Abstract
Patients with the most common and aggressive form of high-grade glioma, glioblastoma multiforme, have poor prognosis and few treatment options. In 2 immunocompetent mouse brain tumor models (CT26-BALB/c and Tu-2449-B6C3F1), we showed that a nonlytic retroviral replicating vector (Toca 511) stably delivers an optimized cytosine deaminase prodrug activating gene to the tumor lesion and leads to long-term survival after treatment with 5-fluorocytosine (5-FC). Survival benefit is dose dependent for both vector and 5-FC, and as few as 4 cycles of 5-FC dosing after Toca 511 therapy provides significant survival advantage. In the virally permissive CT26-BALB/c model, spread of Toca 511 to other tissues, particularly lymphoid tissues, is detectable by polymerase chain reaction (PCR) over a wide range of levels. In the Tu-2449-B6C3F1 model, Toca 511 PCR signal in nontumor tissues is much lower, spread is not always observed, and when observed, is mainly detected in lymphoid tissues at low levels. The difference in vector genome spread correlates with a more effective antiviral restriction element, APOBEC3, present in the B6C3F1 mice. Despite these differences, neither strain showed signs of treatment-related toxicity. These data support the concept that, in immunocompetent animals, a replicating retroviral vector carrying a prodrug activating gene (Toca 511) can spread through a tumor mass, leading to selective elimination of the tumor after prodrug administration, without local or systemic pathology. This concept is under investigation in an ongoing phase I/II clinical trial of Toca 511 in combination with 5-FC in patients with recurrent high-grade glioma (www.clinicaltrials.gov NCT01156584).
Keywords: brain cancer, cytosine deaminase, 5-fluorocyosine, gene therapy, glioblastoma multiforme
Several replicating viruses have been evaluated in preclinical studies of brain cancer, and some are under clinical investigation.1–3 Selectivity, potency, and durability of response are critical attributes of successful cancer therapy, and we show here that retroviral replicating vectors (RRVs) based on amphotropic murine leukemia virus (MLV) have properties that fulfill these criteria in animal models.
Toca 511 (vocimagene amiretrorepvec) is a replicating amphotropic MLV that infects and integrates only in dividing cells, preferentially in cells with defective immunity, such as malignant cells, and, therefore, has built-in specificity for replicating malignant cells in adult animals.4 The virus stably encodes an optimized yeast cytosine deaminase (CD) that converts subsequently administered courses of prodrug, 5-fluorocytosine (5-FC), into the potent antineoplastic drug 5-fluorouracil (5-FU).5,6 Because the virus is nonlytic and 5-FU selectively kills dividing cells, infected cells may remain and act as a reservoir of virus capable of infecting new malignant cells during subsequent viral proliferation.
The 5-FU produced in Toca 511–infected cells is diffusible through cellular membranes7 and has the potential to kill neighboring uninfected cancer cells, enhancing the therapeutic potential via metabolic cooperation and creating a bystander effect.8,9 The use of yeast CD instead of bacterial CD was shown to increase 5-FC to 5-FU conversion by ∼15 fold.10 The yeast CD used here has been optimized for thermostability to produce an ∼3-fold increase in activity, compared with naive yeast CD. These modifications give Toca 511 an advantage over previously tested strategies using CD in brain cancer and other cancer models.
A key feature of the retroviral replicating vectors is reported to be the ability to spread nonlytically and without significant inflammatory response.11 An important criticism of a number of replicating virus experiments in preclinical models using human xenografts has been that, although allowing testing against human tumors, such models do not allow for the role of the immune system to be evaluated.1 For example, the contribution of the immune system toward clearance of the virus or tumor would not be evaluable. Therefore, it is important to investigate fully the behavior of Toca 511 with 5-FC in one or more immunocompetent models.
We present here the experimental findings investigating Toca 511 in combination with 5-FC therapy in 2 immunocompetent murine orthotopic brain cancer models (CT26 in BALB/c mice and Tu-2449 in B6C3F1 mice). The objectives of this series of studies were to (1) demonstrate that Toca 511 can efficiently transduce and kill CT26 and Tu-2449 cells in vitro, (2) determine the optimal intracranial (IC) doses of Toca 511 in combination with 5-FC that increase survival in orthotopic murine brain cancer models, (3) demonstrate that Toca 511 antineoplastic effects are associated with the conversion of 5-FC to 5-FU, (4) determine the biolocalization of Toca 511 after IC administration, and (5) investigate the long-term survival of brain cancer-implanted mice treated with IC Toca 511 followed by various 5-FC dose cycles.
We show that transduction with Toca 511, in combination with 5-FC administration, is effective in treating implanted IC tumors resulting in tumor eradication and a significant survival advantage. We also show that Toca 511 is able to convert 5-FC to 5-FU in vivo and that repeated cycles of 5-FC administration shrink and prevent further growth of the IC tumor. We have demonstrated survival benefit without treatment-related toxicity in mouse strains that are naturally permissive for MLV replication (BALB/c) and restrictive for MLV infection (B6C3F1).12,13 On the basis of previous data from monkeys with MLV infection,14 the lack of replication of amphotropic MLV in human peripheral blood mononuclear cells in vitro,15 and the presence of effective anti-MLV APOBEC3 in humans and C57BL/6 but not BALB/c mice,16 it is reasonable to expect that humans will more closely resemble nonpermissive mouse strains and control Toca 511 spread outside the tumor without sustained infection of normal tissue. Currently, Toca 511 and 5-FC are being investigated in a phase I/II clinical trial of recurrent high-grade glioma in the United States (see NCT01156584 at clinicaltrials.gov).
Materials and Methods
Toca 511
A full description of the modifications and methods used to generate the Toca 511 vector has been submitted for publication (O. D. Perez, C. R. Logg, K. Hiraoka, O. Diago, R. Burnett, A. Inagaki, D. Jolson, K. Amundson, T. Buckley, D. Lohse, A. Lin, C. Burrascano, C. Ibaþez, N. Kasahara, H. E. Gruber, D. J. Jolly unpublished data). In brief, modifications were made to the plasmid pACE-GFP17 to improve stability and increase convenience of transgene insertion to yield the vector pAC3-GFP. Genetic enhancements to the wild-type yeast cytosine deaminase gene were made as follows: (1) the codon use was optimized for protein synthesis in human cells, and (2) 3 amino acid changes were introduced (A23L, I140L and V108I) to increase thermal stability of the yeast cytosine deaminase protein.18 The plasmid pAC3-yCD2 was generated by substituting the modified CD gene into pAC3-GFP. Toca 511 is the vector produced from this plasmid using the production and formulation methods developed for clinical use.
Mice
Female B6C3F1 mice (age, ∼8 weeks) were purchased from Harlan. BALB/c mice (age, ∼8 weeks) were purchased from The Jackson Laboratory. Mice were acclimated for 7–14 days after arrival. Mice underwent surgical placement of an indwelling guide cannula with a 3.0-mm projection implanted into the right striatum and fitted with a cap containing a 3.5-mm projection. The stereotaxic coordinates were AP = 0.5 mm, ML = 1.8 mm (from bregma).
