Abstract
Leucine (Leu) is an essential branched-chain amino acid, which activates the mammalian target of rapamycin (mTOR) signaling pathway. The effect of Leu on cell differentiation during embryonic development is unknown. Here, we show that Leu supplementation during pregnancy significantly increased fetal body weight, caused fetal hyperglycemia and hypoinsulinemia, and decreased the relative islet area. We also used rat embryonic pancreatic explant culture for elucidating the mechanism of Leu action on β-cell development. We found that in the presence of Leu, differentiation of pancreatic duodenal homeobox-1–positive progenitor cells into neurogenin3-positive endocrine progenitor cells was inefficient and resulted in decreased β-cell formation. Mechanistically, Leu increases the intracellular levels of hypoxia-inducible factor 1-α, a repressor of endocrine fate in the pancreas, by activating the mTOR complex 1 signaling pathway. Collectively, our findings indicate that Leu supplementation during pregnancy could potentially increase the risk of type 2 diabetes mellitus by inhibiting the differentiation of pancreatic endocrine progenitor cells during a susceptible period of fetal life.
Type 2 diabetes mellitus (T2DM) is a progressive disease in which glucose homeostasis is compromised as a result of impaired insulin secretion. Results from human and animal studies support the idea that the β-cell mass in T2DM cannot adapt insulin secretion in spite of increased insulin needs (1). Thus, understanding the underlying mechanisms that regulate β-cell mass could be used to develop new strategies to treat T2DM.
T2DM is frequently associated with body weight gain and obesity and a high nutritional state, and it is now well established that nutrients regulate β-cell mass and function (2). Nutrients not only provide energy but also function as signaling molecules that directly influence feeding behavior, energy production, cell growth, and differentiation (3). For example, nutrients, such as folate and taurine, are now used as dietary supplements during gestation to protect the fetus from developing birth defects (4,5). Leucine (Leu), an essential branched-chain amino acid (BCAA), is a nutrient that increases the birth weight of newborns and prevents the development of a fetal growth defect in pregnancies where fetal growth is abnormal (6,7). In addition to being a crucial amino acid for protein synthesis, Leu is a potent activator of the mammalian target of rapamycin (mTOR), a Ser/Thr kinase, which is involved in many cellular processes that include protein synthesis, cell growth, and metabolism (8). In mature pancreatic β-cells, Leu has many functions, such as stimulating insulin release and regulating gene expression and protein synthesis (9). However, the function of Leu in pancreatic cell development has not been elucidated.
The mature pancreas contains exocrine acinar cells that secrete digestive enzymes into the intestine and endocrine islets that synthesize hormones, such as insulin (β-cells), glucagon (α-cells), somatostatin (δ-cells), and pancreatic polypeptide (PP cells). The pancreas originates from the dorsal and ventral regions of the foregut endoderm directly posterior to the stomach. Signals that originate from adjacent mesodermal structures control pancreatic development and the formation of endocrine and exocrine tissue (10). Results from studies in genetically engineered mice have identified a hierarchy of transcription factors that regulate pancreatic specification, growth, and differentiation (11). First, the pancreas-committed endodermal region of the foregut expresses the homeodomain factor pancreatic duodenal homeobox-1 (PDX-1) (12). Next, the basic helix-loop-helix transcription factor, neurogenin3 (NGN3), initiates the endocrine differentiation program in epithelial pancreatic progenitor cells. Indeed, Ngn3-deficient mice fail to generate any pancreatic endocrine cells (13), and the results from lineage-tracing experiments have provided direct evidence that NGN3-expressing cells are islet progenitors (14). Subsequently, additional transcription factors then determine the specific endocrine cell fate (15).
Here, we evaluated the effect of dietary Leu supplementation on the regulation of β-cell mass during pancreatic development. We report that increasing Leu intake in pregnant rats resulted in hyperglycemic hypoinsulinemic fetuses with increased body weight without an adaptation in fetal β-cell mass. To elucidate the effect of Leu on β-cell mass regulation, we used an in vitro bioassay that mimics the major steps that occur during β-cell development from fetal pancreatic progenitor cells (16). Our data demonstrate that Leu increased the expression of the hypoxia-inducible factor 1-α (HIF-1α), a repressor of the development of NGN3-positive pancreatic endocrine progenitor cells (17), by activating the mTOR pathway, which, in turn, prevented β-cell development.
Previous results demonstrate that mTOR has a crucial role in adult β-cell proliferation (18). The results of the current study delineate a new role for the mTOR signaling pathway in the control of β-cell differentiation during prenatal life. Our results also indicate that dietary Leu supplementation and other mTOR activators during a short sensitive period of fetal pancreatic development can increase the risk of developing T2DM in later life.
RESEARCH DESIGN AND METHODS
Pregnant Wistar rats were purchased from CERJ (Le Genest, France). The first day post coitum was taken as embryonic day 0.5 (E0.5). The pregnant rats were killed by CO2 narcosis at predetermined days of gestation (E13.5, E16, or E20), according to the French Animal Care Committee’s guidelines. Fetuses were dissected at E13.5 for the pancreatic bud culture assay. For the in vivo analyses that were done at E16 and E20, the pregnant rats on gestational day 13.5 were divided into two groups. In one group, the drinking water was replaced with a 4% sucrose solution that contained 2% Leu. In the other group (control), the drinking water was replaced with a 4% sucrose solution. Two-week-old rats were injected with bromodeoxyuridine (100 mg/kg i.p.) 1 h before killing.
