Abstract
The native extracellular matrix (ECM) consists of an integrated fibrous protein network and proteoglycan-based ground (hydrogel) substance. We designed a novel electrospinning technique to engineer a three dimensional fiber-hydrogel composite that mimics the native ECM structure, is injectable, and has practical macroscale dimensions for clinically relevant tissue defects. In a model system of articular cartilage tissue engineering, the fiber-hydrogel composites enhanced the biological response of adult stem cells, with dynamic mechanical stimulation resulting in near native levels of extracellular matrix. This technology platform was expanded through structural and biochemical modification of the fibers including hydrophilic fibers containing chondroitin sulfate, a significant component of endogenous tissues, and hydrophobic fibers containing ECM microparticles.
Keywords: Electrospin, fibers, hydrogel, cartilage, extracellular matrix, biomimetic, tissue engineering
Introduction
The extracellular matrix is a framework of proteins and polysaccharides that provides not only the physical framework for cells and tissues but also contains numerous instructive signals critical for tissue development, homeostasis, and repair (Gregory and Annette 2009, Tsang 2010). While the chemical, biological, and physical composition of the extracellular matrix varies from tissue to tissue (Chiara and Ranieri 2009, Wagenseil and Mecham 2009), the fundamental building blocks are similar and include a fibrous protein network embedded within a gelatinous polysaccharide ground substance (Figure 1a) (Mow VC 1992). Similarly, tissue engineering biomaterial scaffolds are designed to serve as a framework for culturing cells and developing tissues while providing the appropriate instructive signals (Awad 2004, Engler 2006, Place 2009). However, most scaffolds to date fall short of being truly biomimetic with respect to mimicking the architecture of the native extracellular matrix. Few research groups have utilized fibrous scaffolds which have been layered or infused with hydrogels containing cells for cartilage tissue engineering (Hannouche 2007, Marijnissen 2002, Moutos 2007, Moutos and Guilak 2010). These composites have limitations which include a highly density fiber phase, lamellar fibrous structure and/or minimal control over fiber composition. In addition, a requisite for successful clinical translation is that the biomaterial must be practical and functional in the surgical arena.
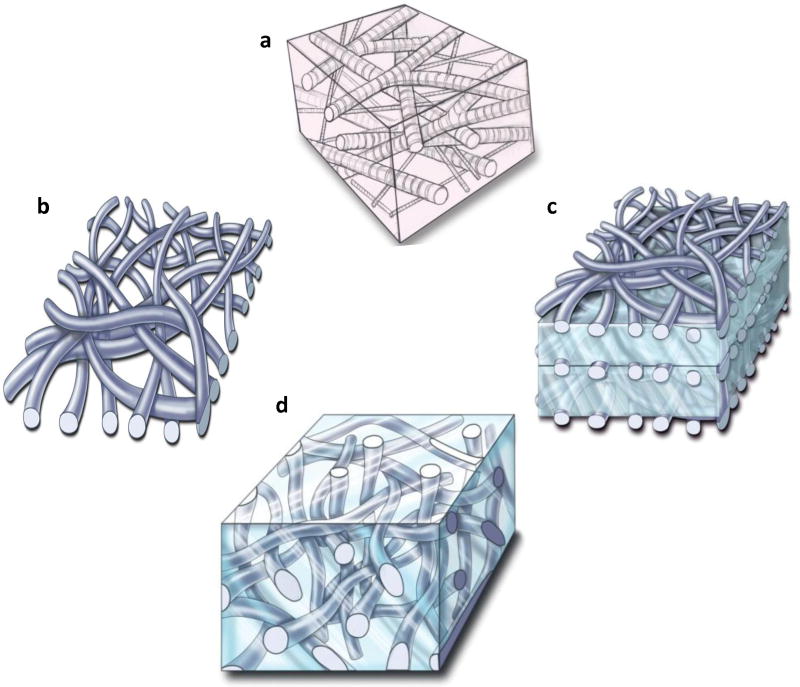
Figure 1. Architectural framework of a native extracellular matrix (ECM) and biomimetic fiber-hydrogel scaffolds.
a, The native ECM is composed of a fibrous protein network encapsulating a proteoglycan ground substance. b, Current electrospinning technologies produce a dense mat of fibers. c, The dense mats of fibers can be stacked with hydrogels to create a more three dimensional biomaterial scaffold. d, Novel electrospinning techniques allow synthesis of three-dimensional, low density fibers that can be combined with hydrogels to mimic the native ECM.
