Abstract
Peptidomimetic-based macrocycles typically have improved pharmacokinetic properties over those observed with peptide analogs. Described are the syntheses of 13 peptidomimetic derivatives that are based on active Sansalvamide A structures, where these analogs incorporate heterocycles (triazoles, oxazoles, thiazoles, or pseudoprolines) along the macrocyclic backbone. The syntheses of these derivatives employ several approaches that can be applied to convert a macrocyclic peptide into its peptidomimetic counterpart. These approaches include peptide modifications to generate the alkyne and azide for click chemistry, a serine conversion into an oxazole, a Hantzsch reaction to generate the thiazole, and protected threonine to generate the pseudoproline derivatives. Furthermore, we show that two different peptidomimetic moieties, triazoles and thiazoles, can be incorporated into the macrocyclic backbone without reducing cytotoxicity: triazole and thiazole.
Keywords: Peptide, Macrocycle, Peptidomimetic, Sansalvamide A, Triazole, Oxazole, Thiazole, Pseudoproline
1. Introduction
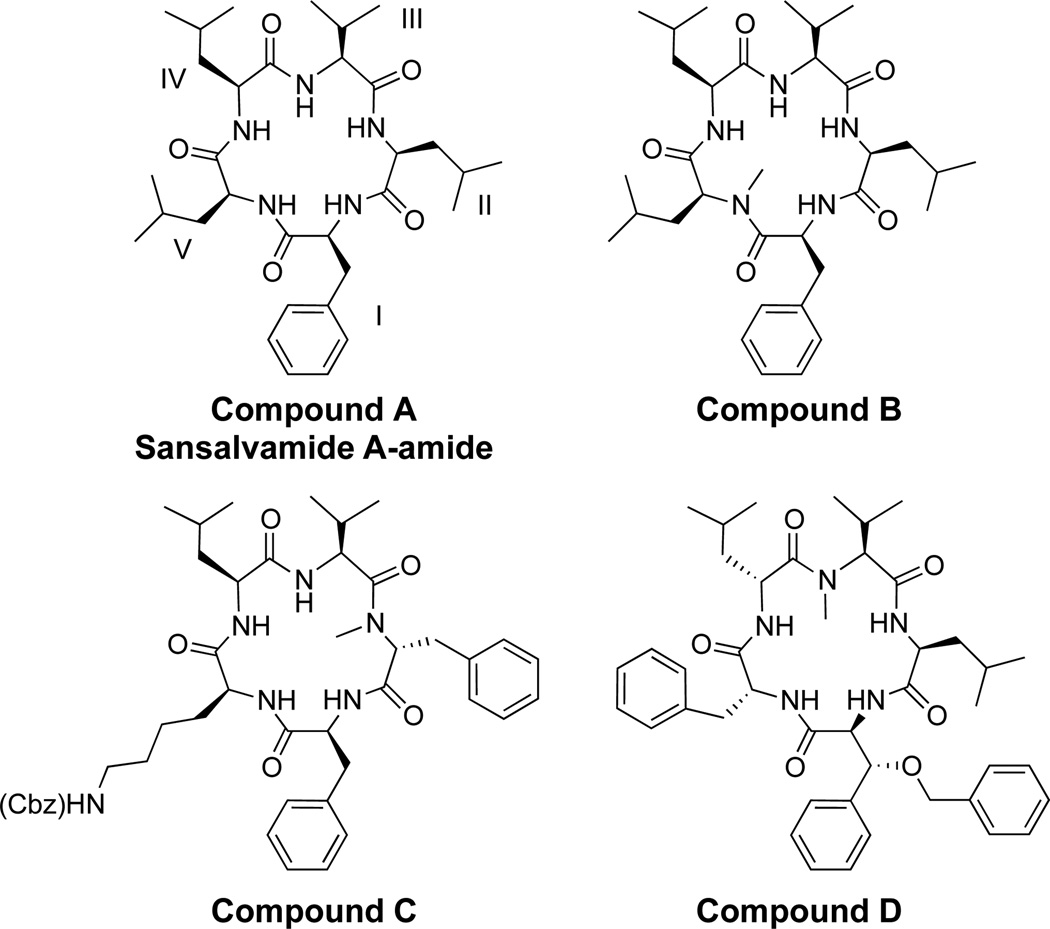
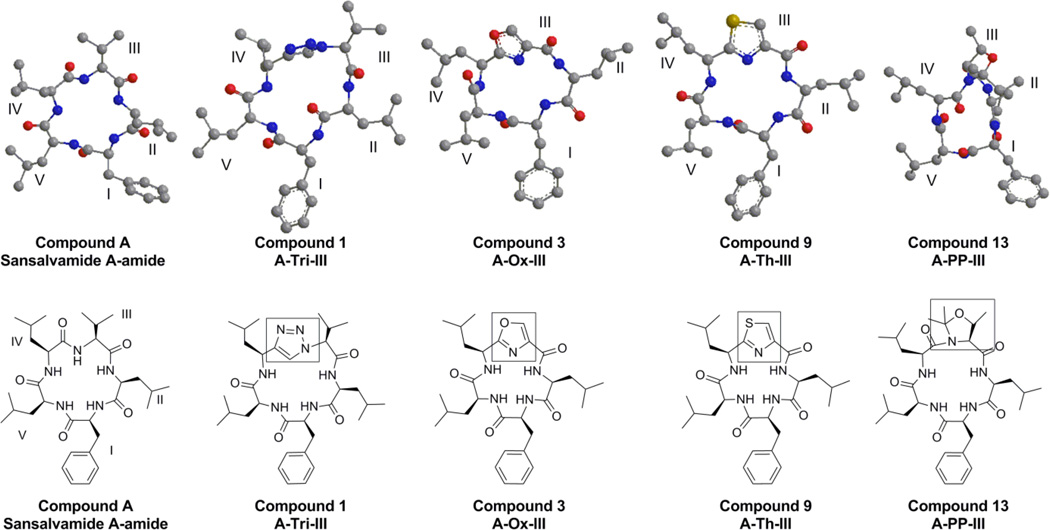
Recent work describing the synthesis and biological activity of pentapeptide derivatives that are based on the natural product Sansalvamide A (San A), has brought attention to this compound class.1–4 San A was isolated from a marine fungus of the genus Fusarium by Fenical and co-workers.3 The pentapeptide structure (San A-amide, compound A, Figure 1) has been used extensively as a template for the synthesis of compounds, where a number of these molecules exhibit cytotoxicity.1,5–7 We have discovered that in addition to San A-amide, three derivatives (compounds B, C, and D, Figure 1) are cytotoxic against numerous cancer cell lines, and these compounds inhibit a key protein that enables many proteins involved in tumor progression: Heat shock protein 90 (Hsp90).1,8
Figure 1.
San A compounds
Hsp90 is a well-established chemotherapeutic target that modulates client proteins involved in cellular growth, angiogenesis, and apoptosis.9–14 The redundancy of pathways involved in cancer cell growth means targeting multiple mechanisms simultaneously improves it's chances as a successful therapy. Hsp90 controls approximately 200 client proteins and co-chaperones, many of which are involved in multiple cancer-related cell signaling pathways.15–17 There are currently fifteen Hsp90 inhibitors in development, with two of these in phase III clinical trials.18–23 We have previously reported that San A-amide (A, Figure 1) is a cytotoxic molecule that modulates the activity of Hsp90. This modulation of Hsp90 acts via an allosteric effect, where San A-amide binds to the N-middle region and inhibits C-terminal client proteins.8 This mechanism of action is unique to Hsp90 inhibitors, making San A and its derivatives valuable molecular tools and potential lead structures for future chemotherapeutic studies.
In order to prepare these compounds to move into the next stage of development (mice models) the pharmacokinetic (PK) properties of these molecules should be improved as they are relatively poor.1 One mechanism for improving PK is to introduce peptidomimetic features, or structural motifs that mimic the peptide backbone, where these mimics often improve the solubility and stability of the molecule without impacting the cytotoxicity.24,25 Introduction of these motifs into the macrocyclic backbone has been shown to rigidify the macrocycles, as well as improve the absorption, distribution, metabolism and excretion (ADME) properties.26–29 Some common heterocycles that are known to improve stability of the peptide backbone include: triazoles, oxazoles, thiazoles and pseudoprolines.30–33
The inclusion of a triazole, particularly in cyclic peptide backbone, has demonstrated an improvement in biological activity.27,32 Further, triazoles induce a rigid conformation by mimicking trans amide bonds.27,30 Studies have shown that a single N-methyl, D-aa or N-methyl D-aa play a critical role in locking the San A-amide macrocyclic analogs into a single conformation.34,35,5–7,36,37,1 If this conformation induces an advantageous presentation of the side chains to their biological target, locking it into place via one of these structural features will likely improve binding between the compound and the protein target. Likewise, there is also precedence for oxazole and thiazole pepidomimetic moieties improving biological stability when substituted within peptide backbones.27 Similarly to triazoles, pseudoprolines induce a rigid conformation by mimicking cis amide bonds, and thus make structurally interesting comparisions to triazoles.38,39,26,40
Herein we describe the synthesis of 13 peptidomimetics that are based on San A-amide and the potent analogs B–D (Figure 1). These compounds were chosen because they have demonstrated appropriate cytotoxicity, and they inhibit Hsp90.1,8 The synthesis of compounds that incorporate a triazole, oxazole, thiazole, or pseudoproline involved both solution and solid-phase approaches. These peptidomimetic residues are substituted for different features within the San-A structure. The triazole replaces an amide bond, whereas the oxazole and thiazole replace both an amide bond as well as the adjacent amino acid side chain, and the pseudoproline replaces only the amino acid side chain. We discuss a series of synthetic strategies for these four unique classes of Sansalvamide peptidomimetics, where these methods can be applied as general approaches for the conversion of macrocyclic peptides into peptidomimetic compounds. Further, biological testing of our peptidomimetic compounds allowed us to evaluate which of these heterocyclic features are ideal for incorporation into future potent analogs and how their position in the macrocycle affects their cytotoxicity.
2. Results and discussion
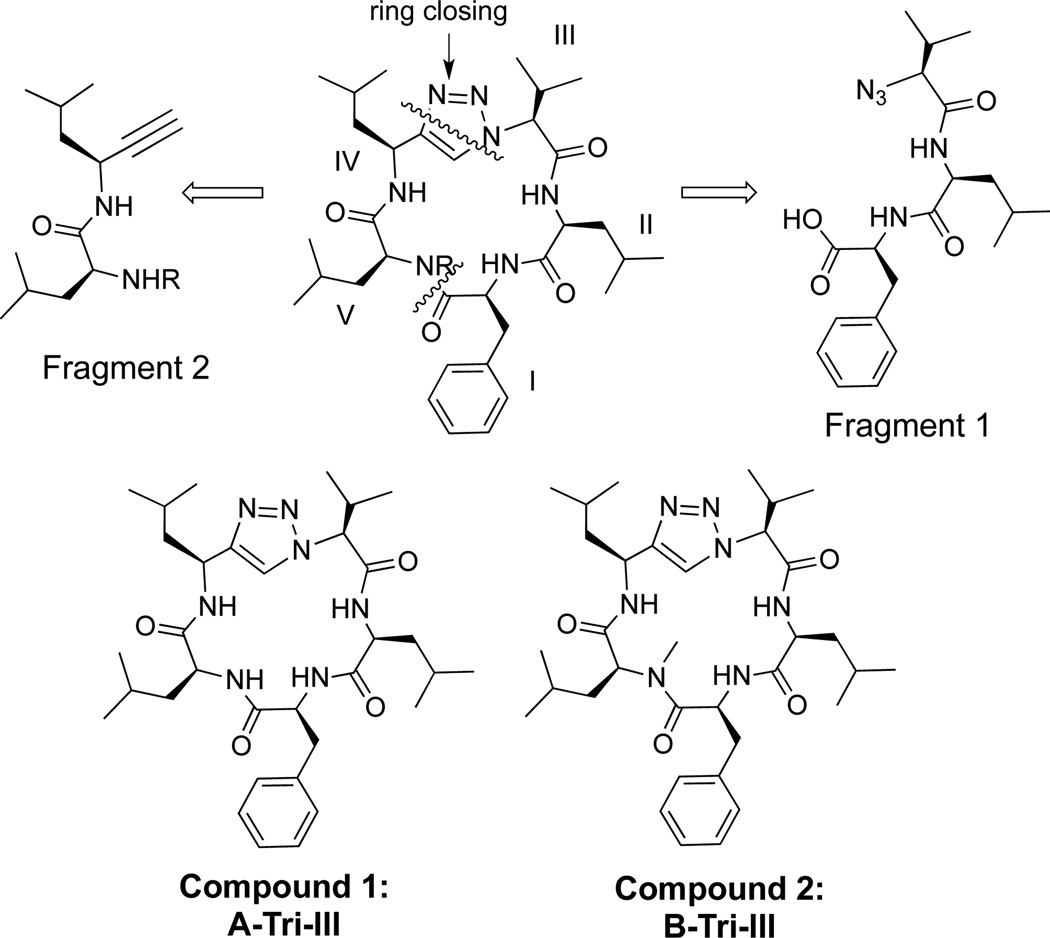
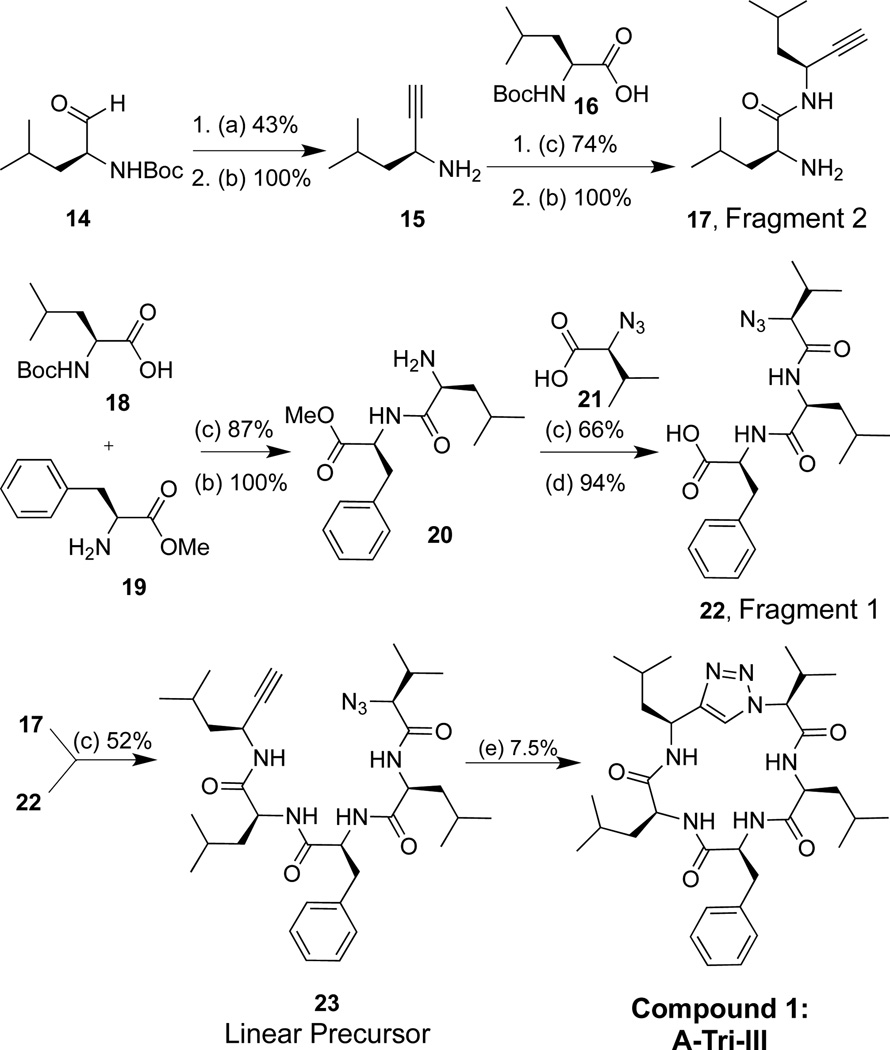
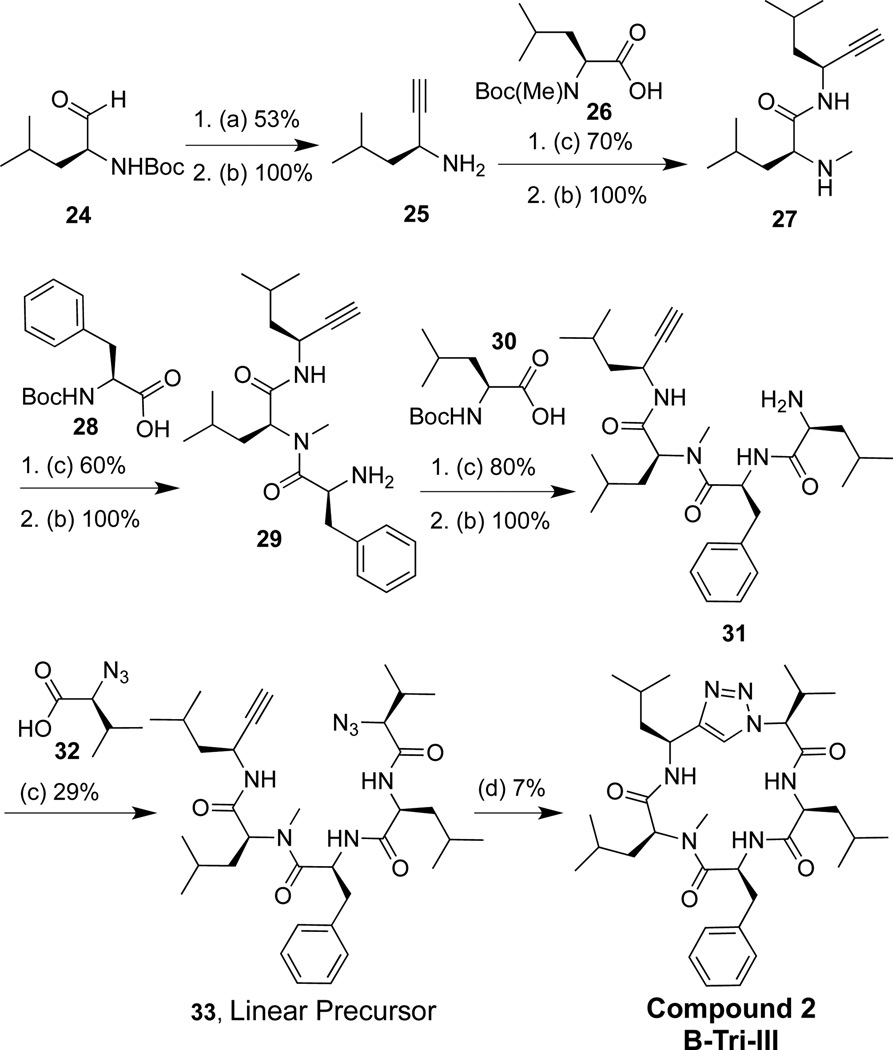
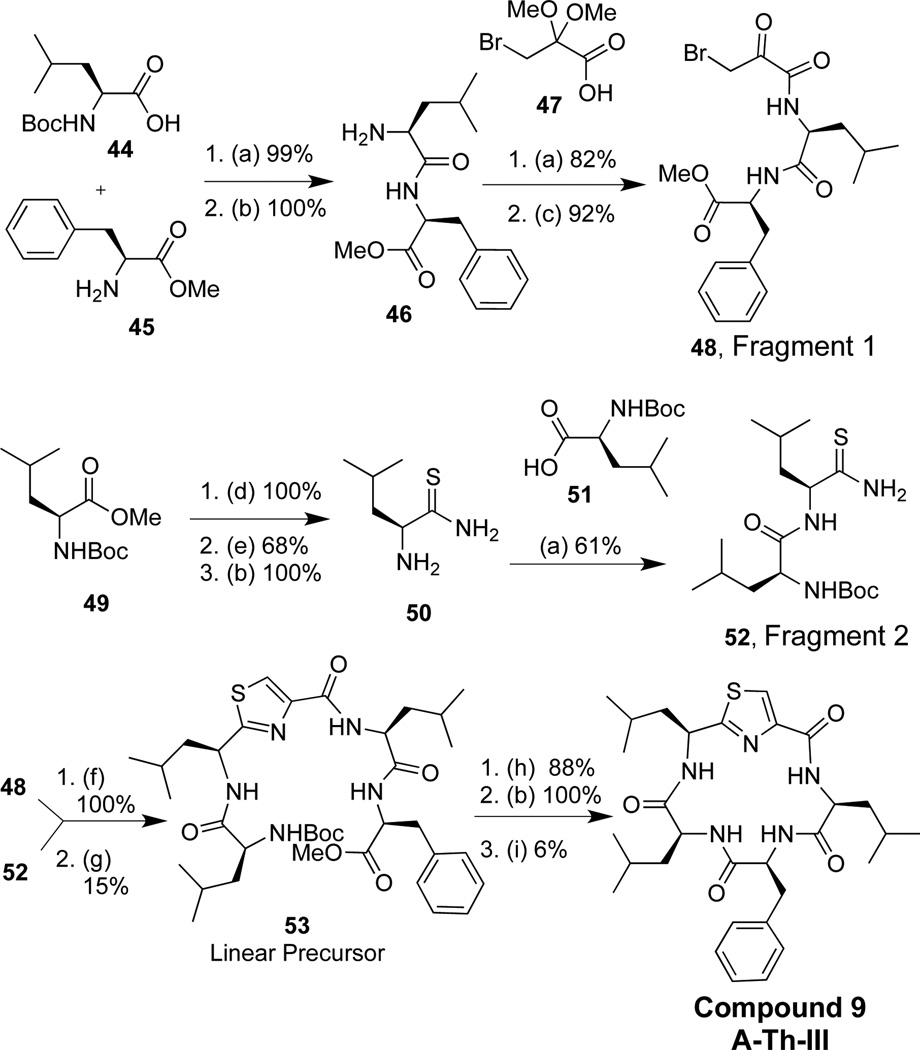
Two triazole peptidomimetics (Tri), compounds 1 and 2 (Figure 2), were synthesized via a convergent solution phase approach. Forming the triazole at the cyclization step has been reported as a successful strategy to synthesize cyclic triazole peptidomimetics.33,41–43 The synthetic strategy involved making two fragments, a tri- and dipeptide; conversion of the amine moiety of the tripeptide to an azide yielded fragment 1, and formation of an alkyne on the dipeptide yielded fragment 2. Both fragments were coupled via a peptide bond to form the linear molecule between residues I and V, and the macrocycle was then clicked shut to generate a single 1,4-disubstituted triazole analog.42
Figure 2.
