Abstract
Diffusion tensor imaging (DTI) tractography enables the in vivo visualization of the course of white matter tracts inside or around a tumor, and it provides the surgeon with important information in resection planning. This study is aimed at assessing the ability of preoperative DTI tractography in predicting the extent of the resection achievable in surgical removal of gliomas. Patients with low-grade gliomas (LGGs; 46) and high-grade gliomas (HGGs; 27) were studied using a 3T scanner according to a protocol including a morphological study (T2, fluid-attenuated inversion-recovery, T1 sequences) and DTI acquisitions (b = 1000 s/mm2, 32 gradient directions). Preoperative tractography was performed off-line on the basis of a streamline algorithm, by reconstructing the inferior fronto-occipital (IFO), the superior longitudinal fascicle (SLF), and the corticospinal tract (CST). For each patient, the relationship between each bundle reconstructed and the lesion was analyzed. Initial and residual tumor volumes were measured on preoperative and postoperative 3D fluid-attenuated inversion-recovery images for LGGs and postcontrast T1-weighted scans for HGGs. The presence of intact fascicles was predictive of a better surgical outcome, because these cases showed a higher probability of total resection than did subtotal and partial resection. The presence of infiltrated or displaced CST or infiltrated IFO was predictive of a lower probability of total resection, especially for tumors with preoperative volume <100 cm3. DTI tractography can thus be considered to be a promising tool for estimating preoperatively the degree of radicality to be reached by surgical resection. This information will aid clinicians in identifying patients who will mostly benefit from surgery.
Keywords: Diffusion tensor imaging, DTI tractography, extent of resection, glioma surgery, presurgical planning
Cerebral gliomas are highly invasive neoplasms involving both cortical and subcortical structures. These tumors are frequently located in or close to eloquent areas, and they are characterized by a diffuse and infiltrative pattern of growth, because invasive glioma cells are frequently found to migrate along myelinated fiber tracts of white matter (WM).1 WM is crucial for brain function, and its pathological involvement correlates with neurological or neuropsychological findings;2,3 however, low-grade gliomas (LGGs) typically grow slowly and often spare neural function as they infiltrate eloquent brain areas, and patients usually have normal neurological examination findings.4
Diffusion tensor imaging (DTI) tractography is widely applied in patients with glioma,5,6 because this advanced magnetic resonance (MR) technique is the only noninvasive method allowing the in vivo identification of the trajectories of WM tracts adjacent to or inside the tumor.6 DTI tractography can show the various effects exerted by the tumor on WM tracts, providing information about the normal course, the displacement, or the infiltration of the fascicles.5–7 Intraoperative subcortical brain mapping studies8,9 recently showed that DTI tractography data are reliable and accurate in describing the trajectories of the tracts and their modifications induced by the lesions, because tracking results correlated with those obtained by direct electrical stimulation. At present, DTI tractography is specifically used as a preoperative examination to improve neurosurgical planning,9–13 and it is usually uploaded to the neuronavigation system together with morphological and functional images.14 This means that patients undergo DTI tractography when a surgical procedure has already been planned.
The aim of this study was to investigate whether DTI tractography could be useful, even a step earlier, as a tool to select which patients will benefit the most from surgery, obtaining a total resection, and thus, having longer overall survival.15–17 The major WM tracts responsible for eloquent functions were preoperatively reconstructed in patients with supratentorial brain gliomas involving speech or motor areas or pathways. The corticospinal tract (CST) and 2 of the main fiber bundles involved in the phonologic and semantic loop for language (superior longitudinal fascicle [SLF] and inferior fronto-occipital fascicle [IFO]) were chosen for this analysis. Surgical resection was performed according to functional boundaries identified by brain mapping. The involvement of the fascicles evaluated by DTI tractography, the preoperative tumor volumes, and the extent of residual tumor after surgical resection were retrospectively analyzed, investigating the predictive value of DTI tractography to assess the odds of an extensive surgical resection.
Materials and Methods
Patient Population
Seventy-three consecutive patients (27 women, 46 men; mean age, 44.2 years; range, 9–70 years) who underwent surgery in our institutions for gliomas located in eloquent areas from January 2006 through January 2009 were included in this retrospective study. Demographic and clinical data of patients are herein reported (Table 1). The study was approved by the local ethical committee, and patients gave informed consent to have their data used.
Table 1.
Patients’ clinical data
| Characteristic | No. of patients | % | |
|---|---|---|---|
| Sex | |||
| Male | 46 | 63 | |
| Female | 27 | 37 | |
| Age at diagnosis (years) | |||
| <40 | 27 | 37 | |
| 40–60 | 38 | 52 | |
| >60 | 8 | 11 | |
| Median | 42 | ||
| Range | 9–70 | ||
| Side of tumor | |||
| Left | 53 | 73 | |
| Right | 20 | 27 | |
| Tumor location | |||
| One lobe | 44 | 60 | |
| Frontal | 36 | ||
| Temporal | 5 | ||
| Parietal | 3 | ||
| Two lobesa | 21 | 29 | |
| Insula | 10 | ||
| Three lobesb | 8 | 11 | |
| Insula | 7 | ||
| Tumor grade | |||
| LGG | 46 | 63 | |
| HGG | 27 | 37 | |
| Tumor histology | |||
| Oligodendroglioma | 47 | ||
| Astrocytoma | 19 | ||
| Oligoastrocytoma | 4 | ||
| DNT | 3 | ||
| Preoperative deficits | |||
| Pts with one or more | 25 | 34 | |
| Motor | 16 | ||
| Language | 12 | ||
| Sensory | 4 | ||
| Urinary | 2 | ||
| Apraxia | 2 | ||
| Amnesia | 1 | ||
| Preoperative seizures | |||
| Partial | 24 | 33 | |
| Generalized | 27 | 37 | |
| Secondary generalized | 14 | 19 | |
| Total of pts with seizures | 65 | 89 | |
| Time to surgery | |||
| Median (months) | 21,1 | ||
| Median (days) | 635 | ||
| Range (days) | 9–7300 | ||
| Brain mapping | |||
| Motor | 72 | 99 | |
| Language | 48 | 65 | |
| Early postoperative deficitsc | |||
| Pts with one or more | 62 | 85 | |
| Language | 34 | ||
| Motor | 11 | ||
| Motor and language | 12 | ||
| Seizures | 8 | ||
| Sensory | 1 | ||
| Urinary | 2 | ||
| Mortality | 0 | 0 | |
| Preoperative tumor volume (cm3) | |||
| <100 cm3 | 67 | 92 | |
| >100 cm3 | 6 | 8 | |
| Median | 36,2 | ||
| Range | 1,1–224,3 | ||
| Postoperative tumor volume (cm3) | |||
| <1 cm3 (total resection) | 23 | 32 | |
| 1–10 cm3 (subtotal resection) | 34 | 46 | |
| >10 cm3 (partial resection) | 16 | 22 | |
| Median | 4 | ||
| Range | 0–130,8 |
aF + Ins = 4; F + T = 1; F + P = 3; T + Ins = 6; T + P = 6; P + O = 1.
