Abstract
Seizure is a common presenting manifestation and plays an important role in the clinical presentation and quality of life for patients with low-grade gliomas (LGGs). The authors set out to identify factors that influence preoperative seizure characteristics and postoperative seizure control. Cases involving adult patients who had undergone initial surgery for LGGs in a single institution between 2005 and 2009 were retrospectively reviewed. Univariate and multivariate logistic regression analyses were used to identify factors associated with preoperative seizures and postoperative seizure control. Of the 508 patients in the series, 350 (68.9%) presented with seizures. Age less than 38 years and cortical involvement of tumor were more likely to be associated with seizures (P = .003 and .001, respectively, multivariate logistic analysis). For the cohort of 350 patients with seizures, Engel classification was used to evaluate 6- and 12-month outcome after surgery: completely seizure free (Engel class I), 65.3% and 62.5%; not seizure free (Engel classes II, III, IV), 34.7% and 37.5%. After multivariate logistic analysis, favorable seizure prognosis was more common in patients with secondary generalized seizure (P = .006) and with calcification on MRI (.031). With respect to treatment-related variables, patients achieved much better seizure control after gross total resection than after subtotal resection (P < .0001). Ki67 was an independent molecular marker predicting poor seizure control in the patients with a history of seizure if overexpressed but was not a predictor for those without preoperative seizures. These factors may provide insight into developing effective treatment strategies aimed at prolonging patients' survival.
Keywords: calcification, extent of resection, Ki67, low-grade glioma, seizure
Seizure is a common presenting symptom of primary brain tumors, particularly slow-growing ones. The pathogenesis of tumor-related epilepsy seems to be different from that of idiopathic epilepsy, leading to some distinct clinical characteristics and treatment. Glioma, accounting for more than half of brain tumors, is one of the most common causes of tumor-related seizures. Although most patients (65% to 90%) with low-grade gliomas (LGGs) experience symptomatic seizures,1–7 many do not have seizures in spite of similar histology and tumor location. It is possible that the variability in seizure occurrence cannot be explained solely by tumor-related or peritumoral factors, but rather that there is a complex interaction between genetic factors of the tumor and the microenvironment of the surrounding tissue. The main goals of this study were: (1) to identify pre- and postsurgical characteristics of seizures in patients undergoing primary resection of LGGs, (2) to determine the factors associated with preoperative seizures, and (3) to identify the predictors of seizure control after surgical resection.
Seizures play an important role in patients' quality of life after surgery. Although more than half of the patients with LGGs may have favorable seizure prognosis after surgery, about 30% of these patients suffer from uncontrolled seizures in spite of treatment with different antiepileptic drugs (AEDs).
Based on previous studies, it seems to be widely accepted that tumor-related seizures are generally induced by peritumoral epileptic focus. Alterations in the excitation/inhibition balance are the main mechanism, including local changes in perfusion, metabolism, electrolytes, and enzymes. Current studies lack further investigation of how the tumor itself causes seizure. Only in a recent study1 have the authors found that patients with histological subtypes of oligodendroglioma and oligoastrocytoma are significantly more likely to present with seizures than patients with astrocytoma. This may suggest that the biological properties of gliomas, such as the genetic component, may be closely related to seizure occurrence. In the same study, the authors also found that seizure recurrence after initial postoperative seizure control was associated with tumor progression. Therefore, it is possible that there might be some relationship between genetic factors and postoperative seizure outcome. Currently, the correlations between tumor markers and surgical outcomes are the most popular research topics. Because of the powerful influence on tumor-suppressor gene mutation and tumor proliferation and infiltration, and potential adverse impact on chemotherapies, overexpression of some biomarkers (P53, Ki67, matrix metalloproteinase–9, and O6-methylguanine methyltransferase) correlated with poor survival in patients with gliomas. However, little is known regarding how expression of these biomarkers may influence tumor-related seizure. To provide a molecular genetic basis to each glioma patient who may need chemotherapy, we routinely measured 11 molecular markers (Table 6) based on immunohistochemical staining. In this study, we investigated the possible relationship between the expression of the 11 molecular markers of brain tumor and tumor-related seizures.
Table 6.
Univariate analysis of molecular pathological markers predicting preoperative seizures and seizure control 6 months after surgerya
|
P-value |
||||||
|---|---|---|---|---|---|---|
| Markerb | No. of Cases | Seizures |
No. Seizures, n (%) | Factors Predisposing to Any Seizureb | Factors Predisposing to Seizure Controlc | |
| Engel I | Engel II–IV | |||||
| P53 | 462 | 88 (44.7) | 37 (35.9) | 73 (49.7) | 0.358 | 0.144 |
| PTEN | 462 | 176 (89.3) | 90 (87.4) | 132 (89.8) | 0.770 | 0.611 |
| Ki67 | 462 | 4 (2.0) | 10 (9.7) | 11 (7.4) | 0.244 | 0.003e |
| PCNA | 190 | 66 (91.7) | 39 (86.7) | 61 (88.4) | 0.857 | 0.386 |
| MMP9 | 462 | 123 (62.4) | 60 (58.3) | 87 (59.2) | 0.669 | 0.480 |
| EGFR | 453 | 138 (71.5) | 76 (75.2) | 103 (71.5) | 0.720 | 0.493 |
| VEGF | 272 | 68 (54.4) | 27 (46.6) | 45 (57.7) | 0.400 | 0.323 |
| P170 | 462 | 121 (61.4) | 67 (65.0) | 90 (61.2) | 0.736 | 0.537 |
| MGMT | 462 | 104 (52.8) | 45 (43.7) | 71 (48.3) | 0.856 | 0.134 |
| TOPO-II | 462 | 21 (10.7) | 6 (5.8) | 18 (12.2) | 0.314 | 0.165 |
| GST-π | 462 | 126 (64.0) | 56 (54.4) | 86 (58.5) | 0.711 | 0.106 |
Abbreviations: PTEN,phosphatase and tensin homolog; PCNA, proliferating cell nuclear antigen; MMP9, matrix metallopeptidase 9; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; MGMT, O6-methylguanine methyltransferase; TOPO, topoisomerase; GST, glutathione S-transferase.
aValues are numbers of patients, with percentages in parentheses unless otherwise indicated.bSetting at a high expression level when analyzing.
cComparing patients with no history of seizures with those with a history of seizures.
dComparing uncontrolled with controlled seizure subgroups.
eStatistically significant.
Materials and Methods
Subjects
This study was approved by the hospital ethics committee, and informed consent was obtained from all patients. We searched the database of the Glioma Treatment Center of Tiantan Hospital, Beijing, China, to find patients who had undergone primary resection of cerebral LGGs between September 2005 and June 2009. This study included the 508 patients who were 17 years of age or older and had World Health Organization (WHO) grade II astrocytoma, oligodendroglioma, and oligoastrocytoma diagnosed by histopathology. We excluded patients who had undergone secondary resection or needle biopsy only and those with infratentorial and occipital lobe LGGs. Applying these criteria, we included a total of 508 patients. This patient population also contained 183 LGGs that had been analyzed for 1p/19q loss of heterozygosity in our unpublished study.8
There were no universally accepted guidelines for the use of AEDs. The choice of a specific AED was based on the clinician's preference. Treatment with AEDs was typically initiated only in patients in whom seizures developed. Patients who experienced seizures despite a therapeutic level of a specific AED were prescribed additional AEDs. Patients without seizures were routinely administered one AED for seizure prophylaxis prior to surgery and were then weaned off the medication at discharge. Patients with preoperative seizures typically continued AED treatment for 3–6 months after surgery and were weaned off gradually. If the patients had any evidence of seizures, AED treatment was continued.
