Abstract
The modification of peripheral positions of corroles by introduction of nitro groups is an important functionalization of this macrocycle. The nitro substituent strongly influences the corrole behavior leading to the preparation of macrocycles with different properties, which can be of interest for their exploitation as catalysts, sensing layers in chemical sensors or in the field of supramolecular chemistry. In the last few years we have developed different routes for the β-nitration of the corrole ring, and we report here novel synthetic protocols which can allow the formation of tri- and tetranitro derivatives, as demonstrated by X-ray analysis. In all of the methodologies used, the presence of isocorrole species as reaction intermediates was established, which regenerated the corresponding corrole by metal insertion.
Keywords: corrole, nitration, isocorrole
INTRODUCTION
Since the seminal papers on the preparation of arylcorroles [1, 2], in the last decade the number of publications related to corrole significantly expanded, allowing a more detailed study of the chemistry of the macrocycle. The synthetic routes for the preparation of corrole are now significantly improved [3, 4], allowing also studies of functionalization of the corrole [5], a fundamental step for exploitation of such a macrocycle in practical applications. The first examples of the potentialities of corrole derivatives in different fields have been reported in the literature [6].
The peripheral functionalization of corroles obviously took advantage of the routes already developed for porphyrins, although the reactivity of the contracted macrocycle could be very unexpected. For this reason, it is not surprising to isolate novel compounds when corroles are reacted under the same conditions used for porphyrins. Furthermore, the functionalization of corrole is complicated by its lower symmetry compared with porphyrin, which could lead to the formation of different substituted regioisomers. However, pyrroles A and D of corrole (Fig. 1) are endowed with enhanced reactivity than are pyrroles B and C, thereby allowing the formation of a few or even a single product among the huge number of potential regioisomers.
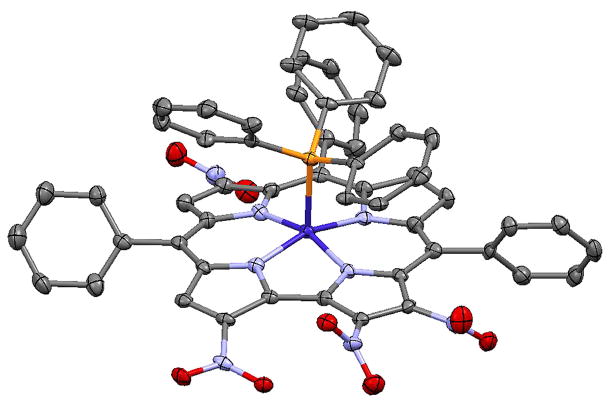
Fig. 1.
X-ray structure of [2,3,12,18-(NO2)4-TPCorr]Co(PPh3) (1) with 40% ellipsoids. Only one of the four independent molecules is illustrated, and H atoms and solvent are not shown
The most studied functionalization reactions reported for corroles are the chlorosulfonation [7, 8], formylation [8, 9], nitration [8, 10–12], and bromination [13–17]. In the case of chlorosulfonation, the 2,17- and 3,17-isomers have been isolated, while in the case of nitration the 3-nitro, and the 3,17-dinitro derivatives could be obtained, together with the 2,3,17-trinitro analog, depending on the nitrating system chosen. Also the formylation of corrole mainly led to the insertion of the carbonyl moiety at positions-3 and -17, together with a different compound, produced by attack of the Vilsmeier reagent to the macrocycle inner core [9].
A different behavior occurs in the case of bromination, because this reaction is more difficult to control than those mentioned above, and the fully brominated corrole is the most usual product, because otherwise a complex and impossible to separate mixture of regioisomers is obtained. Only recently the first examples of partially brominated Ge-corroles [15], and a tetrabrominated free-base corrole have been reported in the literature [16, 17].
Among these different functionalizations, we have been particularly interested in the definition of synthetic protocols for the β-nitration of corroles. The usefulness of the nitro group comes from the possibility to further modify the porphyrinoid macrocycle in a selective way [18, 19]; indeed the ability of this substituent to drive the attack of another functional group on corrole has been reported, making it possible to prepare functionalized compounds not achievable by straightforward synthetic pathways; a second interesting capability of –NO2 is its reduction to the amino group, which could be the first step toward exploitation of aminocorroles and the construction of covalently linked porphyrinoid moieties.
However, in our previous work the preparation of β-nitrocorroles was limited to the introduction of one or two nitro groups, probably because of the deactivating nature of the nitro group towards the further substitution; for this reason we have been interested to study the exploitation of more effective nitrating systems able to increase the level of the nitration above that so far achieved. Furthermore it is also interesting to investigate the possible concomitant nitration of the meso-phenyl groups, which has not yet been observed in the case of corrole.
We report here the results obtained by reacting 5,10,15-triphenylcorrole (TPCorrH3) in TFA/NaNO2 mixture, showing the formation of poly β-nitrated derivatives, obtained as Co triphenylphosphine complexes upon metalation of the corresponding isocorrole species, which were formed under the reaction conditions.
