PubMed] [Google Scholar]
- 33.Graham LN, Smith PA, Stoker JB, Mackintosh AF, Mary DA. Sympathetic neural hyperactivity and its normalization following unstable angina and acute myocardial infarction. Clin Sci (Lond) 2004;106:605–11. doi: 10.1042/CS20030376. [DOI] [PubMed] [Google Scholar]
- 34.Mehra R, Principe-Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med. 2006;7:521–8. doi: 10.1016/j.sleep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Kashyap R, Hock LM, Bowman TJ. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001;5:167–72. doi: 10.1007/s11325-001-0167-5. [DOI] [PubMed] [Google Scholar]
- 36.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–24. [PubMed] [Google Scholar]
- 37.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jennum P, Hein HO, Suadicani P, Gyntelberg F. Cardiovascular risk factors in snorers: a cross-sectional study of 3,323 men aged 54 to 74 years. The Copenhagen Male Study. Chest. 1992;102:1371–6. doi: 10.1378/chest.102.5.1371. [DOI] [PubMed] [Google Scholar]
- 39.Jennum P, Sjol A. Snoring, sleep apnoea and cardiovascular risk factors: the MONICA II Study. Int J Epidemiol. 1993;22:439–44. doi: 10.1093/ije/22.3.439. [DOI] [PubMed] [Google Scholar]
- 40.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep patterns in patients with acute coronary syndromes. Sleep Med. 2010;11:149–53. doi: 10.1016/j.sleep.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Feinsilver SH. A sleeping giant: sleep-disordered breathing in the coronary care unit. Chest. 2005;127:4–5. doi: 10.1378/chest.127.1.4. [DOI] [PubMed] [Google Scholar]