Abstract
Vorinostat, a histone deacetylase (HDAC) inhibitor, has shown evidence of single-agent activity in glioblastoma (GBM), and in preclinical studies, we have demonstrated significant synergistic cytotoxicity between HDAC inhibitors and proteasome inhibitors in GBM cell lines. We therefore conducted a phase II trial to evaluate the efficacy of vorinostat in combination with the proteasome inhibitor bortezomib in patients with recurrent GBM. Vorinostat was administered at a dose of 400 mg daily for 14 days of a 21-day cycle, and bortezomib was administered at a dose of 1.3 mg/m2 intravenously on days 1, 4, 8, and 11 of the cycle. A total of 37 patients were treated, and treatment was well tolerated: grade 3, 4 nonhematologic toxicity occurred in 30% of patients and consisted mainly of fatigue (14%) and neuropathy (5%); grade 3, 4 hematologic toxicity occurred in 37% of patients and consisted of thrombocytopenia (30%), lymphopenia (4%), and neutropenia (4%). The trial was closed at the predetermined interim analysis, with 0 of 34 patients being progression-free at 6 months. One patient achieved a partial response according to the Macdonald criteria. The median time to progression for all patients was 1.5 months (range, 0.5–5.6 months), and median overall survival (OS) was 3.2 months. Patients who had received prior bevacizumab therapy had a shorter time to progression and OS, compared with those who had not. On the basis of the results of this phase II study, further evaluation of the vorinostat-bortezomib combination in GBM patients in this dose and schedule is not recommended.
Keywords: bortezomib, phase II trial, recurrent glioblastoma, vorinostat
Glioblastoma (GBM) is a refractory malignancy. Despite surgical resection, external beam radiation therapy, and chemotherapy, median survival is only 15–18 months.1,2 There is an urgent need to develop novel therapeutic strategies for this disease.
Vorinostat (suberoylanilide hydroxamic acic; MW = 264) is a linear hydroxamic acid that inhibits class I and class II histone deacetylases (HDACs),3,4 causing hyperacetylation of core histone proteins H2A, H2B, H3, and H4.5 Hyperacetylation of nuclear histones causes changes in chromatin structure that ultimately result in changes in gene expression. In addition, the acetylation state of several other proteins, including α-tubulin and hsp90, increases after treatment with vorinostat.6 The biological effects of HDAC inhibition by vorinostat are widespread. Indeed, treatment with HDAC inhibitors results in changes in 2%–10% of all expressed genes.7,8 Vorinostat and other HDAC inhibitors have demonstrated preclinical activity in a broad range of solid tumor and hematological malignancies, both in in vitro and animal models.6
Several HDAC inhibitors, including vorinostat, have demonstrated preclinical activity against GBM cell lines and glioma models.9–11 On the basis of this strong preclinical rationale, North Central Cancer Treatment Group (NCCTG) completed a phase II trial of vorinostat in patients with recurrent GBM.12 Vorinostat was well tolerated in this patient population and met its primary end point, with 9 of the first 52 patients being progression-free at 6 months (15% progression-free at 6 months [PFS6], compared with a <10% PFS6 of historic controls). Paired baseline and post–vorinostat treatment tumor samples demonstrated increased histone acetylation consistent with the expected target effect. Vorinostat-based combination regimens therefore represent a rational next step to build on the modest single agent activity, while capitalizing on the favorable toxicity profile.