Metabolic studies.
Blood samples from live pregnant rats were collected from the tail vein, and blood samples from the E20 pups were collected from the umbilical cord vein. Whole glucose levels were measured using OneTouch Ultra2 blood glucose meter (LifeScan, Milpitas, CA). Plasma insulin levels were determined by ELISA kit (Ultrasensitive Mouse/rat Insulin ELISA; Mercodia AB, Uppsala, Sweden). Glucose tolerance tests were performed in 18-h fasted animals by injecting glucose (2 mg/g i.p.) as described previously (18). Insulin tolerance tests were done in 6-h fasted rats followed by glucose measurements after intraperitoneal insulin injection using 0.75 units/kg as described previously (19).
Dorsal pancreatic bud culture.
Dorsal pancreatic buds from control E13.5 rat embryos were dissected using a previously described protocol (20). The buds were then laid on 0.45-μm filters (Millipore, Billerica, MA) at the air-medium interface in petri dishes that contained RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with penicillin (100 units/mL), streptomycin (100 μg/mL), HEPES (10 mmol/L), l-glutamine (2 mmol/L), nonessential amino acids (1×; Invitrogen), and 10% heat-inactivated FCS (16). The pancreatic buds were incubated for 1, 3, 5, or 7 days at 37°C in a humidified 95% air–5% CO2 gas mixture. The medium was changed every 2nd day. In some experiments, Leu, Val, Ala (Sigma-Aldrich, Saint Louis, MO), or rapamycin (Santa Cruz Biotechnology, Santa Cruz, CA) were added to the culture medium.
Immunohistochemistry and quantification.
Immunohistochemistry and quantification was performed as previously described (18,21). Antibodies and dilution are listed in the Supplementary Data.
For cultured fetal pancreata, all images of the 40–60 sections of each pancreas were digitized using cooled three–charge coupled device cameras (C7780; Hamamatsu Photonics, Hamamatsu, Japan) attached to a fluorescence microscope (Leitz DMRB; Leica, Weitzlar, Germany). For every image, the areas of each immunostaining were quantified using National Institutes of Health (NIH) Image J software (v1.31, which is freely available at http://rsb.info.nih.gov/ij/index.html) and then summed to obtain the total area per explant in millimeters squared as previously described (22).
For E20 and 4-week-old pancreata, quantification of insulin staining was performed on five equally separated sections. In each section, the β-cell area and the pancreatic area were determined using NIH Image J software. The percent area of β-cells in each pancreatic section was determined by dividing the area of all insulin-positive cells by the total surface area of the section. The β-cell mass was calculated by multiplying the pancreas weight by the percent area of the β-cells (18).
RNA extraction and real-time PCR.
Total RNA was extracted from pools of three fetal pancreata using an RNeasy Microkit (Qiagen, Courtaboeuf, France) and then reverse transcribed using Superscript reagents (Invitrogen). Real-time PCR was performed with the 7300 Fast real-time PCR system (Applied Biosystems, Courtaboeuf, France) using a previously described protocol (22). The oligonucleotide sequences are available upon request. Cyclophilin A was used as the internal reference control.
Western blotting.
Protein lysates from pooled fetal pancreata were subjected to immunoblotting as described (17,18). Antibodies used included HIF-1α (Novus Biologicals, Littleton, CO); phospho-rpS6 (Ser240/244), rp-S6, 4E-BP1, and phospho-Akt (Ser473) (all from Cell Signaling, Beverly, MA); and actin (Sigma) at dilutions that were recommended by the manufacturer. Immunoblotting experiments were performed at least three times. Quantification of the Western blots by densitometry was done using NIH Image J software and normalized against that of actin.
Statistical analysis.
Quantitative data are presented as the mean ± SEM from at least three independent experiments, unless otherwise indicated. Interactions among the variables were investigated by two-way ANOVA, and an unpaired Student t test was used to compare the independent means. Statistical significance was set at 5%.
RESULTS
In utero Leu supplementation induces a β-cell mass defect.
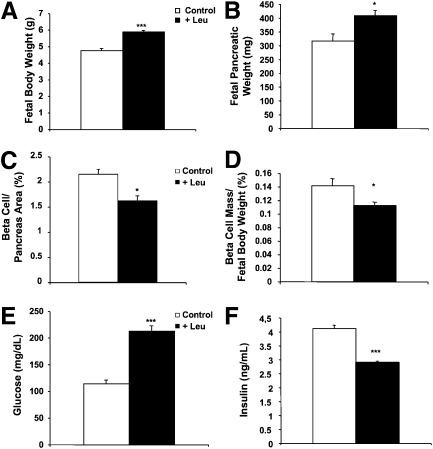
Leu was added to the drinking water of the pregnant dams on E13. At gestational day 20, the body weights and blood glucose levels of and the number of pups/litter in the Leu-supplemented pregnant females were not different than those of the females who did not receive Leu supplementation (data not shown). On the other hand, Leu supplementation increased fetal body weight (5.9 ± 0.08 vs. 4.8 ± 0.12 g; P < 0.005) (Fig. 1A) and fetal pancreatic weight (408.7 ± 17.62 vs. 317.3 ± 24.53 mg; P < 0.05) (Fig. 1B). Leu supplementation had no effect on the fetal β-cell mass in absolute terms but resulted in a reduction in the relative fetal β-cell mass, as measured by the decreased β-cell area–to–pancreatic area and β-cell mass–to–fetal body weight ratios (Fig. 1C and D). This relative defect in the β-cell mass in pups of the Leu-supplemented dams was associated with an increase in fetal whole blood glucose levels (213 ± 6.4 vs. 114.3 ± 9.4 mg/dL; P < 0.005) (Fig. 1E) and a decrease in fetal plasma insulin levels (2.90 ± 0.05 vs. 4.12 ± 0.11 ng/mL; P < 0.005) (Fig. 1F).
