Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive primary brain cancer, with a median survival of less than 2 years after diagnosis. The tumor microenvironment plays a critical role in tumor invasion and progression. Microglia and infiltrating macrophages are the most abundant immune cells in the tumor. In the present study, we demonstrate that systemic propentofylline (PPF), an atypical methylxanthine with central nervous system (CNS) glial modulating and anti-inflammatory actions, significantly decreased tumor growth in a CNS-1 rat model of GBM by targeting microglia and not tumor cells. Rats received tumor injections of 1 × 105 CNS-1 cells in the right striatum with daily intraperitonial injections of PPF (50 mg/kg) or saline beginning the day of tumor injection. PPF did not cause apoptosis or decrease proliferation of CNS-1 tumor cells. Furthermore, we demonstrate, using in vitro methods, that PPF decreased microglial migration toward CNS-1 tumor cells and decreased MMP-9 expression. The effects of PPF were shown to be specific to microglia and not peripheral macrophages. These results support a differential functional role of resident microglia and infiltrating macrophages in the brain tumor environment. Our data highlight microglia as a crucial target for future therapeutic development and present PPF as a possible drug for treatment of human GBM.
Keywords: Glioma, macrophages, microglia, migration, MMP-9
Glioblastoma multiforme (GBM; high-grade or World Health Organization [WHO] grade IV glioma) is the most common and aggressive primary brain cancer, with a median survival of less than 2 years after diagnosis.1 Glioblastomas are histologically heterogeneous tumors derived from glia.2 Current treatment focuses on surgical resection, chemotherapy, and radiation.3 None of these therapies are curative or without possible devastating adverse effects, such as neurocognitive deficits and radiation necrosis.4 These therapeutic modalities are ultimately ineffective because of the radioresistance and infiltrative capacity of glioma cells, resulting in high recurrence rates.5 In the past few years, several phase II clinical trials have focused on single targeted agents (i.e., epithelial growth factor receptor and vascular endothelial growth factor), which have proven to be ineffective.6,7 Because of the highly aggressive nature and lack of effective treatment options for GBM, the development of alternative, novel therapies beyond traditional tumor oncogenic pathways is warranted.
The tumor microenvironment plays a critical role in tumor initiation, invasion, and progression. Once thought to play an antitumor role against established tumors, microglia (central nervous system [CNS] cells of monocytic lineage) are now known to contribute to GBM's progression and growth.8 Microglia and macrophages are highly abundant in GBM, compared with normal brain, and provide 10%–34% of the tumor mass.9 Research suggests a direct positive correlation between the number of macrophages and microglia and the grade of the tumor.9 Microglia and macrophages have been shown to promote GBM cell growth and invasion in vitro.10,11 Mice depleted of both microglia and macrophages (in ex vivo brain slices) demonstrate significantly slower GBM tumor growth.11 Macrophages and microglia share the same immune surface markers; thus, it is not possible to differentiate these cell populations easily. As a result, the current literature has rarely distinguished peripheral infiltrating macrophages from resident microglia when studying the role of immune cells in the GBM tumor microenvironment.
Microglia and macrophages secrete a variety of mediators that promote tumor growth and invasion. They also provide the main source of tumor-promoting molecules, such as matrix metalloproteinases (MMPs).12–14 The MMP family consists of more than25 structurally related, zinc-dependent endopeptidases, with MMP-2 and MMP-9 considered to be the key enzymes involved in glioma invasion.15,16 MMP-9 expression, in particular, has been shown to correlate with high malignant progression of the disease.14,17 The importance of targeting the MMP family has not gone unrecognized, and several MMP inhibitors have been developed.18,19 Unfortunately, clinical development of MMP inhibitor drugs has been very problematic, with severe musculoskeletal toxicities and lack of efficacy.20 Our studies are directed to better understanding the relationship between microglia, macrophages, and tumor cells to identify alternative, safer, more effective therapies.
Propentofylline (PPF) is an atypical synthetic methylxanthine (1-[50-oxohexyl]-3-methyl-7-propylxanthine). PPF has been studied extensively in several CNS disease models, including stroke, opioid tolerance, and acute and chronic pain.21 Clinically, PPF has demonstrated efficacy in degenerative and vascular dementia and as an adjuvant treatment for schizophrenia.22,23 An important clinical feature of PPF is its minimal adverse effect profile, demonstrated in multiple clinical trials.21 Known mechanisms include inhibition of cyclic AMP (cAMP) and cyclic GMP phosphodiesterases and action as a weak antagonist of the adenosine A1 receptor.24,25 More generally, PPF is a glial modulator with direct actions on microglia. PPF dose dependently decreases microglial proliferation and expression of inflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in vitro (tumor necrosis factor–α [TNF-α], interleukin-1β [IL-1β], interleukin-6 [IL-6]).26,27
In the present study, we investigated whether systemic PPF decreased in vivo brain tumor growth with use of the CNS-1 rat model of GBM. In addition, we explored the differential role of microglia and macrophages in the tumor microenvironment with use of in vitro cell culture methods. CNS-1 cells were chosen for their fast growth rate, invasiveness, and histological similarities to human GBM.28 In addition, the absence of strong immunogenicity in the CNS using a syngeneic rodent model offers an excellent system to study the tumor immune microenvironment.29 Here, we show that propentofylline decreases CNS-1 tumor growth by specifically targeting microglia. These data also support a differential functional role of resident microglia and infiltrating macrophages in the brain tumor microenvironment.
Materials and Methods
Clinical Samples
Following review by the Dartmouth College Committee for the Protection of Human Subjects it was determined that further institutional review board review was not required, because the identity of the human subjects were not easily ascertainable. All samples from patients undergoing tumor resection for GBM were determined to be astrocytoma WHO grade IV. Histopathologic diagnosis was assessed under standard light-microscopic evaluation of the sections stained with hematoxylin and eosin stain, based on the WHO criteria for tumors of the CNS. All of the tumor tissues were obtained at primary resection, and none of the patients had been subjected to chemotherapy or radiation therapy before resection. Tissue samples were obtained at the time of surgery and immediately washed with phosphate-buffered saline (PBS). Meninges and large blood vessels were removed, and tissue was incubated with trypsin/EDTA (Mediatech) for 15 min at 37°C. The supernatant was then replaced with DMEM (Mediatech) supplemented with 10% fetal bovine serum (FBS; Hyclone), 1.1% GlutaMax (Invitrogen), and 1% penicillin/streptomycin (100 U/mL penicillin, 100 μg/mL streptomycin; Mediatech) containing 2000 units DNase (Sigma). The tissue was mechanically disrupted by titration, the cell suspensions were centrifuged, and the cells were resuspended in media without DNase. A small aliquot of cells was stained with trypan blue exclusion for counting; then cells were plated at 1 × 106 cells per 75 cm2 flask. Cultures were maintained at 37°C with 5% CO2, and media was changed every 3–4 days.