Materials and Methods
Fiber synthesis
Electrospinning solutions were prepared by dissolving 10% (w/v) PCL (Mn: 80,000, Sigma) in 8:2 dichloromethane/methanol. A standard electrospinning setup was used which includes a syringe pump to control the solution flow rate, a high voltage supply and a grounded substrate. PCL solutions were electrospun at 20 kV through a 20 G blunt end needle with a flow rate of 0.75 – 1.5 ml/min. To create fibers, PCL solutions were electrospun onto a grounded aluminum plate (for fiber mats and lamellar composites) or 9:1 ethanol/water solution while using a needle with a 90° bend (low density 3D fibers). The low density fibers were frozen, processed into usable dimensions, and vacuum dried before PEG and/or cellular infiltration.
For the chemically-modified fibers, aqueous electrospinning solutions were made with 9.1% (w/w) and 2% (w/v) PVAMA and CSMA (poly(vinyl alcohol)-methacrylate and chondroitin sulfate-methacrylate), respectively. Poly(vinyl alcohol) was methacrylated as previously described (Martens and Anseth 2000) except the reaction was run at 80°C. Chondroitin sulfate was methacrylated as previously described (Li 2003). Methacrylated polymers were column purified using Sephadex G-25 Fine (Sigma) size exclusion chromatography. The CSMA/PVAMA fibers were collected on a grounded aluminum plate at a flow rate of 0.4 ml/hr. The CSMA/PVAMA fibers were crosslinked with via free radical polymerization by immersing fiber mats in to 100% ethanol containing 10% (w/v) photoinitiator (Irgacure 2959; Ciba Specialty Chemicals, Tarrytown, NY) followed by UV-light exposure (365-nm, 3.2 kW/cm2) for 5 minutes.
Fiber hydrogel formation and characterization
Poly(ethylene glycol)-diacrylate solutions were prepared by dissolving 100 mg PEGDA (MW: 3,400 Da, SunBio, Inc., South Korea) in 1 ml sterile phosphate buffered saline (PBS) followed by the addition of photoinitiator (final concentration 0.05% (w/v)). Poly(ε-caprolactone) fibers collected in organic solvent were mixed with the hydrogel macromer, followed by UV exposure for 5 minutes. Mechanical testing was performed in unconfined uniaxial compression using a Bose ELF 3200 testing system. Samples were strained to 14% and the compressive Young's modulus was obtained from the slope of the stress-strain curve.
Cell encapsulation and characterization
For lamellar fiber-hydrogels, human mesenchymal stem cells (MSCs) were purchased from Cambrex (Walkersville, MD, USA). For fiber-hydrogel composites, goat MSCs were isolated as previously described (Williams, 2003). Cells were grown in high glucose DMEM (Invitrogen Corp, Grand Island, New York, USA) supplemented with 10% fetal bovine serum (Logan, UT, USA), 2 mM L-glutamine (Invitrogen Corp, Grand Island, New York, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Quality Biological, Inc, Gaithersburg, MD, USA). Passage 3 MSCs were suspended in the polymer solution at a concentration of 20 × 106 cells per ml, added to the fibrous scaffold, and photopolymerized in either lamellar or bulk composite constructs.
Cell-seeded bulk fiber-hydrogel scaffolds were generated by combining the cell-containing hydrogel solution with the low density fibers and polymerizing as described above. Two concentrations of fibers (10 and 40% dry weight) were combined with the hydrogel.