Cell Culture and Implantation, Delivery, and In-Life Observations
CT26 colon adenocarcimona cells were chosen over other possible immunocompetent cancer models, because these cells were previously used as a brain cancer model19 and are well characterized for MLV infection and 5-FC/5-FU sensitivity in a multifocal colorectal cancer metastasis model.20 CT26 (ATCC#2639) is a mouse N-nitroso-N-methylurethane–induced colon adenocarcinoma. The CT26 cell line has also been used as an immunocompetent model to test viral therapy in mice.21 Tu-2449 is a well-established orthotopic glioma model in immunocompetent mice.22 Tu-2449 cells were derived originally from spontaneously arising tumors in glial fibrillary acidic protein (GFAP)–v-src transgenic mice.23 Implanted tumors display an invasive phenotype and express both the astroglial marker GFAP and the oncogenic form of signal transducer and activator of transcription–3 (Stat3) found in many human gliomas. Both cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, sodium pyruvate, and GlutaMAX (Hyclone and Invitrogen). Cells were resuspended in phosphate-buffered saline (PBS; Hyclone) for implantation. CT26 or Tu-2449 cells (1E4 [1 × 104] in 1 μL) were infused at 0.2 µL per minute (5 min, followed by a hold of 5 min) IC through an injection cannula with a 3.5-mm projection inserted through the guide cannula. In the experiment shown in Fig. 7, cannulas were not used. Instead, mice underwent IC inoculation of Tu-2449 cells and Toca 511 vector into the right striatum using a Hamilton syringe. The projected stereotaxic coordinates were AP = 1.0 mm, ML = 2.0 mm, DV = 3.5 mm (from midline, bregma, and dura, respectively). For the cell injections in Fig. 7 only, 2 µL of cell suspension were delivered at a rate of ∼0.5 µL per minute (4 min total). In all cases, Toca 511 was delivered in 5 µL over 15 min. Five minutes after completion of infusion of the cells or vector, the infusion assembly was raised out of the skull and the skin closed. Toca 511 doses are defined as transducing units per gram of brain (TU/g) with the mean mouse brain defined as 0.5 g. Virus dosing is described as per gram of brain, because this has been the convention for viruses that replicate in brain tissue.24 It is unclear whether this is the most appropriate way to scale for clinical use of Toca 511, but the obvious alternative, scaling by body weight, gives predicted human doses that are almost identical to human doses scaled by brain weight.
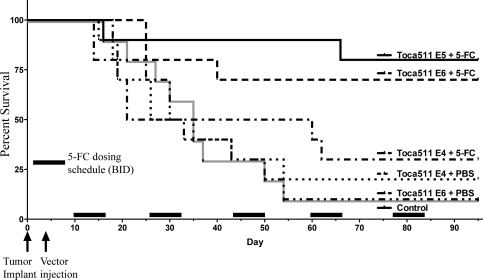
Fig. 7.
Tu-2449 in B6C3F1 glioma model survival analysis after only 4 cycles of 5-FC. Groups of 10 female B6C3F1 mice (8 weeks of age) were implanted IC with 1E4 Tu-2449 tumor cells then dosed IC with vehicle (Control) or IC with Toca 511. Doses of E3, E4, or E5 TU/g of Toca 511 were given as indicated. Following 7 days, to allow spread of Toca 511, mice were treated IP once a day (SID) for 4 consecutive days with either PBS or 5-FC (500 mg/kg) as indicated. This cycle of 4 days on drug followed by 10 days off drug was repeated for only 4 cycles with treatment stopping at day 57. After the last 5-FC dosing regimen, survival analysis was carried out further to day 180.
Routine general health, in-life observations, and body weights were collected throughout the course of the study. In-life observations were scored on a 0–3 point system for severity of each symptom. Mice with a cumulative score of 5 were euthanized. Mice with body weight loss of >20% for >2 days were euthanized. All animal experiments were approved by either the Institutional Animal Care and Use Committee (A4487-01) of Explora (San Diego, California) or the University of Veterinary Medicine Ethics Committee and Austrian government authorities (BMBWK-68.205/232-II/10b/2008).
Infectious Vector Production
Infectious RRV was prepared by standard DNA plasmid transient transfections of 293T cells using the Calcium Phosphate Transfection Kit from Promega. Vector supernatants were transferred onto HT-1080 cells, and the pooled transductants were used as a vector producer cell line. The producer cells were expanded and vector collected and processed using methods analogous to those used previously for nonreplicative retroviral vectors25,26 (US patent 5792643). In brief, cells were expanded and supernatant harvested over 48 h, clarified, and treated with benzonase to digest host cellular DNA. The material was subjected to chromatography through anion exchange and size exclusion columns. The peak fractions from the size exclusion column were pooled, and the vector was formulated in a Tris-sodium chloride isotonic buffer solution containing 1 mg/mL of human serum albumin and 10 mg/mL each of sucrose and mannitol. Titers of the final preparations were determined by quantitative polymerase chain reaction (qPCR) expressed in transducing units (TU)/mL tested on PC-3 cells.
Determination of Titers of Vector Preparations
Titers of vector preparations were determined using several dilutions of vector preparation on PC-3 cells as target for infection, adding azidothymidine (AZT) 24 h after infection to stop vector replication and counting the number of integrated proviruses in the target cell population by qPCR. PC-3 cells were seeded on 12-well plates on day 0 (12–18 h prior to transduction) in 1 mL complete DMEM at a cell confluence targeting 60% on the day of transduction. On day 1, the PC-3 cells were transduced (triplicate transduction for each sample and dilution) with 20 μL of the diluted vector preparations (1:20 and 1:200) prepared in complete DMEM in the presence of 4 µg/mL polybrene. Plates were returned to the incubator for 24 h and on day 2, AZT was added to 40 µM from a 10 mM stock solution to arrest viral replication. Cells were harvested on day 3, and the genomic DNA was prepared from each well of cells using a Promega Maxwell 16 automated purification instrument with the associated cartridges. The concentration of DNA was determined using a Nanodrop ND-1000. The integrity of the DNA was checked by gel electrophoresis on a 0.8% ethidium bromide agarose gel (Invitrogen e-gel). qPCR was performed in triplicate with use of the following primers and probe (Integrated DNA Technologies): forward primer 5–MLV-U3-B: 5′ AGCCCACAACCCCTCACTC; reverse primer 3-MLV-PSI: 5′ TCTCCCGATCCCGGACGA; and probe FAM-5′CCCAAATGAAAGACCCCCGCTGACG- BHQ1. qPCR was performed on a Bio-Rad CFX96 Real Time System. The amplicon size is 192 bp. These primers and detection probe will only detect the integrated provirus with the 3′UTR transposed to the 5′end of the provirus, and will not detect any contaminating plasmid DNA used in the transient transfection step to make the infectious virus. The proviral copy number was determined using a 7-log serial dilution standard curve from a proviral-containing plasmid pAZ3-GFP [21]. DNA from cells infected with a control vector was included as a positive control for transfection and qPCR quantitation; controls without template were run to determine contamination or background. Titers are typically around E8 (1 × 108) TU/mL.
In Vitro Transduction of Tumor Cells and 5-FC Sensitivity Calculation
One hundred percent Toca 511–transduced CT26 and Tu-2449 cells were plated on day 0, and 5-FC (Nantong Jinghua Pharmaceutical) dissolved in PBS was added immediately at different concentrations. The number of live cells was monitored by cell harvest and MTS assay. A 4-parameter curve fit was performed using the Prism 5 statistical package, and 50% inhibitory concentration (IC50) values were calculated.
Comparison of GFP Expression Level in CT26 and Tu-2449 Cells
The CT26 and Tu-2449 cells were infected with vector made from pAC3-GFP at a multiplicity of infection (MOI) of 1.0 or 0.1. After 72 h, cells were resuspended in PBS and analyzed by flow cytometry for GFP expression (BD FACS Canto II).
In vivo Survival Studies
On day 0, mice underwent IC implantation of 1E4 Tu-2449 or CT26 cells. On day 4, mice were injected with Toca 511 or vehicle control (inhibitory concentration, 5 µL/mouse) by IC infusion at 0.33 µL per minute (15 min, followed by a hold of 5 min) through an injection cannula with a 3.5-mm projection inserted through the guide cannula. For CT26 implants, 9 days were allowed for vector spread before the first intraperitoneal injection (IP) cycles of 5-FC (500 mg/kg) or PBS twice per day (BID) dosing were initiated. Each cycle consisted of 7 days of treatment, followed by 10 days without treatment. Cycles were repeated until termination of the study. Tissue sample collection was performed at the end of the survival studies.
For Tu-2449 implants, unless noted otherwise, mice were treated IP BID for 4 consecutive days with PBS or 5-FC (500 mg/kg/dose) starting on day 10. Cycles of 4 days BID treatment with PBS or 5-FC followed by 10 days of no treatment, to allow viral spread, were repeated. Tissue sample collection was performed at the end of the survival studies.