Immunocytochemistry
Human glioma cultures were plated onto sterile 18-mm glass coverslips. After 3 washes in PBS, cells were permeabilized in 5% glacial acetic acid/95% ethanol (acid–alcohol) for 10 min. After washing, cells were incubated in a 1% normal goat serum for 30 min and then 1 h at room temperature in primary mouse anti-GFAP (1:500; Sigma), rabbit anti–MMP-9 (1:500; Affinity BioReagents), and rat anti-CDllb (1:500; Abcam). The following day, cells were washed and then incubated for 2 h at room temperature with goat anti-mouse Alexa Fluor–555, goat anti-rat Alexa Fluor–488, or goat anti-rabbit Alexa Fluor–647 (all at 1:250). Finally, cells were postfixed in acid–alcohol and mounted with Vectashield (Vector Labs) containing 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (Sigma) to visualize cell nuclei. The sections were examined with an Olympus fluorescence microscope, and images were captured with a Q-Fire cooled camera (Olympus). Confocal microscopy was also performed using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss AG; Englert Cell Analysis Laboratory, Dartmouth). Merged color images were processed using Adobe Photoshop, version 7.0 (Adobe Systems). Color labeling was changed for visualization purposes during image processing (red = CDllb, green = MMP-9, blue = GFAP).
Animals and Cell Lines
This work was approved by the Dartmouth College Institutional Animal Care and Use Committee (IACUC protocol 05-07-09). We used adult male Lewis rats (250–300 g) for all animal studies (Harlan Laboratories). For tumor cell studies, we used the CNS-1 cell line, a rat glioma cell line (generously donated by Dr. William F. Hickey, Dartmouth Medical School, Hanover, NH). The cell line has been tested within the past year through verification of GFAP expression with immunohistochemistry.
Tumor Injection
Tumor injection was conducted as previously described.30 For intracranial tumor cell injections, animals were anesthetized with isofluorane and placed in a stereotactic frame. A burr hole in the skull was made with an 18-gauge needle 3 mm to the right of and 1 mm anterior to the bregma. A 10-μL Hamilton syringe was connected to the manipulating arm of a stereotactic frame. A 4-μL volume containing 1 × 105 CNS-1 cells was injected 5.5 mm from the parenchymal surface. After injection, the needle was left in place for 2 min to prevent cell reflux. For subcutaneous tumor studies, rats were anesthetized with isofluorane, and a 200-μL volume of sterile PBS (Mediatech) with 1 × 105 CNS-1 tumor cells was injected into the right flank. Animals were euthanized at various times following tumor cell injection. Intracranial tumor volumes were measured using MRI. Flank tumors were measured with calipers and volumes calculated using the expression 1/2 (D1 × D2 × D3).31 For survival studies, rats were euthanized when they began to experience difficulties in ambulating, eating, or drinking, as indicated by our IACUC protocol.
MRI
MRI was performed on all animals at days 4, 8, 11, and 14 following tumor implantation using a 3.0-Tesla clinical MRI scanner (Philips Healthcare) and a 70-mm diameter transmit/receive solenoid MRI rodent coil (Philips Research Europe). T2-weighted turbo spin-echo (T2W-TSE) (TR, 3000 ms; TE, 80 ms; matrix size, 256 × 256; slice thickness, 1 mm) and T1-weighted TSE (T1W-TSE) before and immediately after gadolinium intravenous injection images of the whole brain (TR, 434 ms; TE, 20 ms; matrix size, 256 × 256; slice thickness, 1 mm; 0.10 mL gadopentatate dimeglumine [Magnevist, Bayer HealthCare Pharmaceuticals]) were acquired. Subtraction images were obtained by subtracting the baseline, pregadolinium injection T1W images from the contrast-enhanced, postgadolinium injection T1W images.
Image Analysis
Tumor size was evaluated using Mimics image analysis software (Materialise) as previously described.32 The tumor region of interest was identified by applying a mask with a minimum intensity threshold in each coronal plane. Manual correction was performed to improve the accuracy of the automated masking. Tumor volumes were calculated in Mimics by summing the number of voxels that exceeded the intensity threshold in each plane on the T1W subtraction images and then multiplying by the appropriate spatial scaling factor (0.35 × 0.35 × 1.00 mm per voxel). Least-squares nonlinear regression analyses were performed to compare the rate of tumor growth between groups. In animals for which MRI data were acquired on at least 3 different days, tumor volumes were fit using an exponential model of the form V = Aekt, where A and k are constant parameters and t is the number of days after implantation. Tumor volume doubling time T1/2 was then calculated for each animal using the equation T1/2 = ln(2)/k.
Cell Culture
Highly purified microglial cultures were prepared as previously described.33 In brief, cortices were harvested from postnatal day 2–3 (P2–P3) Lewis rat pups, minced and incubated with Trypsin/EDTA (Mediatech). The supernatant was then replaced with DMEM (Mediatech) supplemented with 10% fetal bovine serum (Hyclone), 1.1% GlutaMax (Invitrogen), and 1% penicillin/streptomycin (100 U/mL penicillin, 100 μg/mL streptomycin; Mediatech) containing 2000 units DNase (Sigma). The tissue was mechanically disrupted by titration, the cell suspensions were centrifuged, and the cells were resuspended in media without DNase. A small aliquot of cells was stained for trypan blue exclusion for counting; then cells were plated at 1 × 106 cells per 75 cm2 flask. Cultures were maintained at 37°C with 5% CO2, and media was changed every 3–4 days. After 10 days in vitro, microglia were harvested by gently shaking the flasks by hand for 1 min. The resulting cells were found to be more than 98% microglia by staining with CR3/CD11b antibody (a generous gift from Dr. William F. Hickey). Cells were used immediately for migration and Matrigel invasion experiments. Peripheral macrophages were obtained from peritoneal lavage of adult rats. In brief, 10 mL of cold PBS (Mediatech) was injected into the rat peritoneum, the stomach was massaged, and fluid was subsequently removed. Cells were centrifuged, and pellets were resuspended in complete media. Resuspended cells were plated in a 75-mm2 flask and then washed the next day, leaving adherent macrophages on the flask. The resulting cells were found to be more than 98% macrophages by staining with CD11b antibody (mouse mAb clone WT.5; BD) as described by the manufacturer.
Migration
The optimal experimental procedures for microglial migration in Costar Transwell plates have been previously reported.33,34 Cell migration was studied using Costar Transwell plates (6.5-mm diameter insert, 8.0 μm pore size, polycarbonate membrane; Corning). In brief, CNS-1 cells were plated at a density of 3 × 105 cells per 500 μL in the bottom wells 3 days prior to the migration experiment. Microglia, macrophages, or CNS-1 tumor cells were harvested as described above, counted, and resuspended in serum-free media at 1 × 105 cells per 100 μL, placed in siliconized low-adhesion microcentrifuge tubes, and treated with PPF (0.01–100 μM) for 2 h. Pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (Sigma) was used as a positive control for inhibition of CNS-1 migration toward ADP. Cells were counted after treatment with trypan blue to verify survival (>99% viability) and then added (1 × 105 cells per 100 μL) to the top chamber of a transwell plate with (1) fibronectin-coated membranes and 500 μL CNS-1 cells in the bottom well or (2) 500 μL of 10 μM ADP in the bottom well. After a 2-h incubation, any cells remaining on top of the membrane were washed. The membranes were rinsed with PBS, and the migrated cells were fixed with 2% formaldehyde in PBS, permeabilized with 0.01% Triton X-100 (Sigma) in PBS, stained with crystal violet (Sigma), and rinsed twice with dH2O. The membranes were then dried, inverted, and mounted on microscope slides for analysis. Images of 10 random fields (20× magnification) for each membrane were captured at room temperature via a Q-Fire cooled CCD camera attached to an Olympus microscope and counted by hand with the aid of SigmaScan Pro imaging analysis software (SigmaScan). Counts from all 10 fields were averaged to give a mean cell count for each membrane. All experiments were performed at least 3 times with n = 3 per trial. Results are expressed as mean cell migration relative to vehicle control ± standard deviation (SD).