For lamellar fiber-hydrogel studies, two million cells per milliliter were suspended in PEGDA solution containing YRGDS-PEG-acrylate with a final concentration of 15% PEG and 2.5 mM YRGDS. YRGDS-PEG-acrylate synthesis was performed as previously published (Yang, 2005). Thirty microliters of cell suspension was placed into molds and crosslinked using UV light for 1 minute. One 6 mm diameter fiber disk was placed onto the partially polymerized solution and layered with 30 μl cell suspension, crosslinked for 1 minute followed by addition of another layer and crosslinking for 5 minutes. A total of 3 hydrogel layers and 3 fiber disks were incorporated into one scaffold and PEG-RGD hydrogels were used as controls. Cells were cultured for a total 28 days in chondrogenic medium (high-glucose DMEM supplemented with 100 nM dexamethasone, 50 μg/ml ascrobate-2-phosphate, 40 μg/ml proline, 100 μg/ml sodium pyruvate, 1% penicillin streptomycin and 50 mg/ml ITS-Premix (Collaborative Biomedical: 6.25 ng/ml insulin, 6.25 mg transferiin, 6.25 ng/ml selenious acid, 1.25 mg/ml bovine serum albumin, and 5.35 mg/ml linoleic acid), with 10 ng/ml TGF-β1). For mechanical stimulation studies, scaffolds were subjected to dynamic stimulation straining to 10% at a rate of 1 Hz for 2.5 hours (Schmidt, 2006) every day during the first 14 days followed by static culturing for an additional 14 days.
Confocal Microscopy
Cell-laden constructs were fixed in 4% paraformaldehyde (PFA) in PBS (pH 7.4) at 4°C overnight followed by labeling with rhodamine phalloidin and Hoescht 33258 for actin and nuclei, respectively. Constructs were imaged with a Zeiss LSM 510 Meta Confocal. Scanning electron microscopy (SEM) images were obtained using a FEI Quanta 200 Environmental SEM (FEI Comp, Hillsboro, OR, USA). Fiber-ECM samples were sputter coated using an Anatech Hummer 6.2 sputter coater (Anatech Ltd., Springfield, VA, USA) with platinum. For histological analysis, constructs were fixed in 4% PFA and transferred to 70% ethanol until processing. Constructs were processed in paraffin according to standard histological technique and stained with Safranin-O/Fast green. For biochemical analysis, constructs were digested and GAG and DNA contents were measured as previously described (Farndale, 1986, Kim, 1988).
Statistical analysis
Data are presented as the mean with standard deviations. Statistical significance was determined using ANOVA followed by a Tukey's test (SPSS 10.0) and set as P≤0.05.
Results and Discussion
A platform system has been designed to generate biomimetic fiber-hydrogel scaffolds with architectural and chemical flexibility that allows targeting to multiple tissue needs and clinical applications. In contrast to previous fiber-based materials using the electrospinning technique which are effectively a dense sheet on the macroscale and only a few millimeters thick (Figure 1b) (Li 2006), scaffolds have been created with clinically-relevant dimensions (thickness) composed of electrospun fibers and hydrogel. In the first embodiment of this technology, fiber mats were infused with hydrogels and stacked to form a three dimensional, multilayered composite (Figure 1c). The thickness of the hydrogel layer and the number of fiber layers can be tailored for a specific application. This approach is relevant from a biomimetic perspective to replicate tissue structures that comprise layers such as the skin, cornea, and epithelial tissues in general.