FIG. 1.
In utero Leu supplementation induces β-cell mass defect. A and B: Body and pancreatic weights of E20 fetuses from Leu-supplemented pregnant dams and pregnant dams that were not supplemented with Leu. C: Immunohistochemical quantification of the insulin-stained area shows that the fetal β-cell mass–to–fetal pancreatic area ratio at E20 was reduced in fetuses from Leu-supplemented pregnant dams when compared with that in E20 fetuses from pregnant dams that were not supplemented with Leu. D: The β-cell mass–to–body weight ratio is E20 fetuses is significantly lower than that of E20 fetuses from dams that were not supplemented with Leu. E and F: Leu supplementation to pregnant dams increased whole blood glucose levels and reduced plasma insulin levels in the E20 fetuses. Data are mean ± SEM from at least 10 fetuses per condition. *P < 0.05, ***P < 0.005.
We next asked whether pups of Leu-fed experiments have the ability to compensate for the low β-cell mass at birth. We first assessed β-cell proliferation in 2-week-old rats under Leu treatment during prenatal life. The proliferative rate measured by bromodeoxyuridine incorporation was unchanged compared with 2-week-old control rats (Supplementary Fig. 1A). After aged 4 weeks, body weight and glycemia measured after an overnight fast were similar in rats under Leu treatment during prenatal life compared with controls (Supplementary Fig. 1B; some data not shown). However, these rats showed impaired glucose tolerance compared with controls, while insulin resistance was not observed (Supplementary Fig. 1C and D). Finally, in 4-week-old rats under Leu treatment during prenatal life, β-cell mass remained lower compared with 4-week-old control rats (Supplementary Fig. 1E).
Since Leu supplementation of the pregnant dams resulted in a β-cell mass defect in the pups, we decided to investigate the mechanism of Leu action on β-cell development.
In vitro Leu treatment impairs β-cell development.
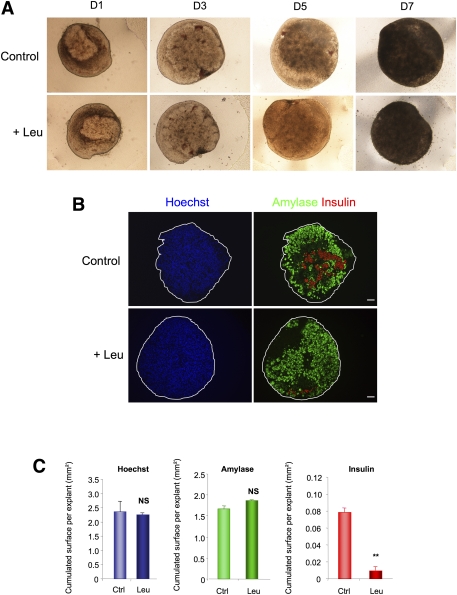
To determine the role of Leu in pancreatic development, we cultured E13.5 pancreata for 7 days under conditions that allowed endocrine and acinar cell development (16). Leu treatment (10 mmol/L) did not modify pancreatic shape and growth (Fig. 2A and C for quantification), or acinar cell development, as measured by amylase immunostaining (Fig. 2B and C for quantification). On the other hand, Leu treatment sharply decreased the β-cell number, as measured by insulin immunostaining (Fig. 2B and C for quantification). Since Leu is a potent insulin secretagogue, the expressions of four other β-cell markers were determined by immunohistochemistry to rule out the possibility that the decreased insulin immunostaining after Leu treatment was due to its potency as an insulin secretagogue. The expressions of prohormone convertase 1/3 (Supplementary Fig. 2A), PDX-1 (Supplementary Fig. 2B), the transcription factor MafA, and the zinc transporter Slc30a8 (Supplementary Fig. 2C) were all reduced. Finally, the effects of l-Leu on β-cell development were not mimicked by d-Leu (data not shown). Such data indicate that l-Leu treatment represses β-cell development.
FIG. 2.