Bortezomib (N-pyrazinecarbonyl-L-phenylalanine-L-leucine boronic acid) is a small, cell-permeable molecule, entering via passive diffusion, that specifically and selectively inhibits the proteasome by binding in a reversible manner. It is a modified dipeptidyl boronic acid derived from leucine and phenylalanine that inhibits the 20S proteasome with a Ki of 0.6 nM. The ordered, temporal degradation of numerous key short-lived regulatory proteins by the proteasome (such as p53, p21, p27, cyclins, cyclin-dependent kinase inhibitors, and tumor suppressors) is required for cell-cycle progression, cell survival, and metastasis.13 The major biological effects of bortezomib include inhibition of NF-κB, CDK activity, angiogenesis, and cellular adhesion and induction of PTEN, p53, p21, p27, ROS, and apoptosis.14,15
There is preclinical evidence that bortezomib has antitumor activity against malignant glioma cell lines. Bortezomib induces growth arrest and apoptosis in established human GBM cell lines.16 The observed growth inhibition is attributable to a G2/M phase cell-cycle arrest and is associated with increased expression of the cell-cycle inhibitors p21WAF1 and p27KIP1 and decreased expression of cyclin dependant kinases. Bortezomib also activated apoptosis in 35%–40% of GBM lines, in part through stimulation of the pro-apoptotic kinase JNK and down regulation of the prosurvival proteins Bcl-2 and Bcl-xl. With use of 2 different human GBM cell lines, bortezomib demonstrated higher cytotoxicity, compared with a panel of anti cancer agents, including temozolamide and carboplatin.17 Early evidence of clinical activity has been demonstrated in a phase I trial in patients with recurrent GBM.18 Although the primary end point of the trial was to determine the maximum tolerated dose of bortezomib, 2/66 patients experienced a partial response. Of importance, proteasome activity was inhibited by 79% in whole blood at bortezomib doses below the maximum tolerated dose.
We have demonstrated that the combination of HDAC and proteasome inhibitors has in vitro synergistic cytotoxicity in GBM cell lines and short-term cultures of patient-derived tumor samples maintained as mouse xenografts.19 Cytoxicity was assessed using multiple assays for cell proliferation and viability, including MTS assays, annexin V/propidium iodide staining with flow cytometry, and morphological analysis of apoptotic cells using DAPI staining. Synergisitic interactions were confirmed using the median effect method of Chou and Talalay,20 which was partially mediated by stimulation of Bax conformational changes and subsequent mitochondrial translocation that initiated the apoptotic cascade. In preparation for this trial, we conducted preclinical in vitro experiments testing the vorinostat-bortezomib combination in short-term cultures derived from tumor samples from patients with GBM and maintained as mouse xenografts. Synergistic in vitro cytotoxicity between vorinostat and bortezomib was also demonstrated (B. Friday and C. Yu, unpublished data). Thus, the synergism between HDAC and proteasome inhibitors against glioma cell lines appears to represent a class effect.
The combination of vorinostat and bortezomib has been tested in 2 phase I trials.21,22 The doses determined to be the MTD in both studies were similar (vorinostat at 400 mg and bortezomib at 1.3 mg/m2). The combination has a reasonable toxicity profile, with thrombocytopenia being the most common toxicity seen in both studies, and demonstrated encouraging phase I activity in heavily pretreated patients with multiple myeloma and sarcoma.
On the basis of the rationale described above, this study was designed to assess the clinical efficacy of vorinostat in combination with bortezomib in patients with recurrent GBM.
Patients and Methods
Patient Eligibility Criteria
Eligible patients were 18 years of age or older and had a histologic confirmation of GBM or gliosarcoma at primary diagnosis or recurrence. Patients were required to be receiving a stable dose of corticosteroids or no corticosteroids for 1 week or more before their baseline imaging. A minimum of 8 weeks from completion of radiation therapy was required for study entry. Patients could have received no more than one prior chemotherapy regimen for progressive or recurrent disease and had to have their last chemotherapy treatment at least 4 weeks or more prior to study entry (≥6 weeks if nitrosourea was administered). They were also required to have an Eastern Cooperative Oncology Group performance score of 0–2; acceptable hematologic function, defined as absolute neutrophil count ≥ 1500 neutrophils/μL, platelet count ≥100 000 platelets/μL, and hemoglobin level ≥8 g/dL; adequate hepatic and renal function, defined as total bilirubin level less than or equal to the upper limit of normal (ULN), aspartate aminotransferase level ≤ 3 × ULN, and creatinine level ≤1.5 × ULN. Patients who were receiving valproic acid (or other HDAC inhibitors) or enzyme-inducing anti-epileptic drugs should have had these discontinued for at least 2 weeks prior to study entry to become eligible. Patients receiving enzyme-inducting anti-epileptic drugs, including carbamazepine, phenytoin, fosphenytoin, phenobarbital, and primidone, were excluded. Patients with grade 2 or greater neuropathy at baseline were excluded from the study. Patients were required to sign an Institutional Review Board–approved consent form prior to study entry.
