Abstract
As patients decline from health to type 2 diabetes, glucose-stimulated insulin secretion (GSIS) typically becomes impaired. Although GSIS is driven predominantly by direct sensing of a rise in blood glucose by pancreatic β-cells, there is growing evidence that hypothalamic neurons control other aspects of peripheral glucose metabolism. Here we investigated the role of the brain in the modulation of GSIS. To examine the effects of increasing or decreasing hypothalamic glucose sensing on glucose tolerance and insulin secretion, glucose or inhibitors of glucokinase, respectively, were infused into the third ventricle during intravenous glucose tolerance tests (IVGTTs). Glucose-infused rats displayed improved glucose handling, particularly within the first few minutes of the IVGTT, with a significantly lower area under the excursion curve within the first 10 min (AUC0-10). This was explained by increased insulin secretion. In contrast, infusion of the glucokinase inhibitors glucosamine or mannoheptulose worsened glucose tolerance and decreased GSIS in the first few minutes of IVGTT. Our data suggest a role for brain glucose sensors in the regulation of GSIS, particularly during the early phase. We propose that pharmacological agents targeting hypothalamic glucose-sensing pathways may represent novel therapeutic strategies for enhancing early phase insulin secretion in type 2 diabetes.
The rise in prevalence of type 2 diabetes reflects a primary medical challenge of the 21st century. The mechanisms underlying glucose homeostasis in general, and glucose-stimulated insulin secretion (GSIS) in particular, are not fully understood. In health, a rise in blood glucose triggers a biphasic pattern of insulin response, consisting of a rapid (<10 min) first phase and a less prominent but sustained second phase (1). The precise mechanisms mediating the early phase of the insulin response are unclear. Nevertheless, impaired first-phase GSIS is a major pathological hallmark of the early stages of type 2 diabetes (2), suggesting that this may be an important determinant of the transition to diabetes in at-risk subjects.
Pancreatic β-cells can directly sense changes in blood glucose and alter insulin release as appropriate. In addition, the pancreas has rich autonomic innervations, and a number of experimental approaches have demonstrated that neural inputs may modulate insulin release (3,4) via muscarinic receptors or α-adrenergic signaling (5,6). A preponderance of the neural inputs that influence pancreatic β-cell activity emanate from the hypothalamus (5); however, the specific nature of hypothalamic pathways regulating insulin secretion is less clear.
In particular, hypothalamic melanocortin signaling (7–9) and inflammation (10) have been implicated in the control of insulin release. Additionally, there is increasing evidence of a role for hypothalamic nutrient-sensing pathways in the control of other facets of peripheral glucose metabolism, in particular for hepatic glucose production (11,12). It is therefore plausible that similar mechanisms might allow central facilitation of insulin secretion in response to a rise in blood glucose.
In order to control peripheral glucose metabolism, the brain must first rapidly and accurately detect changes in glucose availability. The hypothalamus contains glucose-sensing neurons, although the mechanisms used to sense glucose are not fully defined. However, some reports suggest that these hypothalamic neurons may sense products of glucose metabolism such as cellular ATP levels (11,13). To be metabolized, glucose must first be phosphorylated by hexokinases (HKs). The specialized low affinity HK isozyme glucokinase (GK), thought to be central in pancreatic glucose sensing, may also play a key role in hypothalamic glucose sensing (14). Accordingly, here we investigated the effects of acute activation of hypothalamic glucose sensing (by brain glucose infusion) or inhibition (using the competitive GK inhibitors glucosamine [GSN] or mannoheptulose [MH]) on insulin secretion and glucose handling during intravenous glucose tolerance tests (IVGTT) in rats.
RESEARCH DESIGN AND METHODS
Animals.
Healthy adult male Sprague Dawley rats ∼250–350 g were used throughout. For each study, the cohorts were matched for weight and randomized into treatment groups. Procedures were approved in advance by both a local university and a national ethical review process (U.K. Home Office License held under the Animals [Scientific Procedures] Act). Chemicals were from Sigma-Aldrich (Gillingham, U.K.) unless otherwise stated.
Surgical preparation.
Under inhaled anaesthetic, rats underwent stereotaxic insertion of a guide cannula into the base of the third ventricle (coordinates from bregma: 2.2 mm posterior, 0.9 mm lateral, 8.4 mm below skull surface angled at 5° to vertical toward the midline) and placement of jugular vein catheter as previously described (15). Peri- and postoperative injectable analgesia and antibiotic were provided routinely, and only animals that had regained preoperative body weight with no signs of infection or illness were studied 1 week later.
