Abstract
GPR55 is a putative cannabinoid receptor, and l-α-lysophosphatidylinositol (LPI) is its only known endogenous ligand. We investigated 1) whether GPR55 is expressed in fat and liver; 2) the correlation of both GPR55 and LPI with several metabolic parameters; and 3) the actions of LPI on human adipocytes. We analyzed CB1, CB2, and GPR55 gene expression and circulating LPI levels in two independent cohorts of obese and lean subjects, with both normal or impaired glucose tolerance and type 2 diabetes. Ex vivo experiments were used to measure intracellular calcium and lipid accumulation. GPR55 levels were augmented in the adipose tissue of obese subjects and further so in obese patients with type 2 diabetes when compared with nonobese subjects. Visceral adipose tissue GPR55 correlated positively with weight, BMI, and percent fat mass, particularly in women. Hepatic GPR55 gene expression was similar in obese and type 2 diabetic subjects. Circulating LPI levels were increased in obese patients and correlated with fat percentage and BMI in women. LPI increased the expression of lipogenic genes in visceral adipose tissue explants and intracellular calcium in differentiated visceral adipocytes. These findings indicate that the LPI/GPR55 system is positively associated with obesity in humans.
In addition to their ability to store triacylglycerol, adipocytes act as endocrine secretory cells (1). A growing number of adipocyte-derived factors have been described, and their contribution to the pathophysiology of metabolic syndrome is being investigated (2). Patients with type 2 diabetes exhibit increased activity of the endocannabinoid system in visceral fat and higher concentrations of endocannabinoids in the blood, when compared with corresponding controls (3–6). The endocannabinoid system acts through the type 1 and 2 cannabinoid receptors (CB1 and CB2) (4,7,8). GPR55 is a seven-transmembrane G protein–coupled receptor that shares only 13.5% sequence identity with the CB1 receptor and 14.4% with the CB2 receptor (9). The differences between their sequences are in agreement with the distinct relative affinities of ligands established for CB1 or CB2 receptors and GPR55.
The pharmacology of GPR55 has provided intricate results (9–13), and a recent article suggests that l-α-lysophosphatidylinositol (LPI) has GPR55-independent actions in endothelial cells (14). However, it is generally accepted that LPI is an endogenous ligand of GPR55 (15–18) because stimulation of this receptor upon LPI treatment evokes an intracellular calcium concentration ([Ca2+]i) rise in several cell types. LPI belongs to the class of lysophospholipids and is generated by phosphatidylinositol hydrolysis via the action of the calcium-dependent phospholipase A2 (19) and calcium-independent phospholipase A1 (20). LPI is involved in numerous physiological actions, including reproduction, angiogenesis, apoptosis, and inflammation, among others (21), which are closely related to adipose tissue biology.
Most studies on the pharmacological properties of GPR55 have used HEK293 cells transfected with GPR55. However, despite its wide distribution (22,23), the physiological function of GPR55 in vivo remains largely unknown. Recent reports indicate that GPR55 plays an important role in inflammatory pain (24) and in the regulation of bone physiology by regulating osteoclast number and functions as well as bone turnover in vivo (17), and the LPI/GPR55 system also has been involved in cancer (12). To our knowledge, there are no available data on the expression of GPR55 in human tissues or the possible involvement of LPI in energy homeostasis.
Herein, we sought to investigate the potential role of the LPI/GPR55 system in human adiposity. We demonstrate for the first time that GPR55 is present in human visceral and subcutaneous adipose tissue (VAT and SAT, respectively), as well as in the liver. It is important that GPR55 expression in VAT was positively associated with obesity and type 2 diabetes. Consistently, plasma LPI levels were higher in obese patients in comparison with lean subjects. Ex vivo studies using both adipose tissue explants and differentiated primary adipocytes show that LPI increased the expression of genes stimulating fat deposition in VAT explants. Furthermore, in differentiated adipocytes from visceral fat of obese patients, LPI raised [Ca2+]i.