Two different lots of Toca 511 were used for all in vivo studies. Toca 511 lot T511019-FNL had a starting titer of 4.7E8 TU/mL, and Toca 511 lot T511015-FNL had a starting titer of 1.7E8 TU/mL. Figures 2 and 3B used diluted stock from lot T511019-FNL, as indicated. Figures 3A, 6, and 7 used diluted stock from lot T511015-FNL, as indicated. Toca 511 administered intracranially is expressed as TU per gram of brain assuming that the mean mouse brain weighs 0.5 g. As such, an IC dose that is reported as E6 corresponds to 4.7E6 and 1.7E6 for experiments performed with lots T511019-FNL and T511015-FNL, respectively, and so on for doses labeled E3, E4, and E5.
Tumor Processing and HPLC Analysis
Tumors were isolated and portioned if large enough for multiple analyses (>50 mg). Tumor sections for HPLC analysis were crushed in a 1.5 mL centrifuge tube using a plunger from a 1-mL syringe. Crushed samples were mixed with 150 µL RIPA buffer (Thermo Scientific 89900) and vortexed vigorously for 10 min. Samples were spun at 4°C at 20 000 rcf for 10 min. Supernatants were removed and mixed thoroughly with 150 µL of 10% trichloroacetic acid and spun as above. Supernatants were removed for analysis by the Agilent HPLC unit, with a Hypersil BDS C18 column run isocratically at 1 mL/min with 95% buffer A containing 50 mM ammonium phosphate and 0.1% tetra-n-butylammonium perchlorate with pH adjustment of the buffer to 2.1 with phosphoric acid and 5% solvent B, which is 100% methanol. The run time was 5 min with each sample run twice. The photodiode detector array scans 190–350 nm, with chromatograms selected to display at 285 nm for 5-FC and 264 nm for 5-FU. Data were expressed in relative milliabsorbance units (mAU) of peak area from the chromatograms.
Protein Gels, Western Blots, and Tumor PCR
Tumors analyzed by HPLC were also analyzed by Western blot for CD expression. Tumor fragments were mixed with a separate aliquot of RIPA lysis buffer, and 20 µg of total protein from each sample was electrophoresed on polyacrylamide gels and Western blotted as described previously (O. D. Perez, C. R. Logg, K. Hiraoka, O. Diago, R. Burnett, A. Inagaki, D. Jolson, K. Amundson, T. Buckley, D. Lohse, A. Lin, C. Burrascano, C. Ibaþez, N. Kasahara, H. E. Gruber, D. J. Jolly unpublished data). When available, the remaining pellets were extracted for genomic DNA, and the level of vector DNA was determined by PCR, as described in the supplementary materials and methods.
Histological Analysis of Tissues
Individual tissues from nonperfused mice were harvested at sacrifice using disposable instruments to avoid cross contamination. Tissue samples that were placed in formalin were sent for review to a certified pathologist (Histo-Scientific Research Laboratories, Thurmont, MD). Tissues were processed, embedded in paraffin, sectioned, stained with hematoxylin and eosin (H&E) and were evaluated via light microscopy.
Anti-MLV Enzyme-Linked Immunosorbent Assay (ELISA)
A capture ELISA was performed on collected serum samples with MLV capture antigen (viral particle preparation prepared as described in infectious vector production, heat-inactivated). In brief, 100 µL/well of capture antigen (1.56 µg/mL) was incubated overnight at 2–8°C in ELISA 96-well microtiter plates (Corning). Plates were blocked with blocking buffer for 60–90 min at 15–30°C in constant shaking at 150 RPM (1× PBS, 1% BSA, 0.05% TW20, 0.05% sodium azide [PBS; Hyclone], BSA [MP Biomedicals], TMB substrate solution [Southern Biotech], Tween-20 [Sigma], 5% sodium azide solution [VWR]). Serum samples were added in 2-fold dilutions from 1:50 to 1:1000 in PBS and incubated for 90 min at 15–30°C in constant shaking at 150 RPM. After washing with PBS, goat anti-mouse HRP conjugated antibody (Southern Biotech, 1:4000 dilution) was added and the plates were incubated for 70 min at 15–30°C in constant shaking at 150 RPM. After washing for 5 times with PBS, plates were developed with TMB substrate (Southern Biotech), and then TMB stop solution was added after 10 min incubation. Plates were read at 450 nm using an ELISA reader (SpectraMax190; Beckman Coulter).
Statistical Analyses
Survival data were plotted using the Kaplan-Meier method and were compared by the log-rank test or Student's t-test as noted. P values of <.05 were considered to be statistically significant in all analyses, which were done with Prism 5 statistical software (GraphPad Software).
Results
CT26 and Tu-2449 Cells Respond Similarly to Toca 511 with 5-FC Dose-Dependent Killing and MLV Spread In Vitro
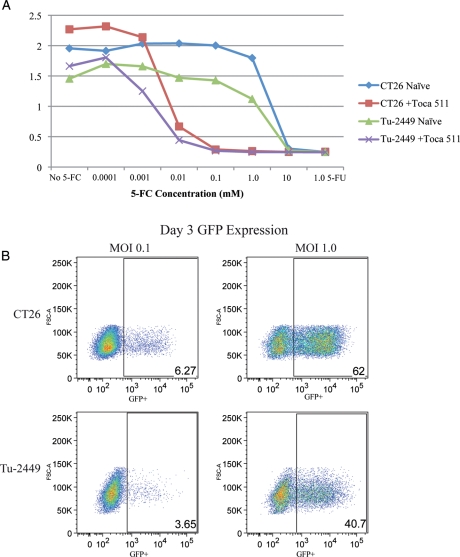
CT26 and Tu-2449 cells were cultured in vitro as either nontransduced control or 100% Toca 511 transduced experimental samples. Sensitivity to 5-FC was determined by measuring cell viability over time in the absence or presence of titrating amounts of 5-FC (Fig. 1A). Both CT26- and Tu-2449–uninfected cells are highly resistant to 5-FC, whereas those that are infected with Toca 511 are killed in a dose-dependent manner. A 4-parameter curve fit was performed using the Prism 5 statistical package and IC50 values calculated. The dose response curves determined that both CT26- and Tu-2449–infected cell lines had similar 5-FC IC50 of 4.2 μM and 1.5 μM, respectively. Analysis of MLV vector spread using a GFP expressing version of Toca 511 shows that the rate of vector spread is similar in both CT26 and Tu-2449 cells at MOI of 0.1 and 1 (Fig. 1B).
Fig. 1.
Comparison of CT26 and Tu-2449 in vitro susceptibility to Toca 511 (511) or MLV expressing GFP (MLV-GFP) vectors. Sensitivity to 5-FC was determined by measuring cell viability (MTS assay) over time in the absence or presence of titrating amounts of 5-FC (A). IC50 values were calculated for CT26 and Tu-2449 cell lines at 4.2 μM (0.5 μg/mL) and 1.5 μM (0.19 μg/mL), respectively. Flow cytometry analysis was done to measure GFP positive cells transduced by MLV-GFP at an MOI of 1.0 or 0.1 (B).
Toca 511 in Combination with 5-FC Significantly Improves Survival in the Syngeneic Murine CT26 Tumor Model
To test the effectiveness of Toca 511 as a therapeutic agent, cannulated BALB/c mice were implanted with 1E4 (1 × 104) CT26 cells intracranially. Various doses of Toca 511 or vehicle were injected into the tumor through the same cannula 4 days after cell injection. After allowing 9 days for vector spread, cycles of 5-FC (500 mg/kg) or PBS BID dosing were initiated. Each cycle consisted of 7 days of treatment, followed by 10 days without treatment. Cycles were repeated until termination of the study.
The lowest Toca 511 dose tested, 1E4 TU/gram of brain (E4), showed an increase in median survival for the 5-FC treatment group (40.5 days), compared with PBS-treated mice (30.5 days) (Fig. 2), that was not statistically significant (P = .665). In contrast, Toca 511 treatment at the mid (E5) and high (E6) dose levels in combination with 5-FC resulted in significantly prolonged survival, compared with the vector plus PBS control (P < .0012 and <.0113, respectively) (Fig. 2).
Fig. 2.