Invasion
Matrigel Matrix BD Bioscience was thawed overnight at 4°C and then diluted with ice-cold dH20 for a final concentration of 0.125 μg/μL. A total of 40 μL of diluted matrigel was added to the top chamber of each well in a Costar Transwell plate (6.5 mm diameter insert, 8.0 μm pore size, polycarbonate membrane; Corning). The plates were left at 37°C overnight, allowing the matrigel to harden and form a barrier. CNS-1 cells were plated at a density of 3 × 104 per 500 μL/well in the bottom wells 3 days prior to the invasion experiment. Microglia or macrophages were harvested as described above, counted, and resuspended in serum-free media at 3 × 105 cells per 100 μL, placed in siliconized low-adhesion microcentrifuge tubes, and treated with PPF (0.01 μM – 100 μM) for 2 h. An MMP-2/MMP-9 inhibitor (100 µM) was used as a positive control for inhibition of CNS-1 invasion (Calbiochem). Cells were counted after treatment with trypan blue to verify survival (>99% viability), then added (3 × 105 cells in 100 μL) to the top chamber of a transwell plate, with 500 μL CNS-1 cells in the bottom well. After a 2-h incubation, any cells remaining on top of the membrane were washed. The membranes were then rinsed with PBS, and the migrated cells were fixed with methanol and stained with crystal violet and rinsed twice with dH2O. The membranes were then dried, inverted, and mounted on microscope slides for analysis. Images of 10 random fields (20× objective) for each membrane were captured via a Q-Fire cooled CCD camera attached to an Olympus microscope and counted by hand with the aid of SigmaScan Pro imaging analysis software. Counts for all 10 fields were averaged to give a mean cell count for each membrane. All experiments were performed at least 3 times with n = 3 per trial. Results are expressed as mean cell migration relative to vehicle control ± SD.
Flow Cytometry
Tumor cells were first labeled with 10 μM carboxyfluorescein succinimidyl ester (CFSE; Sigma) at a concentration of 3 × 105 cells per 1000 μL of PBS at 37°C for 10 min. CNS-1 tumor cells were then incubated at 37°C in 24-well plates (Falcon) and, at various times, were treated with PPF (0.1 μM – 100 μM). For FACS staining, cells were trypsinized, washed, and stained on ice in PBS for 30 min. Fc receptors were blocked using FBS for 15 min before staining. For apoptosis, an Annexin V FITC and PI apoptosis kit was used for staining (eBioscience). All flow cytometry experiments were performed on a FACSCanto (BD Bioscience).
Western Blot Analysis
CNS-1 cells, microglia, macrophages, or a 1:1 (CNS-1:microglia/macrophages) coculture of cells was plated at a cell density of 8 × 104 cells/well in a 12-well plate (Falcon) and treated daily with PPF (0.1 μM – 100 μM). The protein in the supernatant was then quantified using the Lowry method (DC Assay; Bio-Rad). Protein (40 mg) and a standard marker were subjected to SDS-PAGE (10% gels; Bio-Rad), transferred to PVDF membranes (Bio-Rad), and blocked with 5% milk in TBS-Tween 20 (0.05%; Sigma). The membranes were probed with rabbit anti-MMP-9 (1:500; AbCam), rabbit anti-PARP (1:1000; Cell Signaling), and primary antibody for 16 h at 4°C. Membranes were washed 3 times, then incubated with goat anti-rabbit HRP-conjugated secondary antibody for 1 h at 22°C. Visualization was done with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) for 5 min and imaged using the Syngene G-Box (Synoptics). In brief, for cell supernatant, intensity and size of the bands was calculated using analysis software provided by Syngene G-Box. Cellular suspensions were analyzed by stripping membranes and reprobing them with mouse monoclonal anti–β-actin primary antibody (1:1000; Abcam). Band intensities were quantified using the analysis software provided with the Syngene G-Box. The relative intensity of bands was divided by the intensity of the β-actin band and then compared with control. Data are expressed as fold change in band intensity normalized to naive control ± SD.
Gelatin Zymography
Activity of gelatinase MMP-9 was analyzed with the gelatin zymography procedure described previously, using 1% gelatin.35 After electrophoresis, gel was washed and stained with 0.5% Coomassie blue solution for 30 min. The gel was then destained in 10% isoproponol and 10% acetic acid to elucidate bands. The correct MMP-9 band was determined by molecular weight. Visualization was imaged using the Syngene G-Box.
Statistical Analysis
All values are expressed as mean ± SD. Statistical analyses were performed with GraphPad Prism 4 (GraphPad Software), with the significance level set at P < .05. Two-way ANOVA analyses were used for tumor volume changes over time with a Bonferroni post-hoc analysis. One-way ANOVA with Dunnet's post-hoc analysis was used for all migration and invasion experiments.
Results
Propentofylline Decreases CNS-1 Tumor Volume In Vivo
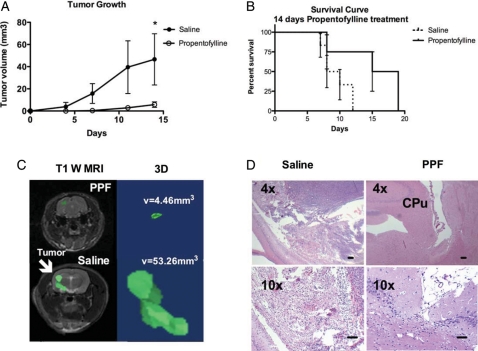
With the knowledge that PPF affects glial function, we investigated the effects of PPF on the growth of CNS-1 cells, a glial-derived brain tumor in a rat model. Lewis rats received tumor injections of 1 × 105 CNS-1 cells in the right striatum with daily intraperitoneal injections of PPF (50 mg/kg) or saline beginning the day of tumor injection.36 PPF treatment resulted in a significant decrease (P < .05) in tumor volume, compared with the saline-treated group at day 14 (Fig. 1A). Tumor volume remained significantly smaller (88% average reduction from saline on day 14). More than 60% of PPF-treated rats never developed a detectable tumor as measured by MRI. PPF treatment for 14 days also resulted in increased survival (P < .05), compared with saline-treated rats (Fig. 1B). When PPF treatment ceased at day 14, the tumors continued to grow and animals were eventually euthanized because of symptoms associated with tumor burden (difficulties eating and drinking). Consistent with human GBM, CNS-1 tumors in the saline group demonstrated an infiltrative, spreading phenotype (Fig. 1C and D). Of importance, in the PPF-treated group, tumor volumes were not only significantly smaller, but remained intact without evidence of dispersal (Fig. 1C).
Fig. 1.