EXPERIMENTAL
General
Silica gel 60 (70–230 mesh, Sigma Aldrich) or neutral alumina oxide (Grade III, Merck) were used for column chromatography. Reagents and solvents (Aldrich, Merck or Fluka) were of the highest grade available and were used without further purification. 1H NMR spectra were recorded on a Bruker AV300 (300 MHz) spectrometer. Chemical shifts are given in ppm relative to residual CHCl3 (7.25 ppm). UV-vis spectra were measured on a Cary 50 spectrophotometer. Mass spectra (FAB mode) were recorded on a VGQuattro spectrometer in the positive-ion mode using m-nitrobenzyl alcohol (Aldrich) as a matrix.
X-ray crystallographic data
Crystals 1 and 2 for X-ray crystallographic analysis were grown from methanol/CDCl3 solutions. Diffraction data were collected at low temperature on a Nonius KappaCCD diffractometer equipped with MoKα radiation (λ = 0.71073 Å) and an Oxford Cryosystems Cryostream chiller. Refinement was by full-matrix least squares using SHELXL [20], with H atoms in idealized positions, guided by difference maps. In 1, one of the four chloroform solvent molecules was disordered, and its electron density was removed using the SQUEEZE [21] procedure. In 2, a disorder exists in which the third NO2 group is on C17 rather than C3 12.2(5)% of the time. The three atoms of the minor site were treated as isotropic. Crystallographic data for 1: C55H34CoN8O8P, CHCl3, triclinic space group P-1, a = 15.9753(15), b = 24.101(3), c = 25.382(3) Å, α = 90.495(4), β = 90.369(7), γ = 90.674(6)°, V = 9771.3(19) Å3, T = 90.0(5) K, Z = 8, ρcalcd = 1.556 g cm−3, μ(MoKα) = 0.62 mm−1. A total of 121,297 data was collected to θ = 25.6°. R = 0.083 for 19,806 data with Fo2 > 2σ(Fo2) of 31,240 unique data and 2739 refined parameters, CCDC 830942. For 2: C55H35CoN7O6P, monoclinic space group P21/n, a = 10.4692(15), b = 23.662(3), c = 18.703(2) Å, β = 103.077(6)°, V = 4513.0(10) Å3, T = 100.0(5) K, Z = 4, ρcalcd = 1.442 g cm−3, μ(MoKα) = 0.48 mm−1. A total of 48,573 data was collected to θ = 22.9°. R = 0.051 for 4380 data with Fo2 > 2σ(Fo2) of 6276 unique data and 642 refined parameters, CCDC 830941.
Syntheses of β-nitrocorroles
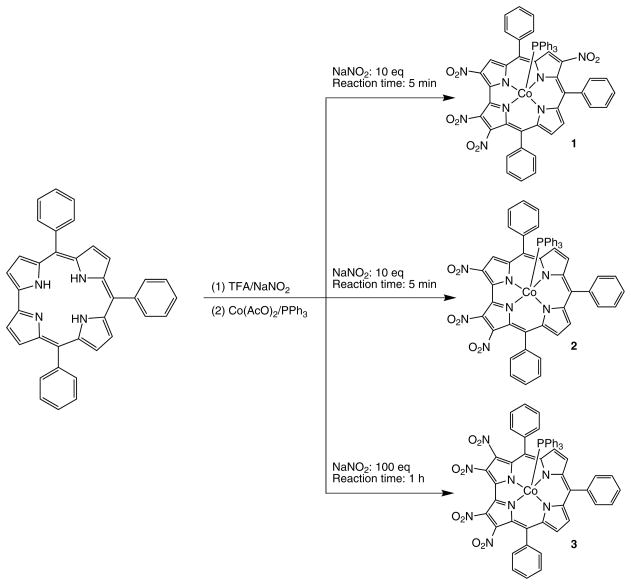
Preparation (method A — compounds 1, 2, 3)
45 mg of TPCorrH3 (0.085 mmol) were dissolved in 6 mL of TFA, and 59 mg (0.85 mmol, 10 equiv.) or 590 mg (8.5 mmol, 100 equiv.) were added. The mixture was stirred at room temperature for 5 min or 1 h (in the case of 3). After that time the reaction was quenched with 60 mL of water and the mixture was extracted with CH2Cl2. The organic phase was washed with aqueous NaHCO3, water and dried over anhydrous Na2SO4, before the solvent was removed under reduced pressure.