Effect of intracerebroventricular infusion of d-glucose versus urea on glucose handling during IVGTT.
Chronically catheterized intracerebroventricular (ICV) and intravenous (IV) rats prepared as above were brought to the study room on 2 subsequent days. Day 1 was for acclimatization, with animals left undisturbed. On day 2, overnight-fasted rats were acclimatized for 2 h before vascular catheters were opened and flushed and an ICV infusion initiated. Nine milligrams of d-glucose (or equimolar urea) was delivered over 30 min (0.5 μl/min, primed for 3 min at 1.5 μl/min). Next, rats underwent IVGTT with 0.35 g/kg delivered over 1 min (glucose dose was reduced by 9 mg in ICV glucose rats so that total glucose dose delivered was similar). The glucose dose in these studies was selected to be toward the lower end of the range used in rodent studies, given that we were anticipating an enhanced response with ICV glucose. Blood samples were collected over the ensuing 60 min for plasma glucose and insulin assays. After 60 min, rats were anesthetized deeply and brains perfused, removed, and examined to verify correct cannula positioning.
Effect of ICV infusion of GSN or MH versus vehicle on glucose handling during IVGTT.
GSN and MH (CMS Chemicals, Abingdon, U.K.) were dissolved in artificial extracellular fluid (aECF; CMA Microdialysis AB, distributed by Linton Instrumentation, Diss, U.K.). Chronically catheterized rats were prepared, acclimatized, and fasted overnight as above. Rats received ICV infusion (0.3 μl/min, primed for 3 min at 0.9 μl/min) of GSN (75 nmol/min or 150 nmol/min), MH (300 nmol/min), or vehicle (aECF). 90 min after the start of ICV infusions, all animals received an IVGTT (0.5 g/kg glucose over 1 min) with blood sampling as above. By design, the glucose dose in these studies was higher than in the ICV glucose studies described above, given that we were anticipating a reduced response with GK inhibition. At the end of the studies, rats were anesthetized deeply and brains perfused with saline then fixative and removed to verify correct cannula positioning.
Hormonal assays.
Glucose was measured using a benchtop glucose analyzer (Analox GM9 [glucose oxidase method]; Analox Instruments, London, U.K.) and insulin by RIA (Linco).
Insulinogenic index.
Insulinogenic index was calculated as the ratio of areas under insulin and glucose excursion curves.
Assay of GK activity.
Sections of hypothalami were dissected from brain samples using the stereotaxic atlas of Paxinos and Watson as a reference guide (16). Thereafter, hypothalamic and liver protein samples were prepared as previously described with minor modification (17). In brief, tissues were homogenized in ice-cold lysis buffer (50 mM HEPES, 150 mM KCl, 5 mM MgCl2, and 1 mM EDTA [pH 7.4], supplemented with 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 10 μM leupeptin HCl) and centrifuged at 13,000 rpm for 20 min at 4°C. Supernatants were collected and stored at −80°C for further analysis. HK and GK activity was assayed spectrophotometrically (340 nm, room temperature, Beckmann DU-64 spectrophotometer) by coupling glucose phosphorylation to a reporter assay, which oxidizes glucose-6-phosphate to 6-phosphogluco-δ-lactone with simultaneous reduction of NAD+ to NADH, as described previously (18). The reaction mixture in 1 mL final volume contained 20 mM HEPES (pH 7.1), 25 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol, 1 mM NAD+, 1mM ATP, 10 units glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides, 1 mM 3-O-methyl N-acetyl GSN (Axxora UK Ltd., Bingham, U.K.), 100 μL liver/hypothalamus protein extract, and glucose in concentrations of 0.5 mM, 1 mM and 20 mM. In all assays for GK activity, 3-O-methyl N-acetyl GSN was incorporated to inhibit N-acetyl GSN kinase (19), except inhibition studies with MH and GSN. GK activity was calculated by subtracting glucose phosphorylation at 0.5 mM (hypothalamus) or 1 mM (liver) glucose from that measured at 20 mM glucose. Data were analyzed by fitting sigmoidal curves to the dose-response studies by nonlinear least square minimization method.
Determination of extent of GSN distribution following ICV infusion.