The second embodiment of the fiber-hydrogel composite is a bulk three dimensional fiber-reinforced hydrogel (Figure 1d). Again, the hydrogel portion can be tailored for a particular application with respect to chemistry, biological properties, and adhesiveness for ease in clinical application (Strehin 2009). The key finding that facilitated development of this technology was the observation that a low density fibrous scaffold could be fabricated using a novel electrospinning technique. Specifically, this technique utilizes a nonaqueous solvent as the medium for collecting the fibers as opposed to a solid surface or water (Tzezana 2008). This process results in a three dimensional fiber mesh with porosity relevant for hydrogel and cell infusion, homogenous tissue growth, and injectability. Utilizing this technology, we constructed three dimensional fibrous composites such that hydrogel and cellular infiltration required only simple mixing, without any external force (Moutos 2007). In fact, Figure 2a pictures a fiber-macromer (pre-hydrogel) mixture that is flexible and can be easily injected through a syringe. In addition, the fiber network can be used as a vehicle for injecting a polymer solution into a wet environment where the fibrous component effectively traps the viscous hydrogel and limits diffusion away from the implant site. After polymerization of the hydrogel component, fibers are distributed in a three-dimensional configuration throughout the bulk of the material. Figure 2b depicts a standard poly(ethylene glycol)-diacrylate (PEGDA) hydrogel (clear) and a fiber-reinforced hydrogel. Importantly, the low density fiber network is distributed throughout the multiple millimeter thick scaffold. This dimensionality represents a significant departure from today's fiber-based tissue engineering applications and allows this system to be truly biomimetic. The fiber-reinforcement of the hydrogels also resulted in a significant increase in mechanical properties in a dose dependent manner (Figure 2c). Ultrastructure of the fiber-reinforced scaffold demonstrated that the fibers are integrated within the hydrogel (Figure 3a). Confocal imaging of the fibers within the hydrogel highlights the three dimensional architecture similar to that of the native extracellular matrix of cartilage (Figure 3b) (Segawa and Takiguchi 1992).
Figure 2. Fiber-hydrogel handling and physical properties.
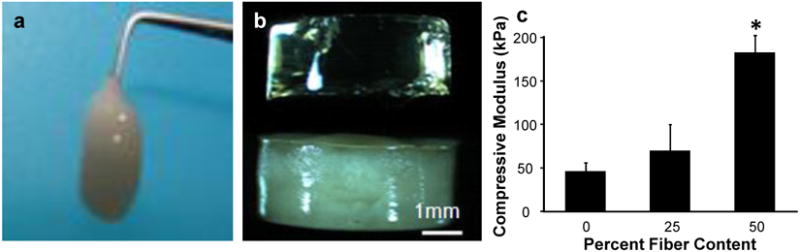
a, Hydrogel macromer solutions can be easily combined with the low density fibers and the resulting three-dimensional fiber-hydrogel composite is injectable before in situ hydrogel polymerization. b, After polymerization, the fibers are visible and homogenously distributed throughout the hydrogel (bottom), compared to the hydrogel alone (top). c, Incorporation of fibers into a hydrogel significantly enhanced compressive strength in a dose-dependent manner. *P≤0.05
Figure 3. Fiber-hydrogel ultrastructure and cellular morphology.
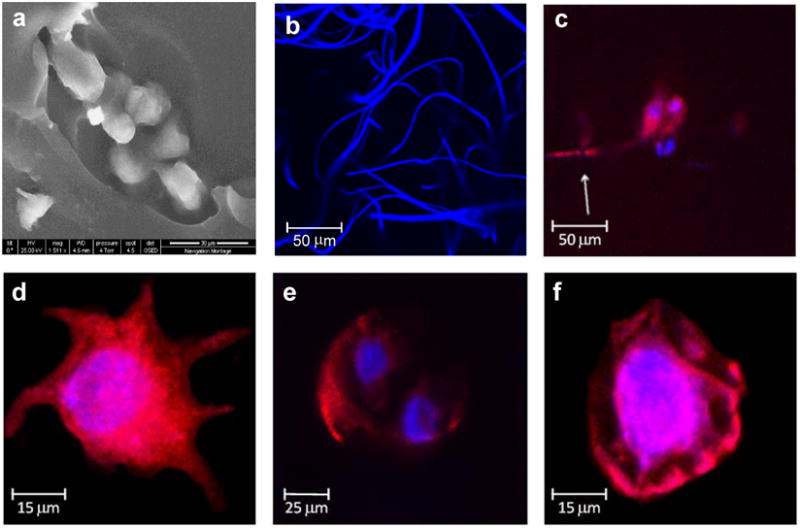
a, Scanning electron microscopy focuses on fibers embedded in the hydrogel. b, Fluorescent staining and confocal microscopy of the fibers highlight the large pore size of the fiber network that creates a three dimensional structure that mimics the native ECM. c-e, Mesenchymal stem cell morphology in the mimetic fiber-hydrogel composites varies to include cells with multiple and long extensions and dividing cells with a round morphology. f, Cells in the hydrogel alone scaffolds have a round morphology.