Assessment and Seizure Outcome Measures
Data on seizure characteristics included date of seizure onset, type of seizure (secondary generalized, simple partial, complex partial, combined partial, and generalized seizures), seizure frequency, and the use of AEDs and steroid medication. The study population was divided into 2 groups based on preoperative seizure status.
The recorded MRI characteristics included tumor size (mean largest diameter in 3 directions based on fluid attenuated inversion recovery [FLAIR] or T2), cortical or subcortical location, specific lobe involvement, and presence or absence of enhancement, edema, cystic change, mass effect, and calcification. The suspectable appearance of calcification on MRI was confirmed by CT images.
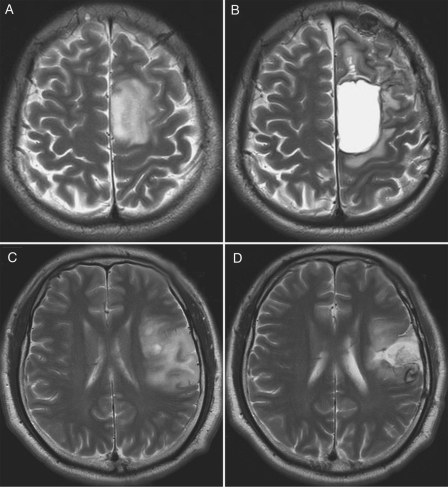
Extent of resection was retrospectively classified from reports on MRIs performed either 3 months postoperatively or less than 72 h after resection as: (1) gross total resection (complete resection of the preoperative T2 or FLAIR signal abnormality as seen from axial, coronal, or sagittal images) or (2) subtotal resection (nodular or thin residual T2 or FLAIR signal abnormality as seen from axial, coronal, or sagittal images) (Fig. 1). All the characteristics above were assessed by an independent neuroradiologist who was blinded to patient outcomes. For patients without pre- or postoperative MRIs, extent of resection was determined by the surgeon's intraoperative impressions.
Fig. 1.
Preoperative and 3-month postoperative T2-weighted MRI scans in patients who underwent resection of grade II astrocytomas. A and B, gross total resection with complete resection of the preoperative T2 signal abnormality. C and D, depiction of subtotal resection with residual nodular T2 signal abnormality.
The primary outcome variable was seizure status, which was evaluated at 6 and 12 months after surgery using the Engel Classification of Seizures: class I = free of disabling seizures (completely seizure free; nondisabling, simple partial seizures only; some disabling seizures, but free of disabling seizures for at least 2 years; generalized convulsion with AED withdrawal only); class II = rare disabling seizures (initially free of disabling seizures, but rare seizures now; rare disabling seizures since surgery; more than rare disabling seizures, but rare seizures for at least 2 years; nocturnal seizures only); class III = worthwhile improvement (worthwhile seizure reduction; prolonged seizure-free intervals amounting to more than half the follow-up period, but not less than 2 years); and class IV = no worthwhile improvement (no significant seizure reduction; no appreciable change; seizures worse).9 Because better follow-up was available for 6 months, seizure control at this time was the primary endpoint used for seizure prognosis analysis.
Surgical Procedures
The goal of surgery was gross total removal of the tumor while protecting functional brain tissue as much as possible. Subtotal resection was performed mainly because of tumor involvement in verbal brain areas as verified by intraoperative electrical stimulation mapping. Extended lesionectomy, which is resection intended to extend beyond the tumor margin, was carried out only when the tumor was small enough in the “nonverbal” frontal or temporal lobe. Electrodiagnostic data, including electrocorticography (ECoG) and cortical direct electrical stimulation (CDES), were collected. The use of ECoG has depended largely upon the preference of the surgeon.
Neuropathological Techniques
Immunoperoxidase staining for the 11 molecular markers (Table 6) was performed on formalin-fixed, paraffin-embedded tissue sections following the standard protocol recommended by the manufacturer. Each slide stained for these markers was individually reviewed and scored by 2 independent observers. Discrepancies in scoring between the 2 observers were resolved by additional review of the specimens and discussion between the reviewers until a consensus was achieved. Approximately 15–20 fields at ×400 magnification were analyzed per specimen. Scoring was done on a 5-point scale from 0 to 4, where 0 = no or rare occurrence of stained nuclei, 1 = <10% of cells had positive staining, 2 = 10%–30% of cells stained positively, 3 = 30%–60% of cells stained positively, and 4 = >60% of cells stained positively. Based on the fact that different markers may have different binding peaks in immunohistochemical staining (markers with nuclear localization generally less easily present positive reactivity than those with nonnuclear specific localization). For purposes of statistical analysis, the percentage of positive cells of each specimen and the cutoffs for dichotomization analysis were determined as follows.10–12 High expressions of P53, phosphatase and tensin homolog, Ki67, and proliferating cell nuclear antigen were defined as strong nuclear staining in at least 10% (scores 2/3/4) of the tumor cells (see Fig. 1 for Ki67). The immunoreactivity of the other 7 markers, which have nonnuclear cellular localization, was evaluated semiquantitatively by estimating the fraction of positive cells, where less than 30% (score 0/1/2) was regarded as low expression, and 30% or more (score 3/4) was regarded as high expression. All immunohistochemical analysis was carried out blind to the clinical and molecular information. The same univariate and multivariate models were used to analyze the markers’ association with preoperative seizures and postoperative seizure control.
Statistical Analyses
All statistical analyses were performed using SPSS version 13.0 and were tailored to address associations between the presence of preoperative seizures and postoperative seizures at 6- and 12-month follow-up. For outcome analyses, Engel classification was dichotomized as class I (completely seizure free) versus class(es) II–IV (not seizure free).1,9 Univariate analyses were carried out using the chi-square test for dichotomous variables and the Mann–Whitney U-test for continuous nonparametric data. All variables associated with seizures in univariate analysis (P < .05) were then included into a backward stepwise multivariate logistic regression model. A probability value of .05 was considered statistically significant after 2-sided P-values were computed.
Results
Seizure Outcomes
The present study included a total of 508 patients who had undergone primary resection of LGGs during the aforementioned period. Of these patients, 350 (68.9%) had preoperative seizures and 158 (31.1%) had no history of seizures. Patients' mean age was 38.1 years (range, 16–72 y) at the time of surgery. Factors that predisposed patients to preoperative seizures were analyzed. Patient information is summarized in Table 1.
Table 1.