Triazole synthetic strategy and compounds synthesized
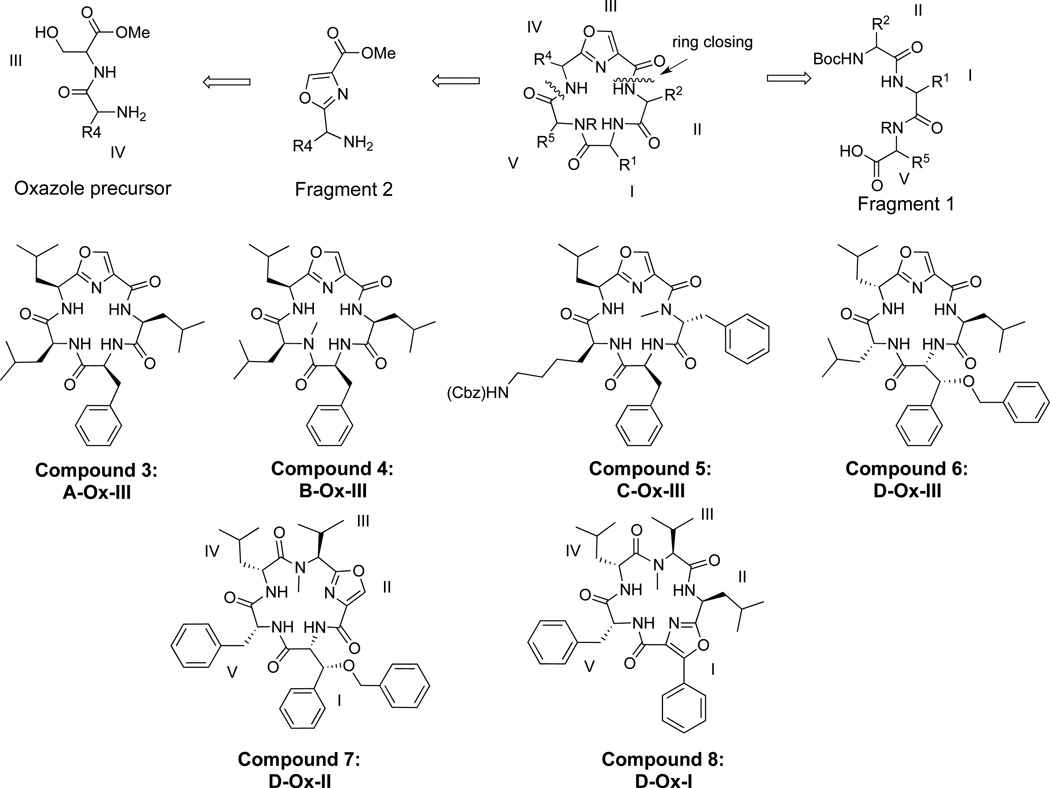
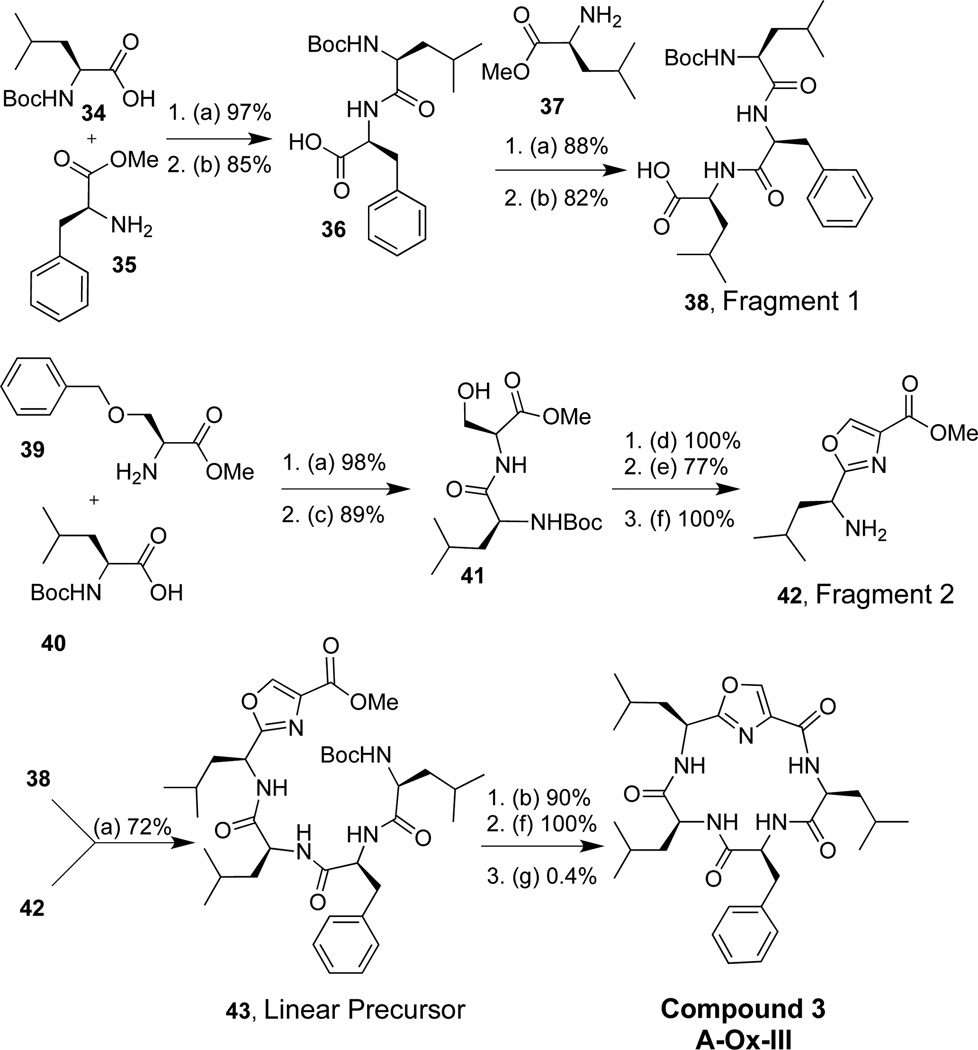
We made six oxazole peptidomimetics (Ox) (Figure 3), which were templated from molecules A–D (Figure 1). Compounds 3–6 were designed with the inclusion of an oxazole at position III based on the macrocycles A–D, whereas compounds 6–8 were only based on molecule D with the oxazole moiety placed at positions I, II, and III. These six oxazole-derived molecules were made using two different approaches, which utilized both a solid and solution phase approach. Initially our synthetic strategy involved oxazole formation after cyclization, whereupon the cyclic peptide was synthesized, and the serine was cyclized and oxidized after the formation of the macrocycle. However, the yield for this approach was extremely low for the oxazole formation (3% final yield for 8) versus an average of 74% yield for oxazole formation prior to cyclization. Presumably this was due to the rigidity of the macrocycle inhibiting the formation of an inflexible heterocycles within the backbone. Thus, the synthetic strategy for the oxazole derivatives 3–7 involved the synthesis of two fragments; fragment 1 consisted of a tripeptide, while fragment 2 incorporated the oxazole moiety. The oxazole was synthesized by coupling a serine to a leucine or valine (compounds 3–6 and 7, respectively). The oxazole was then formed via cyclodehydration upon treatment with DAST and potassium carbonate, then subsequent oxidation using bromochloroform and DBU.32,37,38 Coupling fragments 1 and 2, followed by peptide macrocyclization furnished the desired oxazole peptidomimetic derivatives.
Figure 3.
Oxazole peptidomimetic compounds
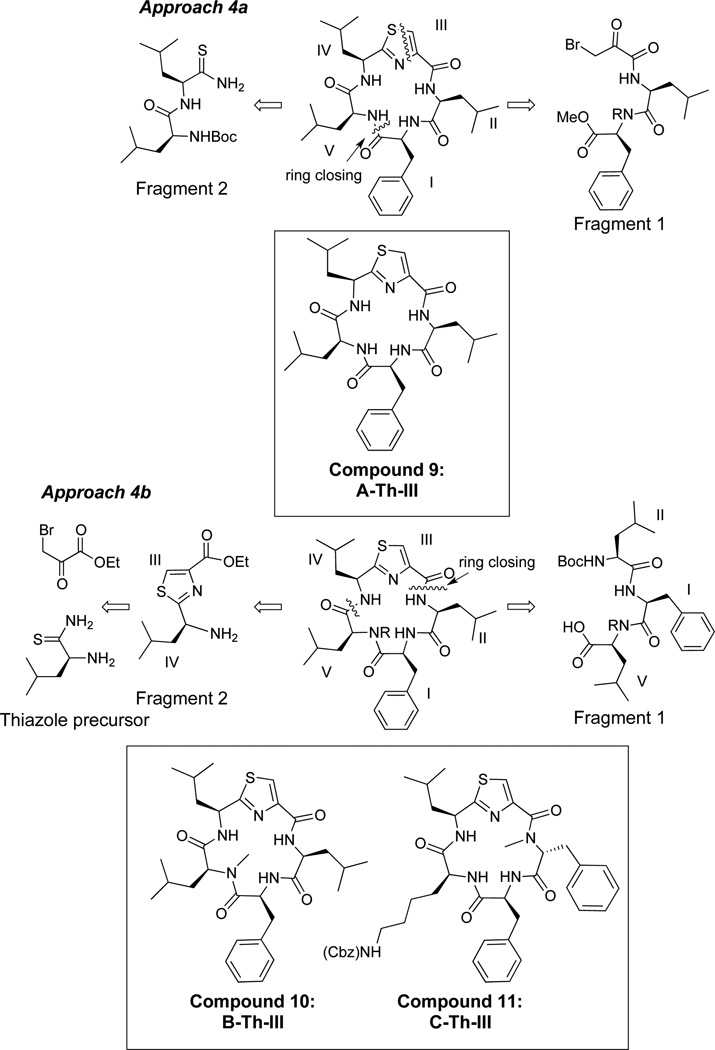
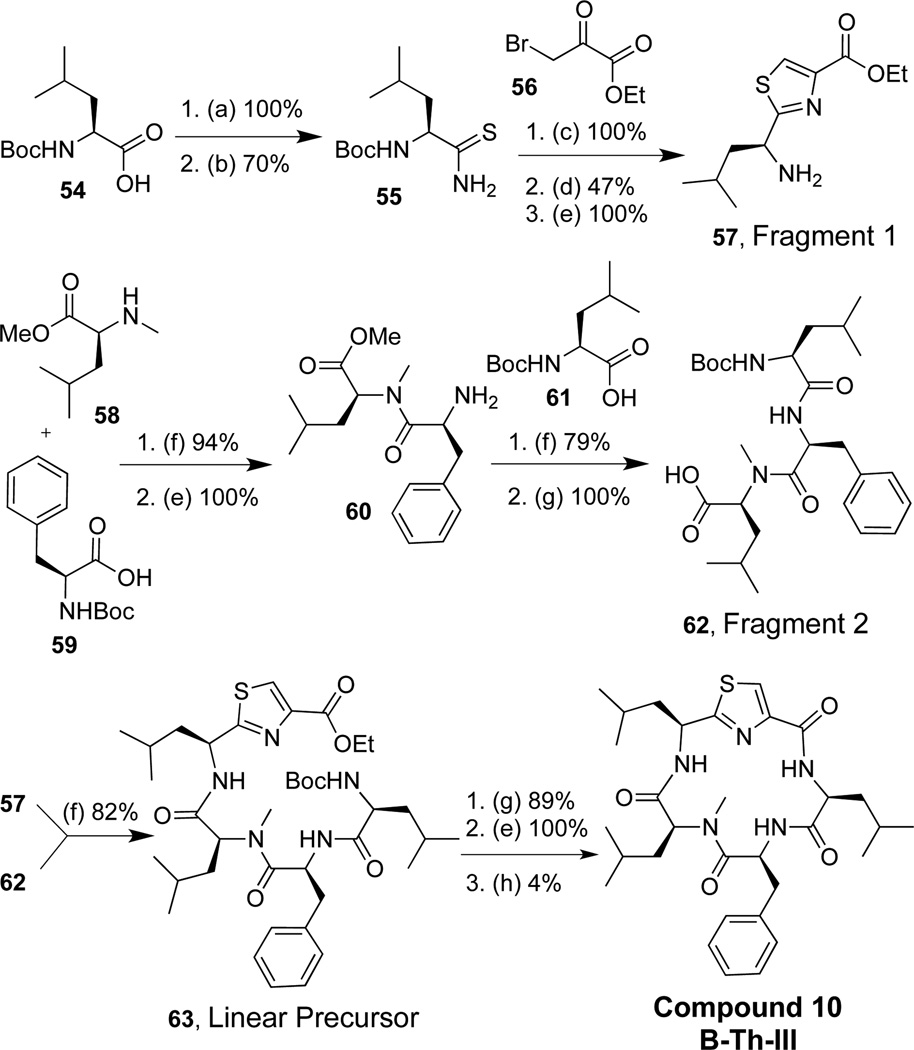
Three thiazole (Th) derivatives were synthesized using two different approaches (Figure 4). The first approach involved the synthesis of fragment 1, which incorporated a bromoketone moiety, while fragment 2 included a thioamide (Figure 4, approach 4a). A linear precursor was formed via the Hantzsch-thiazole reaction43 (Compound 9, A-Th-III), and macrocyclization was performed via peptide bond formation to yield compound 9. Given that the thiazole linear precursor had an extremely low yield (15% for thiazole formation), an alternative synthetic route was used for compounds 10 and 11 (Figure 4, approach 4b). The alternative route involved the same general synthetic approach as the oxazoles, where a solution phase procedure was used to form tripeptide fragment 1. The thiazole moiety was synthesized from two precursors, ethyl bromopyruvate and a thioamide, via the Hantzsch-thiazole reaction to yield fragment 2.44 This proved to be a successful means of synthesizing the thiazole moiety with an average of 62% yield for this reaction. Upon synthesis of these two fragments, amide bond formation between residues IV and V furnished the linear precursor followed by deprotection of the acid and amine and subsequent peptide cyclization between residues II and III generated compounds 10 and 11.
Figure 4.
Thiazole peptidomimetic compounds: 2 approachs
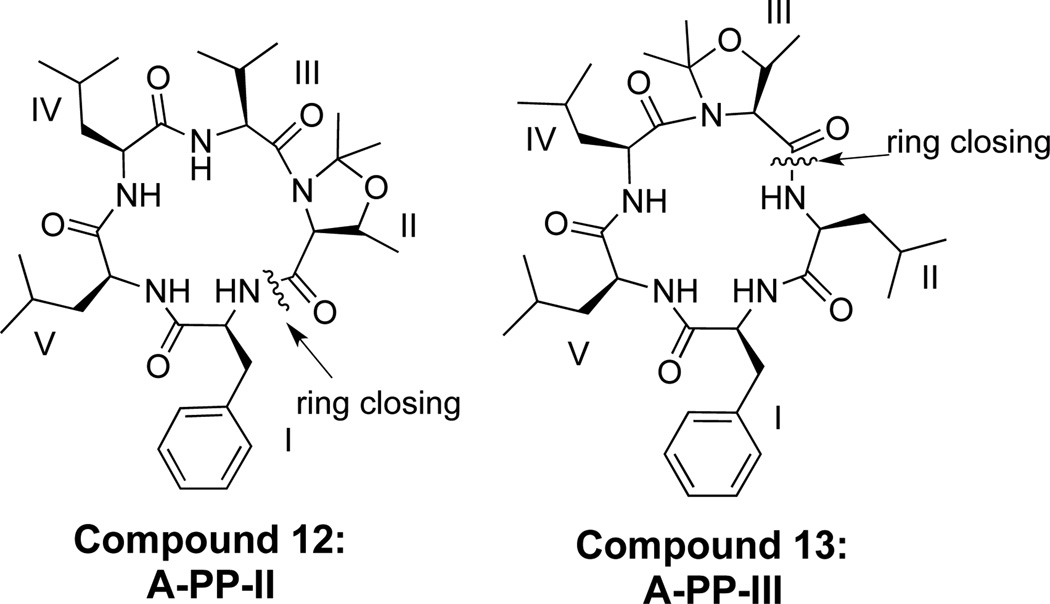
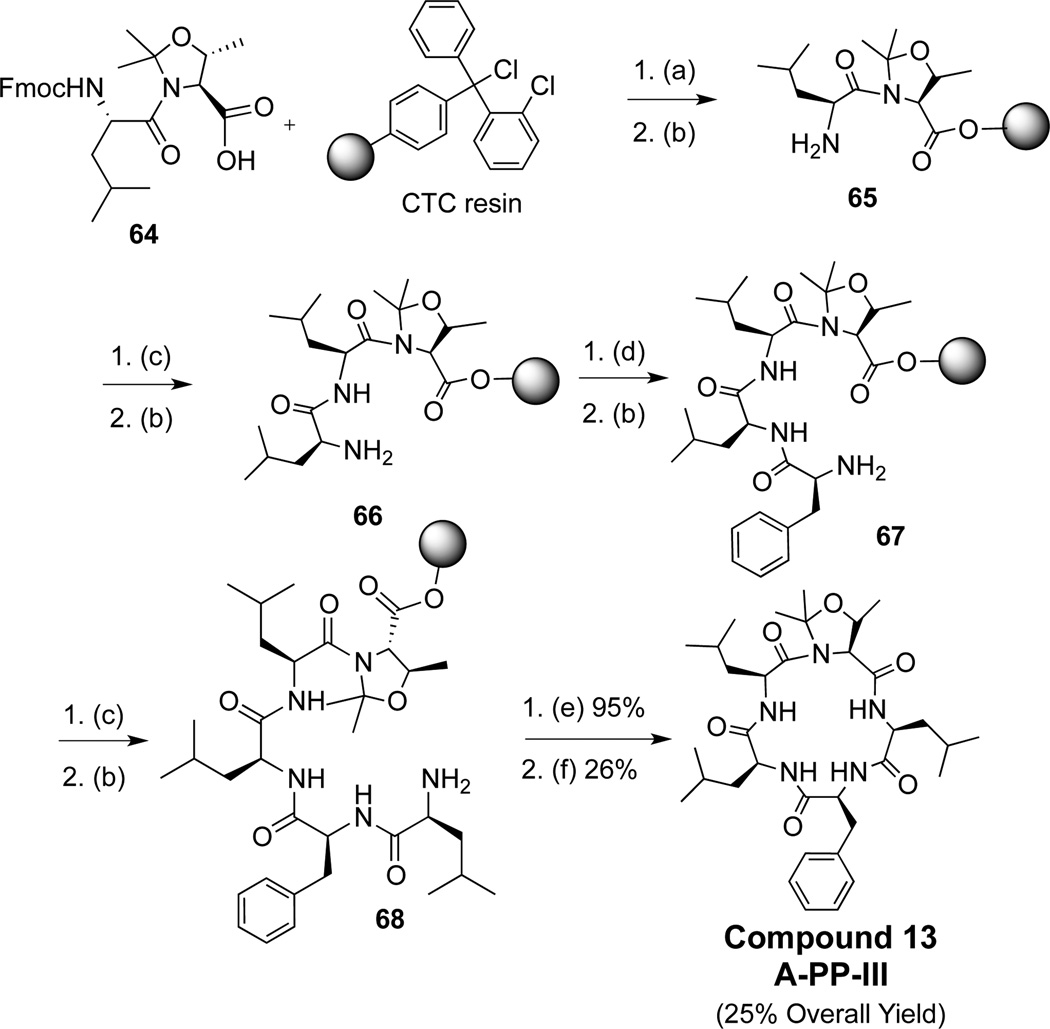
We synthesized two pseudoproline (PP) derivatives based on the compound A scaffold, where the pseudoproline was placed at positions II and III, respectively (12 and 13, Figure 5). These compounds were made via Fmoc solid phase synthesis through sequential peptide coupling from chlorotritylchloride resin loaded with the appropriate pseudoproline dipeptides.43 Upon formation of the linear pentapeptide, the compounds were cleaved, cyclized, and purified.
Figure 5.
Pseudoproline compounds
3. Synthesis
3.1. Synthesis of triazole compound 1
Aldehyde 14 was converted to an alkyne by treatment with p-toluenesulfonyl azide and di-methyl (2-oxopropyl) phosphonate (Scheme 1). Removal of the Boc protecting group with TFA furnished alkyne 15 in a 43% yield over two steps. Alkyne 15 was coupled to amino acid 16 and the Boc group was removed to yield 17, fragment 2 (74% yield over two steps). Standard coupling of amino acids 18 and 19 and deprotection methods produced 20 (87% yield over two steps). Dipeptide 20 was then coupled to azide 21 and subsequently deprotected using lithium hydroxide to yield free acid 22, Fragment 1 (62% yield over two steps). Amine 17 and acid 22 were coupled using peptide coupling conditions to produce linear precursor 23 (52% yield). Treatment of 23 with catalytic amounts of copper produced 1 in 7.5% purified yield of cyclized compound.
Scheme 1.
Synthesis of 1. (a) p-toluenesulfonyl azide (3 equiv), dimethyl (2-oxopropyl) phosphonate (3 equiv), potassium carbonate (3 equiv), 0.25 M in acetonitrile/methanol (1:1); (b) 20% trifluoroacetic acid/dichloromethane (0.1 M); (c) TBTU (1.2 equiv), DIPEA (8 equiv), methylene chloride (0.1 M); (d) LiOH (2 equiv), H2O2 (3.4 equiv), methanol (0.1 M), 0 °C; (e) L-ascorbic acid (9 equiv), NaHCO3 (9 equiv), CuSO4·H2O (0.3 equiv), 0.007 M in methanol/water (1:1)
3.2. Synthesis of triazole compound 2
Compound 2 was synthesized using a linear approach. The yield from this linear approach was then compared to the yield using the convergent approach used for compound 1 (Scheme 2). Aldehyde 24 was converted into an alkyne with the treatment of p-toluenesulfonyl azide and di-methyl (2-oxopropyl) phosphanate in acetonitrile and methanol. The Boc protecting group was removed to produce 25 (53% yield for two steps). Alkyne 25 was coupled to amino acid 26 upon treatement with O-(Benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate (TBTU) and N,N'-Diisopropylethylamine (DIPEA). The Boc protecting group was removed to furnish 27 (70% yield for two steps). This was coupled to amino acid 28, and deprotected to generate alkyne 29 (60% yield over two steps). Coupling 29 to 30 and subsequent deprotection furnished tetrapeptide 31 in good yields (80% yield over two steps). Linear precursor 33 was produced by coupling tetrapeptide 31 to 32 (29% yield). Finally, the macrocyclization step used a copper catalyst to click the 1,4-disubstituted triazole heterocycle and close the macrocyclic ring (7% yield for compound 2).
Scheme 2.
Synthesis of 2. (a) p-toluenesulfonyl azide (3 equiv), dimethyl (2-oxopropyl) phosphonate (3 equiv), potassium carbonate (3 equiv), 0.25 M in acetonitrile/methanol (1:1); (b) 20% trifluoroacetic acid/dichloromethane (0.1 M); (c) TBTU (1.2 equiv), DIPEA (8 equiv), methylene chloride (0.1 M); (d) L-ascorbic acid (9 equiv), NaHCO3 (9 equiv), CuSO4·H2O (0.3 equiv), 0.007 M in methanol/water (1:1)
3.3. Optimal synthesis strategy for oxazole derivatives
The most optimal synthesis for the oxazole derivatives is described. This approach was used on compounds 3–7 and described is the synthesis of analog 3 where the oxazole is formed prior to closing the macorcycle (Scheme 3). Amino acid 34 was coupled to 35 and then underwent methyl ester hydrolysis to furnish 36 (82 % yield over two steps). Coupling 36 and 37 generated a tripeptide, whereupon treatment with lithium hydroxide and hydrogen peroxide formed 38, fragment 1 (66% yield over two steps). Amino acids 39 and 40 were coupled and subjected to hydrogenation to remove the benzyl protecting group, yielding 41 (87% yield). The oxazole was formed on the dipeptide 41 by treatment of DAST and K2CO3, which generated the oxazoline, whereupon treatment with DBU and bromotrichloromethane produced the desired oxazole (in 77% overall yield for two steps). Deprotection of the amine furnished 42 (100% yield), fragment 2. Fragments 1 and 2 were coupled together to form 43, the linear precursor in 72% yield. The acid and amine deprotection and cyclization generated compound 3. Synthesis of compound 8 is described in the supplementary material (page 46–48).
Scheme 3.
Synthesis of 3. (a) TBTU (1.2 equiv.), DIPEA (8 equiv), methylene chloride (0.1 M); (b) LiOH (2 equiv), H2O2 (3.4 equiv), methanol (0.1 M), 0 °C; (c) H2/Pd (0.03 equiv), ethanol (0.1 equiv); (d) DAST (1.1 equiv), K2CO3 (2 equiv), methylene chloride (0.01 M), −78 °C; (e) DBU (2 equiv), BrCCl3 (2 equiv), methylene chloride (0.2 M), −47 °C; (f) 20% trifluoroacetic acid/methylene chloride (0.1 M); (g) TBTU (0.7 equiv), O-(7-Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) (0.6 equiv), 3-(Diethylphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one (DEPBT) (0.7 equiv), DIPEA (10 equiv), 0.007 M in methylene chloride/acetonitrile (1:1)
3.4. Synthesis of thiazole compound 9
Following the synthetic approach described in 4a (Figure 4), we outline the synthetic method used to form thiazole compound 9 (Scheme 4). Dipeptide 46 was produced by the coupling 44 and 45, followed by a Boc removal reaction (99% yield over two steps). Further, coupling 46 to 3-bromo-2,2-dimethyoxypropanoic acid 47 and treatment with formic acid furnished ketone 48, fragment 1 (75% yield over two steps). Amino acid 49 was converted to thioamide 50 (68% yield over three steps), which was subsequently coupled to amino acid 51 to yield fragment 2, 52 (61% yield). Linear precursor 53 was formed using Hantzsch-thiazole conditions, where potassium bicarbonate generated the thiazoline intermediate, followed by an elimination reaction in the presence of pyridine, trifluoroacetic anhydride and triethylamine, which furnished the desired thiazole moiety (15% yield over two steps). Finally, compound 9 was generated by deprotection of the acid and amine of 53, followed by subsequent peptide macrocyclization (5.3% overall yield).
Scheme 4.
Synthesis of 9. (a) TBTU (1.1 equiv), DIPEA (4 equiv), methylene chloride (0.1 M); (b) 20% trifluoroacetic acid/methylene chloride (0.1 M); (c) formic acid (0.1M) (d) (1:1) ammonium hydroxide/methanol (0.1 M);(e) Lawesson’s Reagent (1 equiv), 1,2-dimethoxyethane (.15 M); (f) KHCO3 (8 equiv), 1,2-dimethoxyethane (0.1 M); (g) pyridine (9 equiv), TFAA (4 equiv), TEA (2 equiv), 1,2-dimethoxyethane (0.1 M) 0 °C; (h) LiOH (2 equiv), H2O2 (3.4 equiv), methanol (0.1 M), 0 °C; (i) TBTU (0.7 equiv), HATU (0.7 equiv), DEPBT (0.7 equiv), DIPEA (6 equiv), methylene chloride (0.007 M).
3.5. Synthesis of thiazole compounds 10 and 11
The synthesis of compounds 10–11 was accomplished using the approach described in Scheme 5. Compound 54 was converted to an amide by the treatment of ammonium hydroxide and methanol (100% yield). Thioamide 55 was generated using Lawesson’s Reagent (70% yield), whereupon 55 was reacted with 56 (100% yield) using Hantzsch-thiazole conditions (47% overall yield for thiazole formation). Subsequent amine deprotection furnished 57, fragment 1. Amino acid 58 was coupled to 59, and the amine was deprotected to produce dipeptide 60 (94% over two steps). The free amine on 60 was coupled to acid 61, and the dipeptide underwent methyl ester hydrolysis to furnish 62, fragment 2 (79% yield over two steps). Fragments 1 and 2 (57 and 62 respectively) were coupled together to yield the linear precursor 63 (82% yield). The amine and acid were subsequently deprotected and macrocylization produced compound 10 (3.5% yield for three steps). Synthesis of compound 11 utilizes this strategy and is described in the supplementary data.
Scheme 5.