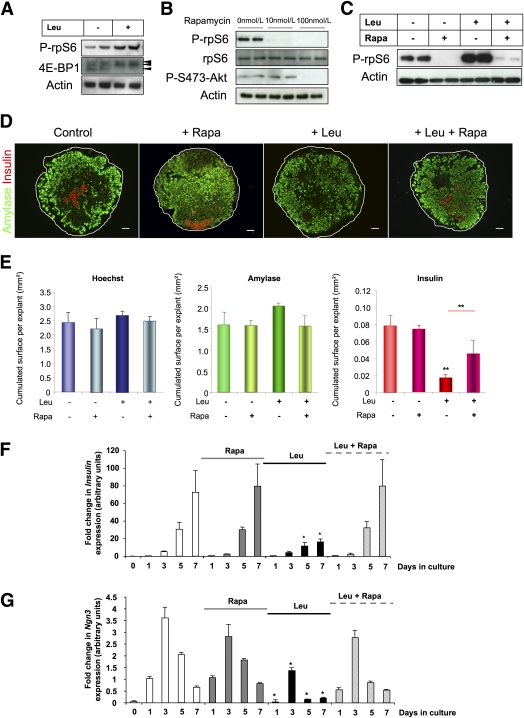
Leu treatment impairs β-cell differentiation. A: Leu did not modify the global growth of E13.5 rat pancreata that were cultured at the air medium/interface for varying durations (day [D]1 to D7). B: Immunohistochemical analyses of E13.5 pancreata after 7 days in culture, with and without Leu treatment. Acinar cell and β-cell development were evaluated using an antibody against amylase (green) and an antibody against insulin (red), respectively. Nuclei were stained with Hoechst 33342 fluorescent stain (blue). Scale bar = 50 μm. C: Absolute areas that were occupied by the nuclei and the amylase- and insulin-positive cells were quantified using NIH Image J software. Data are mean ± SEM from at least three pancreata per condition. NS, not significant; **P < 0.01. (A high-quality digital representation of this figure is available in the online issue.)
Leu treatment impairs endocrine progenitor cell development.
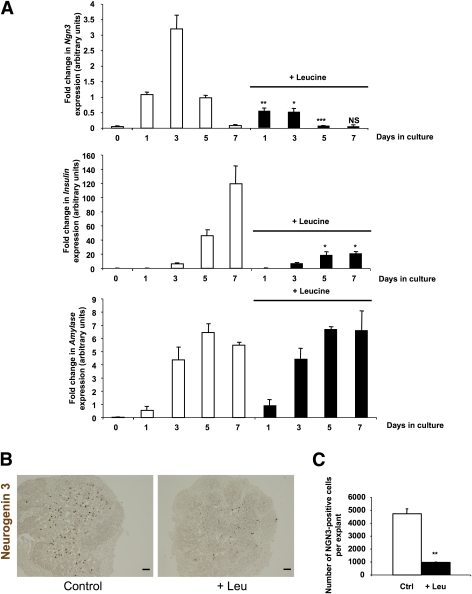
To determine how Leu treatment impairs β-cell development, we cultured E13.5 pancreata with or without 10 mmol/L Leu for 1, 3, 5, and 7 days. Leu treatment did not modify amylase mRNA levels but decreased insulin mRNA levels (Fig. 3A). We next determined the expression levels of Ngn3 mRNA, a specific pancreatic endocrine progenitor marker (13), in cultured E13.5 pancreata. In E13.5 rat pancreatic explants, as previously shown, in absence of added Leu, Ngn3 mRNA levels increased after 1 day of culture, peaked on day 3, and then decreased (16). When Leu was added, we observed a dramatic decrease in Ngn3 mRNA levels (Fig. 3A). This decrease in Ngn3 mRNA levels was paralleled by a decrease in the number of Ngn3-expressing cells (4728 ± 408 vs. 959 ± 28; P < 0.01) (Fig. 3B and C). Finally, Leu also caused a dose-dependent repressive effect on the mRNA levels of the three genes, namely Ngn3, its target Insm1 (23), and insulin (Supplementary Fig. 3). Collectively, these results indicate that Leu treatment decreased both the number of endocrine progenitor cells that express NGN3 and the number of β-cells.
FIG. 3.
Leu treatment impairs β-cell differentiation mainly by causing a loss of NGN3 expression. A: Real-time PCR quantification of Ngn3, insulin, and amylase mRNA in cultured E13.5 pancreatic explants that were treated with Leu (black bars) or not treated with Leu (white bars) after 0, 1, 3, 5, and 7 days. B and C: Immunodetection and quantification of NGN3-positive cells from pancreata that were cultured for 3 days with or without Leu. Scale bar = 100 μm. Data are mean ± SEM of at least three independent experiments. NS, not significant; *P < 0.05, **P < 0.01, ***P < 0.005. (A high-quality digital representation of this figure is available in the online issue.)
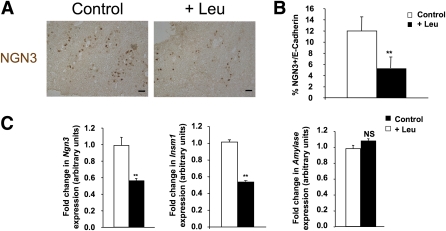
To confirm our in vitro finding that Leu decreases the number of NGN3-positive endocrine progenitors, we then determined NGN3 expression in pancreatic cells of E16 fetuses from the pregnant dams that drank Leu-supplemented water for 3 days, starting on E13.5. At E16, Leu supplementation reduced the number of NGN3-positive cells (Fig. 4A and B), the number of β-cells (Supplementary Fig. 4A and B), and mRNA expression levels of Ngn3 and its target gene, Insm1 (Fig. 4C). Leu supplementation had no effect on amylase mRNA levels in E16 pancreata (Fig. 4C).
FIG. 4.
In utero supplementation with Leu inhibits endocrine cell differentiation by downregulation of Ngn3 expression. A and B: Immunodetection and quantification of NGN3-positive cells (brown) in pancreatic sections that were prepared from E16 fetuses from Leu-supplemented pregnant dams and E16 fetuses from pregnant dams that were not supplemented with Leu. Scale bar = 12.5 μm. C: Real-time PCR quantification of Ngn3, Insm1, and amylase mRNA in fetal pancreata of E16 pups from Leu-supplemented pregnant dams and E16 pups from pregnant dams that were not supplemented with Leu. Data are mean ± SEM of at least three independent experiments. NS, not significant; **P < 0.01. (A high-quality digital representation of this figure is available in the online issue.)