Nonfasted catheterized rats underwent 90 min ICV infusion of 150 nmol/min GSN or aECF. Plasma samples were collected before and after ICV infusion. After 90 min, rats were rapidly euthanized and their brains removed; the hypothalamus, brain stem, and cortex were dissected rapidly and frozen in liquid nitrogen. Samples were stored at –80°C before being analyzed. Brain samples were homogenized in ice-cold lysis buffer, centrifuged at 13,000 rpm for 20 min at 4°C, and supernatants were collected for further analysis.
GSN concentration in plasma and brain homogenate was assayed by high-performance liquid chromatography–electrospray ionization–tandem mass spectometry (HPLC-ESI-MS/MS). Analysis was performed using an Alliance 2695 chromatograph (Waters, Milford, MA) coupled with a triple-quadrupole MS (Quattro LC; Micromass UK Ltd., Manchester, U.K.). Sample preparation was optimized by a slight modification of a previously published method for direct determination of GSN in plasma (20). Briefly, 45 μL of sample was mixed with 5 μL of d-[1-13C] GSN as the internal standard; then, 25 μL of trichloroacetic acid (200 g/L) were added to achieve protein precipitation. 3 μL of the supernatant was injected into the HPLC-ESI-MS/MS system. A polymer-based amino column (5 μm, 2.0 mm i.d. × 150 mm), supplied by Showa Denko K.K. (Kanagawa, Japan), and mobile phase of 80:20 (volume for volume) acetonitrile:10 mmol/L ammonium acetate (pH = 7.5) at 0.3 mL/min flow rate were used. MS/MS detection, using ESI source in positive ionization, was performed in multiple reaction monitoring mode, selecting the charge/mass ratio transition 180→72 for GSN and charge/mass ratio181→73 for internal standard. The limit of quantification of the method was 0.28 μmol/L for both rat plasma and brain homogenate. Matrix-matched standards were used for calibration, obtaining good linearity up to 56 μmol/L (R2 ≥0.9937). For calculating absolute concentrations, the density of brain tissue was assumed to be 1.04 g/mL (21).
RESULTS
ICV infusion of d-glucose improved glucose handling during IVGTT.
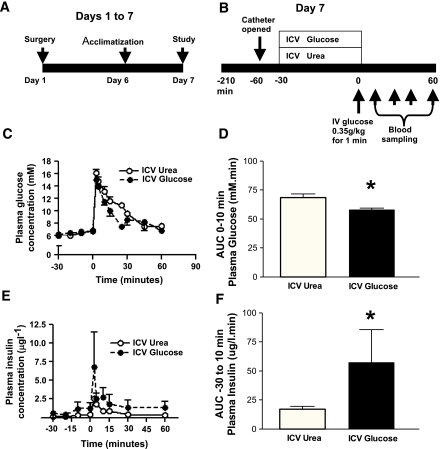
We hypothesized that if the brain plays a role in controlling GSIS, then increasing glucose levels in the hypothalamus would lead to increased insulin secretion. To investigate this, glucose was infused into the third ventricle (ICV) of adult male rats for 30 min followed by an IVGTT (0.35 g/kg) (Fig. 1A and B). ICV infusion of glucose did not change systemic plasma glucose during the 30 min infusion period (−30 to 0 min). However, ICV glucose-infused rats displayed improved glucose handling during the first 10 min of the IVGTT, with a significantly lower (P <0.05) area under the excursion curve (AUC0-10) relative to control urea-infused animals (Fig. 1C and D).
FIG. 1.
ICV infusion of glucose improved glucose handling during IVGTT. A and B: Schematic representation of experimental design. Vascular and third ventricle (ICV) catheters were implanted on day 1. Rats were acclimatized to the study room on day 6 and fasted overnight from 1500 hrs on day 6. On day 7, chronically catheterized rats underwent IVGTT (0.35 g/kg) preceded by ICV infusion of either 9-mg glucose or equimolar urea (n = 6–7) delivered over 30 min. C: ICV glucose rats showed reduced plasma glucose levels particularly during the first few minutes of IVGTT. D: In particular, the AUC0-10 (plasma glucose) was significantly (P < 0.05) lower in ICV glucose-treated rats relative to control urea-treated rats. E and F: Despite exposure to lower glycemia, plasma insulin responses were significantly greater in ICV glucose animals. Data are mean ± SEM. *P < 0.05. (A high-quality color representation of this figure is available in the online issue.)