Cell response to the biomimetic fiber-hydrogel materials was markedly altered compared to the hydrogel alone. The cells encapsulated in the mimetic scaffold showed a variety of shapes and scaffold interactions, similar to the diversity found in a normal tissue environment. Cells with long extensions and stellate morphologies were observed in the fiber-reinforced hydrogels (Figure 3c and d), in addition to dividing cells with a spherical morphology (Figure 3e). This is in contrast to a hydrogel alone where only rounded cells can be found (Figure 3f).
Longer-term biological impact of the biomimetic fiber-hydrogels was evaluated in a cartilage tissue engineering application. Cartilage lines the surface of diarthrodial joints. When damaged, cartilage tissue cannot repair, making it a focus for tissue engineering research. Strategies for cartilage scaffold development range from producing a material with identical mechanical properties to the native cartilage (Gong 2003, Moutos 2007, Nakayama 2004, Slivka 2001, Woodfield 2004) to softer, hydrogel-based scaffolds (Winer 2009). From a developmental biology and materials science perspective, mesenchymal cells undergoing chondrogenesis prefer a softer material (Engler 2006). In this regard, we previously developed PEG-based hydrogel scaffolds for guiding native or exogenous cells to undergo chondrogenesis and develop new cartilage (Lee 2008, Varghese 2008, Williams 2003). However, the limited mechanical properties of hydrogels may negatively affect their survival in the rigorous joint environment. The presence of fibers in the context of a hydrogel environment provides mechanical reinforcement, analogous to the concept of fiber reinforced concrete (Ramadoss and Nagamani 2009, Williams 2010). Furthermore, fibers provide an adhesive substrate for cell attachment and migration. These parameters can be customized to facilitate stem cell proliferation or differentiation.
To characterize the biological significance of the newly developed scaffolds, chondrogenesis of MSCs was evaluated. Mesenchymal stem cells are often employed in tissue engineering because of their robust proliferative and differentiation capacity. These cells were first incorporated into fiber-hydrogel lamellar scaffolds pictured in Figure 1c. The fibrous component of this system consisted of poly(ε-caprolactone) (PCL) with the hydrogel comprised of PEG and PEG-YRGDS. Following 28 days of chondrogenic differentiation, cells in the composite material produced greater levels of proteoglycan (GAG) and total collagen (Figure 4a) compared to hydrogel alone.
Figure 4. Cartilage tissue development in the fiber-hydrogel scaffolds and response to mechanical stimuli.
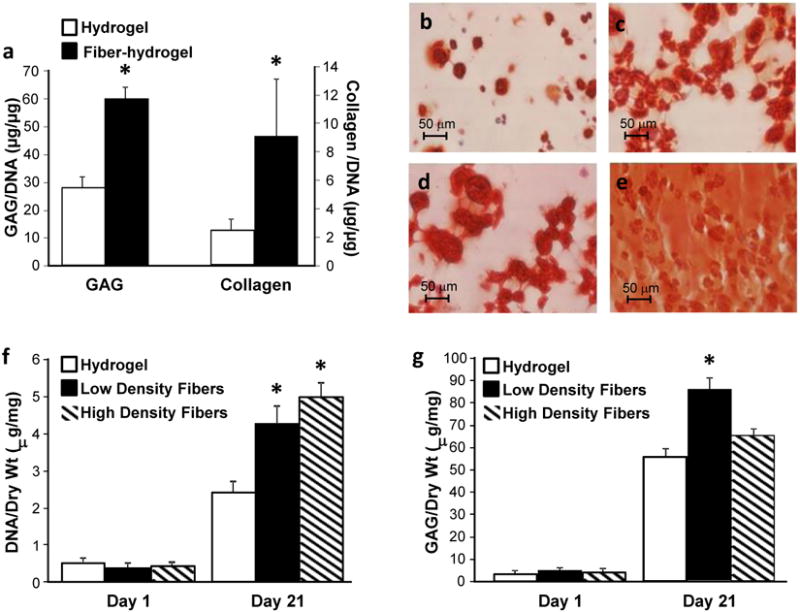
a, MSCs encapsulated in the lamellar fiber-hydrogel scaffolds and cultured in chondrogenic conditions produced significantly greater amounts of extracellular matrix. b, Safranin-O staining confirmed that MSCs secreted more proteoglycans in the fiber-hydrogel composites compared to the c, hydrogel alone scaffolds. d, Dynamic mechanical stimulation (+MS) produced a significantly greater cellular response with increased ECM deposition and homogenous distribution in the fiber-hydrogel composites as visualized by Safranin-O. e, compared to cells in the hydrogel alone. f, MSCs incorporated in the three dimensional fiber-hydrogel composites increased proliferation as the density of fibers increased. g, Proteoglycan secretion, a marker for cartilage formation, was greatest when MSCs were incorporated in fiber-hydrogel composites with a low density of fibers (low and high fiber density: 10% and 40% of dry weight, respectively). *P≤0.05