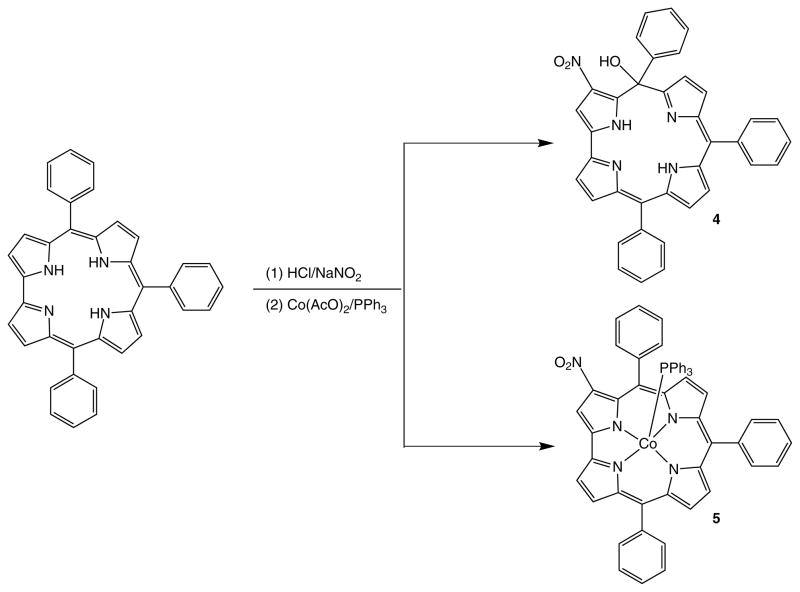
Preparation (method B — compounds 4, 5)
45 mg of TPCorrH3 (0.085 mmol) were dissolved in 30 mL of CH2Cl2, and 189 mg (2.74 mmol, 32 equiv.) dissolved in 30 mL of HCl 0.9 M were added. The mixture was stirred at room temperature for 5 min, monitoring the course of reaction by UV-vis spectrophotometry. After that time acid was neutralized with Na2CO3. The mixture was extracted with CH2Cl2 and the organic phase washed twice with water and dried over anhydrous Na2SO4; the solvent was then removed under reduced pressure.
Insertion of cobalt
The compound was dissolved in 30 mL of CH2Cl2 and 10 mL of CH3OH, and a three fold excess (with respect to the starting TPCorrH3) of Co(AcO)2 and PPh3 were added. The mixture was stirred under reflux for 1 h, and then the solvent was removed under reduced pressure and residue purification by column chromatography then followed. The purification details are described for each case as follows.
[2,3,12,18-(NO2)4-TPCorr]Co(PPh3) (1)
This fraction was collected from silica gel chromatography eluting with CH2Cl2; it was crystallized from CH2Cl2/CH3OH. Yield 8% (7 mg), mp > 300 °C. UV-vis (CH2Cl2): λmax, nm (log ε) 382 (4.20), 528 (3.76), 601 (3.81). 1H NMR (300 MHz, CDCl3): δ, ppm 8.19 (d, 2H, J = 5.07 Hz, β-pyrrole), 8.06 (d, 2H, J = 5.22 Hz, β-pyrrole), 7.74–7.53 (m, 15H, phenyl), 7.30 (m, 3H, p-phosphine), 7.00 (m, 6H, m-phosphine), 5.21 (m, 6H, o-phosphine). Anal. calcd. for C55H34CoN8O8P: C, 64.46; H, 3.34; N, 10.93%. Found: C, 64.48; H, 3.38; N, 10.89.
[2,3,18-(NO2)3-TPCorr]Co(PPh3) (2)
This fraction was collected from silica gel chromatography eluting with diethyl ether and was crystallized from CH2Cl2/CH3OH. Yield 14% (12 mg), mp > 300 °C. UV-vis (CH2Cl2): λmax, nm (log ε) 380 (3.97), 535 (3.52), 606 (3.66). 1H NMR (300 MHz, CDCl3): δ, ppm 8.36 (d, 1H, J = 5.07 Hz, β-pyrrole), 8.29 (s, 1H, β-pyrrole ), 8.23 (d, 2H, J = 5.19 Hz, β-pyrrole), 8.08 (d, 4H, J = 5.04 Hz, β-pyrrole), 7.75–7.65 (m, 9H, phenyl), 7.62–7.49 (m, 7H, phenyl), 7.30 (m, 3H, p-phosphine), 6.93 (m, 6H, m-phosphine), 5.13 (m, 6H, o-phosphine). Anal. calcd. for C55H35CoN7O6P: C, 67.42; H, 3.60; N, 10.00%. Found: C, 67.46; H, 3.58; N, 10.05.
[2,3,17,18-(NO2)4-TPCorr]Co(PPh3) (3)
This fraction was collected from silica gel chromatography eluting with CH2Cl2/diethyl ether 60/40% and was crystallized from CH2Cl2/CH3OH. Yield 5% (4 mg), mp > 300 °C. UV-vis (CH2Cl2): λmax, nm (log ε) 401 (4.47), 452 (4.36), 532 (3.99), 615 (4.21). 1H NMR (300 MHz, CDCl3): δ, ppm 8.69 (s, 1H, β-pyrrole), 8.42 (s, 1H, β-pyrrole), 8.27 (q, 2H, J = 5.71 Hz, β-pyrrole), 7.75 (m, 9H, phenyl), 7.41 (m, 6H, phenyl), 7.73 (m, 3H, p-phosphine), 6.97 (m, 6H, m-phosphine), 5.23 (m, 6H, o-phosphine). Anal. calcd. for C55H34CoN8O8P: C, 64.46; H, 3.34; N, 10.93%. Found: C, 64.42; H, 3.37; N, 10.90.