Despite being exposed to equivalent or lower circulating plasma glucose levels prior to and during IVGTT respectively, plasma insulin levels in ICV glucose-infused rats were significantly higher than controls, seemingly starting to rise even before the delivery of an external glucose load (Fig. 1E). Insulin secretion between the start of the brain infusion and the 10 min time point of the IVGTT (40 min total), which was used as the cutoff between early and late-phase insulin secretion, was significantly higher in ICV glucose-infused rats (Fig. 1F). These data suggest that elevation of hypothalamic glucose leads to a centrally driven insulin secretory response.
ICV infusion of GSN impaired glucose handling and insulin secretion during IVGTT.
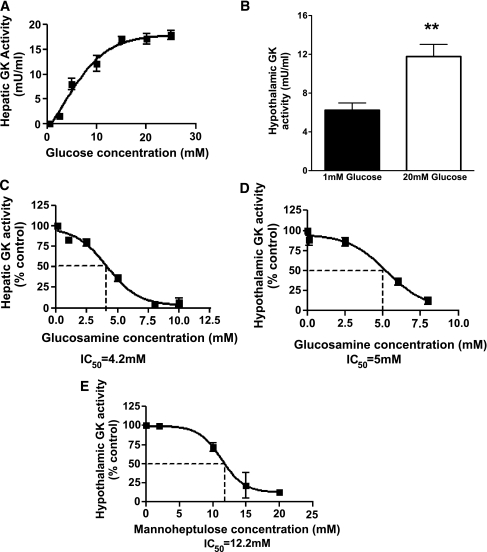
As a possible mechanism underlying this observation, we examined the role of hypothalamic GK-dependent sensing pathways in insulin secretion in response to a systemic glucose load. The competitive inhibitor of glucose phosphorylation, GSN, has previously been shown to inhibit hepatic GK (22). To confirm that GSN inhibits hypothalamic GK, we first examined the effect of GSN on GK activity in hepatic and hypothalamic protein preparations ex vivo. GK activity was detectable in hepatic and hypothalamic protein preparations from healthy Sprague Dawley rats ex vivo (Fig. 2A and B). GSN dose-dependently inhibited rat liver GK activity, with a half-maximal inhibitory concentration (IC50) of 4.2 mM in the presence of 20 mM glucose (Fig. 2C). GSN also dose-dependently inhibited hypothalamic GK activity with an IC50 of 5.0 mM in the presence of 20 mM glucose (Fig. 2D).
FIG. 2.
Ex vivo GK activity assays in protein preparations. A: Sigmoidal dependence of hepatic GK (n = 2) on glucose concentration. B: Glucose increases hypothalamic GK activity. C and D: GSN dose dependently inhibits hepatic (n = 3–5) and hypothalamic (n = 3) GK ex vivo. E: MH dose dependently inhibits hypothalamic GK ex vivo (n = 3), although with reduced potency as compared with GSN (IC50 =12 mM vs. 5 mM, MH vs. GSN, respectively). **P < 0.01.
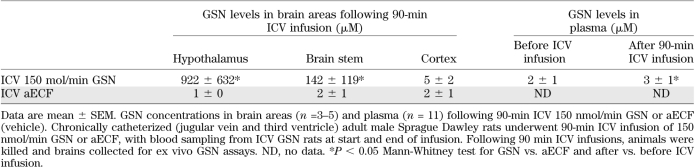
To determine the appropriate infusion regimen to be used in subsequent studies and the sites and concentrations of GSN achieved, the extent of GSN distribution following ICV infusion (150 nmol/min) was measured using HPLC-ESI-MS/MS method. ICV GSN resulted in a marked rise in hypothalamic GSN to levels of approximately 1 mM, a modest rise in brain stem GSN levels (∼15% of hypothalamic levels), but did not alter cerebral cortex levels (Table 1). Importantly, although our sensitive assay was able to detect a small statistically significant change in plasma values, levels of GSN in the blood stream remained at low micromolar levels, three orders of magnitude below the levels required to inhibit GK.
TABLE 1.
GSN concentrations in brain and plasma
Next, we examined whether hypothalamic levels of GSN attained by 90 min ICV infusion (1 mM) would be sufficient to inhibit hypothalamic GK activity. We aimed to measure hypothalamic GK activity in the presence of 1 mM GSN in vitro at a glucose concentration that is likely to simulate ambient hypothalamic glucose levels during the IVGTT. A concentration of 3 mM glucose was selected for in vitro assays based on peak plasma glucose levels observed during IVGTT (17 mM, Fig. 1) and on the fact that accepted approximations of brain extracellular glucose concentrations are roughly 20% of that of plasma glucose (23,24). GK represented ∼5% of total hypothalamic glucose phosphorylation activity at 3 mM glucose and was selectively inhibited by 1 mM GSN (reduced to 48.1 ± 7.3% and total glucose phosphorylating activities was 97 + 1% of control, n = 3). These kinetic studies suggested that ICV GSN in vivo, even at the highest dose used, is unlikely to result in major spillage of GSN outside the brain and that levels achieved might be sufficient to inhibit, at least in part, hypothalamic GK activity.