Leu decreases the number of Ngn3-positive endocrine progenitor cells via mTOR complex 1.
In many tissues, Leu activates the mTOR signaling pathway (24,25). This activation also occurs in the fetal pancreas where Leu treatment enhanced phosphorylation of the p70 S6 kinase substrate, 40S ribosomal protein S6 kinase (rpS6), and 4E-BP1 (Fig. 5A and Supplementary Fig. 5 for quantification). We next used rapamycin, a specific inhibitor of mTOR complex 1 (mTORC1) to interfere with this process. At 10 nmol/L, rapamycin treatment blocked rpS6 phosphorylation in E13.5 rat fetal pancreata without affecting Akt phosphorylation on Ser473 (Fig. 5B). At a higher concentration (100 nmol/L), and as previously described (26), rapamycin was less specific: it repressed both rpS6 and Akt phosphorylation (Fig. 5B) and decreased global pancreatic cell growth (data not shown). Thus, 10 nmol/L concentration of rapamycin was used in subsequent experiments. It is important that rapamycin (10 nmol/L) inhibited Leu-induced rpS6 phosphorylation in cultured E13.5 rat pancreatic buds (Fig. 5C).
FIG. 5.
Leu represses endocrine progenitor cell development via mTORC1. A: Immunoblot for phospho (P)-rpS6 and 4E-BP1 expression in protein lysates from E13.5 pancreatic buds that were treated with Leu for 24 h. B: Immunoblot for P-rpS6, rpS6, and P-S473-Akt expression in protein lysates from E13.5 pancreatic buds that were treated with different concentrations of rapamycin (Rapa) for 24 h. C: Immunoblot for P-rpS6 expression in protein lysates from E13.5 pancreatic buds that were cultured with Leu in the presence or absence of rapamycin (10 mol/L) for 24 h. D: Immunohistological analyses of E13.5 fetal pancreata that were cultured for 7 days with and without Leu or rapamycin. Acinar cell and β-cell development were evaluated using an antibody against amylase (green) and an antibody against insulin (red), respectively. Nuclei were stained with Hoechst 33342 fluorescent stain (blue). Scale bar = 50 μm. E: Absolute areas that were occupied by nuclei and amylase- and insulin-positive cells were quantified using NIH Image J software. F and G: Real-time PCR quantification of insulin and Ngn3 mRNA in pancreata after 0, 1, 3, 5, and 7 days in culture without Leu (white bars), with rapamycin (gray bars), with Leu (black bars), and with both rapamycin and Leu (dashed bars). Data are mean ± SEM of at least three independent experiments. NS, not significant; *P < 0.05, **P < 0.01. (A high-quality digital representation of this figure is available in the online issue.)
We then asked whether rapamycin treatment could reverse the effects of Leu on pancreatic β-cell development in cultured E13.5 rat pancreatic buds. Rapamycin (10 nmol/L) alone did not modify pancreatic growth or β-cell and acinar cell differentiation (Fig. 5D and E). Leu caused a dramatic decrease in insulin expression (Fig. 5F) and β-cell development (Fig. 5D and E), whereas rapamycin reversed Leu-induced repression of β-cell development in the fetal pancreas (Fig. 5D–F). Although rapamycin alone did not modify Ngn3 expression, which was used as a marker of the endocrine progenitor cells, rapamycin did reverse the repressive effects of Leu on Ngn3 expression. (Fig. 5G). These results suggest that Leu activates an mTOR-dependant signaling pathway to repress the development of Ngn3-expressing pancreatic endocrine progenitor cells and inhibit β-cell development.
Other BCAAs, such as Val, also have been shown to activate the mTOR signaling pathway in different cell types (8,27). Supplementary Fig. 6A–C shows the effect of Val on cultured E13.5 rat pancreata. Val mimicked the effects of Leu. In fact, Val treatment (10 mmol/L) for 24 h induced rpS6 phosphorylation (Supplementary Fig. 6A). Val also decreased both Ngn3 and insulin mRNA levels in the cultured E13.5 rat pancreata after 3 and 7 days of exposure to Val (Supplementary Fig. 6B and C), while Ala, one of the simplest amino acids, did not affect mTOR signaling and β-cell differentiation (Supplementary Fig. 6D–F). These findings indicate that BCAAs suppress Ngn3 expression and β-cell development.
Leu induces HIF-1α, an inhibitor of β-cell differentiation.
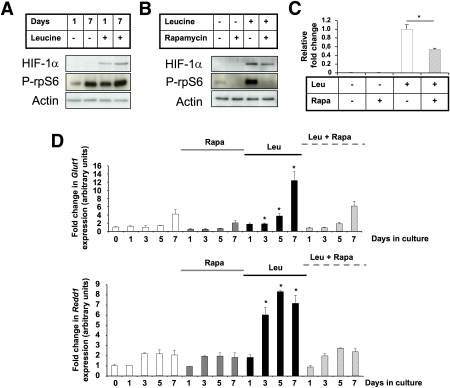
We next determined how Leu, via the mTORC1 pathway, repressed Ngn3 expression. We previously demonstrated that HIF-1α is a repressor of Ngn3 expression (17). Western blot analysis of the protein lysates from pooled E13.5 fetal pancreata indicate that Leu treatment for 1 or 7 days increases HIF-1α expression and mTOR signaling (Fig. 6A). It is interesting that this induction of HIF-1α expression was partly reduced by 10 nmol/L rapamycin, which inhibited the phosphorylation of rpS6 (Fig. 6B and C). We then measured the effect of Leu on the expression of the HIF-1α targets. Leu increased the expression of Glut1 and Redd1 (Fig. 6D), Vegf, Ldha, and Pgk1 mRNA (Supplementary Fig. 7). Rapamycin alone did not modify Glut1 and Redd1 mRNA levels, whereas it reversed the inductive effect of Leu on HIF-1α targets (Fig. 6B).