CT26 in BALB/c brain metastasis tumor model survival analysis. Groups of 10 female BALB/c mice (9 weeks of age) were implanted IC with 1E4 CT26 tumor cells then dosed IC with vehicle (Control) or IC with Toca 511 at 3 dose levels expressed as transducing units per gram of brain (TU/g) ranging from a dose level of E4 to a dose level of E6 TU/g. Following 9 days, to allow spread of Toca 511, mice were treated IP BID for 7 consecutive days with either PBS or 5-FC (500 mg/kg) as indicated. The cycle of 7 days on drug followed by 10 days off drug was repeated until the conclusion of the study. Survival analysis up to day 95 was performed. DNA and RNA PCR analysis to detect integrated vector in various tissues and viral particles in sera were performed on the mice terminated at day 95.
Quantitative PCR analysis to detect integrated vector in various tissues was performed on the surviving mice terminated at approximately day 90 (Supplementary material, Table S1). The qPCR data show a wide range of values in the various organs, but the lymphatic/hematological organs consistently had a high mean vector DNA signal. Because the animals were not exsanguinated, much of the signal in other tissues could be attributed to blood/lymph contamination. A small signal persisted at the site of injection even after apparent tumor clearance. Whether this represents residual quiescent tumor or other type of signal is not clear. A small number of mice in the control groups that survived until the final time point did not show visible tumor.
Histological evaluation of the same tissues analyzed for Toca 511 qPCR was performed. All residual tumors were confined to the central nervous system (brain) and were derived from the CT26 implanted cells. All other microscopic findings were incidental and were not associated with any toxicity due to Toca 511 (as assessed by a qualified pathologist). Three mice with the highest level of Toca 511 signal in the thymus (777 951, 1 193 632, and 2 712 226 copies/μg) (Supplementary material, Table S2) did not have associated pathology despite the high levels of integrated retroviral DNA detected in these animals (as assessed by a qualified pathologist).
Toca 511 Therapy with 5-FC Significantly Improves Survival in the Syngeneic Tu-2449 Murine Glioma Model
The Tu-2449 tumor cell line is derived from tumors that arose spontaneously in GFAP-v-src transgenic mice23 and has been used as an orthotopic, syngeneic, transplantable mouse glioma model in B6C3F1 hybrid mice.22,27 This model more closely mimics human glioblastoma characteristics than some murine models, in that it forms a diffuse infiltrative tumor that quite closely mimics human glioblastoma multiforme (GBM) behavior and has been shown to be efficiently infected with a replicating MLV expressing GFP in an orthotopic model.27 The Tu2449 model was therefore used to study Toca 511 plus 5-FC efficacy in an in vivo preclinical setting.
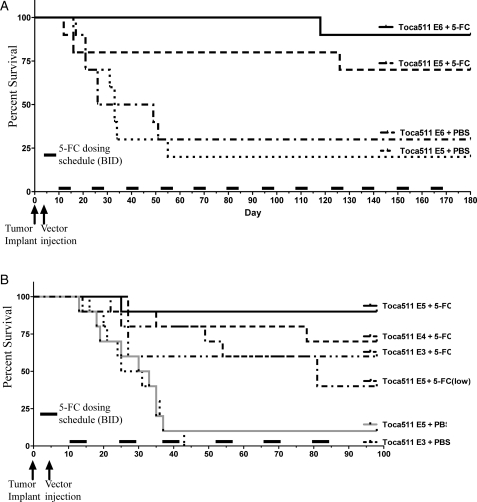
Two separate Tu-2449 glioma model experiments incorporating 4 dose levels of Toca 511 were performed. Toca 511 IC doses ranging from E3 to E6 TU/g were tested (Fig. 3A and B). After allowing 9 days for vector spread, cycles of 5-FC (500 mg/kg) or PBS BID dosing were initiated. Each cycle consisted of 4 days of treatment followed by 10 days without treatment. Cycles were repeated until termination of the study.
Fig. 3.
Tu-2449 in B6C3F1 glioma model survival analysis. Groups of 10 female B6C3F1 mice (8 weeks of age) were implanted IC with 1E4 Tu-2449 tumor cells then dosed IC with vehicle (Control) or IC with Toca 511. Two separate experiments were performed with doses ranging from E3 to a dose level of E6 TU/g (A and B). Following 7 days, to allow spread of Toca 511, mice were treated IP BID for 4 consecutive days with either PBS or 5-FC 500 mg/kg or 50 mg/kg (low) as indicated. This cycle of 4 days on drug followed by 10 days off drug was repeated until the conclusion of the study. Survival analysis up to day 180 (A) or day 100 (B) was performed on each group.
As shown in Fig. 3A, IC Toca 511 dosing at both E6 and E5 dose levels in combination with 5-FC (500 mg/kg) BID resulted in prolonged survival, compared with the Toca 511 plus PBS control (median >189 vs. 33 days), and statistically significant survival (P < .0036 and <.0354, respectively). As shown in Fig. 3B, E5 dose and 2 lower Toca 511 dose levels (E4 and E3) in combination with 5-FC also resulted in prolonged survival, compared with the Toca 511 plus PBS control (E5: P < .0005; E4: P < .002; and E3: P < .01). However, although the E5 vector dose level with the low 50 mg/kg 5-FC BID dose showed some survival advantage, compared with control (P < .0198), it was significantly less effective than the 500 mg/kg BID dose (P < .03).
Quantitative PCR analysis of selected tissues for the 2 highest levels of Toca 511 was performed to detect integrated vector in various tissues in the mice terminated at day 180 (Supplementary material, Table S3). Unlike the high proviral DNA levels detected in multiple tissues in some BALB/c mice, surviving B6C3F1 mice did not show high levels of integrated Toca 511 genome as assessed by qPCR. Only low levels of integrated vector were detected in just a few tissues outside the right side of the brain where vector was injected. Analysis of anti-MLV antibody production in Toca 511–treated BALB/c and B6C3F1 mice over time showed that both strains of mice produced antibody against Toca 511; however, antibody production did not correlate directly to detected vector spread levels in the tissues of the mice (Table 1).
Table 1.
Antivector ELISA activity (positive/total) for CT26/in BALB/c and Tu-2449 in B6C3F1 mouse brain tumor models over time
| Days | 10 | 13 | 30 | 38 | 47 | 52 | 64 | 90 | 98 | 189 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | BALB/c | |||||||||
| E6 | nt | 1/4 | 0/2 | nt | 1/3 | nt | 2/5 | 5/7 | nt | nt |
| E5 | nt | nt | nt | nt | nt | nt | nt | 1/8 | nt | nt |
| E4 | nt | 0/3 | 1/4 | nt | 0/3 | nt | 0/2 | 0/3 | nt | nt |
| B6C3F1 | ||||||||||
| E6 | 0/3 | nt | nt | 1/2 | nt | 2/3 | nt | nt | nt | 8/9 |
| E5 | 0/1 | nt | nt | 0/2 | nt | 0/2 | nt | nt | 5/8 | 4/7 |
Abbreviations: nt, not tested.
BALB/c Mice Are More Permissive to Toca 511 than B6C3F1 Mice after IC Injection of Toca 511
To further characterize the differences seen in the biodistribution of Toca 511 in BALB/c and B6C3F1 mice, tissue profiles of qPCR positivity in tumor-bearing mice (day 90 and 180, respectively) are shown side by side (Fig. 4A and B). This demonstrates a strain-specific susceptibility in normal tissues to Toca 511.
Fig. 4.
Tissue profiles of qPCR positivity from tumor-bearing murine studies after IC administration of Toca 511. Copies/μg of MLV DNA detected per tissue (triplicate measures per mouse sample, averaged for cohorts of n = 10) are presented. Breaks in the X-axis represent the distribution of low (<15 000 copies/μg), medium (15 000–600 000 copies/μg) and high (>600 000 copies/μg). (A) Day 90 tissue positivity profile of tumor bearing BALB/c mice. (B) Day 180 tissue positivity profile of tumor bearing B6C3F1.