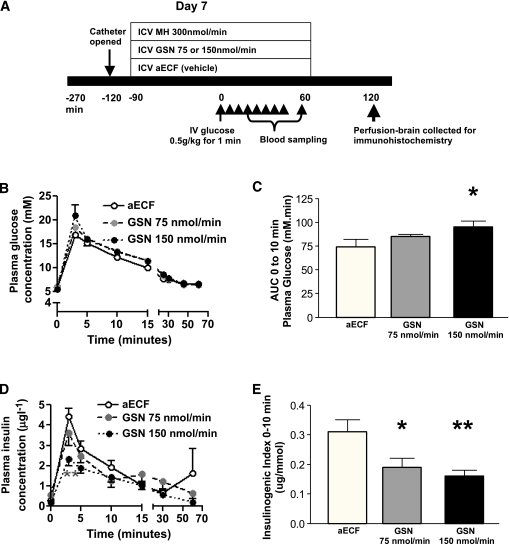
We next investigated the effects of 90 min ICV infusion of GSN at 75 and 150 nmol/min on plasma glucose and insulin responses during IVGTT (0.5 g/kg—a higher dose than that used in ICV-glucose studies above) (Fig. 3A). ICV GSN significantly and dose-dependently impaired glucose handling during IVGTT (Fig. 3B and C). Despite the higher plasma glucose in the ICV GSN rats, insulin responses were reduced, particularly during the first few minutes of the glucose challenge, with peak and AUC0-10 insulin being significantly lower in ICV 150 nmol/min GSN rats compared with controls. As might be expected, ICV 75 nmol/min GSN showed a pattern intermediate between the other two groups. Insulinogenic index was significantly lower in both ICV 150 nmol/min and 75 nmol/min GSN rats relative to controls (Fig. 3D–F). Taken together, these data show that third ventricle infusion of a low dose of a GK inhibitor, GSN, predominantly distributed into the surrounding hypothalamus, impaired insulin secretion and glucose tolerance during the first few minutes of an IVGTT in rats. Moreover, these findings suggest a role for hypothalamic GK mediated glucose sensing in the central regulation of GSIS.
FIG. 3.
ICV infusion of a GK inhibitor, GSN-impaired glucose handling, and insulin secretion during IVGTT. A: Experimental design. On day 7, chronically catheterized (jugular vein and third ventricle) rats, which had been fasted overnight, underwent IVGTT (0.5 g/kg) preceded by 90-min ICV infusion of either GSN at 75 nmol/min or 150 nmol/min, or vehicle (aECF) (n = 9–13). B: ICV GSN rats showed impaired glucose handling, particularly during the first few minutes of IVGTT. C: The AUC0-10 (plasma glucose) increased significantly (P < 0.05) and dose-dependently in ICV GSN-treated rats as compared with ICV vehicle-treated rats. D and E: In spite of higher plasma glucose levels in ICV GSN-infused rats, plasma insulin levels were reduced significantly and dose-dependently relative to vehicle-treated rats. Insulinogenic index was also significantly reduced by both ICV 150 and 75 nmol/min treatment as compared with ICV aECF. Data are mean ± SEM. *P < 0.05; **P < 0.01 (A high-quality color representation of this figure is available in the online issue.)
ICV infusion of MH impaired glucose handling and insulin secretion during IVGTT.