RESEARCH DESIGN AND METHODS
Cohort 1.
A total of 95 Caucasian subjects were recruited from healthy volunteers and patients attending the Departments of Endocrinology and Surgery at the Clínica Universidad de Navarra. Patients underwent a clinical assessment, including medical history, physical examination, body composition analysis, and comorbidity evaluation. Obesity was classified according to BMI (>30 kg/m2). Body fat was estimated by air-displacement plethysmography (Bod-Pod; Life Measurements, Concord, CA) (25). Obese patients were further subclassified according to three established diagnostic thresholds for diabetes: 1) normoglycemia (NGT), fasting plasma glucose (FPG) <100 mg/dL and plasma glucose (PG) <140 mg/dL 2 h after an oral glucose tolerance test (OGTT); 2) impaired glucose tolerance (IGT), FPG between 100 and 125 mg/dL or PG between 140 and 199 mg/dL 2 h after an OGTT; and 3) type 2 diabetes, FPG >126 mg/dL or PG ≥200 mg/dL 2 h after an OGTT (26). Type 2 diabetic subjects were not receiving insulin therapy or medication likely to influence endogenous insulin levels.
The VAT (n = 68) and SAT (n = 59) samples were collected from patients undergoing either Nissen fundoplication (for hiatus hernia repair in lean volunteers) or Roux-en-Y gastric bypass (for morbid obesity treatment in obese subjects). Tissue samples were immediately frozen and stored at −80°C. Hepatic gene expression levels of GPR55 were assessed in a subgroup of subjects (n = 38), from which 25 of 38 corresponded to the same individuals in whom adipose tissue samples were collected. Although an intraoperative liver biopsy can be performed in obese patients undergoing bariatric surgery, this procedure is not clinically justified in lean subjects. The study was approved, from an ethical and scientific standpoint, by the hospital’s ethical committee responsible for research, and written informed consent of participants was obtained.
Cohort 2.
VAT samples from 64 consecutive subjects (35 with NGT, 17 with IFG, and 12 with type 2 diabetes) were obtained at the Endocrinology Service of the Hospital Universitari Dr. Josep Trueta (Girona, Spain). BMI was between 20 and 68 kg/m2. Adipose tissue samples were obtained from visceral depots during elective surgical procedures (cholecystectomy, surgery of abdominal hernia, and gastric bypass surgery). All subjects were of Caucasian origin and reported that their body weight had been stable for at least 3 months before the study. Liver and renal diseases were specifically excluded by biochemical workup. All subjects gave written informed consent after the purpose of the study was explained to them. The study was approved, from an ethical and scientific standpoint, by the ethical committee of the Hospital Universitari Dr. Josep Trueta. Adipose tissue samples were washed, fragmented, and immediately flash frozen in liquid nitrogen before storage at −80°C. To perform the isolation of adipocytes and the stromal vascular fraction (SVF), nonfrozen tissues were washed three to four times with phosphate-buffered saline and suspended in an equal volume of phosphate-buffered saline supplemented with 1% penicillin-streptomycin and 0.1% collagenase type I prewarmed to 37°C. The tissue was placed in a shaking water bath at 37°C with continuous agitation for 60 min and centrifuged for 5 min at 300–500g at room temperature. The supernatant, containing mature adipocytes, was recollected. The pellet was identified as the SVF cell.
Blood assays.
Plasma samples were obtained by venipuncture, after an overnight fast, from individuals of cohort 1, centrifuged at 1,300g for 10 min, and stored at −80°C. Glucose and insulin, total cholesterol, HDL cholesterol, LDL cholesterol, high-sensitivity C-reactive protein, and leptin were measured as previously described (27–29). Please see Supplementary information for details.
LPI analysis.
Plasma samples for the measurement of LPI were obtained from 78 individuals of cohort 1 (Supplementary Table 1). Total LPI was calculated by combining 16:0, 18:0, and 20:4 LPI measurements as previously reported (30). Please see Supplementary information for details.