Treatment Administration
Vorinostat was administered at a dose of 400 mg orally once daily on days 1–14 of a 21-day cycle. Bortezomib was administered at a dose of 1.3 mg/m2 on days 1, 4, 8, and 11 of a 21-day cycle. Toxicity was graded according to the Common Terminology Criteria, version 3.0. Patients with grade 2 or less toxicity continued treatment at the starting dose until progressive disease. For the first occurrence of grade 3 hematological and nonhematological toxicity, vorinostat and bortezomib were reduced by 25% in subsequent cycles, but the toxicity had to improve to less than or equal to grade 1 prior to resuming therapy. For recurrent grade 3 or grade 4 toxicity after initial dose reduction, doses of both medications were reduced by an additional 33% (vorinostat) and 25% (bortezomib). With further grade 3 or greater toxicity after the second dose reduction, patients were withdrawn from study treatment. If the first occurrence of significant toxicity was grade 4, doses were initially reduced by 50% (vorinostat) and 40% (bortezomib). With recurrent grade 3 or higher toxicity, patients were withdrawn from study treatment. For grade 3 or greater neuropathy, only the bortezomib was dose reduced.
Definition of Response
Neuroimaging with MRI was performed at baseline, before the third treatment cycle, and every second cycle thereafter. For patients with measurable disease, a modification of the Macdonald criteria were used for response assessment.23
For patients with evaluable but not measurable disease, regression was defined as unequivocal reduction in size of contrast enhancement or decrease in mass effect as determined by primary physician and quality control physicians and no new lesion, with the patient receiving stable or decreased corticosteroid dose. A complete response was defined as the total disappearance of all tumor with patient off corticosteroids or only receiving adrenal replacement maintenance. Progression was defined as unequivocal increase in size of contrast enhancement or increase in mass effect as assessed by primary physician and quality control physicians or appearance of new lesions. Patients with imaging findings not meeting criteria for complete response, regression, or progression were determined to have stable disease.
Statistical Considerations and Methodology
A 1-stage phase II design with interim analysis based on Simon's MinMax design was used.24 The primary end point of the trial was the percentage of patients alive and PFS6. Secondary end points included confirmed tumor response, overall survival, and time to progression. The study was designed to test the hypothesis that addition of bortezomib to vorinostat would increase the PFS6 rate from 15%, which is the PFS6 rate observed in the NCCTG single agent vorinostat trial,12 to more than 30%. The trial had 91% power, with an α error of 0.10 to declare the regimen active, if the true PFS6 rate met or exceeded 30%. The study design required a total of 53 patients. An interim analysis was planned after the first 34 evaluable patients had been followed up for at least 6 months; the study would continue if more than 5 successes were observed in these 34 patients.
Time to progression was defined as time from study entry to disease progression; patients who died were considered to have disease progression at time of death unless there was documented evidence that no progression occurred before death. Overall survival was defined as time from study entry to death from any cause. Patients who have not died or experienced disease progression were censored at last known follow-up. Associations of categorical baseline outcome and translational data were tested using χ2 and Fisher's exact tests. Comparisons of continuous baseline, outcome, and translational data were tested using Wilcoxon rank-sum test. Survival and time to progression curves were compared using the log-rank test; Cox proportional hazards models were used to assess the relationship between time-to-event end points and outcome.
Results
Patient Characteristics
Thirty-eight patients were enrolled in the study between from August 29, 2008 through February 15, 2010; one patient was excluded from subsequent analysis because of cancellation prior to treatment initiation. Table 1 lists the characteristics of the 37 patients participating in the trial. Most of the patients (59%) had received prior therapy for recurrent disease. Prior therapies for glioblastoma, both as initial therapy and for recurrent disease, included temozolamide (100%), bevacizumab (43%), and nitrosoureas (8%). A significant number of patients were receiving corticosteroids at the time of study enrollment (59%).
Table 1.