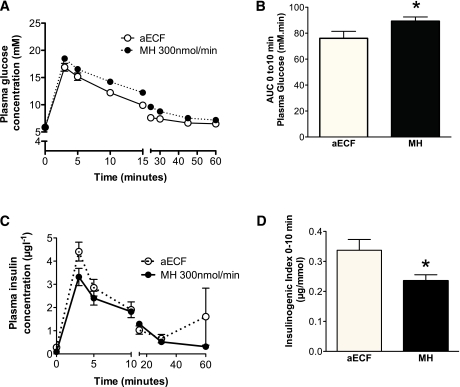
A limitation of the study above is that GSN has other biological actions in addition to inhibiting glucose metabolism via GK, for instance acting through the hexosamine pathway (25). The study was repeated using MH, an alternative GK inhibitor that is structurally unrelated to GSN and does not act through the hexosamine pathway. Again, we first confirmed ex vivo that MH dose-dependently inhibited hypothalamic GK (Fig. 2E). Given the lower potency of MH for GK inhibition, a higher dose of MH was used for in vivo studies. In keeping with an effect mediated by GK inhibition, ICV 300 nmol/min MH-treated rats displayed impaired glucose handling with a significantly higher AUC0-10 glucose relative to control rats (Fig. 4A and B). Consistently, insulinogenic index was significantly lower in ICV 300 nmol/min MH rats relative to controls (Fig. 4C and D). These findings further support a role for hypothalamic GK-mediated glucose sensing in the regulation of GSIS in response to an IV glucose challenge.
FIG. 4.
ICV infusion of a GK inhibitor, MH, impairs insulin secretion during IVGTT. A: ICV MH rats showed impaired glucose handling, particularly during the first few minutes of IVGTT. B: The AUC0-10 (plasma glucose) was significantly (P < 0.05) higher in ICV MH-treated rats as compared with ICV vehicle-treated rats. C and D: In spite of higher plasma glucose levels in ICV GSN-infused rats, plasma insulin levels were reduced relative to vehicle-treated rats. Insulinogenic index was significantly reduced by both ICV 300 nmol/min MH treatment as compared with ICV aECF. Data are mean ± SEM (n = 9–13). *P < 0.05. (A high-quality color representation of this figure is available in the online issue.)
DISCUSSION
Although there is increasing evidence that hypothalamic glucose sensing may contribute to the integrated control of aspects of whole body glucose homeostasis (26) such as hepatic glucose output (11,12) and hypoglycemia counterregulation (13), its role in the regulation of insulin secretion has been less clear. Here, we provide the first direct evidence that hypothalamic glucose sensors play a significant role in the control of insulin secretion, one of the most important systems in the maintenance of whole-body glucose homeostasis.
Specifically, we observed that activation of hypothalamic glucose sensing by ICV infusion of glucose improved glucose handling and insulin secretion during IVGTT. Furthermore, we demonstrated that pharmacological inhibition of hypothalamic glucose sensing by ICV infusion of GK and HK inhibitors, GSN and MH, significantly impaired glucose handling and first-phase insulin secretion during IVGTT. These data suggest a critical role for brain glucose sensing in the regulation of the first phase of pancreatic GSIS and in turn whole-body glucose tolerance. Such a role is consistent with emerging evidence that hypothalamic glucose sensors contribute to the integrated control of peripheral glucose homeostasis (26).
As in humans, glucose infusion in rats elicits a biphasic insulin response (27). In this study, we elected to use an IVGTT rather than oral challenge because it allowed investigation of GSIS without a confounding effect from incretins. Furthermore, IVGTT as a measure of insulin secretion has been used successfully in rats. Similar to the pattern seen in humans, both an early peak response during the first few minutes after a glucose load and a later sustained insulin release can be identified (28). Analogous to humans, the early phase of insulin release is suppressed in some rodent models of diabetes (29).
Although GSN and MH inhibit other HKs in addition to inhibiting GK, the conditions of our studies suggest that these effects are likely mediated via GK. Brain extracellular glucose concentration is ∼20% of that of plasma glucose (23,24). Plasma glucose levels during IVGTT studies attained peak values of approximately 17–20 mM (Figs. 1–3) and 20% of these values are roughly 3–4 mM. However, it is possible that glucose levels sensed by hypothalamic arcuate nucleus (ARC) neurons may be higher than this because of the proximity of the ARC to the median eminence, where the blood-brain barrier is thought to be leaky (30). Since GK is active in the hypothalamus at glucose concentrations ranging from about 3 to 20 mM (31), it is better suited for high capacity glucose phosphorylation necessary for glucose sensing in this range of glucose concentrations than other high affinity HKs (which are easily saturated at glucose concentrations less than 500 μM). Our in vitro simulation studies demonstrating that 1 mM GSN selectively inhibits hypothalamic GK without interfering with other HKs at 3 mM glucose further suggest that ICV GSN’s effects during IVGTT are mediated via inhibition of hypothalamic GK activity.