FIG. 6.
Leu induces HIF-1α, a repressor of β-cell differentiation. A: Representative immunoblot for HIF-1α expression in protein lysates from E13.5 pancreatic buds that were treated with Leu for 1 or 7 days. Immunoblot for phospho (P)-rpS6 indicates that Leu treatment induced mTOR activity. B: Representative immunoblot for HIF-1α in protein lysates from E13.5 pancreatic buds that were treated for 24 h with rapamycin (Rapa), Leu, or Leu and rapamycin. Immunoblot for P-rpS6 indicates that Leu treatment induced mTOR activity. The quantified results are presented in (C). D: Real-time PCR quantification of two HIF-1α target genes. Glut1 and Redd1 mRNA in pancreata after 0, 1, 3, 5, and 7 days in culture without Leu (white bars), with rapamycin (gray bars), with Leu (black bars), or with both rapamycin and Leu (dashed bars). Data are mean ± SEM of at least three independent experiments. *P < 0.05. (A high-quality color representation of this figure is available in the online issue.)
Collectively, our data indicate that Leu increases intracellular HIF-1α levels and activates the HIF-1α signaling pathway, and these two effects are mediated by the mTOR signaling pathway. This process results in Ngn3 repression and, consequently, decreases β-cell differentiation.
DISCUSSION
In this study, we provide both in vivo and in vitro evidence that Leu impairs β-cell development without compensatory process after birth. We also demonstrate that Leu represses β-cell development in the developing pancreas by activating the mTOR signaling pathway and increasing HIF-1α levels, a repressor of pancreatic endocrine progenitor cells (17).
In mature pancreatic β-cells, Leu has many functions. Leu can stimulate insulin release (28). Long-term Leu treatment of islets from T2DM patients improves the insulin secretory function of pancreatic β-cells (29), and Leu administration improves glycemic control in humans and rodents with T2DM (30,31). In pancreatic β-cells, Leu regulates gene transcription and protein synthesis (27,29). Leu also regulates β-cell proliferation, and this effect is mediated by the mTOR signaling pathway (32). Specifically, adult mice with a β-cell–specific deletion of the tuberin/TSC2 protein, the main inhibitor of mTOR activity, have increased β-cell proliferation and are hyperinsulinemic and hypoglycemic (18). Rapamycin, an mTORC1 inhibitor, has been reported as being a repressor of mature β-cell proliferation (33). Thus, the role of Leu and the mTOR signaling pathway in mature β-cell proliferation is now well established. Although it is known that the mTOR signaling pathway regulates the differentiation of many cell types, such as adipocytes or hematopoietic stem cells (34,35), its role in regulating pancreatic cell differentiation still remains undefined.
Our first set of data indicated that Leu supplementation of pregnant dams caused an increase in fetal body weight and fetal hyperglycemia without an adaptive response in fetal β-cell mass. To understand the mechanism of Leu action on fetal pancreatic development, we investigated the actions of Leu in an E13.5 pancreatic explant assay, which we have previously used for defining the different factors and conditions that regulate β-cell development from PDX-1–positive pancreatic progenitors (16,17,22). Using this assay, we demonstrated that Leu, a known activator of the mTOR signaling pathway (24,25), does not modify pancreatic shape and growth or acinar cell development. On the other hand, we demonstrated that Leu regulates a highly specific step in β-cell development, namely, the formation of NGN3-positive endocrine progenitor cells from PDX-1–positive pancreatic progenitor cells. This is a crucial step in endocrine cell development in the pancreas because NGN3-deficient mice lack pancreatic endocrine cells (13). The signals that control the development of NGN3-positive endocrine progenitor cells from PDX-1–positive pancreatic progenitor cells are poorly defined. Specifically, the signals and conditions that underlie the differentiation of embryonic stem cells into PDX-1–positive pancreatic progenitor cells are now well established (36,37). On the other hand, much remains to be discovered regarding the signals that regulate the next steps in the development of the endocrine pathway (i.e., differentiation of PDX-1–positive pancreatic progenitor cells into endocrine progenitor cells and mature endocrine cells) (38). Here, we report that Leu is an inhibitor of β-cell differentiation by repressing Ngn3 induction in pancreatic progenitor cells, and this action is mediated by the mTOR signaling pathway.
mTOR protein exists in two functionally distinct complexes: mTORC1 and mTORC2 (39,40). mTORC1 is sensitive to rapamycin. This complex contains raptor, the regulatory-associated protein of mTOR, which interacts with mTOR, as well as modulates the phosphorylation of rpS6 and 4E-BP1, both of which are key regulators of protein translation (39). In contrast, the rapamycin-insensitive mTORC2 contains rictor, which is involved in Akt phosphorylation at Ser473 (40,41).