Patient Baseline Characteristics (n = 37)
| Characteristic | No. | % |
|---|---|---|
| Sex | ||
| Female | 10 | 27 |
| Male | 27 | 73 |
| Age, years | ||
| Median | 51.5 | |
| Range | 33–80 | |
| Performance Score | ||
| 0 | 8 | 22 |
| 1 | 19 | 51 |
| 2 | 10 | 27 |
| No. of prior chemotherapy regimens for recurrent disease | ||
| 0 | 15 | 41 |
| 1 | 22 | 59 |
| Prior temozolomide | ||
| Adjuvant | 37 | 100 |
| Recurrent | 7 | 19 |
| Prior bevacizumab | ||
| Yes | 16 | 43 |
| No | 21 | 57 |
| Prior nitrosourea | ||
| Yes | 3 | 8 |
| No | 34 | 92 |
| Corticosteroid therapy at enrollment | ||
| Yes | 22 | 59 |
| No | 15 | 41 |
Response and Outcome Assessment
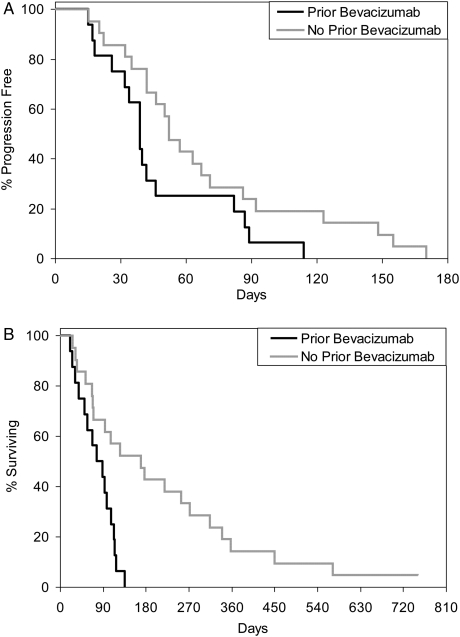
The trial failed to meet the interim analysis threshold for continuation. None of the first 34 patients were progression free at 6 months. All 37 patients completed treatment, with the median number of treatment cycles being 2 (range, 1–8 cycles). Reasons for study withdrawal included disease progression (32 of 37), patient refusal (3 of 27), and adverse events (2 of 37). The median time to progression was 1.5 months (range, 0.5–5.6 months). The median overall survival from study entry was 3.2 months (range, 0.7–24.8 months), with one patient still alive. Patients who had received prior bevacizumab therapy (n = 16) had a shorter median time to progression (1.3 months), compared with those who had no prior bevacizumab therapy (1.7 months; P = .0627). Similarly, median OS was shorter in the group who received prior bevacizumab, as compared with those who did not (2.7 vs 5.6 months; P = .0017). Kaplan-Meier plots demonstrating time to progression and OS relative to prior bevacizumab therapy are presented in Fig. 1. Patient characteristics were analyzed for differences between the bevacizumab-naive and bevacizumab-refractory groups, including sex, age, prior therapies, and corticosteroid use. Only the number of prior chemotherapy regimens was statistically different between these 2 groups, with bevacizumab-pretreated patients having received a significantly higher number of prior chemotherapy regimens (P = .0024). Despite the poor prognosis following progression during bevacizumab therapy and study ptotocol, the patients who had received prior bevacizumab therapy had a longer median overall survival, as measured from initial GBM diagnosis (16.7 vs 13.9 months). Objective responses were infrequent. Only one patient achieved a partial objective response.
Fig. 1.
Kaplan–Meier plots stratified by prior bevacizumab use relative to time to progression (TTP); (A) and overall survival (OS); (B). Prior bevacizumab treatment is associated with a reduced TTP, but the difference was not statistically significant (P = .0627). Prior bevacizumab is associated with a significantly reduced OS (P = .0017).