bF + T + Ins = 7; F + T + P = 1.
cAll the postoperative new deficits were transient; no new permanent deficits appeared.
Topographically, 44 tumors were confined to 1 cerebral lobe and 29 involved >1 lobe. Fifty-three lesions were located in the left hemisphere, and 20 were located in the right hemisphere.
Histology was classified according to the World Health Organization brain tumor classification: 19 patients had a diagnosis of astrocytoma, 47 of oligodendroglioma, 4 of mixed oligoastrocytoma, and 3 of dysembryoplastic neuroepithelial tumor (DNT). Forty-six patients harbored LGGs, and 27 had high-grade gliomas (HGGs). The latter were LGGs that progressed to high grade.
A preoperative neurological and neuropsychological evaluation (language production and comprehension, language dominance, repetition), baseline and volumetric MR studies, functional MR imaging (fMRI), and DTI tractography for motor and language pathways were performed in all patients.
Imaging Protocol
MRI was performed preoperatively on a Philips Intera 3.0T (Best) system with a maximum field gradient strength of 80 mT/m. A multichannel head coil was used for the reception of MR signal. DTI data were obtained using a single-shot echo planar imaging sequence (TR/TE 8986/80 ms) with parallel imaging (SENSitivity Encoding [SENSE] reduction factor R = 2.5). Diffusion gradients were applied along 32 axes, using a b-value of 0 and 1000 s/mm2. A field of view (FOV) of 240 × 240 mm2 and a data matrix of 96 × 96 were used, leading to isotropic voxel dimensions (2.5 × 2.5 × 2.5 mm3). The data were interpolated in-plane to a matrix of 256 × 256 leading to voxel size of 0.94 × 0.94 × 2.5 mm3. Fifty-six slices were obtained, with a thickness of 2.5 mm, with no gap. The sequence was repeated 2 consecutive times, and data were averaged off-line to increase signal-to-noise ratio; thus, total time for diffusion tensor MR imaging was 10 min 46 s.
3D fast field echo T1-weighted imaging (TR/TE 8/4 ms; image resolution equal to DTI) was performed for anatomic guidance. Axial turbo-spin-echo T2-weighted images (TR/TE 3000/85 ms; FOV, 230 mm; 22 slices; section thickness, 5/1 mm gap; matrix, 512 × 512; SENSE reduction factor R = 1.5), 3D axial fluid attenuated inversion recovery (FLAIR) images (TR/TE 10 000/110 ms; FOV, 230 mm; 120 slices; section thickness, 1.5/0 mm gap; matrix, 224 × 256; SENSE reduction factor R = 2), and postcontrast volumetric inversion recovery (IR) T1-weighted images (TR/TE 2000/10 ms; FOV, 230 mm; 22 slices; section thickness, 5/1 mm gap; matrix, 400 × 512; SENSE reduction factor R = 1.5) were acquired for morphologic characterization of the lesion.
DTI Tractography
Images were analyzed using DTI Studio, version 2.4.01, software (H. Jiang and S. Mori, Johns Hopkins University, Kennedy Krieger Institute, Baltimore, MD), obtaining main eigenvector and fractional anisotropy (FA) maps. From their combination, color maps were generated with conventional color-coding.18 Deterministic tractography was performed in all patients to reconstruct subcortical connections using the fiber assignment by continuous tracking method.19 An FA threshold of 0.1 and a turning angle >55° were used as criteria to start and stop tracking. These parameters were chosen according to a previous work8 and to our recent experience on >600 cases (per patient and per fascicle) of combined use of preoperative DTI and intraoperative subcortical stimulation. The CST and some of the major subcortical tracts involved in the phonologic or semantic loop of language—SLF and IFO—were reconstructed, defining for each tract separately regions of interest (ROIs) around areas of WM that all the fibers of each tract must pass through to reach their cortical or subcortical endstations.20,21 Particularly, for the IFO a 2-ROIs approach was used, with a first ROI placed on a coronal section at the level of the anterior part of the external capsule, at the junction of the frontal and temporal lobes, where the tract run in contiguity with uncinate fasciculus; a second posterior ROI was placed on a coronal section at the level of the occipital lobe, to isolate only fibers of IFO with the “AND” operation of DTI Studio software. This option allows us to identify specific fibers that connect more than one anatomical landmark depicted by multiple ROIs. To reconstruct the CST, a ROI was placed on an axial section at the level of subcortical WM of the precentral gyrus. To reconstruct the SLF, a first ROI was placed on a coronal section at the level of a high-anisotropy region laterally to the central part of the lateral ventricle; a second ROI was placed in a peritrigonal site at the level of the descending branch of the tract, to isolate fibers from the arcuate fasciculus. For all the tracts reconstructed, eventual contaminating fibers were removed.