Clinical and demographic characteristics of 508 patientsa
| Parameter | Total | Seizures | No Seizures | P-value |
|---|---|---|---|---|
| Number of patients | 508 | 350 (68.9) | 158 (31.1) | |
| Number of male sex | 306 (60.2) | 217 (62.0) | 89 (56.3) | 0.326 |
| Median age, yrs (range) | 38.1 (16–72) | 37.5 (16–69) | 39.6 (18–72) | 0.016c,d |
| Age <38 years old | 245 (48.2) | 154 (44.0) | 91 (57.6) | 0.005d |
| Tumor location (involvement) | ||||
| Left/right ratio | 1.14 | 1.24 | 0.96 | 0.203 |
| Frontal | 360 (70.9) | 254 (72.6) | 106 (67.1) | 0.208 |
| Temporal | 189 (37.2) | 132 (37.7) | 57 (36.1) | 0.724 |
| Parietal | 46 (9.1) | 29 (8.3) | 17 (10.8) | 0.368 |
| Insula | 107 (21.1) | 77 (22.0) | 30 (19.0) | 0.441 |
| MRI characteristics | 462 (90.9) | 315 (90.0) | 147 (93.0) | |
| Median size, cm (range) | 4.8 (2.0–8.8) | 4.9 (2.5–8.8) | 4.7 (2.0–8.3) | 0.973c |
| Cortex involvement | 143 (31.0) | 113 (35.9) | 30 (20.4) | 0.001d |
| Enhancement | 160 (34.6) | 107 (34.0) | 53 (36.1) | 0.661 |
| Edema | 69 (14.9) | 42 (13.3) | 27 (18.4) | 0.157 |
| Cystic change | 82 (17.7) | 52 (16.5) | 30 (20.4) | 0.307 |
| Mass effect | 359 (77.7) | 244 (77.5) | 115 (78.2) | 0.853 |
| Calcification | 65 (14.4) | 44 (14.0) | 21 (14.3) | 0.927 |
| No. about extent of resection | ||||
| By MRI | 462 (90.9) | 315 (90.0) | 147 (93.0) | |
| By neurosurgeons | 46 (9.1) | 35 (10.0) | 11 (7.0) | |
| Tumor pathology | 0.497 | |||
| Astrocytoma | 229 (45.1) | 154 (44.0) | 75 (47.5) | |
| Oligodendroglioma | 48 (9.4) | 31 (8.9) | 17 (10.8) | |
| Oligoastrocytoma | 231 (45.5) | 165 (47.1) | 66 (41.8) | |
| Median seizure duration in mos (range)b | 10 (0.1–276) | 10 (0.1–276) | NA | |
| Seizure typeb | NA | |||
| Secondary generalized | 235 (67.1) | 235 (67.1) | ||
| Simple partial | 83 (23.7) | 83 (23.7) | ||
| Complex partial | 23 (6.6) | 23 (6.6) | ||
| Combination of partial and generalized | 9 (2.6) | 9 (2.6) | ||
| Seizure frequencyb | NA | |||
| 1 seizure ever | 120 (34.3) | 120 (34.3) | ||
| ≥1/year | 53 (15.1) | 53 (15.1) | ||
| ≥1/month | 105 (30.0) | 105 (30.0) | ||
| ≥1/week | 26 (7.4) | 26 (7.4) | ||
| ≥1/day | 46 (13.1) | 46 (13.1) | ||
Abbreviations: NA, not available.
aUnless otherwise indicated, values are numbers of patients, with percentages in parentheses.
bData on seizure are provided for the 350 patients who had seizures.
cMann–Whitney U-test.
dStatistically significant.
The study population contained 229 (45.1%) astrocytomas, 48 (9.4%) oligodendrogliomas, and 231 (45.5%) oligoastrocytomas. With regard to MRI characteristics, 462 patients were available for analysis. In these 462 patients, the tumor was located cortically in 143 (31%) and subcortically in 319 (69%). The most commonly involved lobe was the frontal (70.9%), followed by the temporal (37.2%), the insula (21.1%), and the parietal (9.1%). Tumors with mass effect (77.7%) and enhancement (34.6%) were more common on MRI than tumors with edema (14.9%), cystic change (17.7%), and calcification (14.4%).
The median duration between seizure onset and admission to the hospital was 10 months (range, 0.1 mo to 23 y). The most common seizure type was secondary generalized (67.1%), followed by simple partial and complex partial (23.7% and 6.6%, respectively); only 9 patients (2.6%) had a combination of partial and generalized seizure. One hundred twenty patients (34.3%) presented with only 1 episode of a preoperative seizure. The most common AED used in our center is valproic acid, followed by carbamazepine, phenytoin, and phenobarbital (Table 2). Only 4 patients without a history of seizures were not prescribed an AED.
Table 2.
Preoperative and postoperative AED use in 508 patients
| Treatment | Total (%) | Preoperative |
Total (%) | Postoperative |
||
|---|---|---|---|---|---|---|
| Seizures (%)a | No. Seizures (%)b | Seizures (%)a | No. Seizures (%)b | |||
| Any AEDs | 502 (98.8) | 348 (99.4) | 154 (97.5) | 480 (94.5) | 333 (95.1) | 147 (97.0) |
| Valproic acid | 444 (87.4) | 306 (87.4) | 138 (87.3) | 431 (84.8) | 300 (85.7) | 131 (82.9) |
| Carbamazepine | 3 (0.6) | 3 (0.9) | 0 (0.0) | 11 (2.2) | 7 (2.0) | 4 (2.5) |
| Phenytoin | 4 (0.8) | 4 (1.1) | 0 (0.0) | 3 (0.6) | 1 (0.3) | 2 (1.3) |
| Phenobarbital | 1 (0.2) | 0 (0.0) | 1 (0.6) | 4 (0.8) | 1 (0.3) | 3 (1.9) |
| Combination | 50 (9.8) | 35 (10.0) | 15 (9.5) | 31 (6.1) | 24 (6.9) | 7 (4.4) |
aPercentage of the 350 patients who had seizures in whole study group.
bPercentage of the 158 patients who did not have seizures in whole study group.
Associations of Preoperative Seizures with Clinical and Laboratory Data
To identify factors that might be associated with preoperative seizures, demographic and tumoral information were compared between patients with seizure and those without (see Table 1 for a complete list). In univariate analysis, only 2 variables were significantly associated with the presence of seizures: age (P = .016, Mann–Whitney; <38 years vs ≥38 years, P = .005, chi-square test) and cortical location (P = .0001). No other clinical, imaging, or pathological variables were found to be associated with increased risk for preoperative seizures in this group of patients. In multivariate analysis, these 2 factors were also found to have a strong association with preoperative seizures (see P-value, odds ratio, and 95% confidence interval inTable 3). There was no association between preoperative seizures and the expression level of the 11 molecular markers (Table 6).
Table 3.
Multivariate predictors of preoperative seizuresa
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Age <38 yr | 1.822 | 1.219–2.721 | 0.003 |
| Cortex involvement | 2.114 | 1.326–3.370 | 0.001 |
aResults of logistic regression analysis.