Toxicity
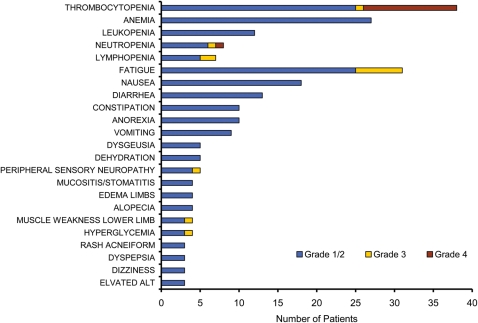
Figure 2 summarizes treatment-related toxicity observed in the trial. Grade 3–4 hematologic toxicity was observed in 37% of the patients, with the most common toxicities being thrombocytopenia (2% grade 3, 28% grade 4), neutropenia (2% grade 3, 2% grade 4), and lymphopenia (4% grade 3). The overall incidence of grade 3–4 nonhematologic toxicity was 30%, with the most common toxicity being fatigue (14% grade 3). Overall, 14 (56%) of the 25 patients who received multiple treatment cycles required dose reduction as a result of toxicity. Adverse events were the reason for study withdrawal for 2 (5%) of the 37 patients treated in the study.
Fig. 2.
Most frequently observed treatment-related toxicities for patients with glioblastoma (GBM) receiving vorinostat and bortezomib. Most toxicities were grade 1 and 2. The most frequent hematologic grade 3 and 4 toxicity was thrombocytopenia, and the most frequent nonhematologic toxicity was fatigue.
Discussion
Combining HDAC inhibition with vorinostat and proteasome inhibition with bortezomib represents a rational therapeutic combination in GBM treatment. A prior single-agent study using vorinostat in recurrent GBM demonstrated modest single-agent activity, with a 15% PFS6 rate, compared with the less than 10% PFS6 rate of historical controls.12 Clinical activity has also been described for the proteasome inhibitor bortezomib in malignant glioma, with 2 patients achieving a partial response in a phase I trial.18 Preclinical synergistic cytoxicity between HDAC inhibitors and proteasome inhibitors has been demonstrated in glioma cell lines,19 which were confirmed in this report using vorinostat and bortezomib. The combination exerts a synergistic cytoxic effect in glioma cell lines that is mediated, in part, through stimulation of Bax translocation to the mitochondria and subsequent apoptotic cell death.19 Preclinical data also support synergisitic cytotoxicity between HDAC and proteasome inhibitors in a variety of other cancer types, including squamous cell head and neck,25 cutaneous T-cell lymphoma,26 hepatoma,27 and multiple myeloma.28 This NCCTG study was undertaken to evaluate the efficacy of vorinostat in combination with bortezomib in patients with recurrent GBM.
Despite a strong scientific rationale supporting the efficacy of the combination, the trial was closed at the first interim efficacy analysis, because none of the first 34 patients were progression free at 6 months. The median overall survival of the 37 patients who received treatment was only 3.2 months. These data compare unfavorably with the single-agent vorinostat study, in which the PFS6 rate was 15% and the median overall survival was 5.7 months.12 Although these differences may just reflect statistical variation because of the relatively small patient numbers, they also may suggest that there is an antagonistic interaction between bortezomib and vorinostat in vivo, despite the in vitro synergy.19 Although this study did not include pharmacokinetic analyses, a phase I study of vorinostat and bortezomib in patient with multiple myeloma using a similar dosing schedule did not find a significant difference between vorinostat pharmacokinetics as a single agent, compared with the combination,21 when vorinostat was administered once daily, as in this trial.
Vorinostat was administered in a twice daily schedule in the prior single-agent study in patients with GBM. Because of the half-life of the agent, ∼2 h for vorinostat, the administration schedule in the 2 trials has likely resulted in different peak and trough vorinostat exposures, which could also have impacted on clinical efficacy.
Compared with other anatomical cancer sites, the brain provides unique therapeutic challenges. Access of drugs through the blood-brain barrier is of particular concern. There is convincing evidence that vorinostat can access the central nervous system (CNS). First, vorinostat was shown to cross the blood-brain Barrier in a mouse model of Huntington's disease.29 Second, vorinostat inhibited the growth of GL26 GBM cells implanted into the brains of mice.11 Finally, vorinostat treatment induced accumulation of acetylated histones H2B, H3, and H4 in patients with recurrent GBM.12 Unlike vorinostat, there is no clear evidence that bortezomib can cross an intact blood-brain barrier or penetrate into CNS tumors. In rats treated with bortezomib using a multidose regimen similar to this trial, bortezomib was found at very low concentrations in the CNS.30 Similarly, bortezomib only reaches low concentrations in the CNS of nonhuman primates.31 These observations are corroborated by clinical experience. In one report, a patient with progressive multiple myeloma was treated with bortezomib and had a good hematological response.32 Unfortunately, the patient developed progressive CNS myeloma despite the ongoing systemic hematological response. The lack of efficacy observed in this trial may therefore also reflect the inability of bortezomib to access the CNS at concentrations needed to exert synergistic cytotoxicity in combination with vorinostat.