[(3-NO2)(5-OH)TPIsoCorr] (4)
This fraction was collected from silica gel chromatography eluting with CH2Cl2 (second fraction eluted) and was crystallized from CH2Cl2/CH3OH. Yield 16% (8 mg), mp > 300 °C. UV-vis (CH2Cl2): λmax, nm (log ε) 390 (4.21), 614 (3.56), 666 (3.53). 1H NMR (300 MHz, CDCl3): δ, ppm 16.36 (s, 1H, NH), 14.81 (s, 1H, NH), 7.64–7.52 (m, 8H, phenyl), 7.45–7-41 (m, 5H, phenyl), 7.31 (m, 2H, phenyl), 7.18 (d, 1H, J = 2.82 Hz, phenyl), 7.10 (d, 1H, J = 4.61 Hz, β-pyrrole), 6.95 (d, 1H, J = 4.61 Hz, β-pyrrole), 6.89 (d, 1H, J = 4.74 Hz, β-pyrrole), 6.67 (d, 1H, J = 4.72 Hz, β-pyrrole), 6.50 (m, 1H, β-pyrrole), 6.31 (s, 1H, β-pyrrole), 6.25 (m, 1H, β-pyrrole). Anal. calcd. for C37H25N5O3: C, 75.62; H, 4.29; N, 11.92%. Found: C, 75.72; H, 4.25 N, 11.98.
[(3-NO2)-TPCorr]Co(PPh3) (5)
This fraction was collected from silica gel chromatography eluting with CH2Cl2 (third fraction eluted) and crystallized from CH2Cl2/CH3OH. Yield 14% (11 mg), mp > 300 °C. UV-vis (CH2Cl2): λmax, nm (log ε) 369 (4.21), 408, shoulder (4.14), 588 (3.81). 1H NMR (300 MHz, CDCl3): δ, ppm 9.09 (s, 1H, β-pyrrole), 8.59 (d, 1H, J = 4.61 Hz, β-pyrrole), 8.35 (d, 1H, J = 4.61 Hz, β-pyrrole), 8.31 (d, 1H, J = 4.96 Hz, β-pyrrole), 8.14 (d, 1H, J = 4.96 Hz, β-pyrrole), 8.42 (s, 1H, β-pyrrole), 8.27 (q, 2H, J = 5.71 Hz, β-pyrrole), 7.75 (m, 9H, phenyl), 8.08 (d, 1H, J = 5.06 Hz, β-pyrrole), 8.00 (d, 2H, J = 5.09 Hz, β-pyrrole), 7.66–7.50 (m, 13H, phenyl), 7.30 (m, 2H, phenyl), 7.15 (m, 3H, p-phosphine), 6.80 (m, 6H, m-phosphine), 5.83 (m, 6H, o-phosphine). Anal. calcd. for C55H37CoN5O2P: C, 74.24; H, 4.19; N, 7.87%. Found: C, 74.31; H, 4.26; N, 7.82.
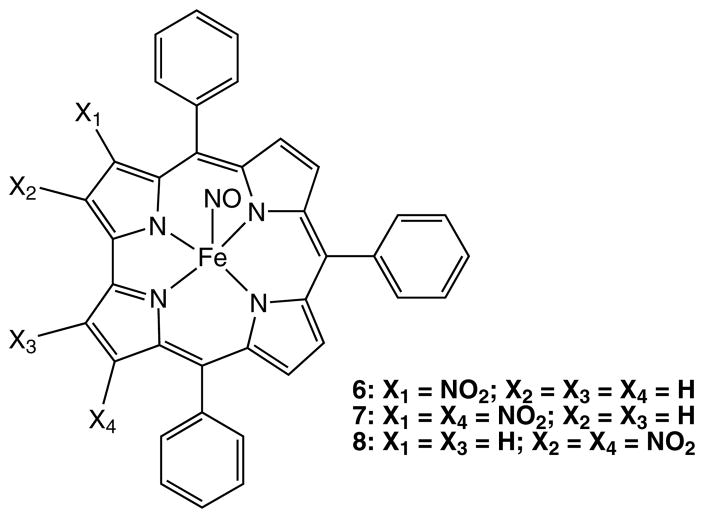
Nitration on iron-corrolates (method B, compounds 6–8)
66 mg of [TPCorr]Fe(Cl) (0.108 mmol) were dissolved in 40 mL of CH2Cl2, and 238 mg (3.45 mmol, 32 equiv.) dissolved in 40 mL of HCl 0.9 M were added. The mixture was stirred at room temperature for 5 min, monitoring the course of reaction by UV-vis spectrophotometry. After that time the acid was neutralized with Na2CO3. The mixture was extracted with CH2Cl2 and the organic phase was washed twice with water and dried over anhydrous Na2SO4; then the solvent was removed under reduced pressure. The residue was purified by column chromatography on silica gel eluting with CH2Cl2/hexane 80/20%.
[(3-NO2)-TPCorr]Fe(NO) (6)
Yield 8% (6 mg). Spectroscopic data are in agreement with those reported in the literature [26].
[(3,17-NO2)2-TPCorr]Fe(NO) (8)
Yield 14% (11 mg). Spectroscopic data are in agreement with those reported in the literature [26].