Prognostication of Seizure Outcomes with Clinical and Imaging Data
The average duration of follow-up was 32.9 months (range, 12.1–58.3 mos). For each of the 350 patients who presented with a history of seizures, seizure control at 6 and 12 months after surgery was evaluated according to Engel classification (Table 4). The majority of patients with preoperative seizures remained seizure free (class I) postoperatively. Six months after surgery, 4.9% had rare seizures (class II), 9.7% had meaningful seizure improvement (class III), and 20.1% had worsening seizures (class IV). Twelve months after surgery, 3.9% were class II, 10.5% class III, and 23.1% class IV. Among those patients who had no history of preoperative seizures, seizure prognosis was mostly favorable postoperatively (about 80% of patients were seizure free); approximately 20% of these patients reported seizures 6 and 12 months, respectively, postoperatively.
Table 4.
Engel classification of 508 patients 6 and 12 months after surgerya
| Variable | Seizures |
No. Seizures |
||
|---|---|---|---|---|
| 6-Mos Follow-up | 12-Mos Follow-up | 6-Mos Follow-up | 12-Mos Follow-up | |
| Total no. of patients | 329 | 304 | 146 | 139 |
| Engel class | ||||
| I | 215 (65.3) | 190 (62.5) | 115 (78.8) | 111 (79.9) |
| II | 16 (4.9) | 12 (3.9) | 31 (21.2) | 28 (20.1) |
| III | 32 (9.7) | 32 (10.5) | 0 | 0 |
| IV | 66 (20.1) | 70 (23.1) | 0 | 0 |
aValues are numbers of patients (%). Engel class I = seizure free; II = rare seizures; III = meaningful improvement; IV = no improvement/worsening seizures.
Among those patients with preoperative seizures in univariate analysis (Table 5), the factors that were associated with postoperative seizure control were secondary generalized seizure type (P = .003), simple partial seizure type (P = .001), combination of partial and generalized seizure type (P = .037), temporal lobe location (P = .026) with edema (P = .043) and calcification (P = .034) on MRI, extent of resection (P < .0001), and adjuvant chemotherapy (P = .02).
Table 5.
Univariate predictors of seizure control 6 months after surgerya
| Variable | Total | Engel Classification |
P-value | |
|---|---|---|---|---|
| I | II–IV | |||
| No. of patients | 329 | 215 (65.3) | 114 (34.7) | |
| No. of male sex | 202 (61.4) | 130 (60.5) | 72 (63.2) | 0.633 |
| Median age in yrs (range) | 37.5 (16–69) | 37.7 (16–72) | 37.2 (18–65) | 0.796 |
| Dexamethasone therapy | 46 (14.0) | 31 (14.4) | 15 (13.2) | 0.754 |
| Duration from onset >1 year | 102 (31.0) | 64 (29.8) | 38 (33.3) | 0.506 |
| Seizure type | ||||
| Secondary generalized | 222 (67.5) | 157 (73.0) | 65 (57.0) | 0.003b |
| Simple partial | 77 (23.4) | 38 (17.7) | 39 (34.2) | 0.001b |
| Complex partial | 22 (6.7) | 12 (5.6) | 10 (8.8) | 0.27 |
| Combination of partial and generalized | 8 (2.4) | 8 (3.7) | 0 | 0.037b |
| Tumor involvement | ||||
| Frontal | 239 (72.6) | 162 (75.3) | 77 (67.5) | 0.131 |
| Temporal | 126 (38.3) | 73 (34.0) | 53 (46.5) | 0.026b |
| Parietal | 27 (8.2) | 19 (8.8) | 8 (7.0) | 0.567 |
| Insula | 70 (21.3) | 43 (20.0) | 27 (23.7) | 0.437 |
| MRI characteristics (296 patients available) | ||||
| Median size in cm (range) | 4.9 (2.5–8.8) | 4.9 (2.5–8.8) | 4.9 (2.5–8.8) | |
| Cortical involvement | 105 (35.5) | 70 (35.9) | 35 (34.7) | 0.832 |
| Enhancement | 101 (34.1) | 72 (36.9) | 29 (28.7) | 0.158 |
| Edema | 40 (13.5) | 32 (16.4) | 8 (7.9) | 0.043b |
| Cystic | 46 (15.50) | 29 (14.9) | 17 (16.8) | 0.659 |
| Mass effect | 227 (76.7) | 155 (79.5) | 72 (71.3) | 0.114 |
| Calcification | 41 (13.9) | 33 (16.9) | 8 (7.9) | 0.034b |
| Tumor pathology | ||||
| Astrocytoma | 145 (44.1) | 99 (46.0) | 46 (40.4) | 0.322 |
| Oligodendroglioma | 29 (8.8) | 17 (7.9) | 12 (10.5) | 0.425 |
| Oligoastrocytoma | 155 (47.1) | 99 (46.0) | 56 (49.1) | 0.595 |
| Extent of resection | <0.0001b | |||
| Gross total | 120 (36.5) | 98 (45.6) | 22 (19.3) | |
| Subtotal | 209 (63.5) | 117 (54.4) | 92 (80.7) | |
| KPS score ≥80 | 267 (88.1) | 180 (89.6) | 87 (85.3) | 0.279 |
| Adjuvant therapy | ||||
| Radiotherapy | 304 (92.4) | 199 (92.6) | 105 (92.1) | 0.883 |
| Chemotherapy | 39 (11.9) | 19 (8.8) | 20 (17.5) | 0.020b |
aValues are numbers of patients (%) unless otherwise indicated.
bStatistically significant.
Prognostication of Outcomes with ECoG Data
ECoG was used at the discretion of the operating surgeon in 103 cases. Areas with epileptiform activities were identified in 43 of these cases and were resected if they were not located in functionally eloquent areas confirmed by intraoperative functional mapping. When comparing seizure control rate in these 43 patients (56.3%) with the 60 patients (51.0%) in whom no epileptiform activities could be identified, the use of intraoperative ECoG had no impact on postoperative seizure control.
Prognostication of Outcomes with Neuropathological Data
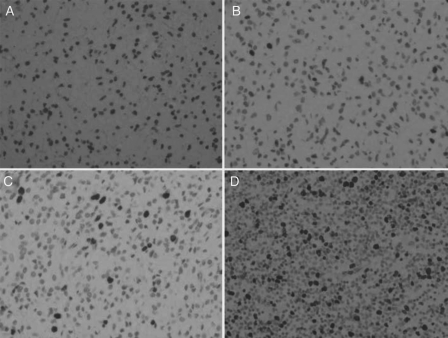
Of note, high-level expression of Ki67 was the only biomarker among the 11 molecular markers we tested that is associated with poor seizure control (Table 6). Figure 2 shows Ki67 immunohistochemistry staining of gliomas removed from patients with lower (A, B) and higher expression (C, D).
Fig. 2.
Photomicrographs of tumor tissue stained for Ki67 with different scores. A, Low Ki67 expression (score 0). B, Low Ki67 expression (score 1). C, High Ki67 expression (score 2). D, High Ki67 expression (score 3). Original magnification: ×400.