PPF treatment decreases tumor volume. Rats were first engrafted with CNS-1 cells, then treated daily with either 50 mg/kg PPF or saline intraperitoneally beginning the day of tumor engraftment. MRI analysis was performed on days 4, 8, 11, and 14. Treatments are significantly different (*P < .05). (A) Tumor growth curve, representative of 2 experiments, 8 rats/group. (B) Survival curve of rats treated daily for 14 days with either 50 mg/kg PPF or saline intraperitoneally beginning the day of tumor engraftment (P < .05). (C) Left is a representative T1-weighted MRI of PPF- or saline-treated rats on day 14. Right is a 3D reconstruction of tumor in saline-treated rat with measured tumor volume. (D) Hematoxylin and eosin staining of PPF- or saline-treated rat brains on day 14 (CPu = caudate putamen). (Scale bar, 100 μm).
Propentofylline Does Not Have a Direct Effect on CNS-1 Tumor Cells
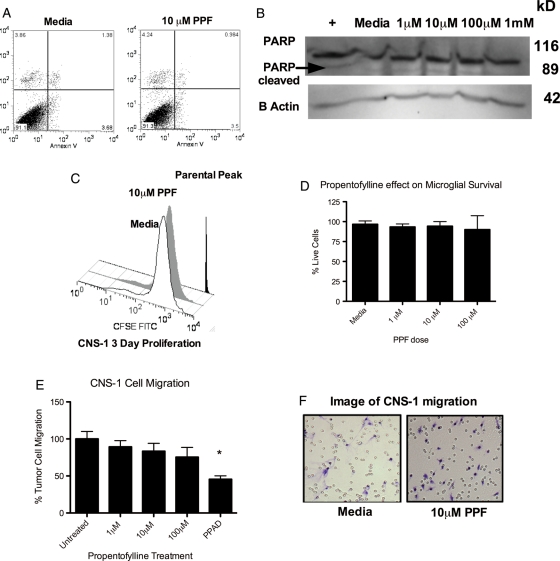
To specifically address the mechanism of action of PPF in the rat model of GBM, we first sought to determine whether PPF has a direct effect on CNS-1 cells. We first examined whether the decreased tumor volume results from PPF-induced apoptosis. CNS-1 cells cultured in vitro were challenged with PPF treatment (1–100 μM) and analyzed for increased apoptosis using Annexin V, a marker for early apoptosis. We found no evidence of enhanced early-stage apoptosis following PPF treatment using Annexin V and Propidium Iodide (marker for necrosis) staining at 24 h (Fig. 2A). No changes were seen at 5 h, 7 h, and 12 h (data not shown). In addition, no evidence of enhanced apoptosis was observed through caspase-dependent poly(ADP-ribose) polymerase (PARP) cleavage at 12 h (Fig. 2B), 5 h, and 24 h (data not shown). To further confirm that direct CNS-1 cell death did not occur as a result of PPF treatment, Hoechst staining was used to visualize condensed chromatin apoptosis. We observed no increase in condensing chromatin in the PPF-treated group at 5, 7, 12, and 24 h (data not shown), in concordance with the absence of apoptosis.
Fig. 2.
PPF does not directly affect CNS-1 tumor cells. (A) CNS-1 cells were treated with dose-dependent PPF and analyzed at 5, 24, and 72 h for Annexin V and PI. No change in Annexin V occurred with PPF treatment at 72 h (above), 24 h, or 5 h (data not shown). (B) Western blot showing no change in PARP cleavage with PPF treatment at 72 h (above), 5 h, or 24 h (data not shown). CNS-1 cells treated with 2 μM vincristine for 2 h were used as a positive control for PARP cleavage. Data are representative of 3 replicates/experiment, experiments were repeated at least twice. (C) CNS-1 cells were CFSE labeled on day 0 and analyzed by FACS anaylsis. There was no change in proliferation on day 3 (above), day 7, and day 10 (data not shown). (D) Graphical representation of the percent of live microglia cells following PPF treatment for 2 h, calculated by trypan blue staining. (E) CNS-1 tumor cells were treated with PPF or 500 nM Pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPAD), a P2X1-3,5-7 inhibitor, as a positive control, then migrated toward ADP. No change in migration occurred with treatment. (F) A representative photomicrograph of tumor cells treated with media alone or 100 μM PPF that have migrated (scale bar, 50 μm). Data are representative of 3 replicates/experimental group, experiment was repeated at least twice (*P < .05).
We then sought to determine whether PPF decreased tumor volume through inhibition of CNS-1 cell proliferation. CNS-1 cells were stained with CFSE dye and cultured in vitro, with proliferation observed after 1 day, 3 days (Fig. 2C), and 5 days of treatment (data not shown for 3 and 5 days). Moreover, PPF was screened for proliferative and apoptotic effects against human tumor cell lines using the National Cancer Institute's in vitro cancer screen, NCI-60 DTP human cell line assay (PPF [94150], NSC:752424). These results demonstrated that PPF has no direct anticancer/proliferative effect on all 60 different human tumor cell lines tested (Supplementary material, Fig. S1).
Previous studies have demonstrated that PPF inhibits glial migration in response to ADP.34 Consequently, we next sought to determine whether PPF inhibited GBM tumor cell migration. Although CNS-1 tumor cells demonstrated a strong migratory response to ADP, PPF did not inhibit tumor cell migration (Fig. 2D and E). Taken together, these results showed that PPF does not induce CNS-1 apoptosis, decrease CNS-1 proliferation, or inhibit CNS-1 cell migration. Our results support a novel mechanism of action in which PPF selectively targets the tumor microenvironment as opposed to directly acting on tumor cells.
Propentofylline Affects the CNS Microenvironment
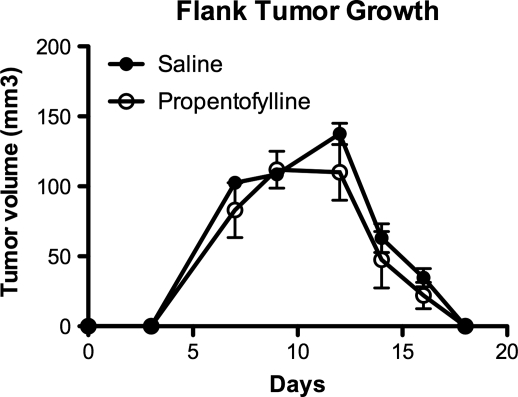
To determine whether PPF requires the CNS tumor microenvironment for its in vivo actions in tumor growth, CNS-1 cell growth was determined in a non-CNS tumor environment. Rats were injected in the right flank with CNS-1 tumor cells (1 × 105), followed by daily intraperitoneal injection of PPF beginning the day of tumor implantation (Fig. 3). Tumor volume was measured for 17 days, with no difference between PPF-treated and saline groups. These results show that PPF treatment has no effect on tumors engrafted in locations external from the CNS microenvironment. Furthermore, unlike the brain, these tumors spontaneously regressed in both PPF and saline groups after 2 weeks of growth (Fig. 3). This is contrary to the original cell line, which demonstrated flank tumor growth without rejection, indicating a phenotypic drift in CNS-1 cell line over time.28 These data demonstrate the importance of the tumor microenvironment for CNS tumor growth in vivo and indicate a role for PPF that is specific to the brain tumor microenvironment.
Fig. 3.