Synthesis of 10. (a) (1:1) ammonium hydroxide/methanol (0.1 M); (b) Lawesson’s Reagent (1 equiv), 1,2-dimethoxyethane (0.15 M); (c) KHCO3 (8 equiv), 1,2-dimethoxyethane (0.1 M); (d) pyridine (9 equiv), TFAA (4 equiv), TEA (2 equiv), 1,2-dimethoxyethane (0.1 M) 0 °C; (e) 20% trifluoroacetic acid/methylene chloride (0.1 M); (f) TBTU (1.1 equiv), DIPEA (4 equiv), methylene chloride (0.1 M); (g) LiOH (2 equiv), H2O2 (3.4 equiv), methanol (0.1 M), 0 °C; (h) TBTU (0.7 equiv), HATU (0.7 equiv), DEPBT (0.7 equiv), DIPEA (6 equiv), methylene chloride (0.007 M).
3.6. Synthesis of pseudoprolines 12 and 13
The synthesis of compound 13 involved loading commercially available compound 64 onto 2-chlorotritylchloride resin, followed by subsequent Fmoc removal from leucine, generating 65. Subsequent coupling and deprotection of three additional amino acids generated compound 66. The linear pentapeptide was cleaved from the resin by treatment with 2,2,2-trifluoroethanol (TFE) providing the double deprotected peptide in an overall yield of 95%. The linear precursor was cyclized to produce the final compound 13 in 25% overall yield from the starting compound 64. Synthesis of compound 12 utilizes this strategy and is described in the supplementary data.
4. Biological and Modeling Data
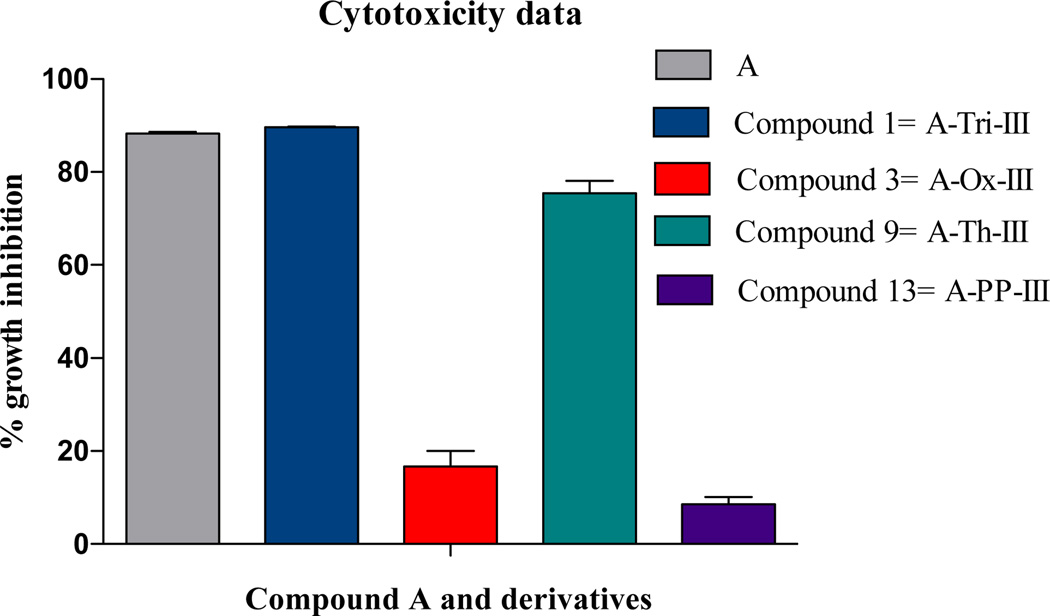
In order to evaluate which of the heterocycles would be ideal for incorporation into the macrocyclic backbone, cytotoxicity data for molecule a peptidomimetic functional group was generated and compared to parent compound A (Figure 6). All four classes of peptidomimetic features and compound A were evaluated for their ability to inhibit growth in HeLa cervical cancer cell lines. The bar graph indicates that potency is maintained with the inclusion of a triazole or thiazole at position III. Interestingly, inclusion of an oxazole or pseudoproline in the peptide backbone decreases cytotoxicity compared to the parent compound. These data likely represent the consequence of altering the macrocyclic backbone, whereby the trans conformation that is induced by the triazole is favorable, and the cis conformation induced by the pseudoproline is not. Further, oxaozles are known to be more rigid than thiazoles, and the additional flexibility of the thiazole must be critical for the macrocycle to maintain its binding affinity with Hsp90.21,1,45
Figure 6.
Peptidomimetic compounds run at 25 µM against HeLa cervical cancer cell lines. Each data point is an average of four wells run in three separate assays using HeLa cancer cell lines. Inhibition is relative to 1% DMSO control.
Given the unique biological data, models of the 4 molecules were completed using ChemBio3D Ultra. The lowest energy structures for each peptidomimetic molecule are shown in Figure 7. Although caution should be employed when drawing conclusions from relatively simple modle systems, these models are useful tools in developing a hypothesis to explain the highly divergent cytotoxicity data we have generated. The inclusion of the triazole provides an additional atom to the macrocycle, and this appears to allow a large ring that is significantly less puckered than the pseudoproline large macrocycle structure, compare 1 versus 13. This is logical given that the triazole induces a trans conformation, while the pseudoproline induces a cis conformation. Although structurally similar, the oxazole places the side chains at orientations that are different from the thiazole, perhaps allowing a compound-protein interaction with residue II that is unique to the thiazole compound (9) versus oxazole analog (3). Indeed, the modeling data below suggestion that compounds 1 and 9 have a conformation that places residues I and II in a similar orientations, whereas 3 and 13 have very different presentations of both residues. Although difficult to visualize, it is also possible that residues IV and V play a role in the improved cytotoxicity observed for compounds 1 and 9 by allowing an improved binding affinity for Hsp90 over that of compounds 3 and 13. Docking studies involving these molecules and Hsp90’s crystal structure are on going and will be published in due course.
Figure 7.
Molecular models of peptidomimetic compounds. Energy was miminized using the Merck Molecular Force Field 94 (MMFF94) with ChemBio3D Ultra (version 12.0) available form CambridgeSoft. Convergence criteria: atomic root mean square force 0.01 kcal/mol; static energy 82.250–115.726 kcal/mol; 500 iterations.
5. Conclusion
In summary, we have outlined the synthesis of thirteen peptidomimetics, all of which are related to a class of sansalvamide cytotoxic agents, with the anticipation that several moieties will have potency equal to that observed with the natural product. Two derivatives A-Tri-III (1) and A-Th-III (9) exhibited cytotoxicity at the same level as the parent San A-amide A. Although it is hard to predict how these peptidomimetic moieties affect the overall macrocyclic conformation, it appears that the triazole, which incorporates an additional carbon into the macrocyclic backbone as well as maintaining the side-chain at position III is a favorable option. This is likely due to its ability to induce a trans-amide conformation, versus a cis amide conformation induced by the pseudoproline.24,27,31,37 The thiazole moiety is also promising, presumably because it is a more flexible heterocycles relative to the oxazole and thus able to accomadate a favorable conformation for binding to Hsp90. Based on modeling, both compounds 1 and 9 may have improved cytotoxicity because of their similar orientation of residues I and II. Future work involves the development of compounds with triazoles and thiazoles in the backbone, with the anticipation that they will maintain their biological activity, while having enhanced PK and ADME properties. Further, docking studies using these two moieties in their backbone will provide insight into the induced conformation, and will be reported in due course.
6. Experimental Section
6.1. Cytotoxicity assays
Proliferation of HeLa cells was tested in the presence and absence of the compounds using CCK-8 assays (Dojindo, catalog# CK04-13). Cells were cultured in 96 well plates at a concentration of 2000 cells/well in DMEM (Gibco) supplemented with L-glutamine, 10% fetal bovine serum and 1% penicillin-streptomycin antibiotic. After overnight incubation, the compounds were added. The compounds were dissolved in DMSO and tested at the concentrations indicated in the manuscript with a final DMSO concentration of 1.0%. The DMSO control was also at 1.0%. The cells were incubated with compound or DMSO for 24 h upon which 10µl of CCK-8 solution was added to each well and allowed to incubate at 37 °C for an additional 2.5 h. The absorbance at 450 nm was measured using a S6 Genios Fluorimeter (Tecan). Percent growth inhibition was calculated as 1 minus the absorbance of compound-treated cells over DMSO-treated cells. All calculations including mean and SEM were performed using Prism software. Each data point is an average of four wells run in three separate assays.
6.2. General solution-phase peptide synthesis
All peptide coupling reactions were carried out under argon with dry solvent, using methylene chloride and/or acetonitrile for dipeptide, tripeptide, and pentapeptide couplings. The amine (1.1 equiv) and acid (1 equiv) were weighed into a dry flask along with 4–8 equiv of DIPEA and 1.1 equiv of TBTU.* Anhydrous methylene chloride and/or acetonitrile were added to generate a 0.1M solution. The solution was stirred at room temperature and reactions were monitored by TLC. Reactions were run for 1 hour before checking via TLC. If reaction was not complete an additional 0.25 equiv of TBTU was added. If reaction was complete then work-up was done by washing with 10% aqueous hydrochloric acid and saturated sodium bicarbonate. After back extraction of aqueous layers with methylene chloride, organic layers were combined, dried over sodium sulfate, filtered and concentrated. Flash column chromatography using a gradient of ethyl acetate-hexane gave our desired peptide.
* Some coupling reactions would not go to completion using only TBTU and therefore 0.2–0.5 equiv of HATU, and/or DEPBT were used. In a few cases up to 0.7 equiv of all three coupling reagents were used.
6.3. General solution-phase amine deprotection
Amines were deprotected using 20% trifluoroacetic acid in methylene chloride (0.1M) with two equiv of anisole. The reactions were monitored by TLC. Reactions were allowed to run for 1–2 h and then concentrated in vacuo.
6.4. General solution-phase acid deprotection
Acids were deprotected using 2 equiv of lithium hydroxide with 3.4 equiv of hydrogen peroxide in methanol (0.1 M). The peptide was dissolved in methanol and cooled to 0 °C. Hydrogen peroxide was added followed by lithium hydroxide. The reaction was monitored by TLC and usually done in 1–2 h. Sodium thiosulfate (3.8 equiv) was added to neutralize the peroxide and 5% hydrochloric acid was added till the solution pH was 1. The aqueous solution was extracted five times with methylene chloride, and the combined organic layer was dried, filtered and concentrated in vacuo.
6.5. General solid-phase synthesis remarks
Stepwise solid phase peptide synthesis was performed in a polypropylene solid-phase extraction cartridge fitted with a 20 µM polyethylene frit purchased from Applied Separations (Allentown, PA). 2-chlorotrityl resins were purchased in pre-loaded form with L-Leu, D-Leu, or D-Phe. In the case of compounds 12 and 13, a commercially available pseudoproline moiety was loaded onto a 2-chlorotrityl resin. Resins were swelled in dimethylformamide for 30 minutes prior to assembly of the linear five-residue peptide sequence. Solid-phase syntheses were performed on a 0.5 mmol scale based on resin-loading. All operations were performed at room temperature under open atmosphere unless stated otherwise.
6.6. General solid-phase peptide synthesis
Fmoc-protected amino acids were coupled using 3 equiv of amino acid, 3 equiv of HOBt, and 6 equiv of DIC. Couplings were performed in dimethylformamide at 0.2 M with respect to the incoming Fmoc-protected amino acid. Couplings were allowed to proceed for a minimum of two h, and were assayed via ninhydrin test to verify competition. Once complete, the coupling reaction solution was drained, and the resin subjected to Fmoc deprotection. (Note: Fmoc and N-methyl amino acids are coupled according to the cycle above, however for subsequent coupling onto the secondary amino terminus, HOBt was substituted with HOAt and the coupling was allowed to proceed overnight).
6.7. General solid-phase amine deprotection
Following coupling completion, the peptide-resin was treated as follows for removal of the Fmoc protecting group: dimethylformamide wash (3 × 1 min), 20% piperdine/dimethylformamide (1 × 5 min), 20% piperdine/dimethylformamide (1 × 10 min), dimethylformamide wash (2 × 1 min), 2-propanol wash (1 × 1 min), dimethylformamide was (1 × 1 min), 2-propanol (1 × 1 min), dimethylformamide (3 × 1 min). An ninhydrin test was performed to verify completion.
6.8. General N-terminal solid-phase deprotection
Once the final N-terminal amino acid residue had been coupled, the peptide-resin was treated as follows for removal of the Fmoc protecting group: dimethylformamide wash (3 × 1 min), 20% piperdine/dimethylformamide (1 × 5 min), 20% piperdine/dimethylformamide (1 × 10 min), dimethylformamide wash (3 × 1 min), 2-propanol wash (3 × 1 min), methanol (3 × 1 min). The fully-assembled peptide-resin was then drained and dried in vacuo overnight.
6.9. Cleavage of linear peptide from solid support
The full-length, linear peptide was cleaved from the resin by swelling and shaking the peptide-resin for 24 h in a 1:1 (v:v) TFE:methylene chloride (10 volumes/gram of dried resin). The cleavage solution was filtered through a Buchner filter, and the drained resin was washed with additional methylene chloride (5 volumes/gram of initial dried peptide-resin) to fully extract the cleaved peptide from the resin. Solvents in the combined filtrates were evaporated by rotary evaporation and the solids dried in vacuo overnight. The solids were then reconstituted in methylene chloride, evaporated by rotary evaporation and dried in vacuo overnight again to remove residual entrapped TFE.
6.10. Macrocyclization procedure (with syringe pump)
Three coupling agents (DEPBT, HATU, and TBTU) were used at ~0.5 to 0.75 equiv each. These coupling agents were dissolved in ¾ of a calculated volume of dry methylene chloride that would give a 0.001 M to 0.0007 M overall concentration when included in the volume used for the deprotected peptide. The crude, dry, double deprotected peptide (free acid and free amine) was dissolved in the other ¼ solvent volume of methylene chloride. DIPEA (8 equivs) was then added to the solution containing coupling reagents dissolved in methylene chloride. The double deprotected peptide was then added to the bulk solution dropwise using a syringe pump at a rate of 30mL/hr. The reaction was monitored via LCMS and generally complete in 1–2 h. Upon completion, the reaction was worked up by washing with aqueous hydrochloric acid (pH 1) and saturated sodium bicarbonate. After back extraction of aqueous layers with large quantities of methylene chloride, the organic layers were combined, dried, filtered and concentrated. All macrocycles were first purified by flash column chromatography using an ethyl acetate/hexane gradient on silica gel. Finally, when necessary, reversed-phase HPLC was used for additional purification using a gradient of acetonitrile and deionized water with 0.1% TFA.
6.11. Alkyne formation (Seyferth-Gilbert)46,47
Dry K2CO3 (3.0 equivs) was weighed into the flask under argon atmosphere. Calculated volume of acetonitrile was added to bring the final concentration of 0.125 M. p-tosyl azide (3.0 equivs) and dimethyl (2-oxypropyl) phosphonate (3.0 equivs) were added to the reaction mixture to generate the Bestmann-Ohira reagent. The reaction mixture stirred at room temperature and was monitored via TLC. After 2 h aldehyde (1.0 equiv dissolved in calculated amount of dry methanol to bring total reaction concentration to 0.25 M) was added. The reaction mixture was left stirring at room temperature overnight. The reaction was usually complete on the next day. Upon completion, confirmed by TLC, the reaction was concentrated in vacuo. The crude dried product was dissolved in 200 mL of ethyl acetate and washed with 150 mL of saturated sodium bicarbonate (× 2 times) and then by 100 mL of saturated sodium chloride (1 time). The organic layer was collected, dried over sodium sulfate, and concentrated in vacuo. Flash chromatography with a gradient of ethyl acetate/hexane was performed to purify the desired alkyne.
6.12. Cu(I)-catalyzed alkyne-azide cycloaddition
Sodium ascorbate was dissolved in 0.5 mL of water and put into round bottom flask. Copper sulfate was dissolved in 0.5 mL of water and added to the flask. The peptide (with azide and alkyne) was dissolved in a mixture of solvents methanol:water (1:1) at a concentration of 0.005M. 10% of this solvent mixture was added to the flask. The remaining solvent mixture was added dropwise via syringe pump to the reaction flask mixture overnight. The concentration of copper was 1.5 mM and concentration of sodium ascorbate was 45 mM for the overall reaction. Upon completion of the reaction, methanol was removed under reduced pressure and the reaction mixture was diluted with 100 mL of methylene chloride. The organic layer was collected and concentrated in vacuo. Flash chromatography with a gradient of ethyl acetate/hexane was performed to purify the desired derivative. Finally, when necessary reverse phase HPLC was used for additional purification using a gradient of Acetonitrile and distilled water with 0.1% trifluoroacetic acid.
6.13. General oxazole synthesis
DAST (1.1 equiv.) was added (0.1mL/min) to a solution of peptidyl-Ser or peptidyl-Phenylserine (1.0 equiv.) in methylene chloride (0.1M) cooled to −78 °C under argon atmosphere. The reaction mixture was stirred for 1 hour and anhydrous K2CO3 (2.0 equiv.) was added to the reaction mixture to stir at −78 °C for 1 hour. The reaction mixture warmed to room temperature and stirred for an additional 1.5 h. Upon reaction completion, confirmed by TLC, the organic solution was poured into saturated aqueous sodium bicarbonate and extracted with methylene chloride. After back-extraction of the aqueous layer with methylene chloride and/or ethyl acetate, the organic layers were combined, dried over sodium sulfate, filtered and concentrated in vacuo to give the oxazoline as an oil. The oxazoline was used without further purification for the oxidation of the oxazoline to yield the desired oxazole. DBU (2.0 equiv.) was added (0.1mL/min) to a solution of oxazoline (1.0 equiv.) in methylene chloride (0.1M) at −47 °C under argon. The reaction was stirred for 20 min and BrCCl3 (2.0 equiv.) was added to the reaction mixture (0.1mL/min). The reaction continued to stir at −47 °C for an additional 2 h and then warmed to room temperature to stir an additional 12 h, or until complete by TLC. Upon reaction completion, a work-up was done by extracting with 10% aqueous hydrochloric acid. After back extraction of aqueous layers with large quantities of methylene chloride and/or ethyl acetate, organic layers were combined, dried over sodium sulfate, filtered and concentrated in vacuo. Flash column chromatography using a gradient of ethyl acetate–hexane gave our desired peptidyl-oxazole. Finally, when necessary, reversed-phase HPLC was used for additional purification using a gradient of acetonitrile and deionized water with 0.1% trifluoroacetic acid.
6.14. General amide formation
Boc-protected amino ester (1 equiv.) was dissolved in 50% ammonium hydroxide and 50% methanol (0.05M). The reaction mixture was stirred overnight or until complete by TLC. Upon completion, the solvent was concentrated in vacuo.
6.15. General thioamide formation
Boc-protected amide (1 equiv.) was converted into Boc-protected thioamide using Lawesson’s Reagent (0.8 equiv.) in 0.4M 1,2-dimethoxyethane at room temperature under argon. The mixture was stirred overnight or until complete by TLC. Upon completion, the solvent was concentrated in vacuo. Boc-protected thioamide was purified by flash column chromatography using an ethyl acetate/methylene chloride gradient on silica gel.
6.16. General bromoketal acid formation
Trimethyl orthoformate (3 equiv.) and sulfuric acid (0.25 equiv.) were used to dissolve bromopyruvic acid (1 equiv.) under argon. The mixture is stirred overnight for less than 24 h. Acid work-up was done by extracting with 10% aqueous hydrochloric acid. After back-extraction of aqueous layers with methylene chloride and/or ethyl acetate, the organic layers were combined, dried over sodium sulfate, filtered and concentrated in vacuo.
6.17. General ketone deprotection
Ketones were deprotected using formic acid (0.1M), heated to 60 °C. The reaction was monitored by TLC and usually done within 30 min. Upon completion, the reaction was washed with saturated aqueous sodium bicarbonate. After back extraction of aqueous layers with methylene chloride, organic layers were combined, dried over sodium sulfate, filtered and concentrated in vacuo.
6.18. General thiazole synthesis (modified Hantzsch)
Thiazole synthesis reaction was carried out under argon with anhydrous 1,2-dimethoxyethane. KHCO3 (8 equiv.) was added to the dry flask containing peptidyl thioamide (1.0 equiv.). Anhydrous 1,2-dimethoxyethane (0.15 M) was added to the reaction, and it was stirred at room temperature for 15 min. α-Bromo ketone residue (3.0 equiv.) was added (0.1mL/min) and the reaction mixture was stirred overnight. Upon reaction completion, confirmed by TLC, the organic solution was poured into pre-prime celite with ethyl acetate. The filtrate was concentrated in vacuo to give the thiazoline intermediate as an oil to be used without further purification. Next, pyridine (9.0 equiv.) was added (0.1mL/min) to a solution of thiazoline in 1,2-dimethoxyethane (0.05M) at 0 °C under argon for the dehydration of the thiazoline to yield the desired thiazole. The reaction was stirred for 15 min and then TFAA (4.0 equiv.) was added to the reaction mixture (0.1mL/min). After 3 h, TEA (2.0 equiv.) was added to the reaction mixture (0.1mL/min) and the reaction continued to stir at room temperature for an additional 2–3 h, or until complete by TLC. Upon completion, the reaction was exctracted with 10% aqueous hydrochloric acid. After back-extraction of aqueous layers with methylene chloride and/or ethyl acetate, the organic layers were combined, dried over sodium sulfate, filtered and concentrated in vacuo. Flash column chromatography using a gradient of ethyl acetate/methylene chloride gave our desired peptidyl-thiazole. Finally, when necessary, reversed-phase HPLC was used for additional purification using a gradient of acetonitrile and deionized water with 0.1% trifluoroacetic acid.
6.19. Benzylation procedure
The cyclized peptide was dissolved in 50% tetrahydrofuran and 50% dimethylformamide to make a 0.1 M solution. The 60% NaH was used at 1.1 equiv and dissolved in the 0.1 M solution. Benzyl bromide (2 equiv.) was then added to the reaction. After 2 h, LC/MS indicated the reaction was developing. The reaction was completed in about 5 h and then worked up by washing with deionized water. After that, the organic layer was collected, dried and preliminarily purified by flash column chromatography. Finally, reverse-phase HPLC was used for further purification by using a gradient of acetonitrile and deionized water with 0.1 % trifluoroacetic acid.
6.20. Synthesis of Compound 1 (A-tri-III)
6.20.1. Dipeptide MeO-Phe-Leu-NHBoc
Dipeptide MeO-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 1.50 g (6.96 mmol) of amine 19 MeO-Phe-NH2·HCl, 1.46 g (6.32 mmol) of acid 18 HO-Leu-NHBoc, 3.95 mL (27.8 mmol) of DIPEA, 2.44 g (7.58 mmol) of TBTU, in 66.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (2.26 g, 87% yield). Rf: 0.6 (EtOAc: Hex 1:1). Physical and spectroscopic data are consistent with those reported in the literature.30
6.20.2. Dipeptide MeO-Phe-Leu-NH2 (20)
Dipeptide MeO-Phe-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (445 mg, 100% yield).
6.20.3. Monomer HO-Val-N3 (21)
Monomer 21 HO-Val-N3 was synthesized utilizing 500 mg (4.27 mmol) of HO-Val-NH2, 2.82 g (8.54 mmol) of triflic anhydride, 2.78 g (42.7 mmol) of sodium azide, 885 mg (6.41 mmol) of potassium carbonate, 10.6 mg (42.5 µmol) of CuSO4·5H2O, in 48 ml of DCM:MeOH:H2O (2:1:1) solvent system. The crude reaction was purified using an aqueous acidic wash to yield the pure monomer (600 mg, 97% yield).
6.20.4. Tripeptide MeO-Phe-Leu-Val-N3
Tripeptide MeO-Phe-Leu-Val-N3 was synthesized following the “General solution-phase peptide synthesis”. Utilizing 444 mg (1.53 mmol) of amine 20 MeO-Phe-Leu-NH2, 200mg (1.39 mmol) of acid 21 HO-Leu-N3, 1.80 mL of DIPEA, 534 mg (1.66 mmol) of TBTU, in 66.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the triipeptide (420 mg, 66% yield). Rf: 0.5 (EtOAc: Hex 1:2); 1H NMR (400 MHz, CDCl3): δ 0.83–0.91 (9 H, m, CH(CH3)2), 1.08 (3 H, d, J 6.6 Hz, CH(CH3)2), 1.47–1.51 (2 H, m, CHCH2(CH3)2), 2.24–2.33 (1 H, m, CH(CH3)2), 3.02–3.20 (2 H, dq, J 86.5, 14.2, 5.5, Hz, CHCH2Ph), 3.72 (3 H, s, OCH3), 3.73 (1 H, d, J 4.1 Hz, αCH), 4.37–4.42 (1 H, m, αCH), 4.90–5.02 (1 H, m, αCH), 6.43 (1 H, d, J 7.7 Hz, NH), 6.62 (1 H, d, J 7.7 Hz, NH), 7.09–7.32 (5 H, m, Ph)
6.20.5. Tripeptide HO-Phe-Leu-Val-N3 (22)
Tripeptide HO-Phe-Leu-Val-N3 was synthesized following the “General solution-phase acid deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization. (382 mg, 94% yield).
6.20.6. Monomer Alkyne-Leu-NHBoc
Monomer Alkyne-Leu-NHBoc was synthesized following “Alkyne formation”. Utilizing 165 mg (0.787 mmol) of 14 Leucinial-NHBoc, 0.353 mL (2.30 mmol) of pTsN3, 0.310 mL (2.30 mmol) of di-methyl (2-oxopropyl) phosphonate, and 317 mg (2.30 mmol) K2CO3 in 3.22 mL of ACN:MeOH (1:1). The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the monomer (70.0 mg, 43% yield). Rf: 0.5 (EtOAc: Hex 1:9).