These variables were entered into multivariate analysis using logistic regression (Table 7). The factors significantly associated with favorable seizure control were secondary generalized seizure type (odds ratio [OR]: 2.234, 95% CI: 1.295–3.965, P = .006), calcification on MRI (OR: 2.561, 95% CI: 1.036–6.330, P = .031), and gross total resection (OR: 3.482, 95% CI: 1.891–6.411, P < .0001). High-level expression of Ki67 remained independently associated with poor seizure control (OR: 0.179, 95% CI: 0.043–0.748, P = .011). Subsequently, these 4 independent predictors were involved in analysis (chi-square test) for possible correlation with seizure prognosis in the patients without history of preoperative seizures (completely seizure free vs 2 or more episodes of seizures). Unfortunately, none of them showed significance (Table 8).
Table 7.
Multivariate predictors of postoperative seizure control 6 months after surgerya
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Secondary generalized seizure | 2.234 | 1.295–3.965 | 0.006 |
| Calcification on MRI | 2.561 | 1.036–6.330 | 0.031 |
| Gross total resection | 3.482 | 1.891–6.411 | <0.0001 |
| High-level expression of Ki67 | 0.179 | 0.043–0.748 | 0.011 |
aResults of logistic regression analysis.
Table 8.
Univariate analysis of seizure control in patients without preoperative seizures 6 months after surgery (according to Table 7)a
| Variable | Total | Seizure Control |
P-value | |
|---|---|---|---|---|
| Free | Not Free | |||
| No. of patients | 146 | 115 (78.8) | 31 (21.2) | |
| Calcification on MRIb | 19 (14.1) | 16 (15.2) | 3 (10.0) | 0.467 |
| Extent of resection | 0.936 | |||
| Gross total | 65 (44.5) | 51 (44.3) | 14 (45.2) | |
| Subtotal | 81 (55.5) | 64 (55.7) | 17 (54.8) | |
| High-level expression of Ki67c | 10 (7.3) | 8 (7.4) | 2 (6.9) | 0.925 |
aOne hundred thirty-three patients available for MRI analysis.
bOne hundred thirty-seven patients available for Ki67 analysis.
Tumor Progression, Ki67, and Recurrent Seizures
Approximately 95% and 90% of the 508 patients were progression free at 6 and 12 months, respectively, after surgery. By the latest time of follow-up, 109 patients had had tumor progression and 42 patients had died. Given the strong association between seizures and LGGs, we sought to determine the prognostic significance of postoperative seizures for tumor recurrence for the patients who initially had good seizure control after surgery (completely seizure free at 6 months). At 6-month follow-up, 306 patients were seizure free and had not experienced tumor progression; 87 of these patients had a known time of tumor progression. Only 23 of these 87 patients had had seizures prior to progression. Postoperative seizure recurrence was not found to be associated with tumor progression (time-dependent Cox proportional hazards model, P = .217).
We then analyzed the possible association between seizure occurrence and Ki67 expression in some cases with tumor progression. Seven patients underwent both primary and secondary craniotomy in our center (Table 9). All of them were seizure free at 6-month follow-up. Three of them (Nos 2, 5, 7) had seizures prior to tumor progression, and 2 of these 3 patients (66.7%) had increased expression of Ki67, while of the other 4 patients (Nos 1, 3, 4, 6) who had not experienced seizures prior to tumor progression, only 1 (25%) had increased expression of Ki67. This may to some extent suggest a possible prognostic significance of Ki67 in predicting seizure recurrence.
Table 9.
Analysis of the association between Ki67 and seizure recurrence in 7 cases with tumor progression
| Patients | Preoperative Seizure | Age (y) | Extent of Resection | PFS (mos) | Seizure Prior to Tumor Progression | First Operation |
Second Operation |
||
|---|---|---|---|---|---|---|---|---|---|
| Pathology | Ki67 Score | Pathology | Ki67 Score | ||||||
| 1 | No | 51 | GTR | 15 | No | II OA | 1 | III AOA | 1 |
| 2 | No | 41 | STR | 17 | Yes | II A | 0 | IV GBM | 1 |
| 3 | Yes | 58 | GTR | 9 | No | II A | 2 | IV GBM | 2 |
| 4 | Yes | 32 | STR | 15 | No | II A | 1 | II A | 2 |
| 5 | Yes | 43 | GTR | 33 | Yes | II A | 1 | II A | 1 |
| 6 | Yes | 33 | GTR | 18 | No | II O | 1 | II AO | 1 |
| 7 | Yes | 47 | GTR | 21 | Yes | II A | 0 | II A | 2 |
Abbreviations: A, astrocytoma; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; O, oligodendroglioma; OA, oligoastrocytoma; GBM, glioblastoma; GTR, gross total resection; STR, subtotal resection; PFS, progression free survival.
Discussion
We retrospectively analyzed a data set of 508 patients who had undergone primary resection of cerebral hemispheric LGGs to identify the clinical factors associated with pre- and postoperative seizures. This is a large series of patients who were treated at a single institute in China over a period of 5 years.
The percentage of our patients with seizures (68.9%) as the presenting symptom is consistent with that (65%–85%) identified in previously published reports.1,13–15 Prior studies1,6 have indicated that oligodendroglial tumors, including oligodendrogliomas and oligoastrocytomas, are the most common tumor types to present with seizures. In our series, there was no significant difference in seizure incidence between patients with oligodendroglial tumors and patients with astrocytomas (70.3% and 67.2%, respectively). This may be to some degree due to different histological diagnostic criteria in different hospitals. In other words, a significant number of patients may be classified differently from where they would be in other studies or hospitals. Different authors suggested various cut-off values for the oligodendroglial component to separate oligoastrocytomas from astrocytomas. In our center, an oligoastrocytoma was pathologically diagnosed only if the proportion of oligodendroglial components reached 25% in at least 5–10 different planes. Otherwise, we would consider it to be a “pure” astrocytoma. Conclusively, we confirmed the same finding that seizure occurrence and types were closely related to the location of i.c. masses, as Chang et al. described before.1 Lesions with more cortical involvement tend to be associated with higher incidence of preoperative seizures. Interestingly, we also demonstrated a relationship between age and seizure occurrence: patients with younger age are more likely to present with seizures. It may be explained that elderly patients have a predisposition to malignant brain tumors that are less likely to cause seizures.6,16–18
In a recent study,19 investigators in our institute analyzed 103 patients with LGGs and argued that patients without loss of heterozygosity of chromosome19q were more likely to present with seizures (P = .033) than those with this alteration, especially in those patients with secondary generalized seizures (P = .005). They hypothesized that some genes on 19q might be susceptibility genes of glioma-related seizures. This finding suggests that a genetic component may be associated with secondary generalized seizures in tumoral epilepsy.
Seizures play an important role in patients' postoperative quality of life,20 especially the patients with LGGs because of the duration of their survival. Of the 329 patients who presented with preoperative seizures and were followed up at 6 months after surgery in our study, 215 (65.3%) were completely seizure free 6 months after surgery. However, about one third of them had seizures postoperatively, and some developed medically intractable seizure (most in the Engel class IV group). We identified 4 important factors significantly associated with freedom from seizures: secondary generalized seizure type, calcification on MRI, gross total resection of tumor, and low expression level of Ki67.