Finally, reconstructed WM tracts were superimposed on volumetric postcontrast T1-weighted or FLAIR images, previously coregistered to the mean of all diffusion-weighted images using the SPM5 software (Statistical Parametric Mapping software, Wellcome Trust Centre for Neuroimaging, University College London, UK; http://www.fil.ion.ucl.ac.uk/spm). This allowed comparison of the trajectories of the tracts in the involved hemisphere with those of the contralateral unaffected hemisphere and evaluation of the anatomical relationship between the tract and the tumor mass and the effect exerted by the tumor on the tract of interest.
DTI tractography images were systematically reviewed by 2 experienced neuroradiologists (A.C. and A.F.). Tracts were then classified as unchanged, displaced, or infiltrated/disrupted, as described in previous articles.6,7
Unchanged reconstructed tracts showed normal anisotropy, location, and orientation, compared with homologous contralateral tracts.
Displaced tracts had a normal or only slightly decreased anisotropy and showed abnormal location or trajectories when compared with those of contralateral unaffected hemisphere.
Infiltrated tracts showed substantially decreased FA with abnormal hues on directional color maps, because infiltrating tumor disrupts the directional organization of fiber tracts causing altered color patterns on directional maps. In these cases, DTI tractography reconstructions superimposed on morphological series showed that tracts passed through the area of altered signal intensity on volumetric postcontrast T1-weighted or FLAIR images; they may still have a normal location if compared with contralateral unaffected tracts. Disruption represented an extreme case of infiltration, with near-zero anisotropy due to destruction of fibers, tracts unidentifiable on directional color maps, and interruption of DTI tractography reconstructions.
Intraoperative Setting and Stimulation Protocol
Surgery was performed in all patients with the aid of intraoperative cortical and subcortical mapping for motor and language functions. Brain mapping was undertaken during asleep craniotomy when only a motor mapping was performed or under asleep awake anesthesia when motor, language, and/or visuospatial functions were tested.22,23 It was planned to resect tumor tissue according to functional boundaries, to maximally preserve functions. The finding of subcortical motor or language tracts determined the limit of resection. Motor and language mapping was performed at both cortical and subcortical level (intraoperative subcortical mapping). Motor responses were evaluated both clinically and by the use of a multichannel electromyographic recording. Language mapping was also performed by the aid of a neuropsychologist who was present in the theater.
Tumor Volume Measurements
Volumetric scan analysis was used for establishing tumor location and topography and the volume of the lesion. Initial and residual tumor volumes were measured on preoperative and postoperative 3D-FLAIR images for LGGs and postcontrast T1-weighted MRI scans for HGGs via a computerized system (BrainLab iPlan Cranial 2.6 software; BrainLab AG). Extent of resection (EOR) was measured on postoperative baseline and postcontrast MRI performed immediately after surgery or at 3 months and classified as previously reported.15,22 The original Berger classification was modified to include, in the category of total resection, all the residual signal abnormalities <1 cm3, to account for postoperative FLAIR changes due to edema and contusion of brain tissue surrounding the resection cavity, because of surgical insult. Therefore, surgical outcome was categorized as total resection (postoperative volume, <1 cm3), subtotal resection (postoperative volume, 1–10 cm3), and partial resection (postoperative volume, >10 cm3). Postoperative diffusion-weighted MRI to check for ischemic damage was also performed.
Statistical Analysis
Multinomial logistic regressions were used to evaluate the potential predictors of total resection, by assessing the association between surgical outcome and presence of infiltration and displacement and the likelihood of total resection over subtotal resection and over partial resection before and after covariating the effects for preoperative volume. Covariating the analysis for preoperative tumor volume allows for establishing associations between infiltration and displacement after removing possible confounding effects of the tumor volume. Furthermore, with use of logistic regression, the probability of total resection for different values of preoperative volume was estimated as for cases with intact fascicles and damaged fascicles, in general and for specific fascicles.
Results
Patient Population
Characteristics and clinical data of the 73 patients included in the study are shown in Table 1. Sixty-five patients had a clinical history of seizures (89%), and 25 patients had ≥1 preoperative deficit (34%). Motor and speech mapping were performed in 72 (99%) and 48 (65%) cases, respectively. There was no operative mortality. In the early postoperative period, deficits were noted in 62 patients (85%); however, no new permanent deficits appeared, because all the postoperative new deficits were transient. A single surgeon (L.B.) performed all the surgical procedures.
Median preoperative and postoperative tumor volumes were 36.2 cm3 (range, 1.1–224.3 cm3) and 4 cm3 (range, 0–130.8 cm3). Twenty-three cases (32%) showed a total resection, 34 (46%) a subtotal resection, and 16 (22%) a partial resection. As established with a series of multinomial logistic regressions, surgical outcome did not depend on the patient’s age (χ2(2) = 1.20; P = .550), sex (χ2(2) = 0.276; P = .251), recurrence (χ2(2) = 0.147; P = .701), grade (χ2(2) = 0.090; P = .956), or histological type (χ2(6) = 5.83; P = .442). In particular, surgical outcomes were distributed very similarly in the low-grade group and in the group of low grade who progressed to high grade (high-grade group). A total resection was achieved in 33.3% of high-grade tumors and in 30.4% of LGGs. Histological types were not statistically different either in the distribution of the 3 surgical outcomes (χ2(6) = 5.83; P = .442) or in the probability of total resection (χ2(3) = 4.367; P = .224).
Fascicles and Surgical Outcome
Intact, displaced, and infiltrated fascicles were found in 14 (20.2%), 19 (26%), and 40 (54%) cases, respectively. Infiltration and displacement were assessed for CST (31.5% infiltrated, 33% displaced), IFO (infiltration found in 41% of patients, displacement for 30%), and SLF (54.7% infiltrated, 24.6% displaced).
Multinomial logistic regressions showed that the presence of intact fascicles increased the odds of a better surgical outcome (Table 2), because patients with intact fascicles showed a higher probability of total resection than subtotal (χ2(1) = 5.31; P = .02) and partial resection (χ2(1) = 4.11; P = .04). Conversely, when evaluated for the specific fascicles, the involvement of the CST was associated with a greater likelihood of subtotal or partial resection, because a total resection was less likely in patients with dislocated or infiltrated CST (Table 2 and Fig. 1). Similarly, infiltration of the IFO was predictive of subtotal or partial resection, because in these patients, total resection was less likely, compared with partial resection (χ2(2) = 5.25; P = .02) (Table 2 and Fig. 2). No significant results were obtained for either displacement or infiltration of SLF and the EOR.