It is important to note that our studies do not permit us to exclude a potential contribution from extrahypothalamic brain regions influenced by third ventricle infusion in mediating centrally driven GSIS responses. However, given that the relative levels of GSN achieved by our third ventricle infusion in hypothalamus were approximately 5-fold higher than in the brain stem, the data suggest that the hypothalamus received the highest concentration of GSN. Furthermore, even though the data clearly demonstrate a direct effect of brain glucose sensors on pancreatic GSIS, the involvement of additional synergistic effects to improve glucose handling by hypothalamic efferents, for example, altering hepatic glucose output directly, cannot be excluded. To delineate further the specific role of the hypothalamus and specific hypothalamic subnuclei in the effects of GSIS, localized injections of glucose and inhibitors of GK should be used. In addition, many elegant genetic and pharmacogenetic tools are now available which will enable further refined probing of the discrete role of specific chemically defined neurons, such as those expressing the melanocortin neuropeptides in the ARC, in GSIS.
Overall, our data support a role for hypothalamic glucose sensing in the integrated control of peripheral glucose homeostasis. In particular, our findings suggest a novel model for the regulation of GSIS. We identified for the first time that GK-dependent glucose phosphorylation in the hypothalamus may play a facilitatory role in the regulation of the first phase of insulin secretion in response to a systemic glucose load. GK activators have been highlighted as potential therapeutic candidates for type 2 diabetes (32), in particular focusing on effects mediated by activation of hepatic and pancreatic GK. Our data suggest that a further beneficial action might be targeting GK-mediated glucose sensing in the hypothalamus.
In conclusion, our findings delineate a novel central mechanism in the control of glucose-stimulated insulin release and suggest that this may offer a future therapeutic target for improving glycemic control in type 2 diabetes.
ACKNOWLEDGMENTS
This study was supported by Juvenile Diabetes Research Foundation regular grants (1-2003-78 and 1-2006-29) and Diabetes UK (BDA: RD05/003059) to M.L.E. and National Institute of Diabetes and Digestive and Kidney Diseases (DK065171), the Wellcome Trust (WT081713), and the American Diabetes Association to L.K.H. and by the Medical Research Council Centre Grant (MRC-CORD) to all authors. M.A.O. was supported by a Diabetes Research and Wellness Foundation Ph.D. studentship, D.D.L. by a Gates Cambridge Trust studentship, and S.P.M. by MRC Studentship.
No potential conflicts of interest relevant to this article were reported.
M.A.O., D.D.L., P.H., S.P.M., and M.L.E. conceived and designed the experiments; performed the experiments; analyzed the data; contributed reagents, materials, and analysis tools; and wrote the manuscript. J.S. conceived and designed the experiments; performed the experiments; contributed reagents, materials, and analysis tools; and wrote the manuscript. C.-Y.Y. conceived and designed the experiments, performed the experiments, and analyzed the data. L.K.H. conceived and designed the experiments; analyzed the data; contributed reagents, materials, and analysis tools; and wrote the manuscript. C.C. and A.R. performed the experiments, analyzed the data, and contributed reagents, materials, and analysis tools. M.L.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 42nd European Association for the Study of Diabetes annual meeting, Copenhagen, Denmark, 14–17 September 2006 and at the 67th Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 22–26 June 2007.
The authors are grateful to Keith Burling, Department of Clinical Biochemistry, Addenbrooke’s Hospital, Cambridge, for performing hormonal assays.