RESULTS AND DISCUSSION
Our previous investigations on the nitration of meso-arylcorrole, either free-base or metal complexes, allowed us to optimize the procedure for the preparation of mono- and dinitro-derivatives; to investigate the possibility to introduce additional nitro groups on the peripheral positions of the corrole ring we decided to test the effectiveness of different nitrating systems.
Our attention was attracted by the exploitation of the TFA/NaNO2 system; it is interesting to note that under these conditions the nitration of porphyrin meso-phenyl rings has been reported [22]; in this case TFA is not only involved in the formation of the nitrating agent, but it also drives the nitration on the phenyl rings, deactivating the pyrrolic positions toward substitution through protonation of the macrocycle inner core. However, differently from porphyrin, corrole has demonstrated to be reactive also after protonation, as in the case of Vilsmeier formylation; corrole free-base, in fact, can be directly formylated, while in the case of porphyrin it is necessary to carry out the reaction on appropriate metal complexes to avoid the deactivation due to the formation of the macro-cycle dication.
Furthermore, in the case of corrole it would be interesting to investigate the possibility of nitration at the meso-phenyls rings, by analogy with the case of porphyrins. We decide to test this reaction on corrole free-base, using TPCorrH3 as starting material, in order to compare the reactivity of pyrrole versus phenyl groups.
TPCorrH3 was dissolved in a few mL of TFA and 10 equivalents of NaNO2 were added (Scheme 1); the initial green solution, corresponding to the cationic [TPCorrH4]+, became brownish, and the resulting UV-vis spectrum completely changed. The reaction was quenched with water and TLC analysis of the mixture showed two green bands, together with a consistent amount of starting material decomposition (non-mobile red-brown spot).
Scheme 1.
Products of nitration with TFA/NaNO2
The purification of the reaction products was quite difficult: column chromatography separation using polar eluants was not effective, while reduction of the polarity of the eluting solvents caused the compounds to stick on the column, further decreasing the reaction yields, as indicated by the small amount of products collected. However, subsequent purification by preparative thin-layer chromatography allowed us to readily isolate the products.
The 1H NMR spectrum of the first green band showed two singlets (1H each) at 15.5 and 14.4 ppm for the inner hydrogens; this chemical shift is a signature of an isocorrole species, as already observed in the case of similar compounds [23]; furthermore, the presence of two signals suggested the formation of an isocorrole derivative where the interruption of the aromatic system takes place at position 5. The absence of the ring current led to a high field shift of the β-pyrrolic hydrogens; from 6.35 to 7.00 ppm two doublets and two multiplets (total integral 4H) pointed to the formation of a poly-nitrated derivative; moreover, a fifth signal for pyrrolic protons was detected at lower field (7.70 ppm), among the unresolved multiplets related to the resonance of the meso-phenyl protons. This chemical shift is a typical signature for β-nitroisocorrole derivatives, as already observed in the case of demetalation of the Ag mononitro corrole complex [24], which afforded the (3-NO2)(5-OH)TTiso CorrH2, and it can be attributed to the electron withdrawing influence of the –NO2 group. All these data are consistent with the formation of a trinitroisocorrole; taking in account the larger reactivity of the directly linked pyrrole subunits, we hypothesized this compound to be the 2,3,18-(NO2)3(5-OH)TPisoCorrH2.
The second band, olive green in color, showed similar behavior upon 1H NMR analysis; a signal at 14.92 ppm and the β-pyrrolic protons upfielded in the region 6.7–7.2 ppm indicated the formation of a different nitroisocorrole. The presence of a single resonance for the inner protons (2H) supported the formation of the 10- substituted isocorrole. Since this fraction showed one singlet (1H) and two doublets (2H each), we assumed it was the second isocorrole isomer of the trinitro derivative: 2,3,18-(NO2)3(10-OH)-TPisoCorrH2. A more accurate identification of the structures was not possible, since we did not obtain crystals good enough for X-ray diffraction analysis.
Having in mind to improve the purification and the characterization of the products, we repeated the reaction, and after the work up the metalation of the mixture with Co(AcO)2 and PPh3 was carried out. We choose this metal because its coordination to the macrocycle drives the rearomatization of isocorrole to corrole [25], and because of the diamagnetic behavior of the pentacoordinated cobalt complex. The crude reaction mixture was purified by silica gel column chromatography, first eluting with CH2Cl2, then with CH2Cl2-diethyl ether 1:1 and finally with diethyl ether.
The first green band was characterized by 1H NMR; the formation of the Co complex was confirmed by the disappearance of the signals around 14–15 ppm, while three new groups of signals belonging to the ortho, meta and para protons of the coordinated phosphine confirmed complex formation and, more interesting for the our objectives, we observed that the pyrrolic protons were shifted over 8 ppm; this is tangible evidence of the restored aromaticity. Two singlet (1H each) and a multiplet (2H) showed the presence of a polysubstituted corrole in an asymmetric way; all these data are consistent with the formation of a tetranitro derivative.
No similar compound was isolated during the previous experiment, probably because of a lower stability of the free-base of this compound, which mainly decomposed during the purification step. Crystals obtained by slow diffusion of methanol into a CDCl3 solution of this compound allowed us to unambiguously identify the compound as [2,3,12,18-(NO2)4-TPCorr]Co(PPh3) (1, Fig. 1).