Ex vivo experiments using SAT and VAT explants.
Samples of VAT and SAT were immediately transported to the laboratory (5–10 min), cut into small pieces, and processed as previously described (31). The experiment was performed in five replicates for each adipose tissue sample and treatment. In these experiments, SAT and VAT explants were used. To evaluate cell integrity, lactate dehydrogenase activity released from damaged cells was analyzed by the Cytotoxicity Detection Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions in all treatments. The doses were selected based on previous reports (32,33). Please see Supplementary information for details.
Effect of LPI on [Ca2+]i in visceral and subcutaneous adipocytes.
Human SVF cells were isolated from VAT and SAT from obese subjects undergoing open abdominal surgery (gastrointestinal bypass) as previously described (34). Please see Supplementary information for details.
RNA extraction and real-time PCR.
Adipose tissue and liver RNA isolation was performed as previously described (35). Primers or TaqMan probes encompassing fragments of the areas from the extremes of two exons were designed to ensure the detection of the corresponding transcript, avoiding genomic DNA amplification (Supplementary Table 2). The cDNA was amplified as previously reported (35). Please see Supplementary information for details.
Western blotting.
White adipose tissue (WAT) was homogenized, and protein was extracted as previously reported (36). Please see Supplementary information for details.
Statistical analysis.
Statistical analyses were performed using the SPSS 12.0 software statistical package (SPSS, Chicago, IL). Unless otherwise stated, descriptive results of continuous variables are expressed as mean ± SE for Gaussian variables. Variables that did not fulfill a normal distribution were logarithmically transformed for subsequent analyses. The relation between variables was analyzed by simple correlation (Pearson χ2 test) and multiple regression analyses. ANOVA was used to compare NGT, IGT, and type 2 diabetic subjects followed by pairwise post hoc tests. For [Ca2+]i measurements, unpaired t test was used. Levels of statistical significance were set at P < 0.05.
RESULTS
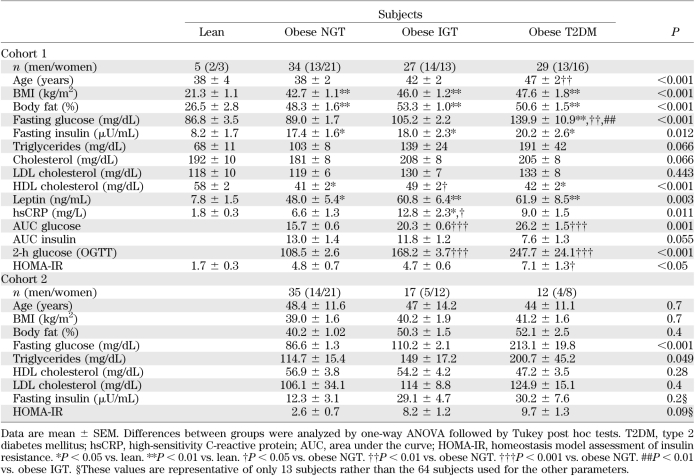
The main anthropometric and biochemical characteristics of the two cohorts are shown in Table 1.
TABLE 1.
Anthropometric and analytical characteristics of subjects in cohorts 1 and 2
Relative GPR55, CB1, and CB2 mRNA expression in WAT.