6.20.7. Monomer Alkyne-Leu-NH2 (15)
Monomer 15 Alkyne-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This monomer was taken on to the next reaction without further purification or characterization. (83mg, 100% yield).
6.20.8. Dipeptide Alkyne-Leu-Leu-NHBoc
Dipeptide Alkyne-Leu-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 83.0 mg (0.743 mmol) of amine 15 alkyne-Leu-NH2, 204 mg (0.817 mmol) of acid 16 HO-Leu-NHBoc, 1.00 mL (6.53 mmol) of DIPEA, 286 mg (0.896 mmol) of TBTU, in 7.40 mL of methylene chloride. The crude reaction was by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (241mg, 74% yield). Rf: 0.6 (EtOAc: Hex 1:4); 1H NMR (400 MHz, CDCl3): δ 0.85–0.90 (12 H, m, CH(CH3)2), 1.37 (9 H, s, OC(CH3)3), 1.45–1.50 (1 H, m, CH2CH(CH3)2), 1.57–1.64 (4 H, m, CHCH2CH), 1.64–1.74 (1 H, m, CH2CH(CH3)2), 2.17 (1 H, s, C≡CH), 3.91–4.02 (1 H, br m, αCH), 4.65–4.78 (1 H, m, αCH), 4.72 (1 H, br s, CHNHCOOtBu), 7.72 (1 H, br d, J 6.19, NH).
6.20.9. Dipeptide Alkyne-Leu-Leu-NH2 (17)
Dipeptide Alkyne-Leu-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (145mg, 100% yield).
6.20.10. Pentapeptide Alkyne-Leu-Leu-Phe-Leu-Val-N3 (23)
Pentapeptide Alkyne-Leu-Leu-Phe-Leu-Val-N3 was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 145 mg (0.649 mmol) of amine 17 alkyne-Leu-Leu-NH2, 237 mg (0.591 mmol) of acid 22 HO-Phe-Leu-Val-N3, 0.82 mL (4.73 mmol) of DIPEA, 189 mg (0.591 mmol) of TBTU, 67.0 mg (0.177 mmols) of HATU, and 34.2 mg (0.118 mmol) of DEPBT, in 5 mL of methylene chloride and 2 mL acetonitrile. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (186 mg, 52% yield). Rf: 0.5 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CD3OD): δ 0.88–0.96 (24 H, m, CH(CH3)2), 1.26–1.36 (1 H, m, CHCH(CH3)2), 1.47–1.65 (6 H, m, CHCH2CH), 1.72–1.83 (1 H, m, CH2CH(CH3)2), 2.05–2.19 (1 H, m, CH2CH(CH3)2), 2.63 (1 H, s, C≡CH), 2.89–316 (2 H, dq, J 80.6, 8.3, 5.6 Hz, CHCH2Ph), 3.55 (1 H, d, J 6.9 Hz, N3CHC=O), 4.32–4.44 (2 H, m, αCH), 4.57–4.70 (2 H, m, αCH), 7.17–7.26 (5 H, m, Ph), 8.01 (1 H, d, J 8.05 Hz, NH), 8.07 (1 H, d, J 7.7 Hz, NH), 8.11 (1 H, d, J 7.8 Hz, NH), 8.21 (1 H, d, J 8.4 Hz, NH). LCMS: m/z calcd for C33H51N7O4 (M+1) = 610.8, found 611.6
6.20.11. Macrocycle Phe-Leu-Val-Triazole-Leu-Leu (1)
Macrocycle Phe-Leu-Val-Triazole-Leu-Leu was synthesized following the “Cu(I)-catalyzed alkyne-azide cycloaddition”. Utilizing 150 mg (0.252 mmol) of linear pentapeptide 23, 389 mg (2.21 mmol) of L-ascorbic acid, 185 mg (2.21 mmol) of NaHCO3, and 18.4 mg (74.2 µmol) CuSO4·5H2O in 35.0 mL MeOH:H2O (1:1). The crude reaction was purified by reverse phase-HPLC to yield the macrocycle (12.3 mg, 7.5% yield). Rf: 0.25 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CD3OD): δ 0.89–1.0 (24 H, m, CH(CH3)2), 1.49–1.54 (2 H, m, CHCH2CH), 1.58–1.63 (2 H, m, CHCH2CH), 1.65–1.69 (1 H, m, CH2CH(CH3)2), 1.75–1.80 (2 H, m, CHCH2CH), 1.98–2.06 (1 H, m, CH2CH(CH3)2), 2.15–2.22 (1 H, m, CH2CH(CH3)2), 3.02–3.16 (2 H, m, CH2Ph), 3.60–3.69 (1 H, m, αCH), 3.80–3.87 (1 H, m, αCH), 4.02–4.09 (1 H, m, αCH), 4.19–4.27 (1 H, m, αCH), 5.18–5.24 (1 H, m, αCH), 7.19–7.32 (5 H, m, Ph), 8.04 (1 H, s, NCH=C). LCMS: m/z calcd for C33H51N7O4 (M+1) = 611.8, found 611.9; HRMS (ESI-TOF): MH+, found 610.4078, requires 610.4075. >95% pure by HPLC.
6.21. Synthesis of Compound 2 (B-tri-III)
6.21.1 . Monomer HO-Val-N3 (32)
Monomer HO-Val-N3 was synthesized utilizing 500 mg (4.27 mmol) of HO-Val-NH2, 2.82 g (8.54 mmol) of triflic anhydride, 2.78 g (42.7 mmol) of sodium azide, 885 mg (6.41 mmol) of potassium carbonate, 10.6 mg (42.5 µmol) of CuSO4·5H2O, in 48.0 ml of DCM:MeOH:H2O (2:1:1) solvent system. The crude reaction was purified using an aqueous acidic wash to yield the pure monomer (600 mg, 97% yield).
6.21.2 . Monomer Alkyne-Leu-NHBoc
Monomer Alkyne-Leu-NHBoc was synthesized following “Alkyne formation”. Utilizing 310 mg (1.39 mmol) of 24 Leucinial-NHBoc, 0.635 mL (4.18 mmol) of pTsN3, 0.572 mL (4.18 mmol) of di-methyl (2-oxopropyl) phosphonate, and 577 mg (4.18 mmol) of K2CO3, in 5.50 mL of ACN:MeOH (1:1). The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the monomer (160 mg, 53% yield). Rf: 0.5 (EtOAc: Hex 1:9).
6.21.3 . Monomer Alkyne-Leu-NH2 (25)
Monomer Alkyne-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This monomer was taken on to the next reaction without further purification or characterization. (83.2 mg, 100% yield).
6.21.4. Dipeptide Alkyne-Leu-Leu-N(Me)Boc
Dipeptide Alkyne-Leu-Leu-N(Me)Boc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 83.2 mg (0.748 mmols) of amine 25 alkyne-Leu-NH2, 169 mg (0.689 mmol) of acid 26 HO-Leu-N(Me)Boc, 0.902 mL (5.51 mmol) of DIPEA, 265 mg (0.823 mmol) of TBTU, in 7.05 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (245 mg, 70% yield). Rf: 0.6 (EtOAc: Hex 1:4); 1H NMR (400 MHz, CDCl3): δ 0.77–0.84 (12 H, m, CH(CH3)2), 1.36 (9 H, s, COOC(CH3)3), 1.37 (2 H, bur m, CH(CH3)2), 1.50–1.66 (4 H, bur m, CHCH2CH), 2.15 (1 H, s, C≡CH), 2.61 (3 H, s, MeNCH), 4.40–4.52 (1 H, br s, αCH), 4.55–4.67 (1 H, q, J 7.3 Hz, αCH).
6.21.5. Dipeptide Alkyne-Leu-Leu-N(Me)H (27)
Dipeptide Alkyne-Leu-Leu-N(Me)H was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (173 mg, 100% yield).
6.21.6. Tripeptide Alkyne-Leu-Leu-N(Me)-Phe-NHBoc
Tripeptide Alkyne-Leu-Leu-N(Me)-Phe-NHBoc was synthesized following the “General solution-phase peptide synthesis”. Utilizing 302 mg (1.13 mmol) of acid 28 HO-Phe-NHBoc, 299 mg (1.25 mmol) of amine 27 Alkyne-Leu-Leu-N(Me)H, 1.54 mL (9.04 mmol) of DIPEA, 362 mg (1.13 mmol) of TBTU, and 128 mg (0.339 mmol) of HATU in 11.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the triipeptide (328 mg, 60% yield). Rf: 0.5 (EtOAc: Hex 1:3); 1H NMR (400 MHz, CDCl3): δ 0.80–0.92 (12 H, m, CH(CH3)2), 1.39 (9 H, s, COOC(CH3)3), 1.48–1.58 (2 H, m, CHCH2CH), 1.63–1.72 (2 H, m, CHCH2CH), 1.81–1.90 (1 H, m, CH2CH(CH2)3), 1.81 (1 H, s, C≡CH), 2.71 (3 H, s, CH3N), 2.84–2.96 (2 H, m, CHCH2Ph), 4.64–4.74 (1 H, m, αCH), 5.05–5.11 (1 H, m, αCH), 5.31–5.51 (1 H, dd, J 50.8, 7.7 Hz, αCH), 5.95 (1 H, d, J 8.17 Hz, NH), 7.23–7.32 (5 H, m, Ph), 7.92 (1 H, d, J 8.4 Hz, NH).
6.21.7. Tripeptide Alkyne-Leu-Leu-N(Me)-Phe-NH2 (29)
Tripeptide Alkyne-Leu-Leu-N(Me)-Phe-NH2 was synthesized following the “General solution-phase amine deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization. (262 mg, 100% yield).
6.21.8. Tetrapeptide Alkyne-Leu-Leu-N(Me)-Phe-Leu-NHBoc
Tripeptide Alkyne-Leu-Leu-N(Me)-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis”. Utilizing 262 mg (0.674 mmol) of amine 29 Alkyne-Leu-Leu-N(Me)-Phe-NH2, 152 mg (0.612 mmol) of acid 30 HO-Leu-NHBoc, 0.805 mL (4.89) of DIPEA, 235 mg (0.734 mmol) of TBTU, in 6.70 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the tetrapeptide (332 mg, 80% yield). Rf: 0.4 (EtOAc: Hex 1:3); 1H NMR (400 MHz, CDCl3): δ 0.86–0.95 (18 H, m, CH(CH3)2), 1.42 (9 H, s, COOC(CH3)3), 1.46–1.56 (2 H, m, CHCH2CH), 1.56–1.68 (2 H, m, CHCH2CH), 1.69–1.80 (1 H, m, CH(CH3)2), 2.84–2.93 (2 H, m, CHCH2Ph), 2.99 (3 H, s, NCH3), 3.04–3.12 (1 H, m, CH(CH3)2), 3.28 (1 H, d, C≡CH), 4.02–4.16 (1 H, m, αCH), 4.59–4.69 (1 H, m, αCH), 4.92 (1 H, m, αCH), 5.09 (1 H, m, αCH), 7.17–7.30 (5 H, m, Ph), 7.94 (1 H, d, J 8.2 Hz, NH), (1 H, d, J 8.13 Hz, NH).
6.21.9. Tetrapeptide Alkyne-Leu-Leu-N(Me)-Phe-Leu-NH2 (31)
Tripeptide Alkyne-Leu-Leu-N(Me)-Phe-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization. (233 mg, 100% yield).
6.21.10. Pentapeptide Alkyne-Leu-Leu-N(Me)-Phe-Leu-Val-N3 (33)
Pentapeptide Alkyne-Leu-Leu-N(Me)-Phe-Leu-Val-N3 was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 326 mg (0.655 mmol) of amine Alkyne-Leu-Leu-N(Me)-Phe-Leu-NH2 (31), 237 mg (0.594 mmol) of acid HO-Val-N3 (32), 0.852 mL (4.75 mmol) of DIPEA, 199 mg (0.623 mmol) of TBTU, and 71.2 mg (0.193) of HATU in 6 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (110 mg, 29% yield). Rf: 0.5 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CD3OD): δ 0.88–0.98 (24 H, m, CH(CH3)2), 1.40–1.49 (1 H, m, CH(CH3)2), 1.51–1.61 (6 H, bur m, CHCH2CH), 1.70–1.81 (1 H, m, CH(CH3)2), 2.10–2.14 (1 H, m, CH(CH3)2), 2.64 (1 H, s, C≡CH), 2.91 (2 H, m, CH2Ph), 3.31 (3 H, s, MeNC=O), 3.50–3.57 (1 H, m, αCH), 4.42–4.48 (1 H, m, αCH), 4.60–4.69 (1 H, m, αCH), 4.80–4.89 (1 H, m, αCH), 7.18–7.31 (5 H, m, Ph). LCMS: m/z calcd for C34H53N7O4 (M+1) = 624.9, found 646.5
6.21.11. Macrocycle Phe-Leu-Val-Triazole-Leu-Leu-N(Me) (2)
Macrocycle Phe-Leu-Val-Triazole-Leu-Leu-N(Me) was synthesized following the “Cu(I)-catalyzed alkyne-azide cycloaddition”. Utilizing 110 mg (0.176 mmol) of linear pentapeptide (33), 1.75g (8.79 mmol) of L-ascorbic acid, 730 mg (8.79 mmol) of NaHCO3, and 124 mg (0.502 mmol) CuSO4·H2O in 35 mL MeOH:H2O (1:1). The crude reaction was purified by reverse phase-HPLC to yield the macrocycle (8.20 mg, 7% yield). Rf: 0.4 (EtOAc: Hex 1:1) 1H NMR (400 MHz, CD3OD): δ 0.71–0.80 (3 H, m, CH(CH3)2), 0.83–1.12 (18 H, m, CH(CH3)2), 1.19–2.01 (1 H, m, CH(CH3)2), 1.30–1.35 (1 H, m, CH(CH3)2), 1.50–1.57 (2 H, m, CHCH2CH), 1.64–1.68 (2 H, m, CHCH2CH), 1.69–1.73 (2 H, m, CHCH2CH), 1.84–1.88 (1 H, m, CH(CH3)2), 2.52–2.56 (1 H, CH(CH3)2), 2.89–2.95 (2 H, m, CH2Ph), 2.99 (s, 3H, MeNC=O), 3.45–3.50 (1 H, m, αCH), 3.70–3.80 (1 H, m, αCH), 4.90–4.98 (1 H, m, 1 H, m, αCH), 5.01–5.12 (1 H, m, 1 H, m, αCH), 7.15–7.31 (5 H, m, Ph), 7.99 (1 H, d, J 7.7 Hz, O=CNHCH), 8.06 (1 H, s, NCH=C), 8.37 (1 H, d J 8.1 Hz, O=CNHCH). LCMS: m/z calcd for C34H53N7O4 (M+1) = 624.8, found 625.3; HRMS (ESI-TOF): MH+, found 624.4257, C34H53N7O4 requires 624.4232. >95% pure by HPLC.
6.22. Synthesis of Compound 3 (A-Ox-III)
6.22.1. Dipeptide MeO-Ser(Bzl)-Leu-NHBoc
Dipeptide MeO-Ser(Bzl)-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure utilizing 756 mg (3.39 mmol) of amine MeO-Ser(Bzl)-NH2 (39), 713 mg (3.08 mmol) of acid HO-Leu-NHBoc (40), 2.10 mL (12.3 mmol) of DIPEA, 1.19 g (3.70 mmol) of TBTU, in 31.0 mL of methylene chloride. This dipeptide was taken on to the next reaction without further purification (1.31 g, 98% yield). Rf: 0.73 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.85 (6 H, d, J 8.0 Hz, CHCH3), 1.36 (9 H, s, CCH3), 1.41–1.48 (1 H, m, CHCH3), 1.55–1.65 (2 H, m, CH2CH), 3.56 (1 H, d, J 4.0 Hz, CH2O), 3.65 (3 H, s, OCH3), 3.76 (1 H, dd, J 4.0, 7.8 Hz, CH2O), 4.22 (1 H, br s, NH), 4.38 (2 H, m, PhCH2O), 4.66–4.72 (1 H, m, αCH), 5.49 (1 H, d, J 7.8 Hz, αCH), 7.14 – 7.21 (5 H, m, Ph).
6.22.2 Dipeptide MeO-Ser-Leu-NHBoc (41)
Dipeptide MeO-Ser-Leu-NHBoc was synthesized by dissolving 1.31 g (3.01 mmol) of dipeptide MeO-Ser(OBn)-Leu-NHBoc in 30 mL of ethyl alcohol, after purging the reaction vessel several times with hydrogen gas, the reaction was run overnight. Upon completion by TLC, this dipeptide was taken on to the next reaction without further purification or characterization. (902 mg, 89% yield).
6.22.3. Dipeptide MeO-Oxazole-Leu-NHBoc
Dipeptide MeO-Oxazole-Leu-NHBoc was synthesized following the “General oxazole synthesis” procedure utilizing 0.902 g (2.70 mmol) of dipeptide MeO-Ser-Leu-NHBoc, 0.390 mL (2.97 mmol) of DAST, 746 mg (5.40 mmol) of K2CO3, in 40 mL of methylene chloride. The intermediate was oxidized into product by using 0.810 mL of DBU (5.40 mmol), 0.53 mL of CBrCl3 (5.40 mmol), in 14 mL of methylene chloride. This dipeptide was taken on to the next reaction without further purification. (649 mg, 77% yield). Rf: 0.55 (EtOAc: Hex 1:1). Physical and spectroscopic data are consistent with those reported in the literature.48
6.22.4. Dipeptide MeO-Oxazole-Leu-NH2 (42)
Dipeptide MeO-Oxazole-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization (210 mg, 100% yield).
6.22.5. Dipeptide MeO-Phe-Leu-NHBoc
Dipeptide MeO-Phe-Leu-NHBoc was synthesized following the “General soltuion-phase peptide synthesis” procedure utilizing 850 mg (4.76 mmol) of amine MeO-Phe-NH2 (35), 1.00 g (4.32 mmol) of acid HO-Leu-NHBoc (34), 2.90 mL (17.3 mmol) of DIPEA, 1.66 g (5.18 mmol) of TBTU, in 43.0 mL of methylene chloride. This dipeptide was taken on to the next reaction without further purification (1.64 g, 97% yield). Rf: 0.85 (EtOAc: Hex 1:1). Physical and spectroscopic data are consistent with those reported in the literature.48
6.22.6. Dipeptide HO-Phe-Leu-NHBoc (36)
Dipeptide HO-Leu-D-Leu-NHBoc was synthesized following the “General solution-phase acid deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization (1.40 g, 85% yield).
6.22.7. Tripeptide MeO-Leu-Phe-Leu-NHBoc
Tripeptide MeO-Leu-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure utilizing 757 mg (2.00 mmol) of acid HO-Phe-Leu-NHBoc, 320 mg (2.20 mmol) of amine MeO-Leu-NH2 (37), 1.40 mL (8.00 mmol) of DIPEA, 706 mg (2.40 mmol) of TBTU, in 20 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the tripeptide (890 mg, 88% yield). Rf: 0.55 (EtOAc: Hex 1:1); Physical and spectroscopic data are consistent with those reported in the literature.49
6.22.8. Tripeptide HO-Leu-Phe-Leu-NHBoc (38)
Dipeptide HO-Leu-D-Leu-NHBoc was synthesized following the “General solution-phase acid deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization (709 mg, 82% yield).
6.22.9. Pentapeptide MeO-Oxazole-Leu-Leu-Phe-Leu-NHBoc (43)
Pentapeptide MeO-Oxazole-Leu-Leu-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure utilizing 469 mg (0.952 mmol) of acid HO-Leu-Phe-Leu-NHBoc (38), 223 mg (1.05 mmol) of amine MeO-Oxazole-Leu-NH2 (42), 0.700 mL (3.81 mmol) of DIPEA, 366 mg (1.08 mmol) of TBTU, in 11.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (468 mg, 72% yield). Rf: 0.35 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.70–0.97 (18 H, m, CHCH3), 1.40 (9 H, s, CCH3), 1.47–1.68 (9 H, m, CHCH3, CH2-CH), 2.98–3.24 (2 H, m, CH2O), 3.84 (3 H, s, OCH3), 4.50–4.78 (2 H, m, αCH), 4.96–5.02 (1 H, m, αCH), 5.17–5.31 (1 H, m, αCH), 7.07–7.28 (5 H, m, Ph), 8.09 (1 H, s, OCH=C).
6.22.10. Macrocycle Phe-Leu-Oxazole-Leu-Leu (3)
Macrocycle Phe-Leu-Oxazole-Leu-Leu was synthesized following the “Macrocyclization procedure” utilizing 349 mg (0.611 mmol) of linear pentapeptide (43), 1.10 mL (6.11 mmol) of DIPEA, 138 mg (0.431 mmol) of TBTU, 141 mg (0.375 mmol) of HATU, and 129 mg (0.432 mmol) of DEPBT, in 9.20 mL methylene chloride and 9.00 mL acetonitrile. The crude reaction was purified by reverse phase-HPLC to yield the macrocycle (1.3 mg, 0.4% yield). Rf: 0.21 (EtOAc: Hex 1:1); 1H NMR (600 MHz, CD3OD): δ 0.69–0.96 (18 H, m, CHCH3), 1.18–1.65 (9 H, m, CHCH3, CH2CH), 2.97–3.27 (2 H, m, CH2O), 3.52–3.77 (2 H, m, αCH), 4.03–4.19 (1 H, m, αCH), 4.38–4.57 (1 H, m, αCH), 6.63 (1 H, br s, NH), 7.03 (1 H, br s, NH), 7.17–7.23 (5 H, m, Ph), 7.79 (1 H, s, OCH=C). LCMS: m/z calcd for C30H43N5O5 (M+1) = 554.6, found 554.3; HRMS (ESI-TOF): MH+, found 554.3313, requires 554.3337. >90% pure by HPLC.
6.23. Synthesis of Compound 4 (B-Ox-III)
6.23.1. Dipeptide MeO-Phe-Leu-NHBoc
Dipeptide MeO-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 850 mg (4.80 mmol) of amine MeO-Phe-NH2, 1.00 g (4.30 mmol) of acid HO-Leu-NHBoc, 2.90 mL (17.2 mmol) of DIPEA, 1.66 g (5.20 mmol) of TBTU, in 43.2 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (1.64 g, 97% yield). Rf: 0.3 (EtOAc: Hex 1:3); 1H NMR (400 MHz, CDCl3): δ 0.78–0.84 (6 H, m, CH(CH3)2), 1.33 (9 H, s, C(CH3)3), 1.35–1.40 (1 H, m, CH(CH3)2), 1.45–1.60 (2 H, m, βCH2), 2.90–3.05 (2 H, m, βCH2), 3.59 (3 H, s, OCH3), 4.00–4.10 (1 H, br, αCH), 4.70–4.78 (1 H, m, αCH), 5.25–5.35 (1 H, br, NH), 6.86–6.96 (1 H, d, J 7.8 Hz, NH), 7.00–7.20 (5 H, m, Ph).
6.23.2. Dipeptide HO-Phe-Leu-NHBoc
Dipeptide HO-Phe-Leu-NHBoc was synthesized following the “General solution-phase acid deprotection”. This Dipeptide was taken on to the next reaction without further purification or characterization. (1.35 g, 85% yield).
6.23.3. Tripeptide MeO-Leu-N(Me)-Phe-Leu-NHBoc
Tripeptide MeO-Leu-N(Me)-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 338 mg (2.10 mmol) of amine MeO-Leu-N(Me)H, 731 mg (1.90 mmol) of acid HO-Phe-Leu-NHBoc, 1.35 mL (5.70 mmol) of DIPEA, 744 mg (2.30 mmol) of TBTU, in 19.3 mL of Methylene Chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the tripeptide (602 mg, 60% yield). Rf: 0.22 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.84–0.98 (12 H, m, CH(CH3)2), 1.34–1.42 (2 H, m, CH(CH3)2), 1.47 (9 H, s, C(CH3)3), 1.54–1.75 (4 H, m, βCH2), 2.80 (3 H, s, NCH3), 2.94–3.20 (2 H, m, βCH2), 3.68 (3 H, s, OCH3), 4.05–4.15 (1 H, br, NH), 4.76–4.84 (1 H, br, NH), 4.84–4.88 (1 H, m, αCH), 5.10–5.20 (1 H, m, αCH), 5.25–5.32 (1 H, m, αCH), 7.10–7.33 (5 H, m, Ph).
6.23.4. Tripeptide HO-Leu-N(Me)-Phe-Leu-NHBoc
Tripeptide HO-Leu-N(Me)-Phe-Leu-NHBoc was synthesized following the “General solution-phase acid deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization. (470mg, 80% yield).
6.23.5. Dipeptide MeO-Ser(Bzl)-Leu-NHBoc
Dipeptide MeO-Ser(Bzl)-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 756 mg (3.40 mmol) of amine MeO-Ser(Bzl)-NH2, 713 mg (3.10 mmol) of acid HO-Leu-NHBoc, 2.10 mL (12.4 mmol) of DIPEA, 1.19 g (3.70 mmol) of TBTU, in 30.8 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (1.31 g, 97 % yield). Rf: 0.76 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.95–0.96 (3 H, d, J 3.5 Hz, CHCH3)2), 0.96–0.97 (3 H, d, J 3.5 Hz, CHCH3)2), 1.43 (9 H, s, C(CH3)2), 1.61–1.63 (1 H, m, CH(CH3)2), 1.63–1.78 (2 H, m, βCH2), 3.66–3.70 (1 H, dd, J 6.9, 1.5 Hz, βCH), 3.75 (3 H, s, OCH3), 3.88–3.92 (1 H, dd, J 7.1, 1.5 Hz, βCH), 4.14–4.21 (1 H, br, αCH), 4.46–4.59 (2 H, CH2OBn), 4.72–4.76 (1 H, m, αCH), 4.84–4.91 (1 H, br, NH), 6.76–6.80 (1 H, br, NH), 7.26–7.40 (5 H, m, Ph).