REFERENCES
- 1.Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003;46:1029–1045 [DOI] [PubMed] [Google Scholar]
- 2.Brunzell JD, Robertson RP, Lerner RL, et al. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab 1976;42:222–229 [DOI] [PubMed] [Google Scholar]
- 3.Porte D, Jr, Girardier L, Seydoux J, Kanazawa Y, Posternak J. Neural regulation of insulin secretion in the dog. J Clin Invest 1973;52:210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kita H, Niijima A, Oomura Y, et al. Pancreatic nerve response induced by hypothalamic stimulation in rats. Brain Res Bull 1980;5(Suppl. 4):163–168
- 5.Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 2000;43:393–410 [DOI] [PubMed] [Google Scholar]
- 6.Boschero AC, Szpak-Glasman M, Carneiro EM, et al. Oxotremorine-m potentiation of glucose-induced insulin release from rat islets involves M3 muscarinic receptors. Am J Physiol 1995;268:E336–E342 [DOI] [PubMed] [Google Scholar]
- 7.Cases JA, Gabriely I, Ma XH, et al. Physiological increase in plasma leptin markedly inhibits insulin secretion in vivo. Diabetes 2001;50:348–352 [DOI] [PubMed] [Google Scholar]
- 8.Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology 2000;141:3072–3079 [DOI] [PubMed] [Google Scholar]
- 9.Muzumdar R, Ma X, Yang X, et al. Physiologic effect of leptin on insulin secretion is mediated mainly through central mechanisms. FASEB J 2003;17:1130–1132 [DOI] [PubMed] [Google Scholar]
- 10.Calegari VC, Torsoni AS, Vanzela EC, et al. Inflammation of the hypothalamus leads to defective pancreatic islet function. J Biol Chem 2011;286:12870–12880 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 2005;1:53–61 [DOI] [PubMed] [Google Scholar]
- 12.Ross R, Wang PYT, Chari M, et al. Hypothalamic protein kinase C regulates glucose production. Diabetes 2008;57:2061–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans M, McCrimmon R, Flanagan D, et al. Hypothalamic ATP-sensitive K + channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 2004;53:2542–2551 [DOI] [PubMed] [Google Scholar]
- 14.Kang L, Dunn-Meynell AA, Routh VH, et al. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes 2006;55:412–420 [DOI] [PubMed] [Google Scholar]
- 15.Flanagan DE, Keshavarz T, Evans ML, et al. Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes 2003;52:605–613 [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 2nd ed. New York, NY, Academic Press, 1998 [Google Scholar]
- 17.Jetton TL, Magnuson MA. Heterogeneous expression of glucokinase among pancreatic beta cells. Proc Natl Acad Sci USA 1992;89:2619–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niswender KD, Postic C, Jetton TL, et al. Cell-specific expression and regulation of a glucokinase gene locus transgene. J Biol Chem 1997;272:22564–22569 [DOI] [PubMed] [Google Scholar]
- 19.Miwa I, Mita Y, Murata T, et al. Utility of 3-O-methyl-N-acetyl-D-glucosamine, an N-acetylglucosamine kinase inhibitor, for accurate assay of glucokinase in pancreatic islets and liver. Enzyme Protein 1994-1995;48:135–142 [DOI] [PubMed] [Google Scholar]
- 20.Roda A, Sabatini L, Barbieri A, et al. Development and validation of a sensitive HPLC-ESI-MS/MS method for the direct determination of glucosamine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2006;844:119–126 [DOI] [PubMed] [Google Scholar]
- 21.Gredell JA, Turnquist PA, Maciver MB, Pearce RA. Determination of diffusion and partition coefficients of propofol in rat brain tissue: implications for studies of drug action in vitro. Br J Anaesth 2004;93:810–817 [DOI] [PubMed] [Google Scholar]
- 22.Balkan B, Dunning BE. Glucosamine inhibits glucokinase in vitro and produces a glucose-specific impairment of in vivo insulin secretion in rats. Diabetes 1994;43:1173–1179 [DOI] [PubMed] [Google Scholar]
- 23.Silver IA, Erecińska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol 1998;79:1733–1745 [DOI] [PubMed] [Google Scholar]
- 24.de Vries MG, Arseneau LM, Lawson ME, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes 2003;52:2767–2773 [DOI] [PubMed] [Google Scholar]
- 25.Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J Clin Invest 1997;99:2173–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parton LE, Ye CP, Coppari R, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007;449:228–232 [DOI] [PubMed] [Google Scholar]
- 27.Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology 1968;83:572–584 [DOI] [PubMed] [Google Scholar]
- 28.Frangioudakis G, Gyte AC, Loxham SJG, Poucher SM. The intravenous glucose tolerance test in cannulated Wistar rats: a robust method for the in vivo assessment of glucose-stimulated insulin secretion. J Pharmacol Toxicol Methods 2008;57:106–113 [DOI] [PubMed] [Google Scholar]
- 29.Jeppesen PB, Gregersen S, Rolfsen SED, et al. Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat. Metabolism 2003;52:372–378 [DOI] [PubMed] [Google Scholar]
- 30.Ganong WF. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin Exp Pharmacol Physiol 2000;27:422–427 [DOI] [PubMed] [Google Scholar]
- 31.Roncero I, Alvarez E, Vázquez P, Blázquez E. Functional glucokinase isoforms are expressed in rat brain. J Neurochem 2000;74:1848–1857 [DOI] [PubMed] [Google Scholar]
- 32.Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003;301:370–373 [DOI] [PubMed] [Google Scholar]