Table 2.
Association between presence of infiltrated and displaced fascicles and surgical outcome
| Total vs Subtotal resection |
Total vs Partial resection |
|||
|---|---|---|---|---|
| χ2 | P | χ2 | P | |
| Intact Fascicles | 5.31 | .02 | 4.11 | .04 |
| Displacement | 4.5 | .03 | 2.91 | .08 |
| Infiltration | 0.23 | .62 | 0.59 | .54 |
| Displaced CST | 4.5 | .03 | 10.06 | <.01 |
| Infiltrated CST | 3.95 | .04 | 4.24 | .03 |
| Displaced IFO | 0.26 | .61 | 1.23 | .72 |
| Infiltrated IFO | 0.15 | .7 | 5.25 | .02 |
| Displaced SLF | 1.43 | .23 | 2.95 | .08 |
| Infiltrated SLF | 1.43 | .23 | 1.64 | .19 |
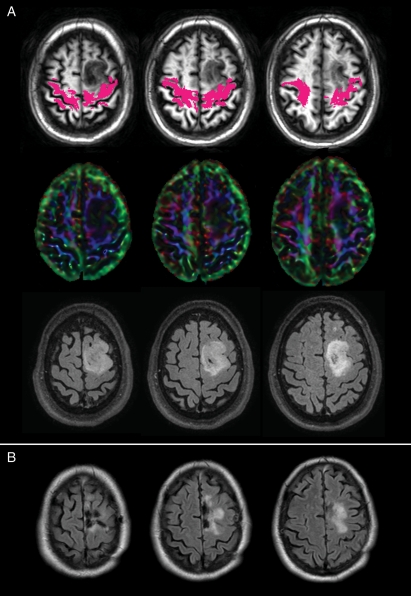
Fig. 1.
(A) A case of a left frontal oligodendroglioma infiltrating the left CST (magenta) both at the level of the subcortical white matter of the precentral gyrus and at the level of the centrum semiovale. Color maps show a reduction of anisotropy due to the presence of the lesion. Preoperative tumor volume was 29 cm3. (B) Postoperative MR shows a residual lesion in the area of deep infiltration of the fascicle. Involvement of CST is predictive of worse surgical outcome, as in patients with infiltrated CST it is less likely to obtain total resection (see Table 1).
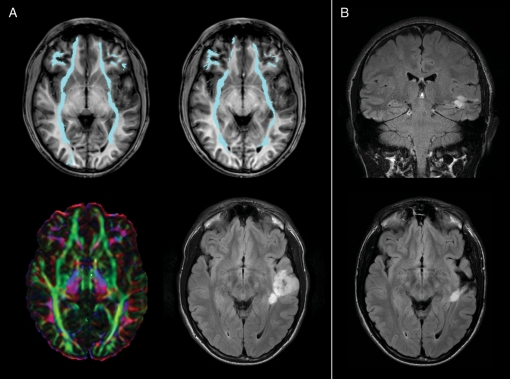
Fig. 2.
(A) A case of a left temporal oligodendroglioma with a deep nodule infiltrating the left IFO (cyan) at a posterior level where it passes from the occipital lobe to the external/extreme capsule. Color maps show a reduction of anisotropy due to the presence of the lesion; tractography shows a narrowing of the fascicle if compared to the contralateral normal IFO. Preoperative tumor volume was 27.5 cm3. (B) Postoperative MR shows the persistence of the deep nodule, resulting in a subtotal resection. Infiltration of IFO is predictive of worse surgical outcome, because in these patients total resection is less likely than is partial resection (see Table 1).
Fascicles, Surgical Outcome, and Tumor Volume
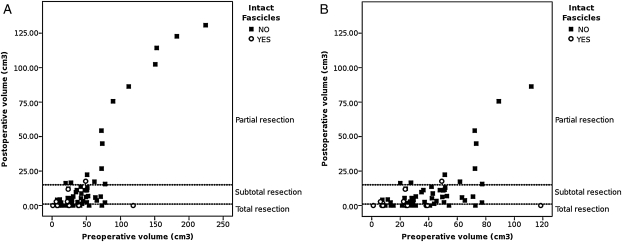
Preliminary analysis showed that preoperative volume was associated with probability of achieving a total resection and with the presence of intact fascicles (χ2(2) = 12.74; P = .001). Figure 3 shows an overview of this relationship. As expected, larger tumors were associated with larger postoperative residual volumes and had an increased likelihood of partial resection (χ2(1) = 11.66; P < .001), whereas smaller tumors (volume < 100 cm3) were equally distributed between cases with total resection and cases with subtotal resection (χ2(1) = 2.23; P = 0.134).
Fig. 3.
(A) Preoperative volume is associated with surgical outcome, as partial resection is more likely to be obtained in larger tumors, whereas total and subtotal resection is more likely to be obtained in smaller tumors (volume, <100 cm3). A detail of the graph is showed in (B): damaged fascicles are more likely to be found in larger tumors, whereas intact fascicles are more likely to be found in smaller tumors, and the size of 100 cm3 appears as a possible threshold.
The same preliminary analysis showed that smaller tumors were generally associated with the presence of intact fascicles: patients with intact fascicles had an average preoperative tumor of 26.6 cm3, with 90% of cases with tumors <50 cm3 (max = 118 cm3), whereas patients with damaged fascicles had larger tumors (average volume, 50.3 cm3, max = 224 cm3, 90th percentile = 89 cm3). Thus, intact fascicles seemed more likely to be found in smaller tumors, whereas damaged fascicles were more frequently associated with larger tumors, and the preoperative volume of 100 cm3 appears to be a possible threshold (Fig. 3).