PPF does not decrease CNS-1 tumor cell growth on the flank of rats, external to CNS microenvironment. Rats were first engrafted in the flank with CNS-1 cells, then treated daily with either 50 mg/kg PPF or saline intraperitoneally beginning the day of tumor engraftment. Tumors were measured daily, with no statistically significant difference in tumor growth observed. Data are representative of 2 experiments, 5 rats/group.
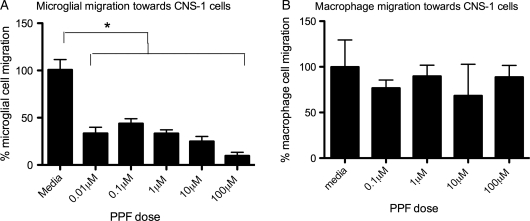
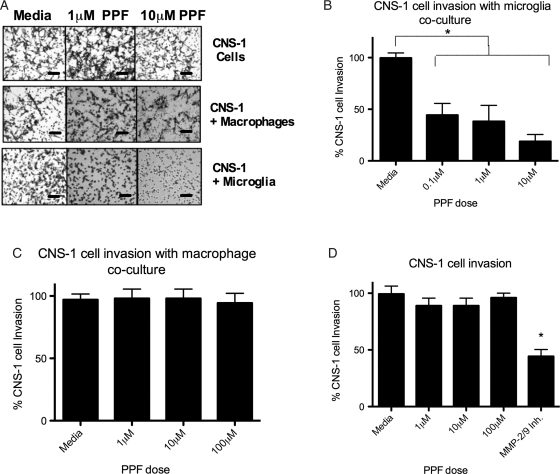
Propentofylline Decreases Microglia but not Macrophage Migration Toward Tumor Cells
Microglia and macrophages represent the predominant immune cells infiltrating gliomas. We next investigated PPF's actions on microglia in response to tumor cells. Microglia treated with PPF migrated toward CNS-1 cells at a lower rate (P< .05), compared with that of microglia treated with saline (Fig. 4A). PPF at a concentration of 0.01 μM decreased microglial migration by 50% after 2 h of treatment. With strong inhibition seen in these lower physiological relevant doses, a higher (100 μM) treatment was not studied. During each experiment, cell viability was determined after PPF treatment by trypan blue staining and cell counting. PPF did not change the viability of microglia or macrophages, indicating that the strong inhibitory effects on migration were not a result of cell death. In contrast, PPF treatment with concentrations as high as 10 μM had no effect on peripheral macrophage migration toward CNS-1 cells (Fig. 4B).
Fig. 4.
PPF decreases microglial but not macrophage migration toward CNS-1 tumor cells in an in vitro transwell migration assay. (A) Microglia were treated with PPF, then migrated toward CNS-1 cells. Migration of microglia in response to CNS-1 cells is significantly decreased with PPF treatment (*P < .05). (B) Peripheral macrophages were treated with PPF, then allowed to migrate toward CNS-1 cells. Data are representative of 3 replicates per experimental group, and the experiments were repeated 5 times with similar statistical results.
Propentofylline Decreases Tumor Invasion Only When Tumor Cells are Cocultured with Microglia
The ability of GBM tumors to invade through the extracellular matrix and migrate large distances is a primary cause of tumor recurrence and is supported by microglia and/or macrophages.37 We next asked whether PPF treatment decreases the invasiveness of CNS-1 tumor cells in the presence of microglia or macrophages. After 2 h of incubation, CNS-1 cells alone migrated through the Matrigel layer and through the transwell membrane pores, demonstrating the highly invasive properties of this cell line (Fig. 5A). CNS-1 cells cocultured with microglia or macrophages with no treatment increased or had no effect on invasion through the Matrigel layer toward CNS-1 supernatant, similar to what has been previously reported (Fig. 5A).11 A decrease in tumor cell invasion was observed only when CNS-1 tumor cells were cocultured with microglia, then treated with PPF for 2 h and migrated toward CNS-1 supernatant (Fig. 5A and B). At a concentration of 0.1 μM PPF, CNS-1 cell invasion is inhibited by 45% after 2 h of treatment in the presence of microglia (Fig. 5B). This inhibition with PPF was not observed with tumor cells alone treated with PPF or when tumor cells were co-cultured with peripheral macrophages and treated with PPF, indicating a specific mechanism targeting microglia (Fig. 5C and D).
Fig. 5.
PPF decreases CNS-1 invasion only when cocultured with microglia in an in vitro transwell matrigel invasion assay. CNS-1 cells were cultured alone or with microglia or macrophages and treated with PPF or media alone. CNS-1 cells then invaded through matrigel in response to CNS-1 cells. (A) A representative photomicrograph of tumor cells invasion with PPF (scale bar, 50 μm). (B) CNS-1 tumor cell invasion was significantly inhibited with PPF treatment when CNS-1 cells were cocultured with microglia (*P < .05). (C) CNS-1 tumor cell invasion was not inhibited with PPF treatment when CNS-1 cells were cocultured with macrophages. (D) CNS-1 tumor cell invasion was not inhibited with PPF treatment when CNS-1 cells were cultured alone. CNS-1 cells were treated with an MMP-2/MMP-9 inhibitor (100 μM) as a positive control for inhibition of CNS-1 invasion. Data are representative of 3 replicates/experimental group; experiment was repeated twice.
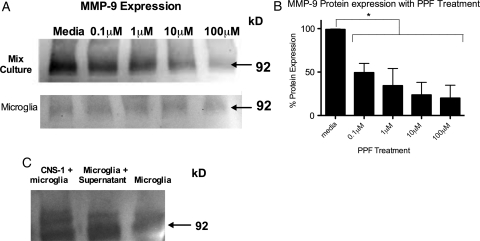
Propentofylline Blocks MMP-9 Expression in Microglia
We sought to address the mechanism of action of PPF on microglia in vitro. We observed that CNS-1 tumor cells alone do not express MMP-9, whereas microglia display a low basal expression, compared with CNS-1 and microglial coculture. When CNS-1 tumor cells and microglia were cocultured, we observed an increase in MMP-9 expression, compared with expression of microglia alone (Fig. 6B) (see Supplementary material, Fig. S2 for control gel). We determined that the increased MMP-9 expression in cocultures was from microglia in response to tumor cell stimulation (Fig. 6C). MMP-9 expression increased when microglia were cultured with CNS-1 cell supernatant (Fig. 6B). PPF treatment decreased MMP-9 expression in CNS-1 and microglia coculture (Fig. 6A). PPF at a dosage of 0.1 μM reduced MMP-9 expression by more than 40% (Fig. 6A). No MMP-9 expression was observed in macrophages cultured with CNS-1 cells. In addition, no MMP-9 was observed from CNS-1 tumor cells, as previously reported (data not shown).38
Fig. 6.
PPF decreases MMP-9 only in CNS-1 and microglia coculture. (A) A graphical representation of differential MMP-9 expression of CNS-1 tumor cells cultured with microglia in vitro and treated with PPF (*P < .05). Graph is representative of 3 Western blots from 3 replicates. (B) MMP-9 Western blot of CNS-1 tumor cells and microglia treated with PPF and microglia cells treated with PPF. (C) Western blot of MMP-9 expression in CNS-1 and microglia cocultured, microglia cultured in CNS-1 supernatant, and microglia cells cultured in media. Images are representative of 3 experiments.