Our data demonstrate that Leu triggers the phosphorylation of both rpS6 and 4E-BP1 in the pancreas and that this action is blocked by rapamycin at a concentration (10 nmol/L) that does not affect Akt phosphorylation. We found that Leu can influence the fate of endocrine progenitor cells by inhibiting the formation of NGN3-positive endocrine progenitor cells from PDX-1–positive pancreatic progenitor cells. Since this inhibition can be prevented by 10 nmol/L rapamycin, it is reasonable to assume that the activity of mTORC1 and the actions of Leu are closely linked. Of interest, rapamycin at a concentration that selectively inhibits activity of mTORC1 did not modify pancreatic development, and this result suggests that basal mTORC1 activity is not required for β-cell differentiation from pancreatic progenitor cells. This lack of need for mTORC1 in cell development has also been described for other cell types, such as hematopoietic stem cells (42). In these cells, activation of mTORC1 leads to loss of stem cell quiescence and stem cell exhaustion, and rapamycin inhibits mTORC1 signaling without affecting cell count or differentiation (42). Rapamycin-insensitive mTORC2 is reported to be sensitive to rapamycin at the 100 nmol/L concentration (26). Since we found that the 100 nmol/L concentration of rapamycin decreased global pancreatic cell growth and inhibited Akt phosphorylation on Ser473, this result supports the notion that mTORC2 regulates pancreatic growth by activating Akt signaling (43).
Recent studies establish an essential role of the Rag (Ras-related GTPase) family of GTPase in the regulation of mTORC1 activity by BCAAs (44), suggesting that Rag GTPases could be involved in the effects of BCAAs on pancreatic development observed here. To the best of our knowledge, information is not available concerning the role of Rag GTPases during pancreatic development. It will now be interesting to define whether Rag GTPases are implicated in the effects of BCAAs on pancreatic endocrine cell development.
We also demonstrate that Leu prevents Ngn3 induction by increasing intracellular HIF-1α levels after activation of the mTOR signaling pathway. HIF-1α is a transcription factor that regulates many hypoxic responses in cells and tissues. HIF-1α is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions and is stabilized by hypoxia (45). It was recently reported that HIF-1α levels are regulated by the mTORC1 pathway under normoxic conditions (46). Specifically, HIF-1α levels are increased when the mTORC1 signaling pathway is activated and reduced when this pathway is inhibited by rapamycin (46). We previously reported that HIF-1α represses β-cell differentiation by inhibiting Ngn3 expression during pancreatic development under hypoxic conditions (17). Thus, our results show that Leu can be added to the list of signals that repress pancreatic β-cell development by increasing HIF-1α levels after activation of the mTOR signaling pathway.
The results of epidemiological studies in humans indicate the existence of a strong association between maternal nutrition (either under- or overnutrition) and the risk of developing chronic diseases, such as T2DM, in the offspring (47). The importance of the relationship between pancreatic β-cell development and nutritional status is highlighted in reports, which have shown that undernutrition of fetal rodents results in a decreased β-cell mass when they become adults (48). Thus, pancreatic β-cell development is highly sensitive to nutritional status. Our findings demonstrate that maternal supplementation during pregnancy with a BCAA, such as Leu, impairs β-cell development in the fetal pancreas by repressing a highly specific step during β-cell differentiation. This new information is important because the results of recent studies indicate that an early defect in β-cell development is not subject to growth compensation and, thus, can lead to the development of T2DM in adults (49).
Finally, nutrient supplementation during pregnancy is an appealing strategy for conferring short- and long-term benefits to the offspring (50). Our findings that BCAA supplementation during pregnancy activates the mTOR signaling pathway and impairs β-cell differentiation in the developing pancreas suggest that a careful evaluation of the potential for adverse consequences of dietary supplementation during pregnancy is now needed.
ACKNOWLEDGMENTS
This work was supported by grants from INSERM (“Junior 5-year Contract” to L.R.), the CODDIM Region Ile de France (to R.S.), and the Beta Cell Biology Consortium (Grant U19-DK-072495 to R.S.).
No potential conflicts of interest relevant to this article were reported.
L.R. designed and performed experiments, analyzed data, and wrote the manuscript. V.A. performed experiments. B.D. provided expertise and reagents. R.S. designed the experiments and wrote the manuscript.
L.R. serves as the guarantor for the article.
The authors thank Dr. Arieh Bomzon, (Consulwrite, Jerusalem, Israel) for his comments and editorial assistance in preparing the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0765/-/DC1.