6.23.6. Dipeptide MeO-Ser-Leu-NHBoc
Dipeptide MeO-Ser-Leu-NHBoc was synthesized by dissolving 1.28 g (3.03 mmol) of dipeptide MeO-Ser(Bzl)-Leu-NHBoc in 30.0 mL EtOH (0.1 M). The reaction mixture was hydrogenated using a catalytic amount of Pd/C and excess H2 for 24 h. The reaction was filtered over Celite to yield pure dipeptide (900 mg, 89% yield). Rf: 0.28 (EtOAc: Hex 1:1).
6.23.7. Dipeptide MeO-Oxazole-Leu-NHBoc
Dipeptide MeO-Oxazole-Leu-NHBoc was synthesized following the “General oxazole synthesis” procedure. The oxazoline intermediate was synthesized utilizing 900 mg (2.72 mmol) of dipeptide MeO-Ser-Leu-NHBoc, 0.390 mL (2.97 mmol) of DAST, 746 mg (5.40 mmol) of K2CO3 in 40.0 mL of methylene chloride. The resulting oxazoline was oxidized utilizing 0.810 mL (5.40 mmol) of DBU, 0.530 mL (5.40 mmol) of CBrCl3 in 40.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the desired peptidyl-oxazole (649 mg,77 % yield over 2 steps). Rf: 0.5 (EtOAc: Hex 7:13); 1H NMR (400 MHz, CDCl3): δ 0.94–0.96 (3 H, d, J 3.5 Hz, CHCH3), 0.96–0.98 (3 H, d, J 3.5 Hz, CHCH3), 1.43 (9 H, s, C(CH3)3), 1.58–1.64 (1 H, m, CH(CH3)2), 1.64–1.80 (2 H, m, βCH2), 3.93 (3 H, s, OCH3), 4.94–5.04 (1 H, br, αCH), 5.04–5.10 (1 H, br, NH), 8.18 (1 H, s, OCH=C).
6.23.8. Dipeptide MeO-Oxazole-Leu-NH2
Dipeptide MeO-Oxazole-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (212 mg, 100% yield).
6.23.9. Pentapeptide MeO-Oxazole-Leu-Leu-N(Me)-Phe-Leu-NHBoc
Pentapeptide MeO-Oxazole-Leu-Leu-N(Me)-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 210 mg (0.990 mmol) of amine MeO-Oxazole-Leu-NH2, 470 mg (0.930 mmol) of acid HO-Leu-N(Me)-Phe-Leu-NHBoc, 0.650 mL (3.72 mmol) of DIPEA, 353 mg (1.10 mmol) of TBTU, in 10.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (281 mg, 43 % yield). Rf: 0.47 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.80–1.02 (18 H, m, CH(CH3)2), 1.22–1.57 (6 H, m, βCH2), 1.35–1.44 (9 H, m, C(CH3)3), 1.57–1.73 (2 H, m, CH(CH3)2), 1.73–1.94 (1 H, m, CH(CH3)2), 2.69 (3 H, m, NCH3), 2.84–3.23 (2 H, m, βCH2), 3.91 (3 H, m, OCH3), 4.04–4.15 (2 H, br, NH), 4.60–4.70 (1 H, br, NH), 4.78–4.96 (2 H, m, αCH), 5.14–5.28 (1 H, m, αCH), 5.28–5.38 (1 H, m, αCH), 7.08–7.30 (5 H, m, Ph), 8.08–8.20 (1 H, m, OCH=C). LCMS: m/z calcd for C37H57N5O8 (M+1) = 699.88, found 699.8
6.23.10. Macrocycle Phe-Leu-Oxazole-Leu-Leu-N(Me) (4)
Macrocycle Phe-Leu-Oxazole-Leu-Leu-N(Me) (4) was synthesized following the “Macrocyclization procedure”. Utilizing 281 mg (0.400 mmol) of linear pentapeptide, 0.420 mL (2.4 mmol) of DIPEA, 90.0 mg (0.280 mmol) of TBTU, 107 mg (0.280 mmol) HATU, and 83.8 mg (0.280 mmol) of DEPBT in 571 mL Methylene Chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) and reverse phase-HPLC to yield the macrocycle (17.3 mg, 6.4% yield). Rf: 0.42 (EtOAc: Hex 1:1) 1H NMR (400 MHz, CDCl3): δ 0.75–0.80 (3 H, d, J 3.5 Hz, CH(CH3)2), 0.84–0.89 (3 H, d, J 3.5Hz, CH(CH3)2), 0.90–1.00 (21 H, bur m, C(CH3)3 & CH(CH3)2), 1.08–1.20 (1 H, m, CH(CH3)2), 1.43–1.50 (2 H, m, CH(CH3)2), 1.50–1.64 (2 H, m, βCH2), 1.65–1.80 (4 H, m, βCH2), 2.56–2.66 (3 H, m, NCH3), 2.98–3.15 (2 H, m, βCH2), 4.15–4.45 (3 H, br, NH & 2αCH), 4.88–4.98 (1 H, br, NH), 4.99–5.10 (2 H, m, αCH Leu-Ox & αCH Phe), 6.93–7.03 (1H, m, Ph), 7.33–7.38 (4 H, m, Ph), 8.15 (1 H, s, OCH=C). LCMS: m/z calcd for C31H45N5O5 (M) = 567.72, found 567.8; HRMS (ESI-TOF): MH+, found 568.3520, C31H45N5O5 requires 568.3493. >90% pure by HPLC.
6.24. Synthesis of Compound 5 (C-Ox-III)
6.24.1 Dipeptide MeO-Lys(Cbz)-Phe-NHBoc
Dipeptide MeO-Lys(Cbz)-Phe-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 294 mg (1.00 mmol) of amine MeO-Lys(Cbz)-NH2, 241 mg (0.910 mmol) of acid HO-Phe-NHBoc, 1.30 mL (7.28 mmol) of DIPEA, 235 mg (0.730 mmol) of TBTU, 209 mg (0.550 mmol) of HATU, in 9.00 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (380 mg, 77% yield). Rf: 0.37 (EtOAc: Hex 1:1) 1H NMR (400 MHz, CDCl3): δ 1.11–1.49 (2 H, m, γCH2 Lys), 1.30–1.36 (9 H, s, C(CH3)3), 1.36–1.64 (2 H, m, δCH2 Lys), 1.51–1.81 (2 H, m, βCH2 Lys), 2.93–3.03 (2 H, m, εCH2 Lys), 3.03–3.16 (2 H, m, βCH2), 3.60–3.65 (3 H, s, OCH3), 4.21–4.35 (1 H, m, αCH), 4.42–4.52 (1 H, m, αCH), 4.85–4.93 (1 H, br, NH), 5.00–5.05 (2 H, s, CH2Ph), 6.39–6.47 (1 H, br, NH), 7.08–7.32 (10H, m, Ph).
6.24.2. Dipeptide MeO-Lys-Cbz-Phe-NH2
Dipeptide MeO-Lys(Cbz)-Phe-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (309mg, 100% yield).
6.24.3. Tripeptide MeO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc
Tripeptide MeO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 309 mg (0.700 mmol) of amine MeO-Lys(Cbz)-Phe-NH2, 215 mg (0.770 mmol) of acid HO-D-Phe-N(Me)Boc, 0.980 mL (4.9 mmol) of DIPEA, 180 mg (0.560 mmol) of TBTU, 133 mg (0.350 mmol) of HATU, in 7.00 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the tripeptide (454 mg, 93% yield). Rf: 0.38 (EtOAc: Hex 7:13); 1H NMR (400 MHz, CDCl3): δ 1.10–1.22 (2 H, m, γCH2 Lys), 1.22–1.30 (9 H, s, C(CH3)3), 1.35–1.48 (2 H, m, δCH2 Lys), 1.51–1.83 (2 H, m, βCH2 Lys), 2.51–2.73 (3H, s, NCH3), 2.82–3.02 (2 H, m, βCH2), 3.02–3.15 (2 H, m, CH2ε Lys), 3.16–3.34 (2 H, m, βCH2), 3.59–3.70 (3 H, s, OCH3), 4.34–4.46 (1 H, br, αCH), 4.47–4.68 (2 H, m, 2αCH), 4.98–5.08 (2 H, s, CH2Ph), 6.22–6.61 (2 H, br-m, NH), 6.98–7.34 (15H, m, Ph).
6.24.4. Tripeptide HO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc
Tripeptide HO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc was synthesized following the “General solution-phase acid deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization. (448mg, 100% yield).
6.24.5. Dipeptide MeO-Ser(Bzl)-Leu-NHBoc
Dipeptide MeO-Ser(Bzl)-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 383 mg (1.83 mmol) of amine MeO-Ser(Bzl)-NH2, 414 mg (1.66 mmol) of acid HO-Leu-NHBoc, 1.20 mL (6.64 mmol) of DIPEA, 588 mg (1.83 mmol) of TBTU, in 17.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (701 mg, 100 % yield). Rf: 0.76 (EtOAc: Hex 1:1). 1H NMR (400 MHz, CDCl3): δ 0.95–0.96 (3 H, d, J 3.5 Hz, CHCH3), 0.96–0.97 (3 H, d, J 3.5 Hz, CHCH3), 1.42–1.46 (9 H, s, C(CH3)3), 1.61–1.63 (1 H, m, CH(CH3)2), 1.63–1.78 (2 H, m, βCH2), 3.66–3.70 (1 H, dd, J 7.2, 1.5 Hz, βCH Ser), 3.73–3.76 (3 H, s, OCH3), 3.88–3.92 (1 H, dd, J 7.9 1.5 Hz, βCH), 4.14–4.21 (1 H, br, αCH), 4.46–4.59 (2 H, m, CH2OBn), 4.72–4.76 (1 H, m, αCH), 4.84–4.91 (1 H, br, NH), 6.76–6.80 (1 H, br, NH), 7.26–7.40 (5 H, m, Ph).
6.24.6. Dipeptide MeO-Ser-Leu-NHBoc
Dipeptide MeO-Ser-Leu-NHBoc was synthesized by dissolving 701 mg (1.66 mmol) of dipeptide MeO-Ser(Bzl)-Leu-NHBoc in 4.00 mL EtOH (0.48 M). The reaction mixture was hydrogenated using a catalytic amount of Pd/C and excess H2 for 24 h. The reaction was filtered over Celite to yield pure dipeptide (521 mg, 95 % yield). Rf: 0.28 (EtOAc: Hex 1:1).
6.24.7. Dipeptide MeO-Oxazole-Leu-NHBoc
Dipeptide MeO-Oxazole-Leu-NHBoc was synthesized following the “General oxazole synthesis” procedure. The oxazoline intermediate was synthesized utilizing 421 mg (1.27 mmol) of dipeptide MeO-Ser-Leu-NHBoc, 0.170 mL (1.40 mmol) of DAST, 351 mg (2.54 mmol) of K2CO3 in 12.7 mL of methylene chloride. The resulting oxazoline was oxidized utilizing 0.380 mL (2.54 mmol) of DBU, 0.250 mL (2.54 mmol) of CBrCl3 in 12.7 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the desired peptidyl-oxazole (298 mg, 76% yield over 2 steps). Rf: 0.5 (EtOAc: Hex 7:13); 1H NMR (400 MHz, CDCl3): δ 0.94–0.96 (3 H, d, J 3.5 Hz, CHCH3), 0.96–0.98 (3 H, d, J 3.5 Hz, CHCH3), 1.42–1.46 (9 H, s, C(CH3)3), 1.58–1.64 (1 H, m, CH(CH3)2), 1.64–1.80 (2 H, m, βCH2), 3.93 (3 H, s, OCH3), 4.94–5.04 (1 H, br, αCH), 5.04–5.10 (1 H, br, NH), 8.18 (1 H, s, OCH=C).
6.24.8. Dipeptide MeO-Oxazole-Leu-NH2
Dipeptide MeO-Oxazole-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (202 mg, 100% yield).
6.24.9. Pentapeptide MeO-Oxazole-Leu-Lys(Cbz)-Phe-D-Phe-N(Me)Boc
Pentapeptide MeO-Oxazole-Leu-Lys(Cbz)-Phe-D-Phe-N(Me)Boc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 152 mg (0.720 mmol) of amine MeO-Oxazole-Leu-NH2, 447 mg (0.650 mmol) of acid HO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc, 0.910 mL (5.2 mmol) of DIPEA, 167 mg (0.520 mmol) of TBTU, 148 mg (0.390 mmol) of HATU in 7.00 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (274 mg, 48% yield). Rf: 0.2 (EtOAc: Hex 1:1) 1H NMR (400 MHz, CDCl3): δ 0.78–0.92 (6 H, m, CH(CH3)2), 1.10–1.31 (2 H, m, γCH2 Lys), 1.23–1.27 (9 H, s, C(CH3)3), 1.33–1.48 (2 H, m, δCH2 Lys), 1.48–1.60 (2 H, m, βCH2 Lys), 1.64–1.87 (3 H, m, CH(CH3)2, CH2β Leu), 2.35 (3H, m, NCH3), 2.85–3.05 (2 H, m, βCH2), 3.05–3.19 (2 H, m, εCH2 Lys), 3.19–3.32 (2 H, m, βCH2), 3.75 (3 H, s, OCH3), 4.21–4.43 (1 H, br, αCH), 4.33–4.55 (1 H, br, αCH), 4.46–4.70 (1 H, m, αCH), 4.95–5.06 (2 H, m, CH2Ph), 5.06–5.26 (2 H, m, αCH, NH), 6.28–6.78 (2 H, m, NH), 6.91–7.44 (15H, m, Ph), 8.09 (1 H, s, OCH=C). LCMS: m/z calcd for C48H62N6O10 (M) = 883.04, found 883
6.24.10. Macrocycle Phe-D-Phe-N(Me)-Oxazole-Leu-Lys(Cbz) (5)
Macrocycle Phe-D-Phe-N(Me)-Oxazole-Leu-Lys(Cbz) (5) was synthesized following the “Macrocyclization procedure”. Utilizing 238 mg (0.310 mmol) of linear pentapeptide, 0.430 mL (2.48 mmol) of DIPEA, 80.0 mg (0.250 mmol) of TBTU, 95.0 mg (0.250 mmol) HATU, and 75.0 mg (0.250 mmol) of DEPBT in 44.3 mL methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) and reverse phase-HPLC to yield the macrocycle (19.8 mg, 8.5% yield). Rf: 0.18 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.82–0.88 (3 H, d, J 15.7 Hz, CH(CH3)2), 0.89–0.94 (3 H, d, J 15.7 Hz, CH(CH3)2), 1.03–1.19 (2 H, br, γCH2 Lys), 1.33–1.58 (2 H, m, δCH2 Lys), 1.58–1.70 (2 H, m, βCH2 Lys), 1.86–2.03 (2 H, m, βCH2), 2.17–2.33 (1 H, m, CH(CH3)2), 2.62–2.67 (3H, s, NCH3), 2.82–3.21 (2 H, m, βCH2), 3.01–3.11 (2 H, m, εCH2 Lys), 3.35–3.44 (1 H, dd, J 8.85, 2.92 Hz, CHaHbβ Phe), 3.46–3.56 (1 H, br, αCH), 4.58–4.65 (1 H, dd, J 8.85, 2.94 Hz, βCHaHb Phe), 4.65–4.78 (2 H, m, 2αCH), 5.01–5.06 (2 H, m, CH2Ph), 5.14–5.24 (1 H, m, αCH), 6.76–6.93 (3 H, m, NH), 7.07–7.33 (15H, m, Ph), 8.00–8.08 (1 H, s, OCH=C). LCMS: m/z calcd for C42H50N6O7 (M) = 750.88, found 751; HRMS (ESI-TOF): MH+, found 751.3821, C42H50N6O7 requires 751.3814. >95% pure by HPLC.
6.25. Synthesis of Compound 6 (D-Ox-III)
6.25.1. Dipeptide NH2-(2R,3R)/(2S,3S)-β-OH-Phe-D-Phe-O-Resin
A mixture of NH2-D-Phe-O-Resin (2.00 g, 1.44 mmol), (2R,3R)/(2S,3S)-racemic Fmoc-β-OH-Phe-OH (1.74 g, 4.32 mmol), HOBt (661 mg, 4.32 mmol) and DIC (1.34 mL, 8.64 mmol) were stirred at room temperature for 3 h following the “General solid-phase peptide synthesis” procedure. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained to leave the amine-protected resin-bound dipeptide. Deprotection of amine was performed following the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal and gave the title compound.
6.25.2. Tripeptide Boc-Leu-(2R,3R)/(2S,3S)-β-OH-Phe-D-Phe-OH
A mixture of NH2-(2R,3R)/(2S,3S)-β-OH-D-Phe-O-Resin (1.44 mmol), Boc-Leu-OH residue (1.00 g, 4.32 mmol), HOBt (661 mg, 4.32 mmol) and DIC (1.34 mL, 8.64 mmol) were stirred at room temperature for 3 h following the “General solid-phase peptide synthesis” procedure. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained and dried in vacuo overnight to leave the amine-protected resin-bound tripeptide. The tripeptide was cleaved from the resin following the “Cleavage of linear peptide from solid support” procedure. A resin slurry of Boc-Leu-(2R,3R)/(2S,3S)-β-OH-Phe-D-Phe-O-Resin (2.50 g), 2,2,2-trifluoroethanol (12.5 mL) and of methylene chloride (12.5) was stirred for 24 h, after which it was filtered, washed with additional methylene chloride, and dried in vacuo for 24 h. (596 mg, 76% yield).
6.25.3. Dipeptide MeO-Ser(Bzl)-D-Leu-NHBoc
A mixture of amine MeO-Ser(Bzl)-NH2 (709 mg, 3.39 mmol), acid HO-D-Leu-NHBoc (767 mg, 3.08 mmol), DIPEA (4.30 mL, 2.46 mmol), TBTU (1.19 g, 3.94 mmol) in methylene chloride (30.8 mL) was stirred at room temperature under an argon atmosphere for 2.5 h following the “General solution-phase peptide synthesis” procedure. The crude reaction was purified by column chromatography (20% EtOAc/Hex) to yield the title compound (1.24 g, 96% yield). Rf: 0.78 (50% EtOAc/Hex); 1H NMR (200 MHz, CDCl3): δ 0.9–0.95 (6 H, m, CH(CH3)2), 1.43 (9 H, s, C(CH3)3), 1.63–1.73 (2 H, m, CHCH2CH), 3.65–3.70 (1 H, dd, J 2.8, 8.9 Hz, OCHaHb), 3.73 (3 H, s, OCH3), 3.86–3.91 (1 H, dd, J 3.0, 9.0 Hz, OCHaHb), 4.18–4.26 (1 H, br, αCH), 4.45–4.54 (2 H, q, J 11.9 Hz, CH2Ph), 4.70–4.75 (1 H, m, αCH), 4.95–5.07 (1 H, br, NH), 7.01 (1 H, br, NH), 7.23–7.36 (5 H, m, Ph).
6.25.4. Dipeptide MeO-Ser-D-Leu-NHBoc
A mixture of dipeptide MeO-Ser(Bzl)-D-Leu-NHBoc (1.24 g, 2.94 mmol) and a catalytic amount of Pd/C in ethanol (29.4 mL) was stirred under a hydrogen atmosphere for 24 h. Upon completion, confirmed by TLC, the reaction mixture was filtered over Celite to yield the title compound (839 mg, 86% yield). Rf: 0.44 (50% EtOAc/Hex); Physical and spectroscopic data are consistent with those reported in the literature.50
6.25.5. Dipeptide MeO-Oxazole-D-Leu-NHBoc
Dipeptide MeO-Oxazole-D-Leu-NHBoc was synthesized following the “General oxazole synthesis” procedure. The oxazoline intermediate was synthesized utilizing dipeptide MeO-Ser-D-Leu-NHBoc (819 mg, 2.46 mmol), DAST (357 µL, 2.71 mmol), K2CO3 (664 mg, 4.92 mmol) in methylene chloride (24.6 mL). The resulting oxazoline was oxidized utilizing DBU (746 µL, 4.92 mmol), CBrCl3 (489 µL, 4.92 mmol) in methylene chloride (12.3 mL). The crude reaction was purified by column chromatography (40% EtOAc/Hex) to yield the desired title compound (516 mg, 70% yield over 2 steps). Rf: 0.80 (50% EtOAc/Hex); 1H NMR (200 MHz, CDCl3): δ 0.86 (3 H, d, J 1.6 Hz, CHCH3), 0.87 (3 H, d, J 1.8 Hz, CHCH3), 1.35 (9 H, s, C(CH3)3), 1.53–1.61 (1 H, m, CH(CH3)2), 1.63–1.71 (2 H, m, CHCH2CH), 3.83 (3 H, s, OCH3), 4.87–4.95 (1 H, br, αCH), 5.08–5.14 (1 H, br, NH), 8.11 (1 H, s, CCHO).
6.25.6. Dipeptide MeO-Oxazole-D-Leu-NH2
A mixture of peptidyl MeO-Oxazole-D-Leu-NHBoc (516 mg, 1.65 mmol) in TFA (3.30 mL) and methylene chloride (13.2 mL) was stirred at room temperature under open atmosphere for 30 min following the “General solution-phase amine deprotection.” Reaction completion was confirmed by TLC and the mixture was concentrated in vacuo with several washes with methylene chloride. This dipeptide was taken on to the next reaction without further purification or characterization. (350 mg, quantitative yield).
6.25.7. Pentapeptide MeO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NHBoc
A mixture of amine MeO-Oxazole-D-Leu-NH2 (257 mg, 1.21 mmol), acid Boc-Leu-(2R,3R)/(2S,3S)-β-OH-Phe-D-Phe-OH (596 mg, 1.1 mmol), TBTU (424 mg, 1.32 mmol), HATU (301 mg, 7.92 mmol) and DIPEA (2.11 mL, 12.1 mmol) in methylene chloride (12.1 mL) was stirred at room temperature under an argon atmosphere for 3 h following the “General solution-phase peptide synthesis” procedure. Upon completion, confirmed by TLC, the reaction mixture was diluted with methylene chloride (200 mL), quenched by the addition of 10% hydrochloric acid solution (200 mL), and further washed with sodium bicarbonate solution (500 mL, sat. aq.) and then brine (200 mL). The organic layer was dried (Na2SO4) and the solvent was evaporated in vacuo. The crude reaction was purified by column chromatography (90% EtOAc/Hex) to yield the title compound (490 mg, 61% yield). Rf: 0.40 (50% EtOAc/Hex); 1H NMR (400 MHz, CDCl3): δ 0.76–0.81 (9 H, m, 3CHCH3), 0.84 (3 H, d, J 4.0 Hz, CHCH3), 0.89 (6 H, d, J 3.4 Hz, 2CHCH3), 0.91 (3 H, d, J 3.5 Hz, CHCH3), 1.38–1.42 (4 H, m, buried, 2CH(CH3)2), 1.39 (9 H, s, C(CH3)3), 1.40 (9 H, s, C(CH3)3), 1.63 (2 H, m, CHCH2CH), 1.74 (2 H, m, CHCH2CH), 2.75 (1 H, dd, J 6.6, 13.9 Hz, PhCHaHb), 3.10 (2 H, m, PhCHaHb), 3.21 (1 H, dd, J 7.7, 13.8 Hz, PhCHaHb), 3.63 (3 H, s, OCH3), 3.87 (3 H, s, OCH3), 4.53 (1 H, m, αCH), 4.63 (2 H, m, 2αH), 4.80 (1 H, m, αCH), 4.88 (1 H, m, αCH), 4.99 (2 H, m, 2αCH), 5.20 (1 H, q, J 7.8 Hz, CHOH), 5.30 (1 H, q, J 7.9 Hz, CHOH), 6.55 (1 H, br, NH), 6.64 (1 H, d, J 8.8 Hz, NH), 6.95 (2 H, m, 2NH), 7.01 (1 H, d, J 8.5 Hz, NH), 7.07 (1 H, d, J 6.1 Hz, NH), 7.12–7.18 (6 H, m, 4CCHCH, 2CHCHCH), 7.22–7.34 (10 H, m, Ph), 7.4 (4 H, m, 4CHCHCH), 7.60 (2 H, d, J 7.2 Hz, NH), 8.19 (2 H, s, CCHO). LCMS (ESI): m/z called for C39H53N5O9 (M+) = 735.4, found 735.8.
6.25.8. Deprotected pentapeptide HO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NH2
A mixture of pentapeptide MeO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NHBoc (100 mg, 0.136 mmol), lithium hydroxide (17.0 mg, 0.408 mmol), and 30% hydrogen peroxide (14.0 µL) in methanol (340 µL) was stirred at room temperature under open atmosphere for 12 h following the “General solution-phase acid deprotection.” Upon completion, the reaction was diluted with methylene chloride (50 mL) and quenched with sodium thiosulfate (154 mg) in pH 1 hydrochloric acid solution (200 mL). The organic layer was separated and the aqueous layer was back extracted with ethyl acetate. The combined organic layers were dried (Na2SO4) and concentrated in vacuo to yield the deprotected acid HO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NHBoc. Deprotection of the amine resulted from a mixture of acid HO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NHBoc (76.0 mg, 0.105 mmol), anisole (23.0 µL, 0.211 mmol) in TFA (211µL) and methylene chlorlide (842 µL) stirred at room temperature for 40 min under open atmosphere following the “General solution-phase amine deprotection.” Reaction completion was confirmed by TLC and the mixture was concentrated in vacuo with several washes with methylene chloride. This deprotected pentapeptide HO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NH2 was taken on to the next reaction without further purification or characterization. (65.0 mg, 77% yield over two steps). LCMS (ESI): m/z called for C33H43N5O7 (M+2H+) = 623.7, found 623.8.