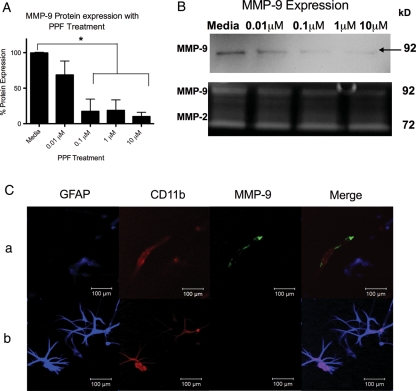
Propentofylline Blocks MMP-9 Expression in Microglia from Human Glioma Tissue
We next sought to determine whether PPF's effects were unique to the CNS-1 rat model or whether these mechanisms translate to human tumor cells. Cell culture from 3 human glioma WHO grade IV tissues, all positive for MMP-9 expression, demonstrated a significant (P < .05) decrease in MMP-9 expression following PPF treatment (Fig. 7A). Furthermore, gelatin zymography confirmed a decrease in active MMP-9, whereas MMP-2 levels did not decrease (Fig. 7B). To confirm that MMP-9 expression was mostly from infiltrated microglia into the tumor, immunocytochemistry was performed on the cell culture. MMP-9 expression was only identified in microglia (positive for CD11b, negative for GFAP) (Fig. 7C). Some tumor cells expressed CD11b, but these cells did not express MMP-9, confirming that expression is from resident microglia and not tumor cells expressing microglial markers (Fig. 7C).
Fig. 7.
PPF decreases MMP-9 in tumor-infiltrating microglia from human GBM tissue. (A) A graphical representation of differential MMP-9 expression of human GBM tissue in vitro and treated with PPF (*P < .05). Graph is representative of 3 Western blots from 3 replicates. (B) MMP-9 Western blot and gelatin zymography demonstrating a decrease in active MMP-9 of cell culture from human GBM tissue treated with PPF. Image is representative of 3 experiments. (C) Immunocytochemistry of cell culture obtained from human GBM tissue. Blue = GFAP, red = CD11b, green = MMP-9.
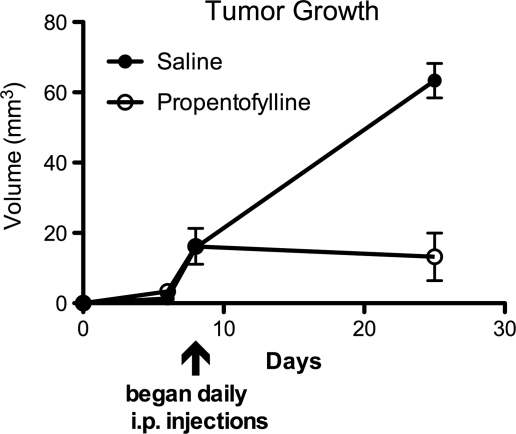
PPF Decreases CNS-1 Tumor Volume of an Established Tumor Model
To test PPF in a more translational model, rats were injected with CNS-1 tumor cells and tumors were allowed to grow until they were measureable by MRI. At this point (day 8), rats were injected daily with 50 mg/kg PPF of saline. PPF-treated rats demonstrated a significant reduction in tumor volume, compared with saline rats by day 25 (Fig. 8).
Fig. 8.
PPF decreases tumor volume of an established tumor. Rats were first engrafted with CNS-1 cells, then treated daily with either 50 mg/kg PPF or saline intraperitoneally beginning day 8 after tumor engraftment. MRI analysis was performed on days 4, 8, and 14. Treatments are significantly different (*P < .05). Tumor growth curve, representative of 2 experiments, 6 rats/group.
Discussion
We demonstrate in these studies that PPF, a glial modulating agent with known anti-inflammatory actions, (1) decreases brain tumor growth in vivo, (2) targets microglia and not tumor cells, (3) decreases microglial migration toward tumor cells, (4) decreases tumor cell invasion by targeting microglia, and (5) decreases MMP-9 expression in microglia. In addition and very importantly, PPF does not act directly on tumor cells to induce apoptosis or necrosis and inhibit brain tumor growth. Thus, it is not a classic chemotherapeutic agent. As such, it is devoid of the inherent toxic adverse effects associated with these drugs.
Beginning in the late 1990s, a link was established between the number of infiltrating macrophages and microglia and tumor grade.9 Presence of high numbers of CNS macrophages is a negative prognostic marker in a murine high-grade glioma model.39 Recently, research in GBM has focused on identifying and understanding the proneoplastic versus antineoplastic actions of microglia and macrophages.40 Postoperative glioma samples demonstrated that microglia and infiltrating macrophages are unable to activate T cells for an immune response.41 Furthermore, N9 microglia exposed to GL261 glioma cells increased expression of inhibitory cytokines, such as IL-10.42 In vivo work has also supported the role of the tumor microenvironment. After irradiation, CD11b-positive bone marrow–derived cells infiltrate tumors and support tumor recurrence in a U251 mouse model.43 It was recently demonstrated that glioma cancer stem cells induce the immunosuppressive phenotype in monocytes.44 These data suggest that microglia and macrophages may play an important role in the high recurrent rates of GBM in humans. We present data that additionally supports microglia's role in promoting tumor growth. Our studies highlight how brain tumor cells interact with microglia, increasing MMP-9 expression and supporting tumor invasion. Furthermore, we demonstrate that targeting microglia and not the tumor cells is sufficient to inhibit tumor cell invasion. Although tumor cells have the ability to mutate and become resistant to therapies, benign glial cells may lack these mechanisms, supporting their role as a therapeutic target.
Our study reveals the important communication between tumor cells and microglia. PPF is able to decrease MMP-9 expression and activation in microglia/tumor cell cocultures or microglia stimulated with tumor supernatant. We also demonstrate that this relationship does not occur with peripheral macrophages. Of importance, we used peritoneal macrophages for our comparative studies and not macrophages from the CNS tumor environment because of the challenges in separating this population. Our work may indicate that microglia play a more influential role in tumor growth and invasion than infiltrating macrophages, because PPF demonstrates specific actions on microglia in vitro. Our findings support the concept that microglia and macrophages possess very different functions even though they express similar surface and cytosolic expression markers. Of interest, a recent publication revealed that adult microglia derive from primitive myeloid progenitors before embryonic day 8, indicating that they are a distinct population from circulating monocytes found in the blood.45 Accordingly, it has been demonstrated that dendritic cells, macrophages, and microglia under steady-state and inflammatory conditions exhibit differential responses.46,47 Human monocytes were discovered to have increased migratory responses to the chemokine CCL2 and decreased IL-12p70 production in response to sphingosine-1-phosphate analog (FTY720) treatment, compared with human microglia.47 In addition, Schmid and colleagues recently discovered variable gene expression in several cytokines between resident microglia and peripheral macrophages in response to LPS.48 Furthermore, Nakagawa and colleagues24 observed differential expression of cell surface TNF in macrophages and microglia in a rodent GBM model. In line with our findings and of particular relevance for our study, it has been shown that the depletion of circulating macrophages, in the same CNS-1 glioma model used in our present study, does not have a significant impact on tumor growth.30 Our results described here add to these data and support the need to further differentiate and investigate the contribution of microglia versus peripheral macrophages to brain tumor growth.