REFERENCES
- 1.Ferrannini E. The stunned beta cell: a brief history. Cell Metab 2010;11:349–352 [DOI] [PubMed] [Google Scholar]
- 2.Lingohr MK, Buettner R, Rhodes CJ. Pancreatic beta-cell growth and survival—a role in obesity-linked type 2 diabetes? Trends Mol Med 2002;8:375–384 [DOI] [PubMed] [Google Scholar]
- 3.Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 2006;3:393–402 [DOI] [PubMed] [Google Scholar]
- 4.Kalhan SC, Bier DM. Protein and amino acid metabolism in the human newborn. Annu Rev Nutr 2008;28:389–410 [DOI] [PubMed] [Google Scholar]
- 5.Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr 2010;30:315–339 [DOI] [PubMed] [Google Scholar]
- 6.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 2008;295:E868–E875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 2007;582:449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 2006;136(Suppl.):227S–231S [DOI] [PubMed] [Google Scholar]
- 9.McDaniel ML, Marshall CA, Pappan KL, Kwon G. Metabolic and autocrine regulation of the mammalian target of rapamycin by pancreatic beta-cells. Diabetes 2002;51:2877–2885 [DOI] [PubMed] [Google Scholar]
- 10.Scharfmann R. Control of early development of the pancreas in rodents and humans: implications of signals from the mesenchyme. Diabetologia 2000;43:1083–1092 [DOI] [PubMed] [Google Scholar]
- 11.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev 2008;22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606–609 [DOI] [PubMed] [Google Scholar]
- 13.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 2000;97:1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 2002;129:2447–2457 [DOI] [PubMed] [Google Scholar]
- 15.Collombat P, Hecksher-Sørensen J, Serup P, Mansouri A. Specifying pancreatic endocrine cell fates. Mech Dev 2006;123:501–512 [DOI] [PubMed] [Google Scholar]
- 16.Attali M, Stetsyuk V, Basmaciogullari A, et al. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes 2007;56:1248–1258 [DOI] [PubMed] [Google Scholar]
- 17.Heinis M, Simon MT, Ilc K, et al. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 2010;59:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rachdi L, Balcazar N, Osorio-Duque F, et al. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci U S A 2008;105:9250–9255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachdi L, Balcazar N, Elghazi L, et al. Differential effects of p27 in regulation of beta-cell mass during development, neonatal period, and adult life. Diabetes 2006;55:3520–3528 [DOI] [PubMed] [Google Scholar]
- 20.Miralles F, Czernichow P, Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development 1998;125:1017–1024 [DOI] [PubMed] [Google Scholar]
- 21.Rachdi L, Marie JC, Scharfmann R. Role for VPAC2 receptor-mediated signals in pancreas development. Diabetes 2003;52:85–92 [DOI] [PubMed] [Google Scholar]
- 22.Guillemain G, Filhoulaud G, Da Silva-Xavier G, Rutter GA, Scharfmann R. Glucose is necessary for embryonic pancreatic endocrine cell differentiation. J Biol Chem 2007;282:15228–15237 [DOI] [PubMed] [Google Scholar]
- 23.Mellitzer G, Bonné S, Luco RF, et al. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J 2006;25:1344–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dann SG, Thomas G. The amino acid sensitive TOR pathway from yeast to mammals. FEBS Lett 2006;580:2821–2829 [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002;110:163–175 [DOI] [PubMed] [Google Scholar]
- 26.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006;22:159–168 [DOI] [PubMed] [Google Scholar]
- 27.Xu G, Kwon G, Cruz WS, Marshall CA, McDaniel ML. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic beta-cells. Diabetes 2001;50:353–360 [DOI] [PubMed] [Google Scholar]
- 28.Newsholme P, Brennan L, Rubi B, Maechler P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin Sci (Lond) 2005;108:185–194 [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Wong RK, Park M, et al. Leucine regulation of glucokinase and ATP synthase sensitizes glucose-induced insulin secretion in pancreatic beta-cells. Diabetes 2006;55:193–201 [PubMed] [Google Scholar]
- 30.Guo K, Yu YH, Hou J, Zhang Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr Metab (Lond) 2010;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr 2006;136(Suppl.):319S–323S [DOI] [PubMed] [Google Scholar]
- 32.Xu G, Kwon G, Marshall CA, Lin TA, Lawrence JC, Jr, McDaniel ML. Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic beta-cells. A possible role in protein translation and mitogenic signaling. J Biol Chem 1998;273:28178–28184 [DOI] [PubMed] [Google Scholar]
- 33.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JE, Chen J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes 2004;53:2748–2756 [DOI] [PubMed] [Google Scholar]
- 35.Gan B, Sahin E, Jiang S, et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A 2008;105:19384–19389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Borowiak M, Fox JL, et al. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 2009;5:258–265 [DOI] [PubMed] [Google Scholar]
- 38.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol 2009;326:4–35 [DOI] [PubMed] [Google Scholar]
- 39.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE 2003;212:re15. [DOI] [PubMed]
- 40.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 2004;14:1296–1302 [DOI] [PubMed] [Google Scholar]
- 41.Jacinto E, Loewith R, Schmidt A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 2004;6:1122–1128 [DOI] [PubMed] [Google Scholar]
- 42.Chen C, Liu Y, Liu R, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med 2008;205:2397–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elghazi L, Weiss AJ, Barker DJ, et al. Regulation of pancreas plasticity and malignant transformation by Akt signaling. Gastroenterology 2009;136:1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim E, Guan KL. RAG GTPases in nutrient-mediated TOR signaling pathway. Cell Cycle 2009;8:1014–1018 [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–594 [DOI] [PubMed] [Google Scholar]
- 46.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 2002;277:27975–27981 [DOI] [PubMed] [Google Scholar]
- 47.Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol 2006;49:270–283 [DOI] [PubMed] [Google Scholar]
- 48.Garofano A, Czernichow P, Bréant B. In utero undernutrition impairs rat beta-cell development. Diabetologia 1997;40:1231–1234 [DOI] [PubMed] [Google Scholar]
- 49.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 2007;445:886–891 [DOI] [PubMed] [Google Scholar]
- 50.Christian P. Micronutrients, birth weight, and survival. Annu Rev Nutr 2010;30:83–104 [DOI] [PubMed] [Google Scholar]