Mechanical forces also play an important role in tissue development and homeostasis. Dynamic mechanical compression, a mimic of walking, stimulates chondrogenesis of MSCs in a number of tissue engineering systems (Janna 2007, Vanessa 2007). The lamellar fiber-hydrogels and control hydrogels were exposed to dynamic mechanical compression for 2.5 hours each day for two weeks, followed by an additional two weeks of static culture. Significantly more new extracellular matrix was produced in the fiber-hydrogel composites compared to hydrogel alone as visualized by Safranin-O staining for proteoglycans (Figures 4b and c). Furthermore, MSCs encapsulated in the fiber-hydrogel composite had a significantly greater response to mechanical stimulation as demonstrated by a drastic increase in tissue production, reaching a nearly uniform distribution of proteoglycan staining throughout the scaffold (Figures 4d and e). The fiber-reinforced hydrogel scaffold enhanced transmission and biological response of the cells with the mechanical signals. This could be attributed to the coupled effects of enhanced matrix production secondary to the incorporated fibers along with more efficient force transduction through the fibers and ECM.
Mesenchymal stem cells were also incorporated into the bulk fiber-hydrogel composites using the fibers formed with the new spinning techniques. Varying levels of fibers were incorporated with hydrogel to create the biomimetic fiber-hydrogel composite pictured in Figure 1d. The density of fibers within the construct influenced tissue formation with the lower (10% dry weight) fiber density resulting in the greatest amount of matrix production. The higher (40% dry weight) fiber density resulted in greater cell proliferation. Scaffold composition can therefore be optimized to select for different cell functions, ie., proliferation versus matrix production.
As a material platform, the biomimetic fiber-hydrogel composite can be further modified for the desired applications. A range of complex physical, chemical and biological cues can be incorporated into this platform. From the physical perspective, fiber diameter (micro to nanometer range) and alignment can be controlled to mimic a variety of native ECM architectures (Figure 5a). The biochemical nature of the fibers can also be manipulated to include biological signals, from various ECM molecules to growth factors and other signaling molecules. For example, nanofibers can be generated from chondroitin sulfate (CS), a molecule that promotes cartilage regeneration (Figure 5b) (Varghese 2008). Even greater biological complexity can be integrated into fibers in the form of actual tissue matrix. To our knowledge, this is the first report to present ECM microparticles engineered into an electrospun fiber. Scanning electron microscopy (SEM) and Safranin-O staining highlight the tissue fragments encapsulated in fibers (Figures 5c and d).
Figure 5. Chemical and biological flexibility of fibers for scaffolding.