A contributory phenomenon that could explain the observed difference in viral distribution between the 2 mouse strains is that BALB/c mice have been shown to carry an allele of APOBEC3 that does not restrict MLV, whereas C57BL/6 mice (from which B6C3F1 mice are derived) carry an allele that does.16 We show that these previously identified allelic differences apply to the mice used in these experiments (Supplementary material, Fig. S1).
Efficient In Vivo Conversion of 5-FC to 5-FU by Toca 511–Infected Tu-2449 Tumors
Highly efficient intratumoral conversion of the 5-FC prodrug into the anticancer drug 5-FU was demonstrated after Toca 511 delivery, followed by intraperitoneal (IP) or oral gavage (OG) with 5-FC (Fig. 5). Mice were implanted with Tu-2449 tumors; Toca 511 was administered at E6 TU/g, and 5-FC dosing was initiated 20 days after Tu-2449 implantation for 2 days BID at either 500 or 250 mg/kg per day and then again 1 h before tumor harvesting and processing on the third day. Brain tumor lysates (2–4/group) as described in Fig. 5B were analyzed by HPLC. In vivo conversion of 5-FC to 5-FU was detected in all groups given Toca 511 and 5-FC (Fig. 5A and C). The Toca 511 no 5-FC group had neither 5-FC nor 5-FU detectable signals as expected (Fig. 5A). The 5-FC only group had detectable 5-FC signals but no signal for 5-FU. Mice given Toca 511 and 5-FC (IP or OG) had comparable levels of 5-FU and very low levels of 5-FC. In all Toca 511 plus 5-FC groups, detection of 5-FC was near background levels, whereas 5-FU was readily detectable, suggesting that the optimized CD gene is rapidly converting almost all of the available 5-FC to 5-FU in the tumor. Conversion of the signal based on a standard curve showed that there was 25–125μg 5-FU per gram of tumor detected at the time of measurement.
Fig. 5.
5-FC to 5-FU conversion analysis (A), CD expression (B), and vector copy number (C). Six groups of female B6C3F1 mice (16 mice, 8 weeks of age) were implanted IC with Tu-2449 glioma tumor cells then injected IC with vehicle (Group 2) or IC with Toca 511 (E6 TU, Groups 1 and 3–6). On day 20, when mice started losing weight, they were treated IP or by oral gavage (OG) BID for 2 consecutive days with either PBS (Group 1) or 5-FC (500 mg/kg, Groups 3 and 6; or 250 mg/kg, Groups 2, 4, and 5). After 2 days of 5-FC dosing the mice were given 1 final 5-FC dose 1 h before sacrifice. Tu-2449 tumors were surgically isolated from the brains for HPLC processing (A) Select tumors (B) were further trimmed for Western blot analysis (tumors greater than 0.05 g). RIPA lysis supernatants were processed for HPLC analysis and, when available, RIPA lysis pellets were analyzed by PCR after DNA extraction. (C) Extracted DNA was obtained from 1, 3, 2, and 2 mice in groups 1, 2, 4, and 5, respectively.
Isolated Tu-2449 tumors were also processed for Western blot analysis of CD expression (Fig. 5B and C). All groups treated with Toca 511 had readily observable CD expression, whereas mice that were not given Toca 511 did not have detectable CD protein.
When available, the remaining pellets, after supernatants were removed for HPLC analysis, were extracted for genomic DNA. Extracted samples were analyzed by qPCR for proviral integration using MLV-LTR primers and probe. The no vector group DNA was negative for proviral sequences as expected, and in the other groups, the copy number/genome ranged from 1.4 copies/genome (208 668 copies/μg) to 15 copies/genome (2 183 971 copies/μg), with a mean of 2.8 copies/genome and a median of 3.7 copies/genome (Fig. 5C). Parallel analyses using the envelope (ENV) and CD gene primers and probes gave similar results.
Toca 511/5-FC Treatment Shrinks IC Tu-2449 Tumors in B6C3F1 Mice
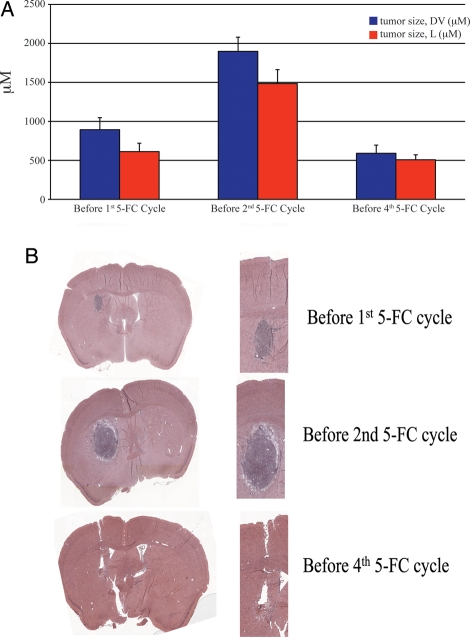
To visualize the in vivo effect of IC Toca 511 treatment on the Tu-2449 tumors, Toca 511–treated brains from Tu-2449 glioma model mice were fixed and stained by H&E for histological analysis at varying times during 5-FC cycles. Mice were sacrificed before the first 5-FC dosing cycle, before the second 5-FC dosing cycle, or before the fourth 5-FC dosing cycle, and brains were fixed and sectioned (Fig. 6). Four mice were analyzed at each time. Tumor area or length was measured and plotted for each group (Fig. 6A). Results show that between the first and second 5-FC dose, the Tu-2449 tumor is still growing (Fig. 6B). However, by the start of the fourth 5-FC dose, most of the tumor is no longer visible and gliosis is evident (Fig. 6B). Full necropsies showed that there was no evidence of tumor spread beyond the immediate vicinity of the skull.
Fig. 6.
Volume and histological analysis of Tu-2449 tumors before 5-FC cycles. (A) Average tumor size (+/− sem, Dorsal-Ventral (DV) or Lateral (L) in μM) from before 1st 5-FC dose, before 2nd 5-FC dose, and before 4th 5-FC dose, N ≥ 4. (B) H&E stains of tumor sections from brains of mice from the three groups analyzed.
Toca 511–Dependent Significant Survival Advantage Out to 180 Days Requires Only 4 Cycles of 5-FC Dosing
Results from the histological analysis in Fig. 6 suggested that, by the end of the fourth 5-FC dosing cycle, we could expect that there would no longer be any viable tumor. To investigate this hypothesis, an additional survival study was conducted using the Tu-2449 glioma model. Groups of 10 mice received IC Toca 511 at E3, E4, or E5 doses and were treated with only 4 cycles of 5-FC (500 mg/kg) once a day (SID). Each cycle consisted of 4 days of treatment (single daily dosing) followed by 10 days without treatment. Survival was assessed until day 180 (Fig. 7). Survival of the mice during the 5-FC treatments was similar to results seen in Fig. 3. At day 67, the last dose of 5-FC (cycle 4), there was no significant difference in mean survival of mice receiving the lowest virus dose (E3) followed by 5-FC or PBS administration (group 1 and 2: 40.4 vs 35.1 days; P = 0.429). However, results from Toca 511 treatment at the E4 and E5 dose levels in combination with 5-FC resulted in significantly prolonged survival, compared with the Toca 511 plus PBS control (P = .025 and P < .0009, respectively, Student's t-test). At the end of 180 days, 80% of the E5 and 40% of the E4 dosed mice survived, compared with 100% death of the control group before the end of 5-FC dosing. The nonoptimal E3 SID 5-FC dosing did not show any survival advantage over control. These data show that treatment with Toca 511 at the mid and high doses results in the elimination of viable cancer in most animals after 4 dosing cycles of 5-FC, because only areas of scarring but no observable tumor were seen in the animals at the end of study.
Discussion
In these studies in mice with implanted brain tumors, we have determined (1) that Toca 511 can efficiently transduce and, in the presence of 5-FC, kill CT26 and Tu-2449 cells in vitro and in vivo; (2) that there is a dose response for Toca 511 in combination with 5-FC; (3) that Toca 511 antineoplastic effects are attributable to infection (typically multiple copies/cell) of the cancer cells, expression of the CD protein, and the intratumoral conversion of 5-FC to 5-FU; (4) that the biolocalization of Toca 511 in tissues after IC administration depends on the murine host but that, in even the Tu-2449 model, where significant viral spread did not occur outside the tumor, there is rapid spread within the tumor; and (5) tumor can be eliminated from a cancer-implanted mouse treated with Toca 511 after only 4 cycles of 5-FC dosing.