To investigate whether the relationship between the EOR and the presence of intact, infiltrated, or displaced fascicles was confounded by the association of the same EOR with the preoperative volume, the relationship between fascicles and surgical outcome (see above) was reassessed with a series of multinomial multiple regressions with preoperative volume as covariate. Table 3 shows the results. Substantial results were confirmed after covariating for preoperative tumor volume: the only difference with the analysis ignoring preoperative volume is found for the effect of infiltration of the IFO that does not appear a significant predictor of surgical outcome when preoperative volume is taken into account. This difference may be attributable to the fact that infiltration of the IFO was predictive of the differential likelihood of total resection over partial resection (see Table 2). Because the comparison between total over partial resection is the comparison most affected by preoperative volume, the effect of infiltration of the IFO may indeed be confounded by the dominant effect of preoperative volume.
Table 3.
Association between presence of infiltrated and displaced fascicles and surgical outcome, covariated for preoperative tumor volume
| Total vs Subtotal resection |
Total vs Partial resection |
|||
|---|---|---|---|---|
| χ2 | P | χ2 | P | |
| Intact Fascicles | 3.95 | .04 | 1.81 | .17 |
| Displacement | 3.89 | .04 | 1.09 | .29 |
| Infiltration | 0.38 | .53 | 0.41 | .52 |
| Displaced CST | 4.09 | .04 | 5.22 | .02 |
| Infiltrated CST | 4.02 | .04 | 3.36 | .06 |
| Displaced IFO | 0.03 | .84 | 3.39 | .06 |
| Infiltrated IFO | 0.01 | .95 | 0.29 | .58 |
| Displaced SLF | 1.32 | .24 | 2.28 | .13 |
| Infiltrated SLF | 1.43 | .23 | 1.64 | .19 |
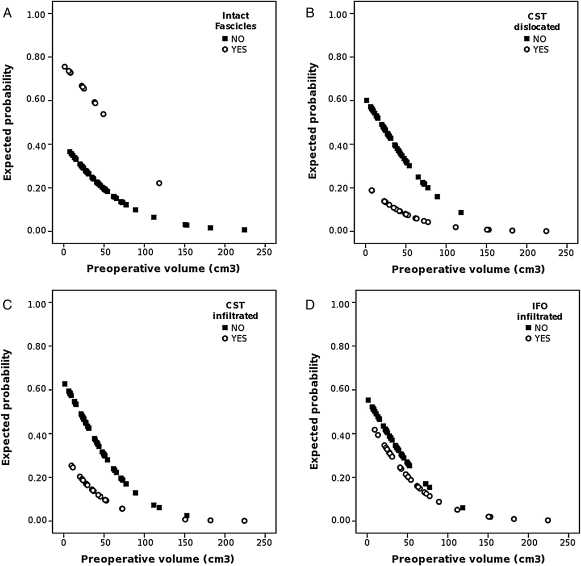
The most intriguing finding was found in tumors with preoperative volume <100 cm3, in which the involvement of fascicles, as documented by preoperative DTI tractography, is a strong predictor of surgical outcome: indeed, although the small preoperative volume should predict per se a favorable surgical outcome in most of cases, the involvement of fascicles could be modified in this outcome, as shown in Fig. 4. The graphs in this figure report the expected probability of total resection for different values of preoperative volume: it is noteworthy that, in tumors smaller than 100 cm3, for the same preoperative tumor volume, the expected probability of total resection is higher when fascicles are intact (Fig. 4A); on the contrary, the expected probability of total resection is substantially lower in the cases of small tumors that showed infiltrated or displaced CST or infiltrated IFO (Fig. 4B–D). The expected probability of total resection decreases with the increase of preoperative volume; consequently, the involvement of WM tracts is less relevant for larger tumors, in which the probability of achieving a total resection per se is low, either in cases with intact fascicles or in tumors in which DTI tractography showed involved fascicles.
Fig. 4.
Expected probability of total resection. (A) The presence of intact fascicles guarantees an high probability of total resection, and those cases are concentrated in the region of small tumors. As concerns with the cases that showed damaged fascicles, the probability of total resection is substantially lower and decreases with preoperative volume. (B and C) A similar pattern is found for infiltrated CST and displaced CST, as the cases with damaged CST show a low probability of total resection, which decreases rapidly to zero as the volume increases, whereas cases with intact CST have a higher probability of total resection, which appears high for small tumors. (D) Also in cases with infiltrated IFO the probability of total resection is higher when the fascicle is not damaged.
Discussion
This retrospective study analyzed the impact of DTI tractography in predicting surgical outcome in patients with gliomas located near or in eloquent structures. This analysis demonstrates that DTI tractography could be a useful tool to estimate the chance of performing a total resection, considering its relevant current role in presurgical planning. In fact, in our study, the presence of intact fascicles was predictive of a higher probability of total resection; conversely, the presence of infiltrated or displaced fascicles was predictive of a lower probability of total resection, especially for tumors with a small preoperative volume (<100 cm3), in which an extensive removal can be foreseen. Although there is growing evidence that DTI tractography is useful and reliable for preoperative surgical planning and for guiding intraoperative subcortical mapping,14 to our knowledge, this is the first study describing a predictive model of the feasibility and the extent of tumor resection based on preoperative DTI tractography data and tumor volumetric assessment.