6.25.9. Macrocycle (2R,3R)-β-benzoxy-Phe-Leu-Oxazole-D-Leu-D-Phe (6)
A mixture of TBTU (41.0 mg, 0.126 mmol), HATU (36.0 mg, 0.948 µmol), and DIPEA (147 µL, 0.842 mmol) in methylene chloride (6.77 mL) and acetonitrile (4.53 mL) stirred at room temperature in a round bottom flask under an argon atmosphere following the “Macrocyclization procedure”. The deprotected pentapeptide HO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NH2 (65.0 mg, 0.105 mmol) was dissolved in methylene chloride (2.26 mL) and acetonitrile (1.49 mL) and added to the reaction flask via syringe pump at the rate of 15 mL/h. After 12 h, the reaction was diluted with methylene chloride (200 mL) and quenched with ammonium chloride solution (200 mL, sat. aq.). The organic layer was separated, dried (Na2SO4) and concentrate in vacuo. The crude reaction was semi-purified by reverse phase-HPLC to yield the macrocycle (2R,3R)/(2S,3S)-β-OH-Phe-Leu-Oxazole-D-Leu-D-Phe (19 mg, 29% yield). Rf: 0.2 (65% EtOAc/Hex); LCMS: m/z called for C33H41N5O6 (M+1) = 604.7, found 603.9 Finally, a mixture of macrocycle (2R,3R)-β-benzoxy-Phe-Leu-Oxazole-D-Leu-D-Phe (18.0 mg, 0.298 µmol), NaH (1.50 mg, 0.596 µmol) and benzyl bromide (14.0 µL, 0.0119 mmol) in THF (1.19 mL) stirred at room temperature for 12 h under an atmosphere of argon following the “Benzylation procedure.” The crude reaction was purified by reverse phase-HPLC to yield the title compound 6 (1.0 mg, 0.4%); stereochemistry was assigned previously.1 Rf: 0.40 (65% EtOAc/Hex); >90% pure by HPLC. 1H NMR (600 MHz, CD3OD): δ 0.79 (3 H, d, J 6.6 Hz, CHCH3), 0.82 (3 H, d, J 6.5 Hz, CHCH3), 0.90 (3 H, d, J 6.4 Hz, CHCH3), 1.05 (3 H, d, J 6.6 Hz, CHCH3), 1.27–1.34 (2 H, m, 2CH(CH3)2), 1.57–1.63 (4 H, m, 2CH2CH), 3.19 (1 H, m, CHaHbPh), 3.43 (1 H, m, CHaHbPh), 3.75–3.80 (2 H, m, OCH2Ph), 4.34–4.38 (2 H, m, 2αCH), 4.70 (1 H, d, J 5.3 Hz, αCH), 4.83 (1 H, m, αCH), 5.06 (1 H, d, J 5.2 Hz, PhCHO), 6.81 (1 H, d, J 8.7 Hz, NH), 7.14–7.34 (10 H, m, 2Ph), 7.37–7.47 (5 H, m, Ph), 7.58 (1 H, d, J 4.3 Hz, NH), 7.59 (1 H, d, J 4.7 Hz, NH), 8.42 (1 H, s, CCHO), 8.52 (1 H, m, NH), 8.77 (1 H, m, NH). LCMS: m/z called for C40H47N5O6 (M+1) = 694.8, found 694.4; HRMS (ESI-TOF): MH+, found 694.3592, C40H47N5O6 requires 694.3599.
6.26. Synthesis of Compound 7 (D-Ox-II)
6.26.1. Dipeptide NH2-D-Phe-D-Leu-O-Resin
A mixture of NH2-D-Leu-O-Resin (1.50 g, 1.10 mmol), Fmoc-D-Phe-OH (1.27 g, 3.29 mmol), HOBt (503 mg, 3.29 mmol) and DIC (1.02 mL, 6.57 mmol) were stirred at room temperature for 3 h following the “General solid-phase peptide synthesis” procedure. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained to leave the amine-protected resin-bound dipeptide. Deprotection of amine was performed following the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal and gave the title compound.
6.26.2. Tripeptide Boc-(2R,3R)/(2S,3S)-β-OH-Phe-D-Phe-D-Leu-OH
A mixture of NH2-D-Phe-D-Leu-O-Resin (1.10 mmol), (2R,3R)/(2S,3S)-racemic Boc-β-OH-Phe-OH residue (923 mg, 3.29 mmol), HOBt (503 mg, 3.29 mmol) and DIC (1.02 mL, 6.57 mmol) were stirred at room temperature for 3 h following the “General solid-phase peptide synthesis” procedure. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained and dried in vacuo overnight to leave the amine-protected resin-bound tripeptide. The tripeptide was cleaved from the resin following the “Cleavage of linear peptide from solid support” procedure. A resin slurry of Boc-(2R,3R)/(2S,3S)-β-OH-Phe-D-Phe-D-Leu-O-Resin (2.07 g), 2,2,2-trifluoroethanol (10.3 mL) and of methylene chloride (10.3) was stirred for 24 h, after which it was filtered, washed with additional methylene chloride, and dried in vacuo for 24 h to yield the title compound (534 mg, 90% yield).
6.26.3. Dipeptide MeO-Ser(Bzl)-Val-N(Me)Boc
A mixture of amine MeO-Ser(Bzl)-NH2 (709 mg, 3.39 mmol), acid HO-Val-N(Me)-Boc (712 mg, 3.09 mmol), DIPEA (5.16 mL, 2.46 mmol), TBTU (1.19 g, 3.69 mmol) in methylene chloride (30.8 mL) was stirred at room temperature under an argon atmosphere for 2.5 h following the “General solution-phase peptide synthesis” procedure. The crude reaction was purified by column chromatography (20% EtOAc/Hex) to yield the title compound (1.22 g, 97% yield). Rf: 0.80 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.82 (3 H, d, J 6.7 Hz, CHCH3), 0.95 (3 H, d, J 6.1 Hz, CHCH3), 1.41 (9 H, s, C(CH3)3), 2.19–2.30 (1 H, m, CH(CH3)2), 2.74 (3 H, s, NCH3), 3.58–3.62 (1 H, dd, J 3.6, 9.4 Hz, CHCHaHbO), 3.70 (3 H, s, OCH3), 4.10 (1 H, br, αCH), 3.77–3.82 (1 H, d, J 7.7 Hz, CHCHaHbO), 4.40–4.56 (2 H, q, J 13.0 Hz, CH2Ph), 4.67 (1 H, m, αCH), 6.93 (1 H, br, NH), 7.20–7.30 (5 H, m, Ph).
6.26.4. Dipeptide MeO-Ser-Val-N(Me)Boc
A mixture of dipeptide MeO-Ser(Bzl)-Val-N(Me)-Boc (1.22 g, 3.01 mmol) and a catalytic amount of Pd/C in ethanol (30.1 mL) was stirred under a hydrogen atmosphere for 24 h. Upon completion, confirmed by TLC, the reaction mixture was filtered over Celite to yield the title compound (723 mg, 75% yield). Rf: 0.5 (EtOAc: Hex 1:1); Physical and spectroscopic data are consistent with those reported in the literature.51
6.26.5. Dipeptide MeO-Oxazole-Val-N(Me)Boc
Dipeptide MeO-Oxazole-Val-N(Me)-Boc was synthesized following the “General oxazole synthesis” procedure. The oxazoline intermediate was synthesized utilizing dipeptide MeO-Ser-Val-N(Me)-Boc (700 mg, 2.11 mmol), DAST (306 µL, 2.32 mmol), K2CO3 (569 mg, 4.21 mmol) in methylene chloride (21.1 mL). The resulting oxazoline was oxidized utilizing DBU (420 µL, 4.21 mmol), CBrCl3 (420 µL, 4.21 mmol) in methylene chloride (21.1 mL). The crude reaction was purified by column chromatography (40% EtOAc/Hex) to yield the desired title compound (494 mg, 75% yield over 2 steps). Rf: 0.82 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.87 (3 H, d, J 6.7 Hz, CHCH3), 0.93 (3 H, d, J 5.6 Hz, CHCH3), 1.43 (9 H, s, C(CH3)3), 2.41–2.52 (1 H, m, CH(CH3)2), 2.76 (3 H, s, NCH3), 3.40 (1 H, s, OH), 3.84 (3 h, s, OCH3), 4.86 (1 H, d, J 10.5 Hz, αCH), 5.12 (1 H, d, J 10.9 Hz, NH), 8.19 (1 H, s, CCHO).
6.26.6. Dipeptide MeO-Oxazole-Val-N(Me)H
A mixture of peptidyl MeO-Oxazole-Val-N(Me)-NBoc (494 mg, 1.58 mmol) in TFA (3.16 mL) and methylene chloride (12.6 mL) was stirred at room temperature under open atmosphere for 30 min following the “General solution-phase amine deprotection.” Reaction completion was confirmed by TLC and the mixture was concentrated in vacuo with several washes with methylene chloride. This dipeptide was taken on to the next reaction without further purification or characterization. (335 mg, quantitative yield).
6.26.7. Pentapeptide MeO-Oxazole-Val-N(Me)-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Phe-NHBoc
A mixture of amine MeO-Oxazole-Val-N(Me)H (230 mg, 1.09 mmol), acid Boc-(2R,3R)/(2S,3S)-β-OH-Phe-D-Phe-D-Leu-OH (534 mg, 0.986 mmol), TBTU (189 mg, 0.592 mmol), HATU (375 mg, 0.986 mmol) and DIPEA (1.38 mL, 7.89 mmol) in acetonitrile (9.86 mL) was stirred at room temperature under an argon atmosphere for 1.5 h following the “General solution-phase peptide synthesis” procedure. Upon completion, confirmed by TLC, the reaction mixture was diluted with ethyl acetate (300 mL), quenched by the addition of 10% hydrochloric acid solution (200 mL), and further washed with sodium bicarbonate solution (500 mL, sat. aq.) and then brine (200 mL). The organic layer was dried (Na2SO4) and the solvent was evaporated in vacuo. The crude reaction was purified by column chromatography (85% EtOAc/Hex) to yield the title compound (158 mg, 22% yield). Rf: 0.30 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.76 (6 H, J 2.7 Hz, CH(CH3)2), 0.78 (6 H, J 1.7 Hz, CH(CH3)2), 0.81–0.79 (6 H, J 3.5 Hz, CH(CH3)2), 0.87–0.83 (6 H, m, 2CH(CH3)2), 1,14 (9 H, s, C(CH3)3), 1.22 (9 H, s, C(CH3)3), 1.32–1.44 (2 H, br, CH(CH3)2), 2.32–2.44 (4 H, m, 2CHCH2CH), 2.73 (2 H, m, CH(CH3)2), 2.84–2.90 (6 H, m, 2NCH3), 3.78 (3 H, s, OCH3), 3.79 (3 H, s, OCH3), 3.82 (4 H, br, 2CH2Ph), 4.09–4.21 (2 H, m, 2αCH), 4.44–4.52 (1 H, q, J 6.4 Hz, αCH), 4.51–4.65 (1 H, m, αCH), 4.78 (2 H, br, 2αCH), 4.86 (1 H, m, αCH), 4.90 (1 H, m, αCH), 5.32 (1 H, d, J 8.6 Hz, CHOH), 5.42 (1 H, t, J 12.3 Hz, CHOH), 6.00 (2 H, d, J 6.7 Hz, 2NH), 6.59 (2 H, d, J 8.4 Hz, 2NH), 6.83–6.87 (2 H, m, 2NH), 7.02–7.32 (20 H, m, 4Ph), 8.05 (1 H, s, CCHO), 8.10 (1 H, s, CCHO).
6.26.8. Deprotected pentapeptide HO-Oxazole-Val-N(Me)-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Phe-NH2
A mixture of pentapeptide MeO-Oxazole-Val-N(Me)-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Phe-NHBoc (158 mg, 0.215 mmol), lithium hydroxide (18.0 mg, 0.429 mmol), and 30% hydrogen peroxide (70.0 µL) in water (134 µL) and THF (403 µL) was stirred at room temperature under open atmosphere for 3 h following the “General solution-phase acid deprotection.” Upon completion, the reaction was diluted with ether (100 mL) and quenched with sodium thiosulfate (175 mg) in pH 1 hydrochloric acid solution (200 mL). The organic layer was separated and the aqueous layer was back extracted with ether (240 mL). The combined organic layers were dried (Na2SO4) and concentrated in vacuo to yield the deprotected acid HO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NHBoc. Deprotection of the amine resulted from a mixture of acid HO-Oxazole-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Leu-NHBoc (155 mg, 0.215 mmol), anisole (50.0 µL, 0.429 mmol) in TFA (537 µL) and methylene chlorlide (1.61 mL) stirred at room temperature for 40 min under open atmosphere following the “General solution-phase amine deprotection.” Reaction completion was confirmed by TLC and the mixture was concentrated in vacuo with several washes with methylene chloride. This deprotected pentapeptide HO-Oxazole-Val-N(Me)-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Phe-NH2 was taken on to the next reaction without further purification or characterization. (135 mg, quantitative yield). LCMS (ESI): m/z called for C33H43N5O7 (M+H+) = 621.7, found 621.8.
6.26.9. Macrocycle (2R,3R)-β-benzoxy-Phe-Oxazole-Val-N(Me)-D-Leu-D-Phe (7)
A mixture of TBTU (69.0 mg, 0.215 mmol), HATU (82.0 mg, 0.215 µmol), and DIPEA (375 µL, 2.15 mmol) in methylene chloride (7.70 mL) and acetonitrile (7.70 mL) stirred at room temperature in a round bottom flask under an argon atmosphere following the “Macrocyclization procedure”. The deprotected pentapeptide HO-Oxazole-Val-N(Me)-D-Leu-D-Phe-(2R,3R)/(2S,3S)-β-OH-Phe-NH2 (134 mg, 0.215 mmol) was dissolved in methylene chloride (7.7 mL) and acetonitrile (7.7 mL) and added to the reaction flask via syringe pump at the rate of 15 mL/h. After 12 h, the reaction was diluted with ethyl acetate (500 mL), quenched with pH 1 hydrochloric acid solution (200 mL, sat. aq.), washed with sodium bicarbonate solution (200 mL, sat. aq.), and then brine (200 mL). The organic layer was separated, dried (Na2SO4) and concentrate in vacuo. The crude reaction was semi-purified by reverse phase-HPLC to yield the macrocycle (2R,3R)-β-OH-Phe-Oxazole-Val-N(Me)-D-Leu-D-Phe (29 mg, 23% yield). Rf: 0.21 (65% EtOAc/Hex); LCMS LCMS: m/z called for C33H41N5O6 (M+1) = 604.7, found 604.3. Finally, a mixture of macrocycle (2R,3R)-β-OH-Phe-Oxazole-Val-N(Me)-D-Leu-D-Phe (18.0 mg, 0.298 µmol), NaH (1.50 mg, 0.596 µmol) and benzyl bromide(14.0 µL, 0.0119 mmol) in THF (1.19 mL) stirred at room temperature for 12 h under an atmosphere of argon following the “Benzylation procedure.” The crude reaction was purified by reverse phase-HPLC to yield the title compound 7 (1.3 mg, 0.6%); stereochemistry was assigned previously.1 Rf: 0.37 (65% EtOAc/Hex); >90% pure by HPLC. 1H NMR (600 MHz, CD3OD): δ 0.79 (3 H, d, J 6.7 Hs, CHCH3), 0.82 (3 H, d, J 6.4 Hz, CHCH3), 1.04 (3 H, d, J 6.8 Hz, CHCH3), 1.37 (3 H, d, J 7.3 Hz, CHCH3), 1.49 (1 H, m, CH(CH3)2), 1.93 (2 H, m, CH2CH), 2.92 (1 H, m, CH(CH3)2), 3.18 (1 H, m, CHaHbPh), 3.32 (3 H, s, NCH3), 3.42 (1 H, m, CHaHbPh), 3.74 (2 H, m, OCH2Ph), 4.69 (1 H, d, J 5.6 Hz, OCH), 4.90 (1 H, m, αCH), 5.04 (1 H, m, αCH), 6.64 (1 H, m, αCH), 6.81 (1 H, m, αCH), 7.12–7.20 (5 H, m, CH2Ph), 7.23–7.31 (10 H, m, 2Ph), 7.43 (1 H, d, J 8.8 Hz, NH), 7.71 (1 H, d, J 8.8 Hz, 1H, NH), 8.41 (1 H, s, CCHO), 8.70 (1 H, d, J 10.3 Hz, NH). LCMS: m/z called for C40H47N5O6 (M+1) = 694.8, found 694.0; HRMS (ESI-TOF): MH+, found 694.3570, C40H47N5O6 requires 694.3599.
6.27. Synthesis of Compound 8 (D-Ox-I)
6.27.1. Dipeptide NH(Me)-Val-Leu-O-Resin
Dipeptide Fmoc-N(Me)-Val-Leu-O-Resin was synthesized following the “General solid-phase peptide synthesis” procedure. Using 1.01 g (0.815 mmol) of NH2-Leu-O-Resin, the residue N(Me)-Val residue was incorporated using 864 mg of Fmoc-N(Me)-Val-OH (2.44 mmol), 374 mg (2.44 mmol, 3 equiv) of HOBt, and 0.756 mL (4.88 mmol) of DIC. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained to leave the amine-protected resin-bound dipeptide. Deprotection of amine was performed following the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal and gave the title compound.
6.27.2. Tripeptide NH2-D-Leu-N(Me)-Val-Leu-O-Resin
Tripeptide Fmoc-D-Leu-N(Me)-Val-Leu-O-resin was synthesized following the “General solid-phase peptide synthesis” procedure. Using the NH(Me)-Val-Leu-O-Resin prepared above, the D-Leu residue was incorporated using 864 mg (2.44 mmol) of Fmoc-D-Leu-OH, 374 mg (2.44 mmol) of HOAt, and 0.756 mL (4.88 mmol) of DIC. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained to leave the amine-protected resin-bound tripeptide. Deprotection of amine was performed following the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal and gave the title compound.
6.27.3. Tetrapeptide NH2-D-Phe-D-Leu-N(Me)-Val-Leu-O-Resin
Tetrapeptide Fmoc-D-Phe-D-Leu-N(Me)-Val-Leu-O-Resin was synthesized following the “General solid-phase peptide synthesis” procedure. Using the NH2-D-Leu-N(Me)-Val-Leu-O-Resin prepared above, the D-Phe residue was incorporated using 947 mg (2.44 mmol) of Fmoc-D-Phe-OH, 374 mg (2.44 mmol) of HOBt, and 0.756 mL of DIC (4.88 mmol). Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained to leave the amine-protected resin-bound tetrapeptide. Deprotection of amine was performed following the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal and gave the title compound.
6.27.4. Pentapeptide NH2-β-OH(2R,3R)(2S,3S)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu-O-Resin
Pentapeptide Fmoc-β-OH(2R,3R)(2S,3S)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu-O-Resin was synthesized following the “General solid-phase peptide synthesis” procedure. Using the NH2-D-Phe-D-Leu-N(Me)-Val-Leu-O-Resin prepared above, the β-OH(2R,3R)(2S,3S)-Phe residue was incorporated using 657 mg (1.63 mmol) of Fmoc-β-OH(2R,3R)(2S,3S)-Phe-OH, 374 mg (2.44 mmol) of HOBt, 0.756 mL of DIC (4.88 mmol). Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was then drained to leave the amine-protected resin-bound pentapeptide. Deprotection of amine was performed following the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal and gave the title compound.
6.27.5. Double Deprotected Pentapeptide NH2-β-OH(2R,3R)(2S,3S)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu-OH
Double Deprotected Pentapeptide NH2-β-OH(2R,3R)(2S,3S)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu-OH was synthesized following the “Cleavage of linear peptide from solid support” procedure. Utilizing the 1.32 g of dried NH2-β-OH(2R,3R)(2S,3S)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu-O-Resin, 6.59 mL of 2,2,2-trifluoroethanol and 6.59 mL of Methylene Chloride. The resin slurry was stirred for 24 h, after which it was filtered, washed with additional methylene chloride, and dried in vacuo for 24 h. (242 mg, 45% yield).
6.27.6. Macrocycle β-OH(2R,3R)(2S,3S)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu (8)
Macrocycle β-OH(2R,3R)(2S,3S)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu was synthesized following the “Macrocyclization procedure”. Utilizing 242 mg (0.363 mmol) of linear pentapeptide, 0.580 mL (3.63 mmol) of DIPEA, 109 mg (0.338 mmol) of TBTU, 128 mg (0.338 mmol) HATU, and 58.0 mg (0.193 mmol) of DEPBT. The crude reaction was purified by reverse phase-HPLC to yield the macrocycle (69.0 mg, 29% yield). 1H NMR (600 MHz, CD3OD): δ 0.84 (3 H, d, J 6.7 Hz, CHCH3), 0.85 (12 H, dd, J 8.4, 6.7 Hz CHCH3), 0.93 (3 H, d, J 6.7 Hz, CHCH3), 1.42–1.51 (2 H, m, CH2CH(CH3)2), 1.74 (2 H, m, CHCH2CH), 1.81 (2 H, m, CHCH2CH), 2.05 (1 H, m, CHCH(CH3)2), 2.83 (3H, s, NCH3), 3.02 (1 H, m, CHCH2C), 3.11 (1 H, m, CHCH2C), 3.85 (1 H, m, αCH), 4.08 (1 H, m, αCH), 4.15 (1 H, m, αCH), 4.56 (1 H, m, 1 H, m, αCH), 4.65 (1 H, m, αCH), 4.91 (1 H, d, J 4.6 Hz, HOCHCH), 7.01–7.45 (10H, m, Ph). LCMS: m/z called for C36H51N5O6 (M+1) = 649.82, found 650.1
6.27.7. Macrocycle Phe-Oxazole-D-Phe-D-Leu-N(Me)-Val-Leu (8)
Macrocycle Oxazole-D-Phe-D-Leu-N(Me)-Val-Leu was synthesized following the “General oxazole synthesis” procedure. The oxazoline intermediate was synthesized utilizing 42.7 mg (66.5 µmol) of Macrocycle β-OH(2R,3R)-Phe-D-Phe-D-Leu-N(Me)-Val-Leu, 11.2 µL (72.0 µmol) of DAST, 11.3 µL (0.131 mmol) of Pyridine in 0.660 mL of Methylene Chloride. The resulting oxazoline was oxidized utilizing 20.1 µL (0.130 mmol) of DBU, 0.130 mL (0.130 mmol) of CBrCl3 in 0.331 mL of methylene chloride. The crude reaction was purified by HPLC to yield the desired Phe-Oxazole-D-Phe-D-Leu-N(Me)-Val-Leu macrocycle (1.30 mg, 3.1% yield over 2 steps). 1H NMR (600 MHz, CDCl3): δ 0.64 (3 H, d, J 7.3 Hz CHCH3), 0.79 (3 H, d, J 6.7 Hz CHCH3), 0.82 (3 H, d, J 7.3 Hz CHCH3), 0.88 (3 H, d, J 4.9 Hz CHCH3), 0.91 (3 H, d, J 6.7 Hz CHCH3), 0.92 (3 H, d, J CHCH3), 1.41–1.50 (2 H, m, CHCH2CH), 1.75 (2 H, m, CHCH2CH), 1.86 (1 H, m, CHCH(CH3)2), 2.40 (1 H, m, CHCH(CH3)2), 2.53 (1 H, m, CHCH(CH3)2), 3.04 (1 H, m, CHCH2C), 3.69 (3 H, s, NCH3), 4.09 (1 H, m, CHCH2C), 4.15 (1 H, m, αCH), 4.22 (1 H, m, αCH), 4.35 (1 H, m, αCH), 5.31 (1 H, m, αCH), 6.78 (1 H, d, J 8.8 Hz, NH), 6.83 (1 H, m, NH), 6.95 (1 H, d, J 8.8 Hz, NH) 7.01–7.38 (10 H, m, Ph) LCMS: m/z called for C36H47N5O5 (M+1) = 629.79, found 630.7; HRMS (ESI-TOF): MH+, found 630.3646, C36H47N5O5 requires 630.3650. >75% pure by HPLC.
6.28. Synthesis of Compound 9 (A-Th-III)
6.28.1. HO-Pyruvic-Ketal-Br (47)
HO-Pyruvic-Ketal-Br was synthesized following the “General bromoketal acid formation” procedure. Utilizing Bromo Pyruvic Acid 806 mg (4.86 mmol) and 0.120 mL (0.122 mmol) of sulfuric acid in 1.60 mL (14.6 mmol) of trimethyl orthoformate. This title compound 47 was taken on to the next reaction without further purification or characterization. (700 mg, 67 % yield).
6.28.2. Dipeptide MeO-Phe-Leu-NHBoc
Dipeptide MeO-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 700 mg (3.90 mmol) of amine MeO-Phe-NH2 (45), 886 mg (3.55 mmol) of acid HO-Leu-NHBoc (44), 2.50 mL (14.2 mmol) of DIPEA, 1.26 g (3.90 mmol) of TBTU, in 35.5 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (1.44 g, 99% yield). Rf: 0.3 (EtOAc: Hex 1:3); 1H NMR (400 MHz, CDCl3): δ 0.78–0.84 (6 H, m, CH(CH3)2), 1.30–1.35 (9 H, s, C(CH3)3), 1.35–1.40 (1 H, m, CH(CH3)2), 1.45–1.60 (2 H, m, CHCH2CH), 2.90–3.05 (2 H, m, CHCH2CH), 3.58–3.60 (3 H, d, J 1.8 Hz, OCH3), 4.00–4.10 (1 H, br, αCH), 4.70–4.78 (1 H, m, αCH), 5.25–5.35 (1 H, br, NH), 6.86–6.96 (1 H, m, NH), 7.00–7.20 (5 H, m, Ph).