This result was quite surprising due to the insertion of –NO2 at C12; we expected for a tetranitro derivative that the susbtitution would take place on pyrroles A and D, since they should be endowed with a larger reactivity; in the compound we identified, the fully functionalization of one pyrrole was accomplished by the insertion of a –NO2 on positions 18 and 12, instead of the presumed more reactive 17.
It is noteworthy that no modification of phenyl rings occurred; despite the protonation of the inner nitrogen, the β-pyrrolic positions retain high reactivity, driving the nitro moieties to attach to the macrocyclic ring instead of the meso-aryl substituents.
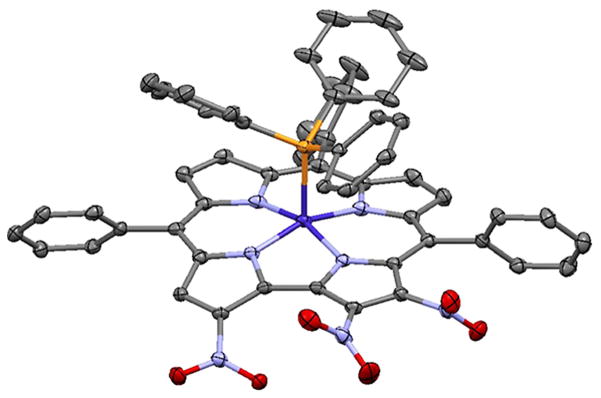
A second fraction, blue-green in color was isolated. 1H NMR spectroscopy suggested the formation of a trinitrocorrole, as indicated by the presence of one singlet corresponding to the proton of the monosubstituted pyrrole. X-ray analysis, carried out on a single crystal obtained by a slow diffusion of methanol into a CDCl3 solution of the corrole derivatives, allowed us to identify the position of the nitro substitution; this compound was identified as [2,3,18-(NO2)3-TPCorr]Co(PPh3) (2, Fig. 2).
Fig. 2.
X-ray structure of [2,3,18-(NO2)3-TPCorr]Co(PPh3) (2) with 40% ellipsoids. H atoms and the partially-occupied NO2 group at C17 are not shown
Also in this case we again observed the anomalous behavior of corrole, where the C18 undergoes the attack of the nitrating agent easier than does C17, as we expected. In the crystal structure, a disorder exists in which C17 is nitrated with population about 12%. While it is possible that this indicates the presence of a small amount of the tetranitro compound 3, it appears that C3 is not nitrated when C17 is. Thus the disorder more likely involves the overlap of two enantiomers of trinitro compound 2.
The crystal of compound 1 contains four independent Co complex molecules. In all, the Co atom is in a square pyramidal coordination geometry with PPh3 in the apical position. The Co–P distances range from 2.2242(17)–2.2432(17) Å, with a mean value of 2.2344 Å. The Co atom lies 0.269 Å (mean of four) out of the N4 basal plane, and Co–N distances lie in the range 1.865(5)–1.896(5) Å, with a mean value of 1.883 Å. In compound 2, the Co coordination is nearly identical to that in 1, with Co–P distance 2.2259(12) Å, Co–N distances in the range 1.871(3)–1.881(3) Å with mean 1.876 Å, and the Co atom lying 0.273(1) Å out of the N4 basal plane.
Even though the products were isolated in low yield, for the first time we were able to prepare different corroles having more than two nitro groups; for this reason we continued to investigate the potential of this reaction by varying some experimental parameters, as follows. The amount of NaNO2 was increased to 100 equivalents; by TLC analysis greater decomposition was observed together with the formation of two green fractions with UV-vis spectra very similar to those reported in the case of the reaction carried out with 10 equivalents of sodium nitrite. The insertion of Co led to the isolation of a main product, corresponding to 2. The reaction time was then extended up to one hour; by the purification of Co derivatives two fractions were collected; subsequent characterization by 1H NMR indicated that when the reaction takes place for longer times, the trinitro corrole continues to react, affording the asymmetric [2,3,12,18-(NO2)3-TPCorr]Co(PPh3) (1) and the symmetric [2,3,17,18-(NO2)3-TPCorr]Co(PPh3) (3).
Unfortunately we did not obtain good crystals of the symmetric nitrocorrole, but the 1H NMR spectra showed a pattern of signals consistent with our identification of the products, due to two singlets (1H each) and a broad doublet (2H) for 1, and only two doublets (2H each) for 3.
The products obtained showed how the nitro group strongly influenced the reactivity of corrole, affording products unexpected in terms of regioselectivity usually observed, inducing also the formation of 1 wherein the further functionalization is directed to the 12-position. However it is also interesting to note that the substitution occurred always at the β-positions of the macrocycle, not involving the meso-phenyl groups in contrast to the case of porphyrins.
The large decomposition we had in the different experiments represents a serious drawback of this method. So we turned our attention to a milder nitrating system, already exploited for the nitration of N-confused porphyrins [26, 27]. The protocol is based on a heterogeneous system, where the porphyrinoid is dissolved in CH2Cl2 and NaNO2 is dissolved in dilute HCl (Scheme 2).