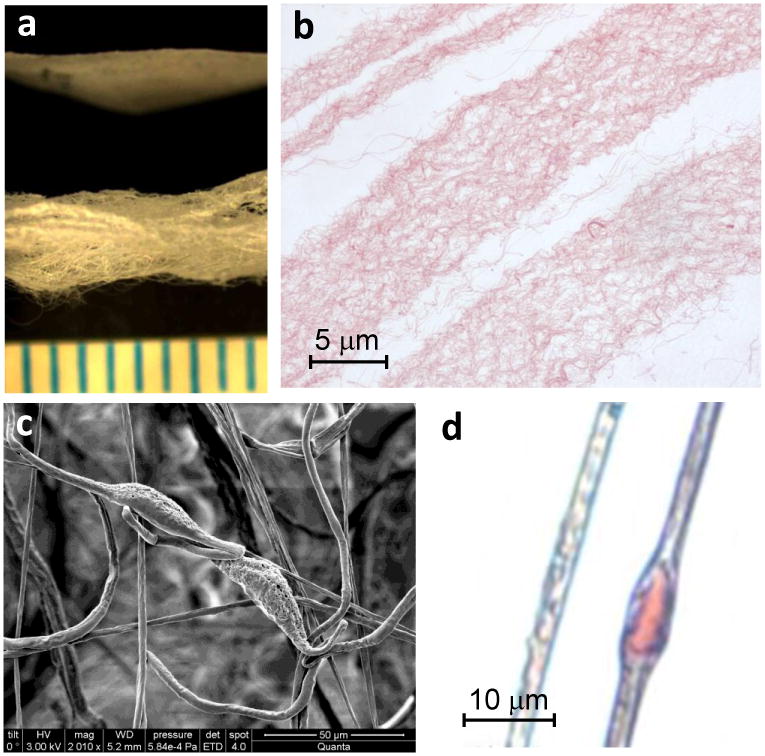
a, Three dimensional nanofibers were generated from a combination of PVA and chondroitin sulfate (CS). b, Histological staining with Safranin-O confirms the presence of the proteoglycan in the fibers. c, Segments of cartilage ECM were encapsulated in the fibers as visualized by scanning electron microscopy and d, Safranin-O staining for proteoglycans.
Conclusions
In summary, a biomimetic fiber-hydrogel composite was created. These scaffolds mimic both the fibrous protein and proteoglycan ground substance of the native extracellular matrix. The resulting integrated fiber-hydrogel composite has significantly greater physical (mechanical) properties and induces enhanced biological responses from adult stem cells to ultimately produce more tissue.
Acknowledgments
The authors gratefully acknowledge NIH grants R01EB005517 and the National Science Foundation Early Career Award DMR-0848340
References
- Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25(16):3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Chiara G, Ranieri C. Cartilage and Bone Extracellular Matrix. Curr Pharm Des. 2009;15:1334–1348. doi: 10.2174/138161209787846739. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta (BBA) Gen Sub. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Gong JP, Katsuyama Y, Kurokawa T, Osada Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv Mater. 2003;15(14):1155–1158. [Google Scholar]
- Gregory SS, Annette W. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17(2):153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Hannouche D, Terai H, Fuchs JR, Terada S, Zand S, Nasseri BA, Petite H, Sedel L, Vacanti JP. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 2007;13(1):87–99. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- Janna KM, John TC, Christopher GW, Kristin EM, Marc EL. Dynamic Compression Regulates the Expression and Synthesis of Chondrocyte-Specific Matrix Molecules in Bone Marrow Stromal Cells. Stem Cells. 2007;25(3):655–663. doi: 10.1634/stemcells.2006-0435. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Sah RLY, Doong JYH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174(1):168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Yu C, Chansakul T, Hwang NS, Varghese S, Yu SM, Elisseeff JH. Enhanced Chondrogenesis of Mesenchymal Stem Cells in Collagen Mimetic Peptide-Mediated Microenvironment. Tissue Eng. 2008;14(11):1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang Da, Elisseeff JH. Heterogeneous-phase reaction of glycidyl methacrylate and chondroitin sulfate: Mechanism of ring-opening-transesterification Competition. Macromolecules. 2003;36(7):2556–2562. [Google Scholar]
- Li W, Cooper JA, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2(4):377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Marijnissen WJ, van Osch GJ, Aigner J, van der Veen SW, Hollander AP, Verwoerd-Verhoef HL, Verhaar JA. Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials. 2002;23(6):1511–1517. doi: 10.1016/s0142-9612(01)00281-2. [DOI] [PubMed] [Google Scholar]
- Martens P, Anseth KS. Characterization of hydrogels formed from acrylate modified poly(vinyl alcohol) macromers. Polymer. 2000;41(21):7715–7722. [Google Scholar]
- Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mat. 2007;6(2):162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- Moutos FT, Guilak F. Functional properties of cell-seeded three-dimensionally woven poly(ε-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng. 2010;16(4):1291–1301. doi: 10.1089/ten.tea.2009.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow VC, Ratcliffe A, Poole AR. Carilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- Nakayama A, Kakugo A, Gong JP, Osada Y, Takai M, Erata T, Kawano S. High Mechanical Strength Double-Network Hydrogel with Bacterial Cellulose. Adv Fun Mat. 2004;14(11):1124–1128. [Google Scholar]
- Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mat. 2009;8(6):457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- Ramadoss P, Nagamani K. Behavior of high-strength fiber reinforced concrete plates under in-plane and transverse loads. Struct Eng Mech. 2009;31(4):371–382. [Google Scholar]
- Schmidt O, Mizrahi J, Elisseeff J, Seliktar D. Immobilized fibrinogen in PEG hydrogels does not improve chondrocyte-mediated matrix deposition in response to mechanical stimulation. Biotechnol Bioeng. 2006;95(6):1061–1069. doi: 10.1002/bit.21072. [DOI] [PubMed] [Google Scholar]
- Segawa K, Takiguchi R. Ultrastructural alteration of cartilaginous fibril arrangement in the rat mandibular condyle as revealed by high-resolution scanning electron microscopy. The Anatomical Record. 1992;234(4):493–499. doi: 10.1002/ar.1092340405. [DOI] [PubMed] [Google Scholar]
- Slivka MA, Leatherbury NC, Kieswetter K, Niederauer GG. Porous, Resorbable, Fiber-Reinforced Scaffolds Tailored for Articular Cartilage Repair. Tissue Eng. 2001;7(6):767–780. doi: 10.1089/107632701753337717. [DOI] [PubMed] [Google Scholar]
- Strehin I, Winnette McIntosh A, Oliver S, Afrah S, Elisseeff JH. Synthesis and characterization of a chondroitin sulfate-polyethylene glycol corneal adhesive. J Cataract Refract Surg. 2009;35(3):567–576. doi: 10.1016/j.jcrs.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Tsang K, Cheung M, Chan D, Cheah K. The developmental roles of the extracellular matrix: beyond structure to regulation. Cell Tissue Res. 2010;339(1):93–110. doi: 10.1007/s00441-009-0893-8. [DOI] [PubMed] [Google Scholar]
- Tzezana R, Zussman E, Levenberg S. A Layered Ultra-Porous Scaffold for Tissue Engineering, Created via a Hydrospinning Method. Tissue Eng. 2008;14(4):281–288. doi: 10.1089/ten.tec.2008.0201. [DOI] [PubMed] [Google Scholar]
- Vanessa T, Nathaniel H, Lorenzo M, Hyung Bin P, Zijun Z, Joseph M, Dror S, Jennifer E. Differential Response of Adult and Embryonic Mesenchymal Progenitor Cells to Mechanical Compression in Hydrogels. Stem Cells. 2007;25(11):2730–2738. doi: 10.1634/stemcells.2007-0228. [DOI] [PubMed] [Google Scholar]
- Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27(1):12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. Vascular Extracellular Matrix and Arterial Mechanics. Physiol Rev. 2009;89(3):957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In Vitro Chondrogenesis of Bone Marrow-Derived Mesenchymal Stem Cells in a Photopolymerizing Hydrogel. Tissue Eng. 2003;9(4):679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- Williams EM, Graham SS, Akers SA, Reed PA, Rushing TS. Constitutive property behavior of an untra-high-performance concrete with and without steel fibers. Computers and Concrete. 2010;7(2):191–202. [Google Scholar]
- Winer JP, Janmey PA, McCormick ME, Funaki M. Bone Marrow-Derived Human Mesenchymal Stem Cells Become Quiescent on Soft Substrates but Remain Responsive to Chemical or Mechanical Stimuli. Tissue Eng. 2009;15(1):147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- Woodfield TBF, Malda J, de Wijn J, Péters F, Riesle J, van Blitterswijk CA. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004;25(18):4149–4161. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Yang F, Williams CG, Wang Da, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26(30):5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]