Two different syngeneic murine brain tumor models were used as models of human brain cancer. The use of immunocompetent models is critical to the functional validation of viral-based cancer gene therapy, because the immune system may have contrasting influences on viral spread and therapeutic response.28 Immunodeficient models using human xenografts may give a somewhat limited view of the effect of viral therapies in immunocompetent models or patients. Both models were successfully treated, and significant survival benefit was achieved at mid (E4) and high (E5 and E6) dose levels of Toca 511. It should be noted that, in all cases, the survival studies at the mid to high Toca 511 dose levels in both tumor models were artificially ended early (approximately days 90 and 180) to conduct analysis of tumor and vector spread. As supported by Figs 6 and 7, in all likelihood, the mid and high Toca 511 dose groups no longer had viable tumors and would have continued to survive with or without further 5-FC dosing past the 3- and 6-month cutoff time. Although Toca 511 was injected 4 days after tumor cell inoculation, this timing did not prevent tumor engraftment (see Fig. 6) and required the vector to spread in the tumor. This approach more closely mimics the clinical procedure of direct, transcranial injection of vector into the tumor, compared with models in which pretransduced vector-producing tumor cells and naive tumor cells are mixed before injection.29 Injection of vector at 4 days after tumor implantation was necessary to allow time for sufficient vector spread before 5-FC treatment. Animals died due to tumor growth as early as 12 days after tumor implantation in both brain cancer models tested. This time frame is more compressed than in our clinical protocol but, nonetheless, demonstrates the effectiveness of Toca 511 against aggressive cancer. 5-FC is converted to 5-FU at an equal molar ratio.30 5-FU is likely produced in excess in the IC Tu-2449 tumor, because the HPLC analysis showed 5-FU concentrations in the 192–960 μM (25–124 μg/mL) range (based on 1 g of tumor being approximately equal to 1 mL volume). This range is 25–620 times greater than the range of IC50 values for 5-FU for several human glioma cell lines in vitro (0.2–1 µg/mL).31
Possible mechanisms that may be acting alone or in concert to destroy the tumors include direct killing of the cells by Toca 511–expressed CD-dependent conversion of 5-FC to 5-FU, bystander effect of the excess 5-FU production killing nontransduced tumor cells,8,9 and immune responses to clear the tumor.32,33 Further studies remain to be done to determine the contribution of each of these mechanisms to the cancer clearance observed in these studies.
Tu-2449 tumors isolated from mice treated with Toca 511 and 5-FC show efficient conversion of 5-FC to 5-FU, and this corresponds with stable transduction of Toca 511 and the CD gene (usually multiple copies) and the subsequent expression of CD. Efficient and rapid 5-FC to 5-FU conversion was seen with both IP and OG delivery of 5-FC, supporting previous reports of efficient 5-FC delivery across the blood-brain barrier.34 Hlavaty et al. screened 9 human glioma cell lines for RRV spread, CD delivery, and conversion of 5-FC to 5-FU and found that all 9 lines supported RRV spread and showed sensitivity toward 5-FC at concentrations observed in human blood after conventional antifungal 5-FC administration.27 Tai et al. demonstrated that a prototype of Toca 511 had therapeutic effect in combination with 5-FC in human glioma U87 orthotopic xenografts, further supporting the potential of Toca 511 and 5-FC in treating GBM.11 Other studies of human glioma xenografts have shown therapeutic response to 5-FU and to 5-FC after CD gene delivery.31,35 At least 1 group has tried treating gliomas with encapsulated 5-FU delivered intracranially. A phase II trial testing 5-FU encapsulated in poly (L-D) lactide-co-glycolide in conjunction with radiation against radiation alone in recurrent malignant glioma showed a trend in favor of the encapsulated 5-FU arm, compared with the radiation alone arm, in median survival (15.2 vs 13.5 months).36 Clear evidence of tumor necrosis was visible where the encapsulated 5-FU was deposited in the tumor. This supports the concept that 5-FU can have an effect against brain tumors in humans if it can be efficiently delivered to the tumor.
Toca 511 spread extensively to lymphoid and other tissues in permissive BALB/c mouse models, whereas the nonpermissive B6C3F1 mouse model largely controlled spread beyond the tumor. This observation is compatible with biolocalization data from non-tumor bearing mice of both strains up to 180 days after IC delivery of Toca 511 (O. D. Perez, D. Valenta, O. Diago, D. M. Jolson, K. Wong, A. P. GalvƉo da Silva, C. Anderson, D. Gammon, A. Lin, K. Amundson, T. Buckley, D. Ostertag, F. Lopez Espinoza, B. Martin, R. Burnett, P. L. Pettersson, C. Burrascano, C. Chen, C. E. Ibanez, J. M. Robbins, H. E. Gruber, D. J. Jolly unpublished data); BALB/c mice exhibited a higher magnitude of qPCR positivity across all tissues than did B6C3F1 mice. In a previous publication, Tai et al.11 did not detect a PCR signal outside the tumor in intracranially infected human U87 IC xenografts in nude mouse models using the previous generation CD-expressing MLV. Difference in mouse strains, because nude mice do not possess many T cells, and the differences in the PCR assays may account for the apparent disparity. The current quantitative qPCR assay is a more sensitive detection method than that used by Tai et al., who used different primers and gel band detection. However, Tai et al. detected a robust signal in the tumor, and as we have shown this is the site of rapid viral DNA sequence accumulation, we believe that the data are qualitatively compatible. Duerner et al.37 used a SCID mouse (CB-17, Charles River) subcutaneous xenograft model either pre-infected with amphotropic MLV or infused afterward. They looked for virus in brain, spleen, bone marrow, liver, lung, kidney, heart, and target tumor and saw results that appear to resemble ours in that there is mostly vector in the lymphocytic compartments in these mice. However, it is not unexpected to see such variation between the different studies when using different mouse strains and different PCR technologies.
Typically, high retroviral copy number has been associated with pathogenicity,38 but we have observed some individual BALB/c mice that have very high vector copy numbers (e.g., up to 2.7 million copies/µg equivalent to 18 copies/diploid genome in 1 thymus; see Supplementary material, Table S2) but that display no visible or histological abnormalities. It has long been known that the capacity for MLV to induce tumorigenesis in mice is dependent on several factors, including the mouse strain and timing of infection, and it is also possible that the 5-FC administration eliminates infected rapidly dividing cells that correspond to an induced tumor, but the high vector copy number is surprising. Mice were not perfused in these experiments, because we judged that perfusion would not have definitively removed all of the blood contamination for assessment of signal in other tissues and that there was a high probability that perfusion could have contaminated other organs during the process. Therefore, the presence of blood could have contributed to positive signals observed in the nonlymphoid tissue, and we believe that much of the broad biodistribution of Toca 511 seen in nonlymphoid tissue was likely the result of blood retained in the tissue. Nevertheless, those tissues with higher Toca 511 signal than that detected in the blood can be considered to be genuinely positive.