Scheme 2.
Products of nitration with HCl/NaNO2
Corrole free-base was reacted with 32 equivalents of NaNO2 for 5 min; when no more variations of the UV-vis spectrum were observed, the acid was neutralized with Na2CO3 and the mixture was extracted with CH2Cl2. TLC analysis of the reaction mixture showed the presence of new products together with some decomposed material.
After purification on a silica gel column eluted with dichloromethane, two green fractions were collected and characterized by 1H NMR; also in this case the products isolated corresponded to isocorrole species, as suggested by the presence of one (2H) or two singlets (1H each) in the region 15–16 ppm, and the upfielded signals of the pyrrolic protons. To facilitate better characterization we repeated the reaction, and the mixture was reacted by metalation with Co ion; the purification afforded three products. The first red band was unreacted Co-corrole as confirmed by comparison of this product with a pure specimen of [TPCorr]Co(PPh3); the second corresponded to the [(3-NO2)(5-OH)TPIsoCorr], while the last band was identified as the [(3-NO2)TPCorr]Co(PPh3). To explain the partial failure in the aromatization of the isocorroles, we should consider that the presence of a nitro group on position 3 deeply affects the stability of isocorroles, making isomer-5 more stable than isomer-10. This is due to the H bond between the OH and NO2 groups, which hampers the rearomatization of the macrocycle. Since this stabilizing effect is not present on the 10-OH-isocorrole, this compound rearranges to form the corresponding Co-complex of nitrocorrole.
This hypothesis was supported by 1H NMR spectra, where the second band (isocorrole-like, emerald green in color) showed the presence of two signals for the inner protons at 16.36 and 14.81 ppm, several signals (doublets and multiplets) of the β-pyrroles resonating in the region 6.24–7.18 ppm and a singlet (1H) for the protons close to the nitro group at 6.31 ppm. For the second dark green band, no signals were observed in the region 14–16 ppm, and the resonance for the β-pyrroles were shifted to lower field than those of the other nitro-derivative, with the singlet located in this case at 9.09 ppm.
When the amount of nitrating agent was increased to 132 equivalent, mainly decomposition of starting material was observed.
Since we investigated the nitration on metallocorroles, to find out the effect of metal ion on corrole chemistry, we decide to test the last nitrating system on corrole complexes (Scheme 3) in order to compare the results with what we have recently reported for nitration on iron corroles [26]. [TPCorr]FeCl was dissolved in dichloromethane, and the mixture reacted with NaNO2 in dilute HCl; subsequent work-up afforded three main fractions. The first result we observed was that only traces of [Corr]Fe(NO) were obtained, while this compound was the major product when the reaction was carried out with the published procedure [26]. Among the other collected compounds, the first red band and the third green band were identified as the [(3-NO2)TPCorr]Fe(NO) (6) and the [(3,17-NO2)2TPCorr]Fe(NO) (7), respectively, as confirmed by comparison of the spectroscopic data with those in the literature [28]. The presence of the axially bonded NO group conferred a diamagnetic character to these compounds, which allowed their characterization by 1H NMR spectroscopy. Between the mono- and the dinitro-derivatives, a small quantity of a reddish compound was isolated.
Scheme 3.
Products of the nitration on iron-corrolates
Traces of a different compound were eluted between the mono-nitro and the dinitro-Fe-corroles. The presence of two singlets at 8.09 and 8.59 ppm at 1H NMR analysis is compatible with the formation of an asymmetric disubstituted corrole 8.
CONCLUSION
The results obtained demonstrate that stable corroles bearing up to four peripheral nitro substituents can be successfully synthesized using our new approach. These species can be of great interest since, on one hand nitro groups greatly influence the behavior of the macrocycle and on the other they can be used for further functionalization of the corrole ring.
Acknowledgments
This research was supported by the Italian MiUR (PRIN project 2007C8RW53), United States National Institutes of Health (K.M.S. grant CA 132861). The purchase of the diffractometer was made possible by Grant No. LEQSF(1999–2000)-ENH-TR-13, administered by the Louisiana Board of Regents.
Footnotes
Dedicated to Professor Karl M. Kadish on the occasion of his 65th birthday
Supporting information
Crystallographic data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under numbers CCDC 830941 and 830942. Copies can be obtained on request, free of charge, via www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223-336-033 or deposit@ccdc.cam.ac.uk).