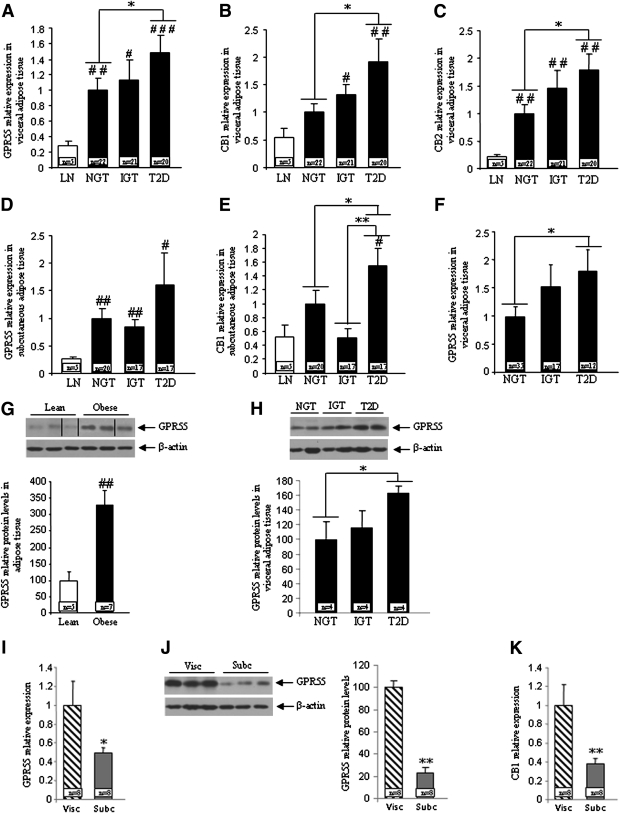
The gene expression of GPR55 (Fig. 1A), CB1 (Fig. 1B), and CB2 (Fig. 1C) was higher in the VAT of obese patients in comparison with lean subjects from cohort 1. Within obese patients, GPR55, CB1, and CB2 mRNA levels were increased in the VAT of subjects with type 2 diabetes when compared with NGT and IGT obese patients (Fig. 1A and C). The expression of the three receptors was also assessed in the SAT, where we failed to detect CB2 gene expression. The pattern of expression of GPR55 and CB1 was quite similar to that observed in VAT. Both GPR55 (Fig. 1D) and CB1 (Fig. 1E) were higher in the SAT from obese diabetic patients when compared with obese NGT and IGT patients in cohort 1. Consistent with results obtained in cohort 1, GPR55 gene expression was increased in the VAT of obese diabetic patients when compared with obese NGT and IGT patients in cohort 2 (Fig. 1F). In accordance with the gene expression data, we found that GPR55 protein levels were significantly increased in the VAT from obese patients in comparison with lean subjects (Fig. 1G). Similar to that observed for GPR55 gene expression, the protein levels of this receptor were increased in type 2 diabetic patients when compared with NGT patients (Fig. 1H). In addition, we compared GPR55 mRNA (Fig. 1I) and protein (Fig. 1J) expression levels between VAT and SAT from the same individuals and found that GPR55 levels were significantly lower in SAT. Finally, CB1 mRNA expression was also higher in VAT when compared with SAT (Fig. 1K).
FIG. 1.
mRNA expression of GPR55 (A), CB1 (B), and CB2 (C) in VAT obtained from lean and obese subjects in cohort 1. mRNA expression of GPR55 (D) and CB1 (E) in SAT obtained from lean and obese subjects in cohort 1. mRNA expression of GPR55 (F) in VAT obtained from lean and obese subjects in cohort 2. Protein levels of GPR55 (G) in VAT obtained from lean and obese subjects in cohort 2. Dividing lines indicate splicings in the figure. Levels of GPR55 in the three different groups of obese patients (H). mRNA expression of GPR55 in the visceral and subcutaneous fat from the same obese patients (I). Protein levels of GPR55 in the visceral and subcutaneous fat from the same obese patients (J). mRNA expression of CB1 in the visceral and subcutaneous fat from the same obese patients (K). Obese subjects were subclassified as NGT, IGT, and type 2 diabetic. The relative amounts of mRNA were normalized to the value of NGT. T2D, type 2 diabetes; Visc, visceral; Subc, subcutaneous. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. lean subjects; *P < 0.05, **P < 0.01 vs. NGT.