The normal route of MLV transmission in mice is vertically from mother to offspring.12 Both BALB/c mice and B6C3F1 mice are considered to have functioning immune systems. However, BALB/c mice express endogenous MLV in young mice, and this at least partially tolerizes the BALB/c mice to MLV.13 This is not the case in C57BL/6 mice,12 which are one of the parental strains of the B6C3F1 mice. This difference in tolerance may allow for a more robust antiviral cellular immune response in the B6C3F1 mice, compared with the BALB/c mice. Finally, it is known that BALB/c mice carry a splice variant of the APOBEC3 gene that is ineffective against MLV infection, whereas an allele effective against MLV infection is expressed in C57BL/616 and, as we show, in B6C3F1 mice. The emerging consensus is that mouse APOBEC3 (mA3) is a major determinant of restriction of MLV infection. The notion that mA3 restriction plays an important but not absolute role in MLV infection is further supported by recent reports comparing the progression of pathogenicity and neutralizing antibody production in mA3+/+ and mA3−/− mice of mA3−/−.16,38,39 Although other factors may contribute to the observed difference in Toca 511 biodistribution, the differences in tolerance and APOBEC3 allelic expression likely account for the observed differences in viral spread outside of tumors. The data from the B6C3F1 mouse model support the original concept11 that retroviral replication is suppressed in normal tissues in immunocompetent adult animals but is not restricted in tumors. We note here that human APOBEC3G (hA3G) has been shown to be somewhat more active against MLV than the active form of mA3.40 Spread of Toca 511 is likely determined by a combination of factors, including inherited viral restriction components, prior MLV exposures, and innate and adaptive immune system defects from mutations acquired during oncogenesis.4
The tumor selectivity of amphotropic MLV is attributable to 2 major factors. First, cancers are hyperproliferative disorders and have a higher mitotic index than most normal tissues where proliferation, if it occurs, is carefully regulated. The mitotic index of rodent tumor models is 10%–77%,41–43 which has been shown to be sufficient for therapeutic spread through the tumor in the studies presented here. Human glioblastomas have mitotic indices that are usually in this range (10%–30%44) that therefore seems sufficient for Toca 511 spread in clinical subjects, with the potential for therapeutic benefit. Second, cancer progression typically creates an internally immune privileged environment within tumors, including GBM tumors, and further prevents the proper signaling of white blood cells to recognize and attack the cancer.6,45 The same mutations in malignant cells that create an immune suppressive microenvironment likely allow increased viral replication within the cancer.6,45,46 Furthermore, in the case of brain cancers, vector spread is likely enhanced by the immune privileged nature of the brain environment. However, although considered to be an immune privileged site, the brain is still capable of mounting an antiviral response against viral infection.47,48 The potential of this response to limit Toca 511 spread is unclear. Our results suggest that whatever antiviral response may be occurring, Toca 511 still spreads sufficiently for therapeutic response in the mouse models reported here. These results in preclinical animal studies suggest that, in humans, the immune system will exert control over the virus in healthy cells and tissues and allow spread in GBM tumors.
To conclude, we report that Toca 511, a replicating MLV-based vector expressing the yeast CD gene, can successfully act as a cancer therapeutic in conjunction with 5-FC to treat both CT26 and Tu-2449 tumors in an orthotopic brain cancer setting in immunocompetent animals. In a formal toxicology study, the combination of Toca 511 and 5-FC was well tolerated and did not cause any apparent excess toxicity over the course of 6 months. These results form part of the preclinical support for the human clinical investigation of Toca 511 plus 5-FC as a treatment for GBM and other cancers. Recently, a first in human phase I/II clinical trials was initiated in patients with recurrent high-grade glioma, such as GBM (NCT01156584, www.clinicaltrials.gov).
Supplementary Material
Conflict of interest statement. D.O., K.A., F.L., B.M., T.B., A.S., A.L., D.V., O.P., C.I., C.C., P.P., R.B., H.G., D.J., and J.R. are employees and/or shareholders of Tocagen. V.D., J.H., and W.G. are recipients of a research grant from Tocagen. N.K. is a consultant, has ownership interest, and is the recipient of a research grant from Tocagen.
Funding
This work was supported by Investors in Tocagen, the Christian Doppler Association (Vienna, Austria), ABC2 Foundation (Washington, DC), the National Brain Tumor Society (Watertown, MA, and San Francisco, CA), and National Institutes of Health (U01 NS059821 to N.K.).
Supplementary Material
Acknowledgments
We thank Maria Rodriguez-Aguirre and Stephanie Gray for administrative and operational support; Dr. Nicholas A. Boyle, Dr. Dan Pertschuk, and Ms. Debra Gessner for their helpful discussions and critical reading of the manuscript; and Dr. Didier Bagnol for his help in evaluating the tumor sections.
References
- 1.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 2.Rainov NG, Heidecke V. Clinical Development of Experimental Virus-Mediated Gene Therapy for Malignant Glioma. [published online ahed of print June 27, 2011]. Anticancer Agents Med Chem. doi: 10.2174/187152011797378724. [DOI] [PubMed] [Google Scholar]
- 3.Stanford MM, Bell JC, Vaha-Koskela MJ. Novel oncolytic viruses: riding high on the next wave? Cytokine Growth Factor Rev. Apr–Jun 2010; 21(2–3):177–183. doi: 10.1016/j.cytogfr.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Dalba C, Klatzmann D, Logg CR, Kasahara N. Beyond oncolytic virotherapy: replication-competent retrovirus vectors for selective and stable transduction of tumors. Curr Gene Ther. 2005;5(6):655–667. doi: 10.2174/156652305774964659. doi:10.2174/156652305774964659. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H, Mullen JT, Chandrasekhar S, Pawlik TM, Yoon SS, Tanabe KK. Multimodality therapy with a replication-conditional herpes simplex virus 1 mutant that expresses yeast cytosine deaminase for intratumoral conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res. 2001;61(14):5447–5452. [PubMed] [Google Scholar]
- 6.Staveley-O'Carroll K, Sotomayor E, Montgomery J, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95(3):1178–1183. doi: 10.1073/pnas.95.3.1178. doi:10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.el-Tahtawy A, Wolf W. In vivo measurements of intratumoral metabolism, modulation, and pharmacokinetics of 5-fluorouracil, using 19F nuclear magnetic resonance spectroscopy. Cancer Res. 1991;51(21):5806–5812. [PubMed] [Google Scholar]
- 8.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA. 1994;91(17):8302–8306. doi: 10.1073/pnas.91.17.8302. doi:10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuriyama S, Masui K, Sakamoto T, et al. Bystander effect caused by cytosine deaminase gene and 5-fluorocytosine in vitro is substantially mediated by generated 5-fluorouracil. Anticancer Res. 1998;18(5A):3399–3406. [PubMed] [Google Scholar]
- 10.Kievit E, Nyati MK, Ng E, et al. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts. Cancer Res. 2000;60(23):6649–6655. [PubMed] [Google Scholar]
- 11.Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12(5):842–851. doi: 10.1016/j.ymthe.2005.03.017. doi:10.1016/j.ymthe.2005.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein E, Klein G. Immunological tolerance of neonatally infected mice to the Moloney leukaemia virus. Nature. 1966;209(5019):163–165. doi: 10.1038/209163a0. doi:10.1038/209163a0. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson JR, Peters RL, Hino S, et al. Natural immunity in mice to structural polypeptides of endogenous type C RNA viruses. J Virol. 1976;19(3):890–898. doi: 10.1128/jvi.19.3.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornetta K, Moen RC, Culver K, et al. Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum Gene Ther. 1990;1(1):15–30. doi: 10.1089/hum.1990.1.1-15. Spring doi:10.1089/hum.1990.1.1-15. [DOI] [PubMed] [Google Scholar]