The correlation between seizure types and seizure control is difficult to explain. Chang et al.1 have demonstrated that simple partial seizure is one of the independent factors predicting poor seizure control. The current study also found significance of simple partial seizures for seizure control after surgery in the univariate analysis. Unfortunately, this variable was excluded after multivariate analysis. However, as a previous study did,21–23 our study also supports the notion that patients with LGGs who had no generalized seizures may have less satisfactory seizure control following surgery. Chaichana and colleagues18 showed that patients with parietal lobe lesions had unfavorable seizure control postoperatively after analysis of 648 malignant glioma patients. These tumors may be in closer proximity to verbal areas, which may make them more difficult to be resected and thus more likely to have seizures.
Recently, characteristics of neuroimages have been frequently analyzed and correlated to surgical outcome. Many studies24–27 have demonstrated that some features on MRI, such as large tumor size, significant enhancement, and edema, could predict shorter survival in patients with LGGs. However, the literature is scarce in addressing the relationship between imaging features and seizures. Lee and colleagues7 showed that in LGGs, lesions tend to grow large without other symptoms and eventually cause seizures. Khan et al.28,29 reported hyperintensity around the tumor cavity on T2-weighted MRI obtained within 72 h after surgery as a risk factor for uncontrolled seizures in a cohort of children with low- and high-grade brain tumors. As a novel marker in our study, calcification on preoperative MRI of LGGs was shown to be a factor predicting favorable seizure prognosis. The pathogenesis of calcification in brain tumor has been studied by different groups.30–33 Calcification is often seen in slow-growing brain tumors. In the immediate vicinity of the lesion, brain tissue reacted with changes consisting of astrocytic gliosis, angiogenesis, inflammatory infiltrates, and a possible small region of demyelination. These changes are gradually transformed into an epileptigenetic zone leading to tumor-related seizures. It is well known that the microenvironment changes surrounding tumors with calcification are relatively focal and closely related to tumor entity. These focal changes may probably lose their epileptigenetic activity once the tumor disappears or may respond better to AEDs after tumor resection. This may explain the fact that the vast majority of these patients remain seizure free postoperatively.
Currently, there are mainly 2 hypotheses regarding the pathogenesis of tumor-related seizures. One potential explanation is from the surrounding normal tissue: the tumor mechanically compresses the surrounding normal tissue, causing a mass effect, and the affected tissue becomes epileptic after suffering from decreasing pH, ischemia, and hypoxia.34,35 The other possibility is from the tumor origin: the tumor may chemically excrete some factors that could change the peritumoral microenvironment into epileptic focus.36–38 These potential effects, among others, may alter the balance between intracortical inhibitory and excitatory mechanisms, thus inducing epileptogenic activity.
Complete seizure control related to gliomas involving different lobes has been described, ranging from 48% to 77%.1,5,18,22,29 Although some studies6,22 have reported that there was no significant association between the type of surgical resection and control of seizures, most of the recent literature1,5,23,39,40 suggests that gross total resection was one of the strongest predictors of tumor-related seizure control. Gross total resection is defined as removal ofmore than 90% of the tumor. Extended lesionectomy, which is a subtype of gross total resection, is resection intended to extend well beyond the tumor margin (>100%). (Extended lesionectomy was rarely performed in our center.) Generally speaking, neurosurgeons are able to perform these 2 procedures better in LGGs than in malignant gliomas. Minimizing the residue volume of tumors with gross total resection could increase the possibility of removing the epileptigenic zone. Thus, patients are more likely to achieve favorable seizure control.
The correlations between tumor markers and surgical outcomes have been the most popular research topics during the past 2 decades. Two recent studies41,42 have presented genetic and molecular tumor marker data in a large number of population-based glioma cases. Other studies on the pathogenesis of both idiopathic and symptomatic epilepsy43–45 have identified a number of seizure-related candidate genes. Nonetheless, little is known regarding how changes in gene expression may influence tumor-related seizure control. In our study, level of Ki67 expression was found to be significantly associated with seizure prognosis, as shown in Table 7. This finding in such a large patient population strongly confirmed the role of Ki67 in predicting seizure control in an unpublished study.8 Prayson46 found that proliferating cells appeared to be primarily astrocytic and endothelial in nature and suggested that malformation-of-cortical-development lesions may not be static. Kee et al.47 provided evidence for Ki67 to be used as a marker of proliferation in the initial phase of adult neurogenesis. These findings are consistent with our results. In addition, Rush and colleagues analyzed the correlation between the expression of the Hedgehog pathway and Ki67 in 20 pilocytic astrocytomas.48 They found that 86% of Ki67-positive cells also expressed protein patched homolog 1 (one component in the Hedgehog pathway), which might be associated with tumor growth. These results indicate that Ki67 may to some extent play a role in this seizure-related tumor by increasing its proliferation. Furthermore, some clinical research1,18 revealed that seizure recurrence was associated with tumor progression. This may suggest that recurrent seizures reflect an increased proliferative activity of tumor cells. Gangliogliomas (GGs), another seizure-related tumor, were reported to have a high seizure incidence of 80%–90%.14,49 Wolf et al.50 first analyzed 61 GGs on the immunohistochemical level in 1994. They found that nuclear labeling for Ki67 was observed exclusively in the astrocytic component. GGs with very large neurons had higher Ki67 labeling indices than GGs with small or intermediate-sized neurons. A subsequent case study51 reported GG and epilepsy, where the authors described a high Ki67 proliferation index (30%) in the tumor. In the present study, the same Ki67 index was seen in several tumors, and these tumors, in spite of grade II morphology, could represent an early stage of anaplastic transformation. Here we suggest that upregulated Ki67 expression plays an important role in postoperative seizure recurrence in patients with LGGs. We also found a possible significance of Ki67 in predicting seizure recurrence in several cases with tumor progression. Ki67 may become a novel diagnostic or treatment target point for tumor-related seizure in the future.
Of note, the pre- and postoperative use of AEDs was not associated with postoperative seizure control in this study (Table 2). The most commonly used AED in our center is valproic acid. We analyzed the effect of AED use on postoperative seizure control. Of patients with preoperative seizures, 61% who were treated with valproic acid were seizure free (Engel class I) postoperatively.
Previous studies of both low- and high-grade gliomas26,52 have found that patients with better functional status have prolonged survival. In this study, we found no significant correlations between postoperative KPS scores and seizure prognosis. Interestingly, in univariate analysis, chemotherapy was associated with better seizure control. This may be to some extent related to the effects of the treatment on tumor progression.
This study, however, has some limitations. First, the AEDs used in our center were all old-generation antiepileptic drugs. The choice of a particular AED, mostly valproic acid in our study, often appeared to be the result of physician preference. Recently, the newly developed AEDs that are devoid of hepatic metabolism, such as levetiracetam and gabapentin,52–54 are now recommended because of good results in many studies. They do not have interactions with anticancer agents. Further studies on the control of seizures with newer-generation AEDs are therefore necessary. Second, this study did not analyze the data by dividing the cohort into a seizure controlled group and an uncontrolled group by AEDs due to the small number of patients who had AEDs before hospital admission. Third, the number of recurrent tumor samples in this study was relatively small, so it presents just a trend that Ki67 may have a prognostic significance in predicting tumor-related seizure control. Further study with a longer follow-up period and more such cases are needed to address this question. Fourth, by design, this study is retrospective. Future prospective studies, particularly on the role of Ki67 in tumor-related seizures, are needed to confirm the findings of this work.