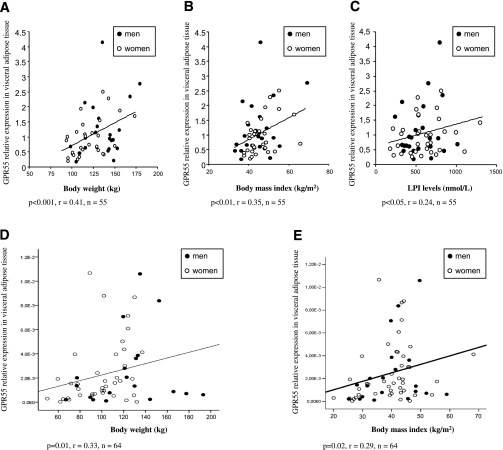
In a multivariate linear regression analysis, BMI emerged as a significant predictor of GPR55 gene expression, except in men from cohort 2 (Supplementary Table 3). The higher expression of GPR55 in obese patients in comparison with lean subjects from cohort 1 was in agreement with a positive correlation between VAT GPR55 levels and body weight (Fig. 2A), BMI (Fig. 2B), circulating LPI levels (Fig. 2C), and percent fat mass (Supplementary Table 4). Given that some groups were not exactly matched for sex, an analysis was performed to verify whether the observed differences were due to sex. A correlation was constructed considering only men or women, and the results showed that VAT GPR55 expression exhibited significantly higher levels with increasing BMI in both sexes (Supplementary Table 4). On the contrary, we did not find a significant correlation between SAT GPR55 mRNA levels with any of the variables studied (Supplementary Table 4). Contrary to GPR55, VAT CB1 expression was not correlated to BMI (Supplementary Table 5), although SAT CB1 expression showed such a correlation, but only in women (Supplementary Table 5).
FIG. 2.
Correlation between VAT GPR55 and body weight (A), BMI (B), and circulating LPI (C) in individuals from cohort 1. For cohort 1, we performed the correlations using only obese individuals, and the cutoff value as a diagnosis of obesity was BMI >30 kg/m2. Correlation between VAT GPR55 and body weight (D) and BMI (E) in individuals from cohort 2.
Similar to cohort 1, we also found that VAT GPR55 mRNA levels were positively associated with body weight (Fig. 2D), BMI (Fig. 2E), and percent fat mass (Supplementary Table 6) in the subjects of cohort 2. Thus, GPR55 gene expression levels were increased in the VAT of obese patients in both cohorts, and the correlation of GPR55 gene expression with weight, BMI, and fat mass was especially significant in women in both cohorts (Supplementary Tables 4 and 7). Therefore, we can conclude that GPR55 gene expression is specifically increased in VAT, but not SAT, of obese subjects.
Relative GPR55 mRNA expression in adipocytes versus the SVF.
We then studied which fraction of the adipose tissue accounted for this increased expression. To address this issue, we used human visceral fat and subcutaneous fat and separated the adipocyte fraction from the SVF. In visceral fat, adipogenic genes (FASN, ACC, and SREBP) were significantly increased in the adipocyte fraction (Supplementary Fig. 1A–C), whereas CD14 (monocyte marker) was significantly increased in the SVF (Supplementary Fig. 1D). The GPR55 gene was similarly expressed in both the adipocyte fraction and the SVF (Supplementary Fig. 1E). Similar results were obtained in SAT (data not shown).
Circulating LPI levels in lean and obese subjects.
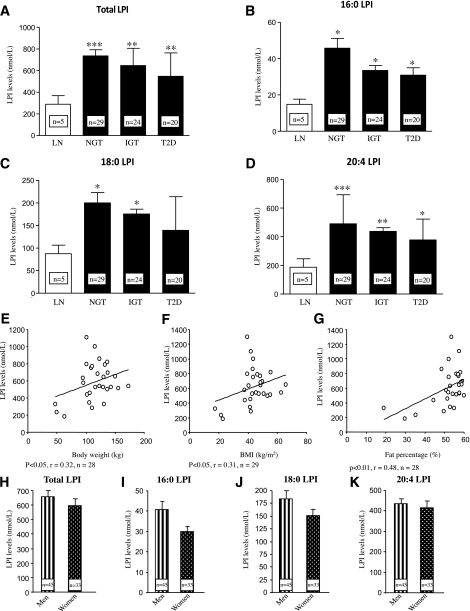
Next, we assessed plasma LPI levels, the only known endogenous ligand of GPR55, in lean and obese subjects from cohort 1. We found that total LPI was significantly increased in obese patients in comparison with lean subjects (Fig. 3A). Similar to plasma total LPI levels, we found that all three individual LPI species measured—16:0 LPI (Fig. 3B), 18:0 LPI (Fig. 3C), and 20:4 LPI (Fig. 3D)—also were increased in the three groups of obese patients when compared with lean volunteers, with the exception of 18:0 LPI in type 2 diabetic patients.