There are growing class III and II evidences showing that a more extensive resection at the time of initial diagnosis is associated with a longer survival time, both in LGGs and HGGs.17,24,25 Particularly for LGGs, Smith et al. provided clear evidence that a significantly improved overall survival is predicted by an EOR near the limits of complete resection.16 Obtaining a maximal ideal cytoreduction while preserving the patient's function are major goals of surgery; considering these premises, it is of prior interest for the patient's counseling and clinical decision making to preoperatively estimate the expected surgical outcome, both in terms of functional outcome and EOR. The eventual functional outcome depends on different factors, such as tumor localization, volume, and involvement of eloquent tissue, particularly at the subcortical level;16,26,27 therefore, a comprehensive preoperative planning is of paramount importance to identify functional brain and to avoid injuring eloquent structures during surgical removal of lesions involving speech or motor areas or pathways.8,23,28 On the other hand, predicting the EOR would also be of great clinical and therapeutical value in the choice of optimal surgical candidates. DTI tractography information may be extremely useful to determine the candidates that maximally benefit from surgery and to refer to other types of treatment for those patients in whom a substantial tumor burden reduction is improbable and the risk of postoperative morbidity is high.15
Our analysis suggests that, to achieve the maximal tumor removal,22,28,29 the characterization of the involvement of WM tracts by the neoplastic tissue and the preoperative tumor volume is of relevance. In LGGs, it is well established that preoperative variables, including larger maximum diameter of the tumor and presumed eloquent location, are the strongest predictors of an incomplete resection.30 Recently, Chang et al.30 thoroughly evaluated these effects by providing a preoperative score based on tumor size and eloquent brain involvement, besides KPS score and patient age, to guide prognosis and clinical management of LGG; because the former variables were both strong predictors of subtotal resection, these factors evidently affected progression-free survival and long-term overall survival. As for the tumor's relationship with specific eloquent cortical and subcortical areas, in a large retrospective series of LGGs, Talos et al.26 demonstrated that tumor involvement of functionally critical structures, as assessed by anatomical intraoperative images, may affect surgical outcome. Of these structures, it was of noteworthy importance the effect of CST involvement, because it was one of the variables most significantly associated with incomplete tumor resection. Our study is in accordance with those results; however, tractography gives a better insight to the organization of fibers of the CST inside or around the tumor, allowing to clearly depict all the trajectory of the tract,31 especially in those cases in which anatomical structures are not clearly identifiable in morphological images because of the disruption of WM architecture by the tumor (see Fig. 1).
Our results are of particular relevance especially for patients harboring tumors with a small volume. In these cases, a DTI finding of infiltrated CST, usually predicts the achievement of a subtotal resection, independently from the small volume of the tumor mass. The CST is the most important motor tract in term of patient quality of life and can be easily reconstructed by DTI tractography in the presurgical clinical setting.8,32,33 It is intuitive that infiltration of the CST reduces the chance of obtaining a total resection,31 because functional fibers involved by the tumor are preserved during resection to minimize morbidity. Furthermore, also the displacement of this fascicle reduces the chance of achieving a total resection, particularly in patients harboring tumors with bulk mass effect.15
Similarly, infiltration of IFO increases the odds of worse surgical outcome, because in these patients, total resection was less likely to obtain as compared with partial resection. This tract runs from the occipital to the frontal lobe, mediating the semantic component of language; previous studies of our group have shown that, among the different language fiber bundles, IFO is anatomically discrete, functionally relevant and with very high DTI tractography specificity for its identification.8 Our analysis suggests that the DTI tractography of the IFO is effectively able to foresee the possibility to achieve a partial resection instead of a total resection, both for smaller tumors and for large lesions located in the frontal, temporal, and/or insular lobe of the dominant hemisphere, when surgery is performed for functional limits. In particular, in cases where the bundle of fibers was located inside the tumor, a complete resection was not achieved, but only subtotal and partial resections; this is because the part of the tumor containing this bundle of fibers was functional, forming the limit of the resection. On the contrary, where the fascicle was located externally to the tumor mass, a considerably large removal of neoplastic tissue was achieved. As shown in Fig. 2, the critical point is the infiltration of the fascicle when it passes through the external capsule, lining the WM of the insular lobe; this anatomical arrangement, along with the complex vascular anatomy of this area,34 determines a low probability of performing a total resection even in cases of smaller tumors.
Globally considered, these first results are in accordance with previously reported data on the basis of probabilistic map for preoperative estimation of the expected EOR of LGGs resected with the aid of intraoperative functional mapping. Recently, Mandonnet et al.27 demonstrated that regions with a high probability of residual tumors are essentially located in or near functional areas and especially in the WM; they clearly included the pyramidal tract, at its origin, just beneath the primary motor area, and deep within the internal capsule, and the IFO. In our series, only the analysis of the involvement of SLF did not show significant correlation with EOR. The SLF is a large fascicle35 running from the parietal to the frontal lobe and mediating the phonemic component of language. As previously reported,36 the anatomical distribution of this tract is usually larger than the functional distribution as identified by subcortical mapping. Therefore, a large part of the tract can be safely resected because it is not essential for the function tested, and this could be the first explanation of our finding. In addition, the arcuate fasciculus was spared from the tumors and preserved during surgery.
As expected, surgical outcome depended on preoperative tumor volume;26,37 this result paralleled published data reporting that the patients who are most at risk for tumor recurrence and malignant progression are those with larger preoperative tumor volume and larger postoperative residue.15 In our population, larger tumors were more likely to be associated with larger postoperative volumes (partial resection), whereas smaller tumors (volume, <100 cm3) were equally distributed between cases with total resection and cases with subtotal resection. Nevertheless, data regarding the involvement of fascicles as depicted by preoperative DTI tractography were confirmed also after covariating preoperative tumor volume. In this analysis, only the effect of infiltration of IFO seems to be less significant; considering that infiltration of IFO was predictive of the differential likelihood of total resection over partial resection (see Table 2), this difference could be explained from a statistical point of view, because the comparison between total over partial resection is the most sensitive to the effect of preoperative volume. In other words, the majority of patients in this series with infiltrated IFO had large fronto-insular or temporo-insular lesions; therefore, the involvement of this fascicle could be still predictive of worse surgical outcome, but its impact could be hidden by the predominant effect of tumor volume on the feasibility of a total resection.