We confirmed that PPF does not induce CNS-1 cell necrosis or apoptosis. Our results are contrary to previous studies that demonstrate apoptosis through Hoechst staining and cellular pathways in the U-251 cell line following PPF treatment.49–51 PPF was administered in vitro up to 3 mM in these studies (0.1 μM – 100 μM were used in our study), a concentration that is very high and beyond clinical relevance. Doses this high can produce toxic and off-target effects, possibly explaining the observed differences in our data.
There are some limitations in conducting these therapeutic studies in a glioma animal model.52 The established CNS-1 glioma cell line in clonotypic animals does not display the extensive heterogeneity of high-grade gliomas in humans. To address some of the shortcomings of an animal model, we performed in vitro studies with human GBM tissue samples in which we observed similar effects using PPF (see Fig. 7). Because of the challenges and difficulties of separating resident microglia and infiltrating macrophage populations in the CNS, we chose to initially perform our studies in vitro with cultured microglia and macrophages. This enabled adequate number of cells for experiments and identification of mechanisms in vitro. In the future, decreases in MMP-9 as a result of PPF treatment targeting microglia will need to be confirmed in vivo. We also are investigating the intracellular signaling that results in the downstream effect of decreased MMP-9 expression with PPF treatment. In the future, we plan to investigate PPF's effects on an established tumor.
Our study not only demonstrates and confirms the protumorigenic role of microglia in the CNS-1 rodent GBM model, but provides evidence that PPF may be a new drug therapy for human GBM that primarily targets microglia. Of most importance, PPF has been administered extensively in patients for different diseases, with minimal adverse effects.21 This is crucial, because adverse effects are often the dose-limiting factor in effective cancer treatments that patients currently face. As a result of its low toxicity, PPF may be useful in combination with chemotherapies that target tumor cells or after surgical resection to decrease recurrence.
In summary, we present a possible new drug for GBM treatment that targets microglia, decreasing brain tumor growth in vivo, and further supports a different functional role of microglia and infiltrating macrophages in the tumor microenvironment.
Supplementary Material
Conflict of interest statement. None declared.
Funding
This work was supported by NIH/NIDA (1RO1DA025211) and NIH (T32 A107363).
Supplementary Material
Acknowledgments
We thank Dr. William Hickey for his expert help with the CNS-1 cell line, Dr. David Roberts for supplying the human GBM tissue, Dr. Christian NDong for his comments on the manuscript, and Dr. Pablo Valdes for his imaging expertise and comments on the manuscript.
References
- 1.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64(6):479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 2.Meyer MA. Malignant gliomas in adults. N Engl J Med. 2008;359(17):1850. doi: 10.1056/NEJMc086380. author reply 1850. [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. doi:10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro WR. Therapy of adult malignant brain tumors: what have the clinical trials taught us? Semin Oncol. 1986;13(1):38–45. [PubMed] [Google Scholar]
- 5.Tuettenberg J, Grobholz R, Seiz M, et al. Recurrence pattern in glioblastoma multiforme patients treated with anti-angiogenic chemotherapy. J Cancer Res Clin Oncol. 2009;135(9):1239–1244. doi: 10.1007/s00432-009-0565-9. doi:10.1007/s00432-009-0565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benesch M, Weber-Mzell D, Gerber NU, et al. Ependymoma of the spinal cord in children and adolescents: a retrospective series from the HIT database. J Neurosurg Pediatr. 2010;6(2):137–144. doi: 10.3171/2010.5.PEDS09553. doi:10.3171/2010.5.PEDS09553. [DOI] [PubMed] [Google Scholar]
- 7.Gabelloni P, Da Pozzo E, Bendinelli S, et al. Inhibition of metalloproteinases derived from tumours: new insights in the treatment of human glioblastoma. Neuroscience. 2010;168(2):514–522. doi: 10.1016/j.neuroscience.2010.03.064. doi:10.1016/j.neuroscience.2010.03.064. [DOI] [PubMed] [Google Scholar]
- 8.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17(1):6–10. doi: 10.1016/j.jocn.2009.05.006. doi:10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92(3):288–293. doi: 10.1007/s004010050520. doi:10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- 10.Bettinger I, Thanos S, Paulus W. Microglia promote glioma migration. Acta Neuropathol. 2002;103(4):351–355. doi: 10.1007/s00401-001-0472-x. doi:10.1007/s00401-001-0472-x. [DOI] [PubMed] [Google Scholar]
- 11.Markovic DS, Glass R, Synowitz M, Rooijen N, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64(9):754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. doi:10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 12.Wagner S, Czub S, Greif M, et al. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82(1):12–16. doi: 10.1002/(sici)1097-0215(19990702)82:1<12::aid-ijc3>3.0.co;2-o. doi:10.1002/(SICI)1097-0215(19990702)82:1<12::AID-IJC3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Huettner C, Czub S, Kerkau S, Roggendorf W, Tonn JC. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 1997;17(5A):3217–3224. [PubMed] [Google Scholar]
- 14.Esteve PO, Chicoine E, Robledo O, et al. Protein kinase C-zeta regulates transcription of the matrix metalloproteinase-9 gene induced by IL-1 and TNF-alpha in glioma cells via NF-kappa B. J Biol Chem. 2002;277(38):35150–35155. doi: 10.1074/jbc.M108600200. doi:10.1074/jbc.M108600200. [DOI] [PubMed] [Google Scholar]
- 15.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–5297. doi: 10.1200/JCO.2009.23.5556. doi:10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SY, Lee EJ, Woo MS, et al. Inhibition of matrix metalloproteinase-9 gene expression by an isoflavone metabolite, irisolidone in U87MG human astroglioma cells. Biochem Biophys Res Commun. 2008;366(2):493–499. doi: 10.1016/j.bbrc.2007.11.178. doi:10.1016/j.bbrc.2007.11.178. [DOI] [PubMed] [Google Scholar]
- 17.Rao JS, Yamamoto M, Mohaman S, et al. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996;14(1):12–18. doi: 10.1007/BF00157681. doi:10.1007/BF00157681. [DOI] [PubMed] [Google Scholar]
- 18.Groves MD, Puduvalli VK, Hess KR, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20(5):1383–1388. doi: 10.1200/JCO.2002.20.5.1383. doi:10.1200/JCO.20.5.1383. [DOI] [PubMed] [Google Scholar]
- 19.Levin VA, Phuphanich S, Yung WK, et al. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J Neurooncol. 2006;78(3):295–302. doi: 10.1007/s11060-005-9098-5. doi:10.1007/s11060-005-9098-5. [DOI] [PubMed] [Google Scholar]
- 20.Fingleton B. MMPs as therapeutic targets–still a viable option? Semin Cell Dev Biol. 2008;19(1):61–68. doi: 10.1016/j.semcdb.2007.06.006. doi:10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweitzer S, De Leo J. Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol. 2011;(200):235–250. doi: 10.1007/978-3-642-13443-2_8. doi:10.1007/978-3-642-13443-2_8. [DOI] [PubMed] [Google Scholar]