The statistical design for this study was based on the results of N047B, a phase II NCCTG trial of vorinostat in recurrent GBM.12 Since completion of N047B, the therapeutic landscape for patients with recurrent GBM has changed substantially with the Food and Drug Administration–accelerated approval of the anti-VEGF antibody bevacizumab. After study entry, bevacizumab pretreatment was associated with significantly shorter PFS and OS. This is unlikely to represent a negative effect of the regimen, because accumulating data suggest that progression after bevacizumab therapy is associated with poor prognosis and rapid clinical decline.33–36 In addition to prior bevacizumab exposure, however, other negative prognostic factors might have contributed to the worse outcome in these patients. For example, bevacizumab-refractory patients had also received a significantly higher number of prior chemotherapy regimens (P = .0024). Although the possible impact of prior bevacizumab therapy may have negatively affected our results, even among the 21 patients treated without prior bevacizumab exposure, none were progression free at 6 months. Nevertheless, these data, in conjunction with data derived from other groups,34–36 support that trials in patients with recurrent GBM should be separately powered depending on prior bevacizumab therapy.
In summary, despite a strong scientific and clinical rationale for combining vorinostat and bortezomib in patients with recurrent GBM, this combination is clinically ineffective, and further development of this combination in treatment of recurrent GBM is not warranted.
Conflict of interest statement. None declared.
Funding
This work was supported in part by National Institutes of Health/National Cancer Institute U10 CA 25224 and R01 CA 154348.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. doi:10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks PA, Rifkind RA, Richon VM, Breslow R. Inhibitors of histone deacetylase are potentially effective anticancer agents. Clin Cancer Res. 2001;7(4):759–760. [PubMed] [Google Scholar]
- 4.Richon VM, Webb Y, Merger R, et al. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc Natl Acad Sci USA. 1996;93(12):5705–5708. doi: 10.1073/pnas.93.12.5705. doi:10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richon VM, Emiliani S, Verdin E, et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci USA. 1998;95(6):3003–3007. doi: 10.1073/pnas.95.6.3003. doi:10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors–development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2(3):150–157. doi: 10.1038/ncponc0106. doi:10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 7.Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2(2):151–163. doi:10.4161/cbt.2.2.349. [PubMed] [Google Scholar]
- 8.Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 1996;5(4–5):245–253. [PMC free article] [PubMed] [Google Scholar]
- 9.Eyupoglu IY, Hahnen E, Buslei R, et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93(4):992–999. doi: 10.1111/j.1471-4159.2005.03098.x. doi:10.1111/j.1471-4159.2005.03098.x. [DOI] [PubMed] [Google Scholar]
- 10.Ugur HC, Ramakrishna N, Bello L, et al. Continuous intracranial administration of suberoylanilide hydroxamic acid (SAHA) inhibits tumor growth in an orthotopic glioma model. J Neurooncol. 2007;83(3):267–275. doi: 10.1007/s11060-007-9337-z. doi:10.1007/s11060-007-9337-z. [DOI] [PubMed] [Google Scholar]
- 11.Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13(3):1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. doi:10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 12.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–2058. doi: 10.1200/JCO.2008.19.0694. doi:10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg AL, Stein R, Adams J. New insights into proteasome function: from archaebacteria to drug development. Chem Biol. 1995;2(8):503–508. doi: 10.1016/1074-5521(95)90182-5. doi:10.1016/1074-5521(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 14.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16(4):433–443. doi: 10.1038/sj.leu.2402417. doi:10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 15.Fujita T, Doihara H, Washio K, et al. Proteasome inhibitor bortezomib increases PTEN expression and enhances trastuzumab-induced growth inhibition in trastuzumab-resistant cells. Anticancer Drugs. 2006;17(4):455–462. doi: 10.1097/01.cad.0000198910.90819.06. doi:10.1097/01.cad.0000198910.90819.06. [DOI] [PubMed] [Google Scholar]