6.28.3. Dipeptide MeO-Phe-Leu-NH2 (46)
Dipeptide MeO-Phe-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (1.09 g, 100% yield).
6.28.4. Tripeptide MeO-Phe-Leu-Ketal-Br
Tripeptide MeO-Phe-Leu-Ketal-Br was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 1.09 g (3.52 mmol) of amine MeO-Phe-Leu-NH2 (46), 688 mg (3.20 mmol) of acid HO-Pyruvic-Ketal-Br (47), 2.46 mL (12.8 mmol) of DIPEA, 1.13 g (3.52 mmol) of TBTU, in 35.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the tripeptide (1.33 g, 82% yield). Rf: 0.55 (EtOAc: Hex 1:1).
6.28.5. Tripeptide MeO-Phe-Leu-Ketone-Br (48)
Tripeptide MeO-D-Phe-Leu-Ketone-Br was synthesized following the “General ketone deprotection” procedure. Utilizing 507 mg (1.00 mmol) of MeO-Phe-Leu-Ketal-Br in 26.0 mL of formic acid. The reaction mixture was refluxed. This tripeptide was taken on to the next reaction without further purification (405 mg, 92% yield). Rf: 0.56 (EtOAc: Hex 1:1); 1H NMR (200 MHz, CDCl3): δ 0.88–0.96 (6 H, m, CH(CH3)2), 1.55–1.65 (2 H, m, CHCH2CH), 1.55–1.76 (1 H, m, CH(CH3)2), 3.05–3.20 (2 H, dq, J 86.5, 14.2, 5.5 Hz, CH2Phe), 3.78 (3 H, s, OCH3), 4.35–4.45 (1 H, m, αCH), 4.39–4.50 (2 H, m, CH2Br), 4.82–4.90 (1 H, m, αCHα), 6.32–6.38 (1 H, m, NH), 7.04–7.10 (2 H, m, Ph), 7.20–7.30 (3 H, m, Ph).
6.28.6. Monomer Amide-Leu-NHBoc
Amide-Leu-NHBoc was synthesized following the “General Amide Formation” procedure. Utilizing 471 mg (1.79 mmol, 1.0 equiv.) of MeO-Leu-NHBoc (49) in 36.0 mL of Ammonium Hydroxide and 36.0 mL of Methanol. The title compound was taken on to the next reaction without further purification (444 mg, 100% yield). Rf: 0.05 (EtOAc: Hex 1:1).
6.28.7. Monomer Thioamide-Leu-NHBoc
Thioamide-Leu-NHBoc was synthesized following the “General thioamide formation” procedure. Utilizing 444 mg (1.79 mmol) of Amide-Leu-NHBoc and 724 mg (1.79 mmol) of Lawesson’s Reagent in 12.0 mL of dimethoxy ethane. This thioamide was purified by column chromatography (silica gel, EtOAc/DCM) to yield the Thioamide-Leu-NHBoc. (322 mg, 68 % yield). Rf: 0.74 (EtOAc: Hex 1:1)
6.28.8. Monomer Thioamide-Leu-NH2 (50)
Thioamide-Leu-NH2 was synthesized following the “General solution-phase amine deprotection”. This Thioamide-Leu-NH2 was taken on to the next reaction without further purification or characterization. (200 mg, 100% yield).
6.28.9. Dipeptide Thioamide-Leu-Leu-NHBoc (52)
Dipeptide Thioamide-Leu-Leu-NBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 199 mg (1.22 mmol) of amine Thioamide-Leu-NH2, 274 mg (1.10 mmol) of acid HO-Leu-NHBoc, 0.801 mL (4.40 mmol) of DIPEA, 391 mg (1.22 mmol) of TBTU, in 11.0 mL methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (265 mg, 61% yield). Rf: 0.6 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.90–0.95 (6 H, d, J 3.5 Hz, CH(CH3)2), 0.95–1.00 (6 H, d, J 3.5 Hz, CH(CH3)2), 1.46 (9H, s, C(CH3)3), 1.62–1.76 (4 H, m, CHCH2CH), 1.76–1.92 (2 H, m, CH(CH3)2), 4.02–4.12 (1 H, m, αCH), 4.75–4.83 (1 H, m, αCH), 4.90–4.98 (1 H, d, J 13.3 Hz, NH), 6.70–6.78 (1 H, d, J 13.3 Hz, NH), 7.48–7.56 (1 H, br, CSNH), 8.04–8.12 (1 H, br, CSNH).
6.28.10. Pentapeptide MeO-Phe-Leu-Thiazole-Leu-Leu-NHBoc (53)
Pentapeptide MeO-Phe-Leu-Thiazole-Leu-Leu-NHBoc was synthesized following the “General thiazole synthesis” procedure. 265 mg (0.670 mmol) of Thioamide-Leu-Leu-NHBoc (52) and 537 mg (5.36 mmol) of Potassium Bicarbonate were dissolved in 6.00 mL dimethoxy ethane. 413 mg (0.940 mmol) of MeO-Phe-Leu-Ketone-Br (48) was dissolved in 7.50 mL dimethoxy ethane and added dropwise to the thioamide mixture to yield thiazoline intermediate. Thiazoline was dehydrated using 0.400 mL (2.68 mmol) of trifluoroacetic acid, 0.490 mL (6.03 mmol) of pyridine, 0.190 mL (1.34 mmol) triethylamine in 13.5 mL dimethoxy ethane. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (71.0 mg, 15% yield). Rf: 0.37 (EtOAc: Hex 2:3); 1H NMR (400 MHz, CDCl3): δ 0.86–1.02 (18 H, m, CH(CH3)2), 1.46 (9 H, s, C(CH3)3), 1.60–1.82 (8 H, bur m, 3CHCH2CH & 2CH(CH3)2), 1.88–2.00 (1 H, m, CH(CH3)2), 3.02–3.18 (2 H, m, CH2Phe), 3.74 (3 H, s, OCH3), 4.05–4.15 (1 H, m, αCH), 4.55–4.62 (1 H, m, αCH), 4.80–4.88 (1 H, m, αCH), 5.30–5.38 (1 H, m, αCH), 6.66–6.70 (1 H, d, J 13.3 Hz, NH), 6.74–6.82 (1 H, br, NH), 7.05–7.32 (5 H, m, Ph), 7.45–7.52 (1 H, d, J 13.3 Hz, NH), 8.00 (1 H, s, SCH=C). LCMS: m/z calcd for C36H55N5O7S (M+23) = 724.92, found 724.3
6.28.11. Macrocycle Phe-Leu-Thiazole-Leu-Leu (9)
Macrocycle Phe-Leu-Thiazole-Leu-Leu (9) was synthesized following the “Macrocyclization procedure”. Utilizing 40.0 mg (70.1 µmol) of linear pentapeptide (53), 0.070 µL (0.420 mmol) of DIPEA, 15.5 mg (50.4 µmol, 0.7 equiv) of TBTU, 18.2 mg (50.4 µmol) HATU, and 15.0 mg (50.2 µmol) of DEPBT in 97.6 mL methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) and reverse phase-HPLC to yield the macrocycle (2.30 mg, 6% yield). Rf: 0.1 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CD3OD): δ 0.84–1.05 (18 H, m, CH(CH3)2), 1.26–1.42 (9 H, m, C(CH3)3), 1.46–1.85 (8 H, m, 3CHCH2CH & 2CH(CH3)2), 1.95–2.05 (1 H, m, CH(CH3)2), 3.02–3.18 (2 H, m, CHCH2CH), 4.38–4.45 (1 H, m, αCH), 4.52–4.55 (1 H, m, αCH), 4.80–4.88 (1 H, m, αCH), 5.23–5.27 (1 H, m, αCH), 7.05–7.32 (5 H, m, Ph), 8.03 (1 H, s, SCH=C). LCMS: m/z calcd for C30H43N5O4S (M+1) = 570.76, found 570.30; HRMS (ESI-TOF): MH+, found 570.3102, requires 570.3108. >95% pure by HPLC.
6.29. Synthesis of Compound 10 (B-Th-III)
6.29.1. Dipeptide MeO-Leu-N(Me)-Phe-NHBoc
Dipeptide MeO-Leu-N(Me)-Phe-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 325 mg (2.04 mmol) of amine MeO-Leu-N(Me)H (58), 494 mg (1.86 mmol) of acid HO-Phe-NHBoc (59), 1.30 mL (7.44 mmol) of DIPEA, 655 mg (2.04 mmol) of TBTU, in 18.6 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (711 mg, 94% yield). Rf: 0.79 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.88–0.92 (6 H, m, CH(CH3)2), 1.22–1.25 (1 H, m, CH(CH3)2), 1.34–1.50 (9 H, s, C(CH3)3), 1.58–1.74 (3 H, m, CHCH2CH), 2.79–2.82 (3 H, s, NCH3), 2.94–3.14 (2 H, m, CH2Phe), 3.65–3.72 (3 H, d, J 2.5 Hz, OCH3), 4.55–4.62 (1 H, br, NH), 4.80–4.88 (1 H, m, αCH), 4.94–5.00 (1 H, br, NH), 5.25–5.32 (1 H, m, αCH), 7.10–7.33 (5 H, m, Ph).
6.29.2. Dipeptide MeO-Leu-N(Me)-Phe-NH2 (60)
Dipeptide MeO-Leu-N(Me)-Phe-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (536 mg, 100% yield).
6.29.3. Tripeptide MeO-Leu-N(Me)-Phe-Leu-NHBoc
Tripeptide MeO-Leu-N(Me)-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 536 mg (1.75 mmol) of amine MeO-Leu-N(Me)-Phe-NH2 (60), 400 mg (1.60 mmol) of acid HO-Leu-NHBoc (61), 1.10 mL (6.40 mmol) of DIPEA, 562 mg (1.75 mmol) of TBTU, in 16.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the tripeptide (683 mg, 79% yield). Rf: 0.22 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.84–0.98 (12 H, m, 2CH(CH3)2), 1.34–1.42 (2 H, m, 2CH(CH3)2), 1.42–1.50 (9 H, s, C(CH3)3), 1.54–1.75 (4 H, m, 2CHCH2CH), 2.79–2.82 (3 H, s, NCH3), 2.94–3.20 (2 H, m, CH2Phe), 3.65–3.72 (3 H, s, OCH3), 4.05–4.15 (1 H, br, NH), 4.76–4.84 (1 H, br, NH), 4.84–4.88 (1 H, m, αCH), 5.10–5.20 (1 H, m, αCH), 5.25–5.32 (1 H, m, αCH), 7.10–7.33 (5 H, m, Ph).
6.29.4. Tripeptide HO-Leu-N(Me)-Phe-Leu-NHBoc (62)
Tripeptide HO-Leu-N(Me)-Phe-Leu-NHBoc was synthesized following the “General solution-phase acid deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization. (665 mg, 100% yield).
6.29.5. Monomer Amide-Leu-NHBoc
Amide-Leu-NHBoc was synthesized following the “General amide formation” procedure. Utilizing 463 mg (1.76 mmol) of MeO-Leu-NHBoc (54) in 35.0 mL of ammonium hydroxide and 35.0 mL of methanol. This amide was taken on to the next reaction without further purification (437 mg, 100 % yield). Rf: 0.05 (EtOAc: Hex 1:1).
6.29.6. Monomer Thioamide-Leu-NHBoc (55)
Thioamide-Leu-NHBoc was synthesized following the “General thioamide formation” procedure. Utilizing 437 mg (1.76 mmol) of Amide-Leu-NHBoc and 712 mg (1.76 mmol) of Lawesson’s Reagent in 12.0 mL of dimethoxy ethane. The title compound 55 was purified by column chromatography (silica gel, EtOAc/DCM) to yield the Thioamide-Leu-NHBoc. (324 mg, 70 % yield). Rf: 0.74 (EtOAc: Hex 1:1).
6.29.7. Dipeptide EtO-Thiazole-Leu-NHBoc
Dipeptide EtO-Thiazole-Leu-NHBoc was synthesized following the “General thiazole synthesis” procedure. 324 mg (1.23 mmol) of Thioamide-Leu-NHBoc (55) and 981 mg (9.80 mmol) of potassium bicarbonate were dissolved in 24.6 mL dimethoxy ethane. 0.310 mL (2.46 mmol) of ethyl-Br-pyruvate (56) was added dropwise to the thioamide mixture to yield thiazoline intermediate. Thiazoline was dehydrated using 0.680 mL (4.92 mmol) of trifluoroacetic acid, 0.890 mL (11.1 mmol) of pyridine, 0.340 mL (2.46 mmol) triethyl amine in 24.6 mL dimethoxy ethane. The crude reaction was purified by column chromatography (silica gel, EtOAc/DCM) to yield the pentapeptide (198 mg, 47% yield). Rf: 0.47 (EtOAc: Hex 1:3); 1H NMR (400 MHz, CDCl3): δ 0.88–0.90 (3 H, d, J 4.3 Hz, CHCH3), 0.90–0.92 (3 H, d, J 4.3 Hz, CHCH3), 1.29–1.35 (3 H, t, OCH2CH3), 1.34–1.39 (9 H, s, C(CH3)3), 1.60–1.74 (1 H, m, CH(CH3)2), 1.77–1.98 (2 H, m, CH2CH(CH3)2), 4.29–4.39 (2 H, q, OCH2CH3), 4.85–5.14 (1 H, br, CONHCH), 7.98–8.00 (1H, s, SCH=C).
6.29.8. Dipeptide EtO-Thiazole-Leu-NH2 (57)
Dipeptide EtO-Thiazole-Leu-NH2 was synthesized following the “General amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (140 mg, 100% yield).
6.29.9. Pentapeptide EtO-Thiazole-Leu-Leu-N(Me)-Phe-Leu-NHBoc (63)
Pentapeptide EtO-Thiazole-Leu-Leu-N(Me)-Phe-Leu-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 140 mg (0.580 mmol) of amine EtO-Thiazole-Leu-NH2 (57), 276 mg (0.530 mmol) of acid HO-Leu-N(Me)-Phe-Leu-NHBoc (62), 0.370 mL (2.12 mmol) of DIPEA, 186 mg (0.580 mmol) of TBTU, in 5.30 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (317 mg, 82% yield). Rf: 0.63 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.74–1.00 (18 H, m, CH(CH3)2), 1.24–1.30 (3 H, t, J 4.2 Hz, OCH2CH3), 1.35–1.44 (13 H, m, C(CH3)3, 2CHCH2CH), 1.46–1.66 (2 H, m, CHCH2CH), 1.66–1.88 (2 H, m, CH(CH3)2), 1.90–2.04 (1 H, m, CH(CH3)2), 2.64–2.90 (3 H, m, NCH3), 2.90–3.23 (2 H, m, CH2Phe), 4.04–4.15 (1 H, br, NH), 4.09–4.15 (2 H, m, OCH2CH3), 4.30–4.44 (2 H, m, αCH, NH), 4.60–4.70 (1 H, br, NH), 4.85–5.01 (1 H, m, αCH), 5.01–5.21 (1 H, m, αCH), 5.25–5.40 (1 H, br, αCH), 7.08–7.30 (5 H, m, Ph), 7.95–8.05 (1 H, m, SCH=C). LCMS: m/z calcd for C38H59N5O7S (M+1) = 730.41, found 730.10
6.29.10. Macrocycle Phe-Leu-Thiazole-Leu-Leu-N(Me) (10)
Macrocycle Phe-Leu-Thiazole-Leu-Leu-N(Me) (10) was synthesized following the “Macrocyclization procedure”. Utilizing 170 mg (0.280 mmol) of linear pentapeptide (63), 0.290 mL (1.68 mmol) of DIPEA, 64.2 mg (50.2 µmol) of TBTU, 76.0 mg (50.5 µmol) HATU, and 60.0 mg (50.2 µmol) of DEPBT in 300 mL methylene chloride and 100 mL acetonitrile. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) and reverse phase-HPLC to yield the macrocycle (6.50 mg, 4% yield). Rf: 0.35 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.81–0.84 (3 H, d, J 3.5 Hz, CH(CH3)2), 0.86–0.89 (3 H, d, J 3.5 Hz, CH(CH3)2), 0.98–1.08 (12 H, m, CH(CH3)2), 1.25–1.36 (2 H, m, CH(CH3)2), 1.58–1.80 (6 H, m, 3CHCH2CH), 2.10–2.30 (1 H, m, CH(CH3)2), 2.53–2.63 (3 H, m, NCH3), 3.03–3.18 (2 H, m, CH2Phe), 4.65–4.79 (1 H, m, αCH), 4.90–4.98 (1 H, m, αCH), 5.10–5.20 (1 H, m, αCH), 5.23–5.32 (1H, m, αCH), 6.42–6.50 (1 H, m, NH), 6.92–7.00 (1 H, m, NH), 7.20–7.33 (5 H, m, Ph), 7.42–7.50 (1 H, m, NH), 8.15 (1 H, s, SCH=C). LCMS: m/z calcd for C31H45N5O4S (M) = 584.3265, found 583.80; HRMS (ESI-TOF): MH+, found 584.3253, C31H45N5O4S requires 610.4075. >95% pure by HPLC.
6.30. Synthesis of Compound 11 (C-Th-III)
6.30.1. Dipeptide MeO-Lys-Cbz-Phe-NHBoc
Dipeptide MeO-Lys(Cbz)-Phe-NHBoc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 392 mg (1.30 mmol) of amine MeO-Lys(Cbz)-NH2, 379 mg (1.43 mmol) of acid HO-Phe-NHBoc, 1.80 mL (10.4 mmol) of DIPEA, 334 mg (1.04 mmol) of TBTU, 297 mg (0.780 mmol) of HATU, in 13.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the dipeptide (578 mg, 80% yield). Rf: 0.37 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 1.11–1.49 (2 H, m, γCH2), 1.30–1.36 (9 H, s, C(CH3)3), 1.36–1.64 (2 H, m, δCH2), 1.51–1.81 (2 H, m, βCH2), 2.93–3.03 (2 H, m, εCH2), 3.03–3.16 (2 H, m, CH2Phe), 3.60–3.65 (3 H, s, OCH3), 4.21–4.35 (1 H, m, αCHα), 4.42–4.52 (1 H, m, αCH), 4.85–4.93 (1 H, br, NH), 5.03 (2 H, s, CH2Ph), 6.39–6.47 (1 H, br, NH), 7.08–7.32 (10H, m, Ph).
6.30.2. Dipeptide MeO-Lys(Cbz)-Phe-NH2
Dipeptide MeO-Lys(Cbz)-Phe-NH2 was synthesized following the “General solution-phase amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (459 mg, 100% yield).
6.30.3. Tripeptide MeO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc
Tripeptide MeO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 459 mg (1.04 mmol) of amine MeO-Lys(Cbz)-Phe-NH2, 320 mg (1.14 mmol) of acid HO-D-Phe-N(Me)Boc, 1.50 mL (8.32 mmol) of DIPEA, 267 mg (0.830 mmol) of TBTU, 198 mg (0.520 mmol) of HATU, in 10.4 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the tripeptide (731 mg, 100% yield). Rf: 0.38 (EtOAc: Hex 7:13); 1H NMR (400 MHz, CDCl3): δ 1.10–1.22 (2 H, m, γ CH2), 1.22–1.30 (9 H, s, C(CH3)3), 1.35–1.48 (2 H, m, δCH2), 1.51–1.83 (2 H, m, βCH2), 2.51–2.73 (3H, s, NCH3), 2.82–3.02 (2 H, m, CH2Phe), 3.02–3.15 (2 H, m, εCH2), 3.16–3.34 (2 H, m, CH2Phe), 3.59–3.70 (3 H, s, OCH3), 4.34–4.46 (1 H, br, αCH), 4.47–4.68 (2 H, m, 2αCH), 4.98–5.08 (2 H, s, CH2Ph), 6.22–6.61 (2 H, br-m, NH), 6.98–7.34 (15H, m, Ph).
6.30.4. Tripeptide HO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc
Tripeptide HO-Lys(Cbz)-Phe-D-Phe-N(Me)Boc was synthesized following the “General solution-phase acid deprotection”. This tripeptide was taken on to the next reaction without further purification or characterization. (716 mg, 100% yield).
6.30.5. Monomer Amide-Leu-NHBoc
Amide-Leu-NHBoc was synthesized following the “General amide formation” procedure. Utilizing 353 mg (1.34 mmol) of MeO-Leu-NHBoc in 27.0 mL of ammonium hydroxide and 27.0 mL of methanol. This amide was taken on to the next reaction without further purification (333 mg, 100% yield). Rf: 0.05 (EtOAc: Hex 1:1)
6.30.6. Monomer Thioamide-Leu-NHBoc
Thioamide-Leu-NHBoc was synthesized following the “General thioamide formation” procedure. Utilizing 333 mg (1.34 mmol) of Amide-Leu-NHBoc and 542 mg (1.34 mmol) of Lawesson’s Reagent in 9.00 mL of dimethoxy ethane. This thioamide was purified by column chromatography (silica gel, EtOAc/DCM) to yield the Thioamide-Leu-NHBoc. (240 mg, 68 % yield). Rf: 0.74 (EtOAc: Hex 1:1)
6.30.7. Dipeptide EtO-Thiazole-Leu-NHBoc
Dipeptide EtO-Thiazole-Leu-NHBoc was synthesized following the “General thiazole synthesis” procedure. 240 mg (0.910 mmol) of Thioamide-Leu-NHBoc and 729 mg (7.30 mmol) of potassium bicarbonate were dissolved in 18.2 mL dimethoxy ethane. 0.230 mL (2 equiv.) of ethyl-Br-pyruvate was added dropwise to the thioamide mixture to yield thiazoline intermediate. Thiazoline was dehydrated using 0.510 mL (3.64 mmol) of trifluoroacetic acid, 0.660 mL (8.19 mmol) of pyridine, 0.250 mL (1.82 mmol) triethyl amine in 18.2 mL dimethoxy ethane. The crude reaction was purified by column chromatography (silica gel, EtOAc/DCM) to yield the pentapeptide (404 mg, 77% yield). Rf: 0.81 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.88–0.90 (3 H, d, J 4.3 Hz, CHCH3), 0.90–0.92 (3 H, d, J 4.3 Hz, CHCH3), 1.29–1.35 (3 H, t, J 4.3 Hz, OCH2CH3), 1.36 (9 H, s, C(CH3)3), 1.60–1.74 (1 H, m, CH(CH3)2), 1.77–1.98 (2 H, m, CH2CH(CH3)2), 4.29–4.39 (2 H, q, J 2.1 Hz, OCH2CH3), 4.85–5.14 (1 H, br, CONHCH), 7.99 (1H, s, SCH=C).
6.30.8. Dipeptide EtO-Thiazole-Leu-NH2
Dipeptide EtO-Thiazole-Leu-NH2 was synthesized following the “General amine deprotection”. This dipeptide was taken on to the next reaction without further purification or characterization. (287 mg, 100% yield).
6.30.9. Pentapeptide EtO-Thiazole-Leu-Lys(Cbz)-Phe-D-Phe-N(Me)Boc
Pentapeptide EtO-Thiazole-Leu-Lys(Cbz)-Phe-D-Phe-N(Me)Boc was synthesized following the “General solution-phase peptide synthesis” procedure. Utilizing 266 mg (1.10 mmol) of amine EtO-Thiazole-Leu-NH2, 688 mg (1.00 mmol) of acid HO-Lys(Cbz)Phe-D-Phe-N(Me)Boc, 1.05 mL (6 mmol) of DIPEA, 257 mg (0.802 mmol) of TBTU, 190 mg (0.501 mmol) of HATU in 11.0 mL of methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) to yield the pentapeptide (497 mg, 49.5% yield). Rf: 0.16 (EtOAc: Hex 1:1); 1H NMR (400 MHz, CDCl3): δ 0.79–0.91 (6 H, m, CH(CH3)2), 1.09–1.30 (12 H, m, C(CH3)3 & OCH2CH3), 1.34–1.60 (2 H, m, γCH2), 1.66–1.87 (6 H, m, 2βCH2 & δCH2), 1.88–2.01 (1 H, m, CH(CH3)2), 2.26–2.44 (3H, m, NCH3), 2.86–3.04 (2 H, m, εCH2), 3.04–3.19 (3 H, m, CH2Phe & CHaHbPhe), 3.19–3.33 (1 H, m, βCHaHb), 3.71–3.91 (2 H, m, OCH2CH3), 4.21–4.70 (2 H, m, 2αCH), 4.96–5.04 (2 H, m, CH2Ph), 5.06–5.23 (2 H, m, 2αCH), 6.29–6.79 (2 H, br-m, 2NH), 6.89–7.36 (15H, m, Ph), 8.02–8.13 (1H, m, SCH=C). LCMS: m/z calcd for C49H64N6O9S (M) = 913.13, found 913.00
6.30.10. Macrocycle Phe-D-Phe-N(Me)-Thiazole-Leu-Lys(Cbz) (11)
Macrocycle Phe-D-Phe-N(Me)-Thiazole-Leu-Lys(Cbz) (11) was synthesized following the “Macrocyclization procedure”. Utilizing 418 mg (0.530 mmol) of linear pentapeptide, 0.750 mL (4.24 mmol) of DIPEA, 138 mg (0.430 mmol) of TBTU, 164 mg (0.430 mmol) HATU, and 129 mg (0.430 mmol) of DEPBT in 533 mL methylene chloride. The crude reaction was purified by column chromatography (silica gel, EtOAc/Hex) and reverse phase-HPLC to yield the macrocycle (50.3 mg, 12.3 % yield). Rf: 0.53 (EtOAc:Hex 9:1); 1H NMR (400 MHz, CDCl3): δ 0.79–0.87 (3 H, d, J 11.4 Hz, CH(CH3)2), 0.87–0.92 (3 H, d, J 11.4 Hz, CH(CH3)2), 1.08–1.30 (4 H, m, γCH2 & δCH2), 1.34–1.68 (3 H, m, CHCH2CH & CHCHaHbCH), 1.83–2.00 (1 H, m, CHaHbCH), 2.21–2.36 (1 H, m, CH(CH3)2), 2.49–2.72 (3H, m, NCH3), 2.87–3.24 (2 H, m, CH2Phe), 3.00–3.12 (2 H, m, εCH2), 3.29–3.39 (1 H, d, J 9.75 Hz, CHaHbPhe), 3.50–3.64 (1 H, br, NH), 3.98–4.10 (1 H, m, αCH), 4.35–4.42 (1 H, d, J 9.75 Hz, CHaHbPhe), 4.76–4.87 (2 H, m, 2αCHα), 4.97–5.08 (2 H, m, CH2Ph), 5.24–5.34 (1 H, m, αCH), 6.66–6.85 (2 H, m, NH), 6.97–7.34 (15H, m, Ph), 7.52–7.64 (1 H, m, NH), 7.94–8.02 (1H, m, SCH=C). LCMS: m/z calcd for C42H50N6O6S (M) = 766.95, found 767; HRMS (ESI-TOF): MH+, found 767.3583, C42H50N6O6S requires 767.3585.>95% pure by HPLC.