- 22.Salimi S, Fotouhi A, Ghoreishi A, et al. A placebo controlled study of the propentofylline added to risperidone in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):726–732. doi: 10.1016/j.pnpbp.2007.11.021. doi:10.1016/j.pnpbp.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Kittner B. Clinical trials of propentofylline in vascular dementia. European/Canadian Propentofylline Study Group. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S166–S171. [PubMed] [Google Scholar]
- 24.Nagata K, Ogawa T, Omosu M, Fujimoto K, Hayashi S. In vitro and in vivo inhibitory effects of propentofylline on cyclic AMP phosphodiesterase activity. Arzneimittelforschung. 1985;35(7):1034–1036. [PubMed] [Google Scholar]
- 25.Fredholm BB, Lindstrom K. The xanthine derivative 1-(5′-oxohexyl)-3-methyl-7-propyl xanthine (HWA 285) enhances the actions of adenosine. Acta Pharmacol Toxicol (Copenh) 1986;58(3):187–192. doi: 10.1111/j.1600-0773.1986.tb00093.x. doi:10.1111/j.1600-0773.1986.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 26.Si Q, Nakamura Y, Ogata T, Kataoka K, Schubert P. Differential regulation of microglial activation by propentofylline via cAMP signaling. Brain Res. 1998;812(1–2):97–104. doi: 10.1016/s0006-8993(98)00954-8. doi:10.1016/S0006-8993(98)00954-8. [DOI] [PubMed] [Google Scholar]
- 27.Si QS, Nakamura Y, Schubert P, Rudolphi K, Kataoka K. Adenosine and propentofylline inhibit the proliferation of cultured microglial cells. Exp Neurol. 1996;137(2):345–349. doi: 10.1006/exnr.1996.0035. doi:10.1006/exnr.1996.0035. [DOI] [PubMed] [Google Scholar]
- 28.Kruse CA, Molleston MC, Parks EP, Schiltz PM, Kleinschmidt-DeMasters BK, Hickey WF. A rat glioma model, CNS-1, with invasive characteristics similar to those of human gliomas: a comparison to 9L gliosarcoma. J Neurooncol. 1994;22(3):191–200. doi: 10.1007/BF01052919. doi:10.1007/BF01052919. [DOI] [PubMed] [Google Scholar]
- 29.Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85(2):133–148. doi: 10.1007/s11060-007-9400-9. doi:10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kielian T, van Rooijen N, Hickey WF. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. J Neurooncol. 2002;56(1):1–12. doi: 10.1023/a:1014495613455. doi:10.1023/A:1014495613455. [DOI] [PubMed] [Google Scholar]
- 31.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. doi:10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 32.Samkoe KS, Chen A, Rizvi I, et al. Imaging tumor variation in response to photodynamic therapy in pancreatic cancer xenograft models. Int J Radiat Oncol Biol Phys. 2010;76(1):251–259. doi: 10.1016/j.ijrobp.2009.08.041. doi:10.1016/j.ijrobp.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nutile-McMenemy N, Elfenbein A, Deleo JA. Minocycline decreases in vitro microglial motility, beta1-integrin, and Kv1.3 channel expression. J Neurochem. 2007;103(5):2035–2046. doi: 10.1111/j.1471-4159.2007.04889.x. doi:10.1111/j.1471-4159.2007.04889.x. [DOI] [PubMed] [Google Scholar]
- 34.Horvath RJ, Nutile-McMenemy N, Alkaitis MS, Deleo JA. Differential migration, LPS-induced cytokine, chemokine, and NO expression in immortalized BV-2 and HAPI cell lines and primary microglial cultures. J Neurochem. 2008;107(2):557–569. doi: 10.1111/j.1471-4159.2008.05633.x. doi:10.1111/j.1471-4159.2008.05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troeberg L, Nagase H. Zymography of metalloproteinases. Curr Protoc Protein Sci. 2004;15:1–15. doi: 10.1002/0471140864.ps2115s33. [DOI] [PubMed] [Google Scholar]
- 36.Hamachi Y, Nakashima MN, Nakashima K. High-performance liquid chromatography with peroxyoxalate chemiluminescence determination of propentofylline concentrations in rat brain microdialysate. J Chromatogr B Biomed Sci Appl. 1999;724(1):189–194. doi: 10.1016/s0378-4347(98)00587-8. doi:10.1016/S0378-4347(98)00587-8. [DOI] [PubMed] [Google Scholar]
- 37.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3(7):489–501. doi: 10.1038/nrc1121. doi:10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 38.Regina A, Demeule M, Berube A, Moumdjian R, Berthelet F, Beliveau R. Differences in multidrug resistance phenotype and matrix metalloproteinases activity between endothelial cells from normal brain and glioma. J Neurochem. 2003;84(2):316–324. doi: 10.1046/j.1471-4159.2003.01521.x. doi:10.1046/j.1471-4159.2003.01521.x. [DOI] [PubMed] [Google Scholar]
- 39.Kong LY, Wu AS, Doucette T, et al. Intratumoral mediated immunosuppression is prognostic in genetically engineered murine models of glioma and correlates to immunotherapeutic responses. Clin Cancer Res. 2010;16(23):5722–5733. doi: 10.1158/1078-0432.CCR-10-1693. doi:10.1158/1078-0432.CCR-10-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. doi:10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 41.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. doi:10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57(13):1458–1467. doi: 10.1002/glia.20863. doi:10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 43.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120(3):694–705. doi: 10.1172/JCI40283. doi:10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. doi: 10.1093/neuonc/noq082. doi:10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. doi:10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durafourt BA, Lambert C, Johnson TA, Blain M, Bar-Or A, Antel JP. Differential responses of human microglia and blood-derived myeloid cells to FTY720. J Neuroimmunol. 2010;230(1–2):10–16. doi: 10.1016/j.jneuroim.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Lambert C, Ase AR, Seguela P, Antel JP. Distinct migratory and cytokine responses of human microglia and macrophages to ATP. Brain Behav Immun. 2010;24(8):1241–1248. doi: 10.1016/j.bbi.2010.02.010. doi:10.1016/j.bbi.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Schmid CD, Melchior B, Masek K, et al. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. J Neurochem. 2009;109(Suppl 1):117–125. doi: 10.1111/j.1471-4159.2009.05984.x. doi:10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamada H, Murakami H, Satone A, et al. [Up-regulation of NGF, trkA, Fas, down-regulation of bcl-2, and induction of apoptosis by propentofylline in human glioma cell lines] No To Shinkei. 1996;48(11):1022–1028. [PubMed] [Google Scholar]
- 50.Satone A, Ukita H, Murakami H, et al. [Neural protective agents, propentofylline (PPF) could induce apoptotic cell death in the human glioma cells: analysis of Bcl-2 and Bax alpha/Bax beta expressions] No To Shinkei. 1998;50(4):330–337. [PubMed] [Google Scholar]
- 51.Yamaguchi H, Ukita H, Murakami H, et al. [Induction of apoptosis through NGF/p75NTR in human glioma cells treated with propentofylline] No To Shinkei. 1998;50(5):407–414. [PubMed] [Google Scholar]
- 52.Jacobs VL, Valdes PA, Hickey WF, De Leo JA. Current review of in vivo GBM rodent models: emphasis on the CNS-1 tumour model. ASN Neuro. doi: 10.1042/AN20110014. [Published online ahead of print July 8, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.