- 16.Yin D, Zhou H, Kumagai T, et al. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24(3):344–354. doi: 10.1038/sj.onc.1208225. doi:10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
- 17.Pedeboscq S, L'Azou B, Passagne I, et al. Cytotoxic and apoptotic effects of bortezomib and gefitinib compared to alkylating agents on human glioblastoma cells. J Exp Ther Oncol. 2008;7(2):99–111. [PubMed] [Google Scholar]
- 18.Phuphanich S, Supko JG, Carson KA, et al. Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. J Neurooncol. 2010;100(1):95–103. doi: 10.1007/s11060-010-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu C, Friday BB, Yang L, et al. Mitochondrial Bax translocation partially mediates synergistic cytotoxicity between histone deacetylase inhibitors and proteasome inhibitors in glioma cells. Neuro Oncol. 2008;10(3):309–319. doi: 10.1215/15228517-2007-063. doi:10.1215/15228517-2007-063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. doi:10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Badros A, Burger AM, Philip S, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15(16):5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. doi:10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schelman WR, Kolesar J, Schell K, et al. A phase I study of vorinostat in combination with bortezomib in refractory solid tumors. J Clin Oncol (Meeting Abstracts) 2007;25(suppl 18):3573. [Google Scholar]
- 23.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. doi:10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Guan J, Chang I, Chen X, Han D, Wang CY. PS-341 and histone deacetylase inhibitor synergistically induce apoptosis in head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2010;9(7):1977–1984. doi: 10.1158/1535-7163.MCT-10-0141. doi:10.1158/1535-7163.MCT-10-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heider U, Rademacher J, Lamottke B, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in cutaneous T cell lymphoma. Eur J Haematol. 2009;82(6):440–449. doi: 10.1111/j.1600-0609.2009.01239.x. doi:10.1111/j.1600-0609.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 27.Emanuele S, Lauricella M, Carlisi D, et al. SAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor Bortezomib. Apoptosis. 2007;12(7):1327–1338. doi: 10.1007/s10495-007-0063-y. doi:10.1007/s10495-007-0063-y. [DOI] [PubMed] [Google Scholar]
- 28.Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin Cancer Res. 2004;10(11):3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. doi:10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 29.Hockly E, Richon VM, Woodman B, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc Natl Acad Sci USA. 2003;100(4):2041–2046. doi: 10.1073/pnas.0437870100. doi:10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemeryck A, Geerts R, Monbaliu J, et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14 C-bortezomib. Cancer Chemother Pharmacol. 2007;60(6):777–787. doi: 10.1007/s00280-007-0424-9. doi:10.1007/s00280-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 31.Bross PF, Kane R, Farrell AT, et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin Cancer Res. 2004;10(12 Pt 1):3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. doi:10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- 32.Mele G, Pinna S, Alloro E, Brocca MC, Coppi MR, Quarta G. Inefficacy of bortezomib therapy for CNS involvement of refractory multiple myeloma. Leuk Res. 2007;31(5):721–723. doi: 10.1016/j.leukres.2006.06.019. doi:10.1016/j.leukres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 33.Zuniga RM, Torcuator R, Jain R, et al. Rebound tumour progression after the cessation of bevacizumab therapy in patients with recurrent high-grade glioma. J Neurooncol. 2010;99(2):237–242. doi: 10.1007/s11060-010-0121-0. doi:10.1007/s11060-010-0121-0. [DOI] [PubMed] [Google Scholar]
- 34.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. doi:10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reardon DA, Vredenburgh JJ, Desjardins A, et al. Bevacizumab (BV) continuation following BV progression: Meta-analysis of five consecutive recurrent glioblastoma (GBM) trials. J Clin Oncol. 2011;29((suppl) abstr 2030. [Google Scholar]
- 36.Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. doi: 10.1215/15228517-2009-006. doi:10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]