References
- 1.Paolesse R, Jaquinod L, Nurco DJ, Mini S, Sagone F, Boschi T, Smith KM. Chem Commun. 1999:1307–1308. [Google Scholar]
- 2.Gross Z, Galili N, Saltsman I. Angew Chem, Int Ed Engl. 1999;38:1427–1429. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1427::AID-ANIE1427>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.a) Paolesse R, Marini A, Nardis S, Froiio A, Mandoj F, Nurco DJ, Prodi L, Montalti M, Smith KM. J Porphyrins Phthalocyanines. 2003;7:25–36. [Google Scholar]; b) Gryko DT, Fox JP, Goldberg DP. J Porphyrins Phthalocyanines. 2004;8:1091–1105. [Google Scholar]; c) Nardis S, Monti D, Paolesse R. Mini-Rev Org Chem. 2005;2:355–374. [Google Scholar]
- 4.Koszarna B, Gryko D. J Org Chem. 2006;71:3707–3717. doi: 10.1021/jo060007k. [DOI] [PubMed] [Google Scholar]
- 5.Paolesse R. Synlett. 2008:2215–2230. [Google Scholar]
- 6.Gross Z, Aviv-Harel I. Chem Eur J. 2009;15:8382–8394. doi: 10.1002/chem.200900920. [DOI] [PubMed] [Google Scholar]
- 7.Mahammed A, Goldberg I, Gross Z. Org Lett. 2001;3:3443–3446. doi: 10.1021/ol0163878. [DOI] [PubMed] [Google Scholar]
- 8.Saltsman I, Mahammed A, Goldberg I, Tkachenko E, Botoshansky M, Gross Z. J Am Chem Soc. 2002;124:7411–7420. doi: 10.1021/ja025851g. [DOI] [PubMed] [Google Scholar]
- 9.Paolesse R, Nardis S, Venanzi M, Mastroianni M, Russo M, Fronczek FR, Vicente MGH. Chem Eur J. 2003;9:1192–1197. doi: 10.1002/chem.200390136. [DOI] [PubMed] [Google Scholar]
- 10.Stefanelli M, Mastroianni M, Nardis S, Licoccia S, Fronczek FR, Smith KM, Zhu W, Ou Z, Kadish KM, Paolesse R. Inorg Chem. 2007;46:10791–10799. doi: 10.1021/ic7014572. [DOI] [PubMed] [Google Scholar]
- 11.Mastroianni M, Zhu W, Stefanelli M, Nardis S, Fronczek FR, Smith KM, Ou Z, Kadish KM, Paolesse R. Inorg Chem. 2008;47:11680–11687. doi: 10.1021/ic801421a. [DOI] [PubMed] [Google Scholar]
- 12.Stefanelli M, Mandoj F, Mastroianni M, Nardis S, Mohite P, Fronczek FR, Smith KM, Kadish KM, Xiao X, Ou Z, Paolesse R. Inorg Chem. 2011;50:8281–8292. doi: 10.1021/ic2008073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolesse R, Nardis S, Sagone F, Khoury RG. J Org Chem. 2001;66:550–556. doi: 10.1021/jo005661t. [DOI] [PubMed] [Google Scholar]
- 14.Wasbotten IH, Wondimagegn T, Ghosh A. J Am Chem Soc. 2002;124:8104–8116. doi: 10.1021/ja0113697. [DOI] [PubMed] [Google Scholar]
- 15.Nardis S, Mandoj F, Paolesse R, Fronczek FR, Smith KM, Prodi L, Montalti M, Battistini G. Eur J Inorg Chem. 2007:2345–2352. [Google Scholar]
- 16.Ruo-Bing D, Chao L, Dong-Mei S, Qing-Yun C. Synlett. 2009:2701–2705. [Google Scholar]
- 17.Tse MK, Zhou ZY, Mak TCW, Chan KS. Tetrahedron. 2000;56:7779–7783. [Google Scholar]
- 18.Jaquinod L. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Vol. 1. Academic Press; San Diego: 2000. pp. 201–232. [Google Scholar]
- 19.Tortora L, Nardis S, Fronczek FR, Smith KM, Paolesse R. Chem Commun. 2011;47:4243–4245. doi: 10.1039/c0cc05837h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheldrick GM. Acta Crystallogr Sect A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 21.Spek AL. Acta Crystallogr Sect D-Biol Crystallogr. 2009;65:148–155. doi: 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luguya R, Jaquinod L, Fronczek FR, Vicente MGH, Smith KM. Tetrahedron Lett. 2004;60:2757–2763. [Google Scholar]
- 23.Nardis S, Pomarico G, Fronczek FR, Vicente MGH, Paolesse R. Tetrahedron Lett. 2007;48:8643–8646. [Google Scholar]
- 24.Stefanelli M, Shen J, Zhu W, Mastroianni M, Mandoj F, Nardis S, Ou Z, Kadish KM, Fronczek FR, Smith KM, Paolesse R. Inorg Chem. 2009;48:6879–6887. doi: 10.1021/ic900859a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pomarico G, Xiao X, Nardis S, Paolesse R, Fronczek FR, Smith KM, Fang Y, Ou Z, Kadish KM. Inorg Chem. 2010;49:5766–5774. doi: 10.1021/ic100730j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toganoh M, Ikeda S, Furuta H. Inorg Chem. 2007;46:10003–10015. doi: 10.1021/ic701208g. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda S, Toganoh M, Easwaramoorthi S, Lim JM, Kim D, Furuta H. J Org Chem. 2010;75:8637–8649. doi: 10.1021/jo102128m. [DOI] [PubMed] [Google Scholar]
- 28.Stefanelli M, Nardis S, Tortora L, Fronczek FR, Smith KM, Licoccia S, Paolesse R. Chem Commun. 2011;47:4255–4257. doi: 10.1039/c0cc05491g. [DOI] [PMC free article] [PubMed] [Google Scholar]