Despite these inherent limitations, our findings offer useful insights into the pathogenesis of seizures in patients with LGGs, especially the finding of a pathological biomarker of Ki67 predicting seizure control after surgery in such a large population of patients with LGGs. Of note, Ki67 was not a predictor of seizure control for the patients without preoperative seizures. Nevertheless, prospective studies are needed to provide better evidence to guide clinical decision making.
In summary, epilepsy is a major issue for patients with LGGs. It significantly impacts patients' quality of life. Secondary generalized seizure type, calcification on MRI, and gross total resection, as well as low-level expression of Ki67, predisposed patients to favorable postoperative seizure control. These factors may therefore provide insight into developing effective treatment strategies aimed at prolonging patients' survival.
Conflict of interest statement. None declared.
Funding
This study was supported by funds from the Science and Technology Star Plan of Fengtai District and the National Basic Research Program of China (No. 2010CB529406).
Acknowledgments
We would like to thank Thomas Henry (Department of Neurology, Medical School, University of Minnesota, USA) for assistance with reviewing this manuscript.
References
- 1.Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. doi:10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 2.Leighton C, Fisher B, Bauman G, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15:1294–1301. doi: 10.1200/JCO.1997.15.4.1294. [DOI] [PubMed] [Google Scholar]
- 3.Schaller B, Rüegg SJ. Brain tumor and seizures: pathophysiology and its implications for treatment revisited. Epilepsia. 2003;44:1223–1232. doi: 10.1046/j.1528-1157.2003.05203.x. doi:10.1046/j.1528-1157.2003.05203.x. [DOI] [PubMed] [Google Scholar]
- 4.Danfors T, Ribom D, Berntsson SG, Smits A. Epileptic seizures and survival in early disease of grade 2 gliomas. Eur J Neurol. 2009;16:823–831. doi: 10.1111/j.1468-1331.2009.02599.x. doi:10.1111/j.1468-1331.2009.02599.x. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65:212–215. doi: 10.1212/01.wnl.0000168903.09277.8f. doi:10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- 6.Lynam LM, Lyons MK, Drazkowski JF, et al. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin Neurol Neurosurg. 2007;109:634–638. doi: 10.1016/j.clineuro.2007.05.017. doi:10.1016/j.clineuro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Lee JW, Wen PY, Hurwitz S, et al. Morphological characteristics of brain tumors causing seizures. Arch Neurol. 2010;67:336–342. doi: 10.1001/archneurol.2010.2. doi:10.1001/archneurol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You G, Huang L, Yang P, et al. Clinical and molecular genetic factors affecting post-operative seizure control of 183 Chinese adult patients with low-grade gliomas. Eur J Neurol. 2011 doi: 10.1111/j.1468-1331.2011.03509.x. doi:10.1111/j.1468-1331.2011.03509.x. [DOI] [PubMed] [Google Scholar]
- 9.Engel J., Jr. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. doi:10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 10.Korshunov A, Golanov A, Sycheva R. Immunohistochemical markers for prognosis of cerebral glioblastomas. J Neurooncol. 2002;58:217–236. doi: 10.1023/a:1016218117251. doi:10.1023/A:1016218117251. [DOI] [PubMed] [Google Scholar]
- 11.Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin Cancer Res. 2005;11:5167–5174. doi: 10.1158/1078-0432.CCR-05-0230. doi:10.1158/1078-0432.CCR-05-0230. [DOI] [PubMed] [Google Scholar]
- 12.Kujas M, Lejeune J, Benouaich-Amiel A, et al. Chromosome 1p loss: a favorable prognostic factor in low-grade gliomas. Ann Neurol. 2005;58:322–326. doi: 10.1002/ana.20543. doi:10.1002/ana.20543. [DOI] [PubMed] [Google Scholar]
- 13.Lote K, Stenwig AE, Skullerud K, Hirschberg H. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34:98–102. doi: 10.1016/s0959-8049(97)00374-2. doi:10.1016/S0959-8049(97)00374-2. [DOI] [PubMed] [Google Scholar]
- 14.Villemure JG, de Tribolet N. Epilepsy in patients with central nervous system tumors. Curr Opin Neurol. 1996;9:424–428. doi: 10.1097/00019052-199612000-00005. doi:10.1097/00019052-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Pace A, Bove L, Innocenti P, et al. Epilepsy and gliomas: incidence and treatment in 119 patients. J Exp Clin Cancer Res. 1998;17:479–482. [PubMed] [Google Scholar]
- 16.LaRoche SM, Helmers SL. Epilepsy in the elderly. Neurologist. 2003;9:241–249. doi: 10.1097/01.nrl.0000087719.64343.be. doi:10.1097/01.nrl.0000087719.64343.be. [DOI] [PubMed] [Google Scholar]
- 17.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. doi:10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 18.Chaichana KL, Parker SL, Olivi A, Quiñones-Hinojosa A. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. J Neurosurg. 2009;111:282–292. doi: 10.3171/2009.2.JNS081132. doi:10.3171/2009.2.JNS081132. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, You G, Jiang T, Li GL, Li SW, Wang ZC. Correlation between tumor-related seizures and molecular genetic profile in 103 Chinese patients with low-grade gliomas: a preliminary study. J Neurol Sci. 2010;302:63–67. doi: 10.1016/j.jns.2010.11.024. doi:10.1016/j.jns.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 20.Jacoby A, Wang W, Vu TD, et al. Meanings of epilepsy in its socio cultural context and implications for stigma: findings from ethnographic studies in local communities in China and Vietnam. Epilepsy Behav. 2008;12:286–297. doi: 10.1016/j.yebeh.2007.10.006. doi:10.1016/j.yebeh.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Luyken C, Blümcke I, Fimmers R, et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44:822–830. doi: 10.1046/j.1528-1157.2003.56102.x. doi:10.1046/j.1528-1157.2003.56102.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosati A, Tomassini A, Pollo B, et al. Epilepsy in cerebral glioma: timing of appearance and histological correlations. J Neurooncol. 2009;93:395–400. doi: 10.1007/s11060-009-9796-5. doi:10.1007/s11060-009-9796-5. [DOI] [PubMed] [Google Scholar]
- 23.Zaatreh MM, Firlik KS, Spencer DD, Spencer SS. Temporal lobe tumoral epilepsy: characteristics and predictors of surgical outcome. Neurology. 2003;61:636–641. doi: 10.1212/01.wnl.0000079374.78589.1b. [DOI] [PubMed] [Google Scholar]