6.31. Synthesis of Compound 12 (A-PP-II)
6.31.1. Fmoc-Val-Thr(ΨMe,Me-Pro)-O-Resin
A mixture of 2-chlorotrityl-chloride resin (100 mg, 0.100 mmol), dipeptide Fmoc-Val-Thr(ΨMe,Me-Pro)-OH (96.0 mg, 0.200 mmol) and DIPEA (3.00 mL) in anhydrous methylene chloride (0.400 M) was stirred under argon at room temperature for 12 h. The reaction mixture was filtered and the collected resin was washed with a solution of CH2Cl2:CH3OH:DIPEA (30.0 mL 17:4:1, v:v:v), followed by methylene chloride (20.0 mL), dimethylformamide (20.0 mL), and finally methylene chloride (30 mL). The resin was drained well and dried in vacuo overnight to give the resin-bound dipeptide. Resin loading was determined via RP-HPLC to be 0.250 mmol/g.
6.31.2. Dipeptide NH2-Val-Thr(ΨMe,Me-Pro)-O-Resin
Dipeptide NH2-Val-Thr(ΨMe,Me-Pro)-O-Resin was synthesized using Fmoc-Val-Thr(ΨMe,Me-Pro)-O-Resin synthesized above and followed the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal.
6.31.3. Tripeptide NH2-Leu-Val-Thr(ΨMe,Me-Pro)-O-Resin
Following the “General solid-phase peptide synthesis” procedure, a mixture of NH2-Val-Thr(ΨMe,Me-Pro)-O-Resin (0.250 mmol), Fmoc-Leu-OH (177 mg, 0.500 mmol), HBTU (190 mg, 0.500 mmol), and DIPEA (3.00 mL, 0.400 M) in DMF was stirred at room temperature for 2 h. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was drained and washed with DMF (30 mL) to give the resin-bound tripeptide. The Fmoc was then removed following the “General solid-phase amine deprotection” procedure to give the title tripeptide. A positive ninhydrin test served to verify Fmoc removal.
6.31.4. Tetrapeptide NH2-Leu-Leu-Val-Thr(ΨMe, Me-Pro)-O-Resin
Following the “General solid-phase peptide synthesis” procedure, a mixture of NH2-Leu-Val-Thr(ΨMe,Me-Pro)-O-Resin prepared above (0.250 mmol), Fmoc-Leu-OH (177 mg, 0.500 mmol), HBTU (190 mg, 0.500 mmol), and DIPEA (3 mL, 0.400M) in dimethylformamide was stirred at room temperature for 12 h. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was drained and washed with DMF (30 mL) to give the resin-bound tetrapeptide. The Fmoc was then removed following the “General solid-phase amine deprotection” procedure to give title tetrapeptide. A positive ninhydrin test served to verify Fmoc removal.
6.31.5. Pentapeptide NH2-Phe-Leu-Leu-Val-Thr(ΨMe,Me-Pro)-O-Resin
Following the “General solid-phase peptide synthesis” procedure, a mixture of NH2-Leu-Leu-Val-Thr(ΨMe,Me-Pro)-O-Resin prepared above (0.250 mmol), Fmoc-Phe-OH (194 mg, 0.500 mmol), HBTU (190 mg, 0.500 mmol), and DIPEA (3.00 mL, 0.400 M) in dimethylformamide was stirred at room temperature for 12 h. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was drained and washed with DMF (30 mL) to give the resin-bound pentapeptide. The Fmoc was then removed following the “General solid-phase amine deprotection” procedure to give the title pentapeptide. A positive ninhydrin test served to verify Fmoc removal. The resin was then dried in vacuo for 24 h in a vacuum desiccator.
6.31.6. Double Deprotected Pentapeptide NH2-Phe-Leu-Leu-Val-Thr(ΨMe,Me-Pro)-OH
Double Deprotected Pentapeptide NH2-Phe-Leu-Leu-Val-Thr(ΨMe,Me-Pro)-OH was synthesized following the “Cleavage of linear peptide from solid support” procedure. Utilizing the 250 mg of dried Resin-O-Thr(ΨMe,Me-Pro)-Val-Leu-Leu-Phe-NH2, 1 mL of hexafluoroisopropanol and 4 mL of methylene chloride. The resin slurry was stirred for 30 min, after which it was filtered, washed with additional methylene chloride, and dried in vacuo to give the double deprotected pentapeptide (150 mg, 95% yield).
6.31.7. Macrocycle Phe-Thr(ΨMe,Me-Pro)-Val-Leu-Leu (12)
Macrocycle Phe-Thr(ΨMe,Me-Pro)-Val-Leu-Leu was synthesized following the “Macrocyclization procedure”. Utilizing linear pentapeptide (59.0 mg, 90.0 µmol), DIPEA (80.0 µL), and DMTMM BF4− (87.0 mg, 0.270 mmol), the linear peptide and coupling reagent were dissolved in dimethylformamide (10 mL) and added dropwise via syringe pump (0.5 mL/hr) to a vigorously stirred solution of DIPEA and dimethylformamide (1 mM) under N2. The reaction mixture was stirred for 3 d, after which time the solvent was removed under reduced pressure and the crude reaction was purified by reverse phase-HPLC to yield the macrocycle 12 (14.5 mg, 26% yield). 1H NMR (CD3CN, 400 MHz): δ 0.80–1.03 (18 H, m, 3CH(CH3)2), 1.19 (3 H, d, J 5.6 Hz, CHCH3), 1.39–1.78 (12 H, m, CHaCHbCH3), 2.88 (1 H, m, CHMe2), 3.30–3.40 (2 H, m, CH2Ph), 3.67 (1 H, m, αCH), 3.85 (1 H, m, 1 αCH), 3.91–4.04 (2 H, m, 2αCH), 4.21–4.46 (2 H, m, 2 αCH), 6.59 (1 H, m, NH), 6.69 (1 H, m, NH), 6.87 (1 H, m, NH), 7.12–7.41 (5 h, m, Ph), 7.48 (1 H, d, J 8.0 Hz, NH). >95% pure by HPLC. HRMS (ESI-TOF): MH+, found 614.3887, C33H51N5O6 requires 614.3912.
6.32. Synthesis of Compound 13 (A-PP-III)
6.32.1. Fmoc-Leu-Thr(ΨMe,Me-Pro)-O-Resin
A mixture of 2-chlorotrityl-chloride resin (3.00 g, 4.74 mmol), dipeptide 64 Fmoc-Leu-Thr(ΨMe,Me-Pro)-OH (4.68 g, 9.48 mmol), N-methyl-2-pyrrolidone (14.04 mL or 3.00 mL per gram of dipeptide), and DIPEA (4.90 mL, 28.4 mmol) in anhydrous methylene chloride (46.8 mL) was stirred under argon at room temperature for 12 h. The reaction mixture was filtered and the collected resin was washed with a solution of CH2Cl2:CH3OH:DIPEA (30.0 mL 17:4:1, v:v:v), followed by methylene chloride (20.0 mL), dimethylformamide (20.0 mL), and finally methylene chloride (30 mL). The resin was drained well and dried in vacuo overnight to give the resin-bound dipeptide. Resin loading was determined via RP-HPLC to be 0.400 mmol/g.
6.32.2. Dipeptide NH2-Leu-Thr(ΨMe,Me-Pro)-O-Resin (65)
Title compound 65 was synthesized utilizing Fmoc-Leu-Thr(ΨMe,Me-Pro)-O-Resin from above and followed the “General solid-phase amine deprotection” procedure. A positive ninhydrin test served to verify Fmoc removal.
6.32.3. Tripeptide NH2-Leu-Leu-Thr(ΨMe,Me-Pro)-O-Resin (66)
Following the “General solid-phase peptide synthesis” procedure, a mixture of NH2-Leu-Thr(ΨMe,Me-Pro)-O-Resin 65 (1.00 g, 0.400 mmol), Fmoc-Leu-OH (420 mg, 1.20 mmol), HOBt (180 mg, 1.20 mmol), and DIPEA (0.370 mL, 2.40 mmol) in DMF was stirred at room temperature for 2 h. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was drained and washed with DMF (30 mL) to give the resin-bound tripeptide. The Fmoc was then removed following the General solid-phase amine deprotection” procedure to give title compound 66. A positive ninhydrin test served to verify Fmoc removal.
6.32.4. Tetrapeptide NH2-Phe-Leu-Leu-Thr(ΨMe, Me-Pro)-O-Resin (67)
Following the “General solid-phase peptide synthesis” procedure, a mixture of NH2-Leu-Leu-Thr(ΨMe,Me-Pro)-O-Resin 66 prepared above, Fmoc-Phe-OH (460 mg, 0.120 mmol), HOBt (180 mg, 1.20 mmol), and DIPEA (0.370 mL, 2.40mmol) in DMF was stirred at room temperature for 12 h. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was drained and washed with DMF (30 mL) to give the resin-bound tetrapeptide. The Fmoc was then removed following the General solid-phase amine deprotection” procedure to give title compound 67. A positive ninhydrin test served to verify Fmoc removal..
6.32.5. Pentapeptide NH2-Leu-Phe-Leu-Leu-Thr(ΨMe,Me-Pro)-O-Resin (68)
Following the “General solid-phase peptide synthesis” procedure, a mixture of NH2-Phe-Leu-Leu-Thr(ΨMe,Me-Pro)-O-Resin 67 prepared above, Fmoc-Leu-OH (420 mg, 1.20 mmol), HOBt (180 mg, 1.20 mmol), and DIPEA (0.370 mL, 2.40 mmol) in DMF was stirred at room temperature for 12 h. Completion of the coupling reaction was verified by a negative ninhydrin test. The reaction mixture was drained and washed with DMF (30 mL) to give the resin-bound pentapeptide. The Fmoc was then removed following the General solid-phase amine deprotection” procedure to give title compound 68. A positive ninhydrin test served to verify Fmoc removal. The resin was then dried in vacuo for 24 h in a vacuum desiccator.
6.32.6. Double Deprotected Pentapeptide NH2-Leu-Phe-Leu-Leu-Thr(ΨMe,Me-Pro)-OH
Dried 68 (206 mg) in a solution of 2,2,2-trifluoroethanol (3.00 mL) and methylene chloride (3.00 mL) was stirred at room temperature for 24 h, after which it was filtered, washed with additional methylene chloride, and the resulting eluent was dried in vacuo for 24 h to give resin-free double-deprotected linear pentapeptide NH2-Leu-Phe-Leu-Leu-Thr(ΨMe,Me-Pro)-OH (65.0 mg, 25%). LCMS: m/z called for C34H55N5O7 (M+1) = 646.4, found 646.3.
6.32.7. Macrocycle Leu-Phe-Leu-Leu-Thr(ΨMe,Me-Pro) (13)
Following the “Macrocyclization procedure,” a mixture of TBTU (23.0 mg, 0.0707 mmol), HATU (27.0 mg, 0.0707 mmol), DEPBT (18.0 mg, 0.0606 mmol) and DIPEA (0.140 mL, 0.808 mmol) were dissolved in 7.20 mL of anhydrous methylene chloride and acetonitrile (1:1, v:v) and stirred under argon at room temperature. Linear double-deprotected pentapeptide (65.0 mg, 0.101 mmol) was dissolved in 7.20 mL of anhydrous methylene chloride and acetonitrile (1:1, v:v) under argon and placed in a 10.0 mL syringe with 15.96 mm diameter and injected into the mixture of coupling reagents and allowed to run at room temperature for 4 h. The reaction was diluted in methylene chloride and quenched with saturated ammonium chloride (100 mL). The organic was then washed with brine (200 mL), dried over sodium sulfate, and the solvent evaporated in vacuo to give the crude product which was then purified by flash chromatography (75% ethyl acetate/hexanes) and reverse-phase HPLC to yield macrocycle 13 (1.01 mg, 1.60% yield). Rf: 0.48 (EtOAc:Hex 3:1) 1H NMR (400 MHz, CD3OD): δ 0.83–1.00 (18 H, m, CH2CH(CH3)2), 1.44–1.46 (3 H, d, J 7.3 Hz, CHCH3), 1.56 (6 H, s, CH3), 1.56–1.78 (9 H, m, CH2CH(CH3)2), 2.98 (1 H, m, CHaHbPh), 3.19 (1 H, m, CHaHbPh), 3.63 (1 H, m, CHCH3), 3.95 (1 H, m, αCH), 4.18 (1 H, m, αCH), 4.30 (1 H, m, αCH), 4.40 (1 H, m, αCH), 7.19–7.35 (m, 5 H, _). HRMS (ESI-TOF): MH+, found 628.4040, C34H53N5O6 requires 628.4068.
Supplementary Material
Scheme 6.
Syntheisis of 13. (a) 3 mL/g resin of N-Methylpyrrolidone (NMP), DIPEA (6 equiv), methylene chloride (10 mL/g AA); (b) 20% piperidine/dimethylformamide (0.2 M); (c) Fmoc-Leu-OH (1.2 equiv), HOBt (3 equiv), DIC (6 equiv), dimethylformamide (0.2M); (d) Fmoc-Phe-OH (1.2 equiv), HOBt (3 equiv), DIC (6 equiv), dimethylformamide (0.2M); (e) TFE/methylene chloride (1:1) (0.2 M); (f) HATU (0.7 equiv), TBTU (0.7 equiv), DEPBT (0.6 equiv), DIPEA (8 equiv), methylene chloride/acetonitrile (1:1) (0.007 M)
Acknowlegdements
We thank the University of New South Wales for support of SRM, the University of Sydney for a Visiting International Fellowship to SRM, the Frasch foundation (658-HF07) for support of MRD, EKS, LDA, and LAN, NIH 1R01CA137873 for support of EKS, LDA, and SRM, and NIH MIRT for support of EKS, LDA, and JBK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Sellers RP, Alexander LD, Johnson VA, Lin C-C, Savage J, Corral R, Moss J, Slugocki TS, Singh EK, Davis MR, Ravula S, Spicer JE, Oelrich JL, Thornquist A, Pan C-M, McAlpine SR. Bioorg. &Med. Chem. 2010;18:6822–6856. doi: 10.1016/j.bmc.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otrubova K, Lushington G, Vander Velde D, McGuire KL, McAlpine SR. Journal of Medicinal Chemistry. 2008;51:530–544. doi: 10.1021/jm070731a. [DOI] [PubMed] [Google Scholar]
- 3.Belofsky GN, Jensen PR, Fenical W. Tetrahedron Lett. 1999;40:2913–2916. [Google Scholar]
- 4.Pan P-S, Vasko RC, Lapera SA, Johnson VA, Sellers RP, Lin C-C, Pan C-M, Davis MR, Ardi VC, McAlpine SR. Bioorganic & Medicinal Chemistry. 2009;17:5806–5825. doi: 10.1016/j.bmc.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otrubova K, Lushington GH, Vander Velde D, McGuire KL, McAlpine SR. J. Med. Chem. 2008;51:530–544. doi: 10.1021/jm070731a. [DOI] [PubMed] [Google Scholar]
- 6.Otrubova K, McGuire KL, McAlpine SR. J. Med. Chem. 2007;50:1999–2002. doi: 10.1021/jm070088s. [DOI] [PubMed] [Google Scholar]
- 7.Pan PS, McGuire K, McAlpine SR. Bioorg. & Med. Chem. Lett. 2007;17:5072–5077. doi: 10.1016/j.bmcl.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Vasko RC, Rodriguez RA, Cunningham CN, Ardi VC, Agard DA, McAlpine SR. ACS Med. Chem. Lett. 2010;1:4–8. doi: 10.1021/ml900003t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neckers L. Trends in Molecular Medicine. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 10.Chiosis G, Huezo H, Rosen N, Mimnaugh E, Whitesell L, Neckers L. Molecular Cancer Therapeutics. 2003;2:123–129. [PubMed] [Google Scholar]
- 11.Sausville EA, Hollingshead M, Alley M, Burger AM, Borgel S, Pacula-Cox C, Fiebig HH. Cancer Chemotherapy and Pharmacology. 2005;56:115–125. doi: 10.1007/s00280-004-0939-2. [DOI] [PubMed] [Google Scholar]
- 12.Sueoka N, Senju M, Sato A, Iwanaga K, Sakao Y, Tomimitsu S, Tominaga M, Irie K, Hayashi S, Sueoka E. Journal of Cancer Research and Clinical Oncology. 2006;132:150–158. doi: 10.1007/s00432-005-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang YS, Lee LC, Sun FC, Chao CC, Fu HW, Lai YK. J Cell Biochem. 2006;97:156–165. doi: 10.1002/jcb.20623. [DOI] [PubMed] [Google Scholar]
- 14.Matei D, Satpathy M, Cao L, Lai YC, Nakshatri H, Donner DB. J Biol Chem. 2007;282:445–453. doi: 10.1074/jbc.M607012200. [DOI] [PubMed] [Google Scholar]
- 15.Usmani SZ, Bona R, Li ZH. Curr. Mol. Med. 2009;9:654–664. doi: 10.2174/156652409788488757. [DOI] [PubMed] [Google Scholar]
- 16.Koga F, Kihara K, Neckers L. Anticancer Res. 2009;29:797–807. [PubMed] [Google Scholar]
- 17.Barginear MF, Van Poznak C, Rosen N, Miodi S, Hudis CA, Budman DR. Curr. Cancer Drug Targets. 2008;8:522–535. doi: 10.2174/156800908785699379. [DOI] [PubMed] [Google Scholar]
- 18.Banerji U. Proc. Am. Assoc. Cancer Res. 2003:677. [Google Scholar]
- 19.Sausville EA, Tomaszewski JE, Ivy P. Curr Cancer Drug Targets. 2003;3:377–383. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
- 20.Pearl LH, Prodromou C. Curr Opin Struct Biol. 2000;10:46–51. doi: 10.1016/s0959-440x(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 21.Dehner A, Furrer J, Richter K, Schuster I, Buchner J, Kessler H. Chembiochem. 2003;4:870–877. doi: 10.1002/cbic.200300658. [DOI] [PubMed] [Google Scholar]
- 22.Hagn FX, Richter K, Buchner J, Kessler H. Proc. Experimental Nuclear Magnetic Resonance Conference. 2005:211. [Google Scholar]
- 23.Jez JM, Chen JC, Rastelli G, Stroud RM, Santi DV. Chem Biol. 2003;10:361–368. doi: 10.1016/s1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 24.von Geldern TW, Kester JA, Bal R, Wu-Wong JR, Chiou W, Dixon DB, Opgenorth TJ. Journal of Medicinal Chemistry. 1996;39:968–981. doi: 10.1021/jm950592+. [DOI] [PubMed] [Google Scholar]
- 25.Bartroli J, Turmo E, Algueró Mn, Boncompte EulÃl, Vericat ML, Conte L, Ramis J, Merlos M, GarcÃa- Rafanell Jn, Forn J. Journal of Medicinal Chemistry. 1998;41:1869–1882. doi: 10.1021/jm9707277. [DOI] [PubMed] [Google Scholar]
- 26.Dumy P, Keller M, Ryan DE, Rohwedder B, Wöhr T, Mutter M. Journal of the American Chemical Society. 1997;119:918–925. [Google Scholar]
- 27.Morgan BA, Gainor JA, Richard CA. Annual Reports in Medicinal Chemistry. Academic Press; 1989. pp. 243–252. [Google Scholar]
- 28.Rizo J, Gierasch LM. Annual Review of Biochemistry. 1992;61:387–418. doi: 10.1146/annurev.bi.61.070192.002131. [DOI] [PubMed] [Google Scholar]
- 29.Horne WS, Olsen CA, Beierle JM, Montero A, Ghadiri MR. Angewandte Chemie International Edition. 2009;48:4718–4724. doi: 10.1002/anie.200805900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen F, Koehler MFT, Bergeron P, Elliott LO, Flygare JA, Franklin MC, Gazzard L, Keteltas SF, Lau K, Ly CQ, Tsui V, Fairbrother WJ. Bioorganic &Medicinal Chemistry Letters. 20:2229–2233. doi: 10.1016/j.bmcl.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Sperry J, Moody CJ. Tetrahedron. 66:6483–6495. [Google Scholar]
- 32.Keller M, Wöhr T, Dumy P, Patiny L, Mutter M. Chemistry – A European Journal. 2000;6:4358–4363. doi: 10.1002/1521-3765(20001201)6:23<4358::aid-chem4358>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen DS, Abell A. European Journal of Organic Chemistry. 2011:2399–2411. [Google Scholar]
- 34.Chatterjee J, Mierke DF, Kessler H. J. Am. Chem. Soc. 2006;128:15164–15172. doi: 10.1021/ja063123d. [DOI] [PubMed] [Google Scholar]
- 35.Heller M, Sukopp M, Tsomaia N, John M, Mierke DF, Reif B, Kessler H. J. Am. Chem. Soc. 2006;128:13806–13814. doi: 10.1021/ja063174a. [DOI] [PubMed] [Google Scholar]
- 36.Pan PS, Vasko RC, Lapera SA, Johnson VA, Sellers RP, Lin C-C, Pan C-M, Davis MR, Ardi VC, McAlpine SR. Bio. Org. Med. Chem. 2009;17:5806–5825. doi: 10.1016/j.bmc.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez RA, Pan P-S, Pan C-M, Ravula S, Lapera SA, Singh EK, Styers TJ, Brown JD, Cajica J, Parry E, Otrubova K, McAlpine SR. J. Org. Chem. 2007;72:1980–2002. doi: 10.1021/jo061830j. [DOI] [PubMed] [Google Scholar]
- 38.Dumy P, Keller M, Ryan DE, Rohwedder B, Wohr T, Mutter M. Journal of the American Chemical Society. 1997;119:918–925. [Google Scholar]
- 39.Wöhr T, Wahl F, Nefzi A, Rohwedder B, Sato T, Sun X, Mutter M. Journal of the American Chemical Society. 1996;118:9218–9227. [Google Scholar]
- 40.Rückle T, de Lavallaz P, Keller M, Dumy P, Mutter M. Tetrahedron. 1999;55:11281–11288. [Google Scholar]
- 41.Sun H, Liu L, Lu J, Qiu S, Yang CY, Yi H, Wang S. Bioorg Med Chem Lett. 20:3043–3046. doi: 10.1016/j.bmcl.2010.03.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolb HC, Finn MG, Sharpless KB. Angewandte Chemie International Edition. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Fairweather KA, Sayyadi N, Luck IJ, Clegg JK, Jolliffe KA. Organic Letters. 12:3136–3139. doi: 10.1021/ol101018w. [DOI] [PubMed] [Google Scholar]
- 44.Nicolaou KC, Zak M, Safina BS, Estrada AA, Lee SH, Nevalainen M. J. Am. Chem. Soc. 2005;127:11176–11183. doi: 10.1021/ja052934z. [DOI] [PubMed] [Google Scholar]
- 45.Deng S, Taunton J. J Am Chem Soc. 2002;124:916–917. doi: 10.1021/ja017160a. [DOI] [PubMed] [Google Scholar]
- 46.Seyferth D, Marmor RS, Hilbert P. The Journal of Organic Chemistry. 1971;36:1379–1386. [Google Scholar]
- 47.Gilbert JC, Weerasooriya U. The Journal of Organic Chemistry. 1982;47:1837–1845. [Google Scholar]
- 48.Mahler SG, Serra G, Viera I, Manta E. Rev. Latinoamer. Quím. 2007;35:74–82. [Google Scholar]
- 49.Saladino R, Mezzetti M, Mincione E, Torrini I, Paradisi MP, Mastropietro G. J. Org. Chem. 1999;64:8468–8474. [Google Scholar]
- 50.Malhotra SV, Chengdong Z, Hao W. Letters in Organic Chemistry. 2010;7:168–171. [Google Scholar]
- 51.Bronner SM, Goetz AE, Garg NK. J. Am. Chem. Soc. 2011;133:3832–3835. doi: 10.1021/ja200437g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.