The results of this study are preliminary, because we have only considered the involvement of 3 of the most representative bundles of subcortical fibers and, therefore, a restricted sphere of possible involvement in each hemisphere. A more comprehensive lesion topography obviously requires that a similar analysis should also include other bundles involved, for example, in the circuits of speech, such as uncinate fascicle38 and inferior longitudinal fascicle,39 or in the visual pathways, such as the optic radiation.40,41
Reconstruction and interpretation of DTI tractography data in a presurgical setting could show some drawbacks, mainly related to the differentiation between the lowering of anisotropy value because of a real neoplastic infiltration or to the peritumoral edema. A detailed definition of this point is out of the scopes of this analysis; however, the relatively low fractional anisotropy threshold of 0.1 used in this study, previous established on the basis of combination of DTI tractography with functional intraoperative data,8 allows to obtain reliable reconstruction of WM tracts also through regions of tumor infiltration or edema, the last one less frequent because of the small amount of edematous lesions in our patients. Strictly related to this point, the inability of DTI tractography to resolve WM architecture where more than one fiber population occupies the same voxel42–44 can affect the reliability of tracking mainly in regions that contain multiple crossing WM pathways. The shortcomings of DTI tractography have driven the development of advanced high angular resolution diffusion imaging techniques and q-ball reconstruction methods, to provide a more accurate presurgical tractography.9 In the future, a more complex algorithm will demonstrate more accurately WM involvement by brain tumors; nevertheless, this preliminary study including a large series of gliomas demonstrates that the assessment of WM involvement is an essential part of an integrate presurgical evaluation, because the analysis of the relationship between tumor and eloquent bundles by DTI tractography allows to estimate preoperatively the degree of radicality of surgical resection, allowing to identify the patients who may maximally benefit from surgery.
Conclusion
From the data presented in this study, it clearly stands out how preoperative DTI tractography data and tumor volumetric assessment can indicate preoperatively the possibility of removing a glial neoplasm and approximate the extent of surgical resection. Particularly in tumors <100 cm3, the involvement of fascicles is a strong predictor of the surgical outcome, because the probability of achieving a total resection for the same preoperative tumor volume is higher when fascicles are intact; conversely, the expected probability of total resection is substantially lower in the cases in which tractography showed infiltrated or displaced CST or infiltrated IFO. This information could be of extremely useful prognostic value, and once integrated with the patient's anesthaesiological, neurological, and neuropsychological evaluation, it could be proposed as a useful tool to help in surgical decision making, contributing to a more accurate selection of therapeutic strategy in cerebral gliomas.
Funding
This work was supported by grants from Associazione Italiana Ricerca sul Cancro and Fondazione Berlucchi and Fondazione Italo Monzino (to L.B.).
Acknowledgments
We would like to thank Dr. Anna Gambini for her helpful and critical support in DTI data processing and interpretation in the first part of this study.
Conflict of interest statement. None declared.
References
- 1.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. discussion 250–252 doi:10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Duffau H. New concepts in surgery of WHO grade II gliomas: Functional brain mapping, connectionism and plasticity–a review. J Neurooncol. 2006;79:77–115. doi: 10.1007/s11060-005-9109-6. doi:10.1007/s11060-005-9109-6. [DOI] [PubMed] [Google Scholar]
- 3.Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: A window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. doi:10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11:681–693. doi: 10.1634/theoncologist.11-6-681. doi:10.1634/theoncologist.11-6-681. [DOI] [PubMed] [Google Scholar]
- 5.Mori S, Frederiksen K, van Zijl PC, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002;51:377–380. doi: 10.1002/ana.10137. doi:10.1002/ana.10137. [DOI] [PubMed] [Google Scholar]
- 6.Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: A pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- 7.Witwer BP, Moftakhar R, Hasan KM, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg. 2002;97:568–575. doi: 10.3171/jns.2002.97.3.0568. doi:10.3171/jns.2002.97.3.0568. [DOI] [PubMed] [Google Scholar]
- 8.Bello L, Gambini A, Castellano A, et al. Motor and language DTI fiber tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. Neuroimage. 2008;39:369–382. doi: 10.1016/j.neuroimage.2007.08.031. doi:10.1016/j.neuroimage.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Berman J. Diffusion MR tractography as a tool for surgical planning. Magn Reson Imaging Clin N Am. 2009;17:205–214. doi: 10.1016/j.mric.2009.02.002. doi:10.1016/j.mric.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Arfanakis K, Gui M, Lazar M. Optimization of white matter tractography for pre-surgical planning and image-guided surgery. Oncol Rep. 2006;15:1061–1064. doi: 10.3892/or.15.4.1061. [DOI] [PubMed] [Google Scholar]
- 11.Yu CS, Li KC, Xuan Y, Ji XM, Qin W. Diffusion tensor tractography in patients with cerebral tumors: A helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol. 2005;56:197–204. doi: 10.1016/j.ejrad.2005.04.010. doi:10.1016/j.ejrad.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Nimsky C, Ganslandt O, Hastreiter P, et al. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery. 2005;56:130–137. doi: 10.1227/01.neu.0000144842.18771.30. discussion 138. [DOI] [PubMed] [Google Scholar]
- 13.Romano A, D'Andrea G, Minniti G, et al. Pre-surgical planning and MR-tractography utility in brain tumour resection. Eur Radiol. 2009;19:2798–2808. doi: 10.1007/s00330-009-1483-6. doi:10.1007/s00330-009-1483-6. [DOI] [PubMed] [Google Scholar]