- 24.Pallud J, Capelle L, Taillandier L, et al. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro Onco. 2009;11:176–182. doi: 10.1215/15228517-2008-066. doi:10.1215/15228517-2008-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiff D, Brown PD, Giannini C. Outcome in adult low-grade glioma: the impact of prognostic factors and treatment. Neurology. 2007;69:1366–1373. doi: 10.1212/01.wnl.0000277271.47601.a1. doi:10.1212/01.wnl.0000277271.47601.a1. [DOI] [PubMed] [Google Scholar]
- 26.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. doi:10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 27.El-Hateer H, Souhami L, Roberge D, et al. Low-grade oligodendroglioma: an indolent but incurable disease? J Neurosurg. 2009;111:265–271. doi: 10.3171/2008.11.JNS08983. doi:10.3171/2008.11.JNS08983. [DOI] [PubMed] [Google Scholar]
- 28.Khan RB, Hunt DL, Thompson SJ. Gabapentin to control seizures in children undergoing cancer treatment. J Child Neurol. 2004;19:97–101. doi: 10.1177/08830738040190020301. [DOI] [PubMed] [Google Scholar]
- 29.Khan RB, Boop FA, Onar A, Sanford RA. Seizures in children with low-grade tumors: outcome after tumor resection and risk factors for uncontrolled seizures. J Neurosurg. 2006;104:377–382. doi: 10.3171/ped.2006.104.6.377. [DOI] [PubMed] [Google Scholar]
- 30.Restrepo BI, Alvarez JI, Castano JA, et al. Brain granulomas in neurocysticercosis patients is associated with a Th1 and Th2 profile. Infect Immun. 2001;69:4554–4560. doi: 10.1128/IAI.69.7.4554-4560.2001. doi:10.1128/IAI.69.7.4554-4560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia. 2000;41:718–726. doi: 10.1111/j.1528-1157.2000.tb00234.x. doi:10.1111/j.1528-1157.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal A, Raghav S, Husain M, Kumar R, Gupta RK. Epilepsy with focal cerebral calcification: role of magnetization transfer MR imaging. Neurol India. 2004;52:197–199. [PubMed] [Google Scholar]
- 33.Murthy JM, Subba Reddy YV. Prognosis of epilepsy associated with single CT enhancing lesion: a long-term follow-up study. J Neurol Sci. 1998;159:151–155. doi: 10.1016/s0022-510x(98)00156-7. doi:10.1016/S0022-510X(98)00156-7. [DOI] [PubMed] [Google Scholar]
- 34.Schaller B. Influences of brain tumor-associated pH changes and hypoxia on epileptogenesis. Acta Neurol Scand. 2005;111:75–83. doi: 10.1111/j.1600-0404.2004.00355.x. doi:10.1111/j.1600-0404.2004.00355.x. [DOI] [PubMed] [Google Scholar]
- 35.Wolf HK, Roos D, Blumcke I, Pietsch T, Wiestler OD. Perilesional neurochemical changes in focal epilepsies. Acta Neuropathol. 1996;91:376–384. doi: 10.1007/s004010050439. doi:10.1007/s004010050439. [DOI] [PubMed] [Google Scholar]
- 36.Bordey A, Sontheimer H. Electrophysiological properties of human astrocytic tumor cells In situ: enigma of spiking glial cells. J Neurophysiol. 1998;79:2782–2793. doi: 10.1152/jn.1998.79.5.2782. [DOI] [PubMed] [Google Scholar]
- 37.Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. doi:10.1016/S0920-1211(98)00059-X. [DOI] [PubMed] [Google Scholar]
- 38.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- 39.Simon M, Neuloh G, von Lehe M, Meyer B, Schramm J. Insular gliomas: the case for surgical management. J Neurosurg. 2009;110:685–695. doi: 10.3171/2008.7.JNS17639. doi:10.3171/2008.7.JNS17639. [DOI] [PubMed] [Google Scholar]
- 40.Duffau H, Capelle L, Lopes M, Bitar A, Sichez JP, van Effenterre R. Medically intractable epilepsy from insular low-grade gliomas: improvement after an extended lesionectomy. Acta Neurochir (Wien) 2002;144:563–572. doi: 10.1007/s00701-002-0941-6. doi:10.1007/s00701-002-0941-6. [DOI] [PubMed] [Google Scholar]
- 41.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. doi:10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 42.Wiencke JK, Aldape K, McMillan A, et al. Molecular features of adult glioma associated with patient race/ethnicity, age, and a polymorphism in O6-methylguanine-DNAalkyltransferase. Cancer Epidemiol Biomarkers Prev. 2005;14:1774–1783. doi: 10.1158/1055-9965.EPI-05-0089. doi:10.1158/1055-9965.EPI-05-0089. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez-Carpintero Abad R, Sanmartí Vilaplana FX, Serratosa Fernández JM. Genetic causes of epilepsy. Neurologist. 2007;13:S47–51. doi: 10.1097/NRL.0b013e31815bb07d. doi:10.1097/NRL.0b013e31815bb07d. [DOI] [PubMed] [Google Scholar]
- 44.Rees MI. The genetics of epilepsy: the past, the present and future. Seizure. 2010;19:680–683. doi: 10.1016/j.seizure.2010.10.029. doi:10.1016/j.seizure.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Pitkänen A, Lukasiuk K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14:16–25. doi: 10.1016/j.yebeh.2008.09.023. doi:10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Prayson RA. Ki67 immunoreactivity in type II malformations of cortical development. Appl Immunohistochem Mol Morphol. 2008;16:357–361. doi: 10.1097/PAI.0b013e31812eef07. doi:10.1097/PAI.0b013e31812eef07. [DOI] [PubMed] [Google Scholar]
- 47.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. doi:10.1016/S0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 48.Rush SZ, Abel TW, Valadez JG, Pearson M, Cooper MK. Activation of the Hedgehog pathway in pilocytic astrocytomas. Neuro Onco. 2010;12:790–798. doi: 10.1093/neuonc/noq026. doi:10.1093/neuonc/noq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology. 2002;59:S21–S26. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 50.Wolf HK, Müller MB, Spanle M, Zentner J, Schramm J, Wiestler OD. Ganglioglioma: a detailed histopathological and immunohistochemical analysis of 61 cases. Acta Neuropathol. 1994;88:166–173. doi: 10.1007/BF00294510. doi:10.1007/BF00294510. [DOI] [PubMed] [Google Scholar]
- 51.Tantbirojn P, Sanpavat A, Bunyaratavei K, Desudchit T, Shuangshoti S. Desmoplastic infantile ganglioglioma with high proliferation index: report of a case. J Med Assoc Thai. 2005;88:1962–1965. [PubMed] [Google Scholar]
- 52.Krex D, Klink B, Hartmann C, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–2606. doi: 10.1093/brain/awm204. doi:10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 53.Dinapoli L, Maschio M, Jandolo B, et al. Quality of life and seizure control in patients with brain tumor-related epilepsy treated with levetiracetam monotherapy: preliminary data of an open-label study. Neuro Sci. 2009;30:353–359. doi: 10.1007/s10072-009-0087-x. doi:10.1007/s10072-009-0087-x. [DOI] [PubMed] [Google Scholar]
- 54.van Breemen MSM, Rijsman RM, Taphoorn MJB, Walchenbach R, Zwinkels H, Vecht CJ. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256:1519–1526. doi: 10.1007/s00415-009-5156-9. doi:10.1007/s00415-009-5156-9. [DOI] [PubMed] [Google Scholar]