- 15.Ebeling SB, Simonetti ER, Borst HP, et al. Human primary T lymphocytes have a low capacity to amplify MLV-based amphotropic RCR and the virions produced are largely noninfectious. Gene Ther. 2003;10(21):1800–1806. doi: 10.1038/sj.gt.3302080. doi:10.1038/sj.gt.3302080. [DOI] [PubMed] [Google Scholar]
- 16.Takeda E, Tsuji-Kawahara S, Sakamoto M, et al. Mouse APOBEC3 restricts friend leukemia virus infection and pathogenesis in vivo. J Virol. 2008;82(22):10998–11008. doi: 10.1128/JVI.01311-08. doi:10.1128/JVI.01311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Logg CR, Logg A, Tai CK, Cannon PM, Kasahara N. Genomic stability of murine leukemia viruses containing insertions at the Env-3′ untranslated region boundary. J Virol. 2001;75(15):6989–6998. doi: 10.1128/JVI.75.15.6989-6998.2001. doi:10.1128/JVI.75.15.6989-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korkegian A, Black ME, Baker D, Stoddard BL. Computational thermostabilization of an enzyme. Science. 2005;308(5723):857–860. doi: 10.1126/science.1107387. doi:10.1126/science.1107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trehin R, Figueiredo JL, Pittet MJ, Weissleder R, Josephson L, Mahmood U. Fluorescent nanoparticle uptake for brain tumor visualization. Neoplasia. 2006;8(4):302–311. doi: 10.1593/neo.05751. doi:10.1593/neo.05751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraoka K, Kimura T, Logg CR, et al. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res. 2007;67(11):5345–5353. doi: 10.1158/0008-5472.CAN-06-4673. doi:10.1158/0008-5472.CAN-06-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toda M, Iizuka Y, Kawase T, Uyemura K, Kawakami Y. Immuno-viral therapy of brain tumors by combination of viral therapy with cancer vaccination using a replication-conditional HSV. Cancer Gene Ther. 2002;9(4):356–364. doi: 10.1038/sj.cgt.7700446. doi:10.1038/sj.cgt.7700446. [DOI] [PubMed] [Google Scholar]
- 22.Smilowitz HM, Weissenberger J, Weis J, Brown JD, O'Neill RJ, Laissue JA. Orthotopic transplantation of v-src-expressing glioma cell lines into immunocompetent mice: establishment of a new transplantable in vivo model for malignant glioma. J Neurosurg. 2007;106(4):652–659. doi: 10.3171/jns.2007.106.4.652. doi:10.3171/jns.2007.106.4.652. [DOI] [PubMed] [Google Scholar]
- 23.Pohl U, Wick W, Weissenberger J, et al. Characterization of Tu-2449, a glioma cell line derived from a spontaneous tumor in GFAP-v-src-transgenic mice: comparison with established murine glioma cell lines. Int J Oncol. 1999;15(4):829–834. doi: 10.3892/ijo.15.4.829. [DOI] [PubMed] [Google Scholar]
- 24.Eldadah AH, Nathanson N, Sarsitis R. Pathogenesis of West Nile Virus encephalitis in mice and rats. 1. Influence of age and species on mortality and infection. Am J Epidemiol. 1967;86(3):765–775. doi: 10.1093/oxfordjournals.aje.a120785. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues T, Carvalho A, Carmo M, Carrondo MJ, Alves PM, Cruz PE. Scaleable purification process for gene therapy retroviral vectors. J Gene Med. 2007;9(4):233–243. doi: 10.1002/jgm.1021. doi:10.1002/jgm.1021. [DOI] [PubMed] [Google Scholar]
- 26.McCormack JE, Edwards W, Sensintaffer J, et al. Factors affecting long-term expression of a secreted transgene product after intravenous administration of a retroviral vector. Mol Ther. 2001;3(4):516–525. doi: 10.1006/mthe.2000.0259. doi:10.1006/mthe.2000.0259. [DOI] [PubMed] [Google Scholar]
- 27.Hlavaty J, Jandl G, Liszt M, et al. Comparative evaluation of preclinical in vivo models for the assessment of replicating retroviral vectors for the treatment of glioblastoma. J Neurooncol. 2011;102(1):59–69. doi: 10.1007/s11060-010-0295-5. doi:10.1007/s11060-010-0295-5. [DOI] [PubMed] [Google Scholar]
- 28.Prestwich RJ, Errington F, Diaz RM, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20(10):1119–1132. doi: 10.1089/hum.2009.135. doi:10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solly SK, Trajcevski S, Frisen C, et al. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther. 2003;10(1):30–39. doi: 10.1038/sj.cgt.7700521. doi:10.1038/sj.cgt.7700521. [DOI] [PubMed] [Google Scholar]
- 30.Stegman LD, Rehemtulla A, Beattie B, et al. Noninvasive quantitation of cytosine deaminase transgene expression in human tumor xenografts with in vivo magnetic resonance spectroscopy. Proc Natl Acad Sci USA. 1999;96(17):9821–9826. doi: 10.1073/pnas.96.17.9821. doi:10.1073/pnas.96.17.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller CR, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62(3):773–780. [PubMed] [Google Scholar]
- 32.Consalvo M, Mullen CA, Modesti A, et al. 5-Fluorocytosine-induced eradication of murine adenocarcinomas engineered to express the cytosine deaminase suicide gene requires host immune competence and leaves an efficient memory. J Immunol. 1995;154(10):5302–5312. [PubMed] [Google Scholar]
- 33.Mullen CA, Coale MM, Lowe R, Blaese RM. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994;54(6):1503–1506. [PubMed] [Google Scholar]
- 34.Diasio RB, Bennett JE, Myers CE. Mode of action of 5-fluorocytosine. Biochem Pharmacol. 1978;27(5):703–707. doi: 10.1016/0006-2952(78)90507-5. doi:10.1016/0006-2952(78)90507-5. [DOI] [PubMed] [Google Scholar]
- 35.Lv SQ, Zhang KB, Zhang EE, et al. Antitumor efficiency of the cytosine deaminase/5-fluorocytosine suicide gene therapy system on malignant gliomas: an in vivo study. Med Sci Monit. 2009;15(1):BR13–20. [PubMed] [Google Scholar]
- 36.Menei P, Capelle L, Guyotat J, et al. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: a randomized phase II trial. Neurosurgery. 2005;56(2):242–248. doi: 10.1227/01.neu.0000144982.82068.a2. discussion 242–248 doi:10.1227/01.NEU.0000144982.82068.A2. [DOI] [PubMed] [Google Scholar]
- 37.Duerner LJ, Schwantes A, Schneider IC, Cichutek K, Buchholz CJ. Cell entry targeting restricts biodistribution of replication-competent retroviruses to tumour tissue. Gene Ther. 2008;15(22):1500–1510. doi: 10.1038/gt.2008.92. doi:10.1038/gt.2008.92. [DOI] [PubMed] [Google Scholar]
- 38.Low A, Okeoma CM, Lovsin N, et al. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology. 2009;385(2):455–463. doi: 10.1016/j.virol.2008.11.051. doi:10.1016/j.virol.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santiago ML, Benitez RL, Montano M, Hasenkrug KJ, Greene WC. Innate retroviral restriction by Apobec3 promotes antibody affinity maturation in vivo. J Immunol. 2010;185(2):1114–1123. doi: 10.4049/jimmunol.1001143. doi:10.4049/jimmunol.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rulli SJ, Jr., Mirro J, Hill SA, et al. Interactions of murine APOBEC3 and human APOBEC3G with murine leukemia viruses. J Virol. 2008;82(13):6566–6575. doi: 10.1128/JVI.01357-07. doi:10.1128/JVI.01357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura K, Suzuki K, Imura N. Effects of methylmercury on mitotic mouse glioma cells. Environ Res. 1978;17(3):453–471. doi: 10.1016/0013-9351(78)90048-8. doi:10.1016/0013-9351(78)90048-8. [DOI] [PubMed] [Google Scholar]
- 42.Morrone FB, Oliveira DL, Gamermann P, et al. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:226. doi: 10.1186/1471-2407-6-226. doi:10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walter MA, Hildebrandt IJ, Hacke K, et al. Small-animal PET/CT for monitoring the development and response to chemotherapy of thymic lymphoma in Trp53-/- mice. J Nucl Med. 2010;51(8):1285–1292. doi: 10.2967/jnumed.109.073585. doi:10.2967/jnumed.109.073585. [DOI] [PubMed] [Google Scholar]
- 44.Mizoguchi M, Inamura T, Shono T, et al. A comparative study of apoptosis and proliferation in germinoma and glioblastoma. Neuro Oncol. 2000;2(2):96–102. doi: 10.1093/neuonc/2.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Critchley-Thorne RJ, Simons DL, Yan N, et al. Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci USA. 2009;106(22):9010–9015. doi: 10.1073/pnas.0901329106. doi:10.1073/pnas.0901329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stojdl DF, Lichty BD, ten Oever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. doi:10.1016/S1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 47.Kaushik DK, Gupta M, Basu A. Microglial response to viral challenges: every silver lining comes with a cloud. Front Biosci. 2011;17:2187–2205. doi: 10.2741/3847. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–227. doi: 10.1038/nrn752. doi:10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.