- 14.Bello L, Fava E, Casaceli G, et al. Intraoperative mapping for tumor resection. Neuroimaging Clin N Am. 2009;19:597–614. doi: 10.1016/j.nic.2009.08.011. doi:10.1016/j.nic.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Berger MS, Deliganis AV, Dobbins J, Keles GE. The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer. 1994;74:1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. doi:10.1002/1097-0142(19940915)74:6<1784::AID-CNCR2820740622>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–1345. doi: 10.1200/JCO.2007.13.9337. doi:10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 17.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. discussion 564–576 doi:10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 18.Pajevic S, Pierpaoli C. Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: Application to white matter fiber tract mapping in the human brain. Magn Reson Med. 1999;42:526–540. doi:10.1002/(SICI)1522-2594(199909)42:3<526::AID-MRM15>3.0.CO;2-J. [PubMed] [Google Scholar]
- 19.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. doi:10.1002/1531-8249(199902)45:2<265::AID-ANA21>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. doi:10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 21.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. doi:10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Bello L, Gallucci M, Fava M, et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60:67–80. doi: 10.1227/01.NEU.0000249206.58601.DE. discussion 80–82. [DOI] [PubMed] [Google Scholar]
- 23.Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. an anatomo-functional study. Brain. 2002;125:199–214. doi: 10.1093/brain/awf016. doi:10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- 24.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–764. doi: 10.1227/01.neu.0000318159.21731.cf. discussion 264–266 doi:10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 25.Berger MS, Rostomily RC. Low grade gliomas: Functional mapping resection strategies, extent of resection, and outcome. J Neurooncol. 1997;34:85–101. doi: 10.1023/a:1005715405413. doi:10.1023/A:1005715405413. [DOI] [PubMed] [Google Scholar]
- 26.Talos IF, Zou KH, Ohno-Machado L, et al. Supratentorial low-grade glioma resectability: Statistical predictive analysis based on anatomic MR features and tumor characteristics. Radiology. 2006;239:506–513. doi: 10.1148/radiol.2392050661. doi:10.1148/radiol.2392050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandonnet E, Jbabdi S, Taillandier L, et al. Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping. Neuro Oncol. 2007;9:63–69. doi: 10.1215/15228517-2006-015. doi:10.1215/15228517-2006-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger MS. Minimalism through intraoperative functional mapping. Clin Neurosurg. 1996;43:324–337. [PubMed] [Google Scholar]
- 29.Black PM. Brain tumors. part 1. N Engl J Med. 1991;324:1471–1476. doi: 10.1056/NEJM199105233242105. doi:10.1056/NEJM199105233242105. [DOI] [PubMed] [Google Scholar]
- 30.Chang EF, Smith JS, Chang SM, et al. Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg. 2008;109:817–824. doi: 10.3171/JNS/2008/109/11/0817. doi:10.3171/JNS/2008/109/11/0817. [DOI] [PubMed] [Google Scholar]
- 31.Berman JI, Berger MS, Mukherjee P, Henry RG. Diffusion-tensor imaging-guided tracking of fibers of the pyramidal tract combined with intraoperative cortical stimulation mapping in patients with gliomas. J Neurosurg. 2004;101:66–72. doi: 10.3171/jns.2004.101.1.0066. doi:10.3171/jns.2004.101.1.0066. [DOI] [PubMed] [Google Scholar]
- 32.Okada T, Mikuni N, Miki Y, et al. Corticospinal tract localization: Integration of diffusion-tensor tractography at 3-T MR imaging with intraoperative white matter stimulation mapping–preliminary results. Radiology. 2006;240:849–857. doi: 10.1148/radiol.2403050916. doi:10.1148/radiol.2403050916. [DOI] [PubMed] [Google Scholar]
- 33.Holodny AI, Schwartz TH, Ollenschleger M, Liu WC, Schulder M. Tumor involvement of the corticospinal tract: Diffusion magnetic resonance tractography with intraoperative correlation. J Neurosurg. 2001;95:1082. doi: 10.3171/jns.2001.95.6.1082. doi:10.3171/jns.2001.95.6.1082. [DOI] [PubMed] [Google Scholar]
- 34.Sanai N, Polley MY, Berger MS. Insular glioma resection: Assessment of patient morbidity, survival, and tumor progression. J Neurosurg. 2010;112:1–9. doi: 10.3171/2009.6.JNS0952. doi:10.3171/2009.6.JNS0952. [DOI] [PubMed] [Google Scholar]
- 35.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. doi:10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 36.Bello L, Castellano A, Fava E, et al. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: Technical considerations. Neurosurg Focus. 2010;28:E6. doi: 10.3171/2009.12.FOCUS09240. doi:10.3171/2009.12.FOCUS09240. [DOI] [PubMed] [Google Scholar]
- 37.Keles GE, Lamborn KR, Berger MS. Low-grade hemispheric gliomas in adults: A critical review of extent of resection as a factor influencing outcome. J Neurosurg. 2001;95:735–745. doi: 10.3171/jns.2001.95.5.0735. doi:10.3171/jns.2001.95.5.0735. [DOI] [PubMed] [Google Scholar]
- 38.Papagno C, Miracapillo C, Casarotti A, et al. What is the role of the uncinate fasciculus? surgical removal and proper name retrieval. Brain. 2011;134:405–414. doi: 10.1093/brain/awq283. doi:10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- 39.Powell HW, Parker GJ, Alexander DC, et al. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry. 2008;79:327–330. doi: 10.1136/jnnp.2007.126078. doi:10.1136/jnnp.2007.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikuta K, Takagi Y, Nozaki K, et al. Early experience with 3-T magnetic resonance tractography in the surgery of cerebral arteriovenous malformations in and around the visual pathway. Neurosurgery. 2006;58:331–337. doi: 10.1227/01.NEU.0000195017.82776.90. discussion 331–337 doi:10.1227/01.NEU.0000195017.82776.90. [DOI] [PubMed] [Google Scholar]
- 41.Shinoura N, Suzuki Y, Yamada R, Tabei Y, Saito K, Yagi K. Relationships between brain tumor and optic tract or calcarine fissure are involved in visual field deficits after surgery for brain tumor. Acta Neurochir (Wien) 2010;152:637–642. doi: 10.1007/s00701-009-0582-0. doi:10.1007/s00701-009-0582-0. [DOI] [PubMed] [Google Scholar]
- 42.Jones DK. Determining and visualizing uncertainty in estimates of fiber orientation from diffusion tensor MRI. Magn Reson Med. 2003;49:7–12. doi: 10.1002/mrm.10331. doi:10.1002/mrm.10331. [DOI] [PubMed] [Google Scholar]
- 43.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 44.Wiegell MR, Larsson HB, Wedeen VJ. Fiber crossing in human brain depicted with diffusion tensor MR imaging. Radiology. 2000;217:897–903. doi: 10.1148/radiology.217.3.r00nv43897. [DOI] [PubMed] [Google Scholar]