FIG. 3.
Circulating levels of total LPI (A), 16:0 LPI (B), 18:0 LPI (C), and 20:4 LPI (D) in the plasma obtained from lean and obese subjects in cohort 1. Correlation between circulating levels (plasma obtained from a subset of individuals from cohort 1) of total LPI and body weight (E), BMI (F), and fat percentage (G) in women. Circulating levels of total LPI (H), 16:0 LPI (I), 18:0 LPI (J), and 20:4 LPI (K) in the plasma obtained from men and women in cohort 1. LN, lean; T2D, type 2 diabetes. *P < 0.05, **P < 0.01, ***P < 0.001.
Plasma LPI levels were not correlated with body weight or BMI when men and women were analyzed together (data not shown). However, when a correlation was constructed considering only men or women, the results showed that plasma LPI exhibited significantly higher levels with increasing body weight (Fig. 3E), BMI (Fig. 3F), and fat percentage (Fig. 3G) in women but not men (Supplementary Table 7). Finally, we also found that plasma LPI correlates with LDL levels in women but not men (Supplementary Table 7). It is important that circulating levels of total LPI (Fig. 3H), 16:0 LPI (Fig. 3I), 18:0 LPI (Fig. 3J), and 20:4 LPI (Fig. 3K) were not significantly different between men and women.
Effects of LPI on human adipose tissue explants.
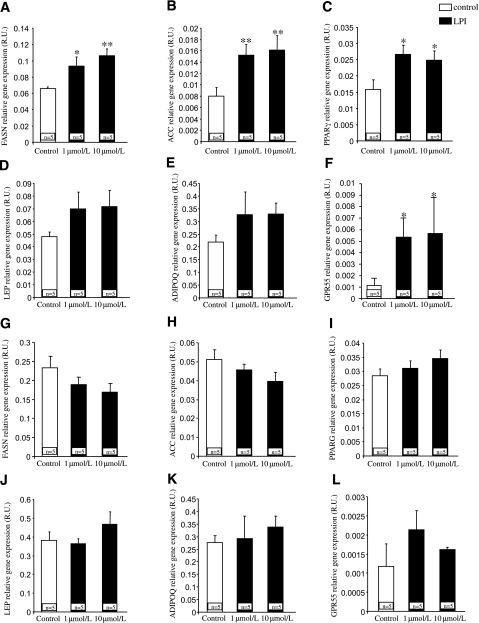
After demonstrating that VAT GPR55 and plasma LPI correlate with obesity, we then assessed the direct effects of LPI on explants obtained from SAT and VAT. LPI (1 and 10 μmol/L) increased the expression of genes promoting the synthesis of fatty acids, including fatty acid synthase (Fig. 4A) and acetyl CoA carboxylase (Fig. 4B), in explants from VAT. Both doses of LPI also triggered the expression of peroxisome proliferator–activated receptor γ (PPARγ) (Fig. 4C), which plays an important role in adipocyte differentiation. Other adipokines, such as leptin (Fig. 4D) and adiponectin (Fig. 4E), tended to show a slight increase in their expression, but the differences were not statistically significant. The expression of GPR55 was significantly upregulated by LPI at both doses (Fig. 4F) in adipocytes from VAT. In contrast to the findings obtained in VAT, LPI did not modify the expression of any of the studied genes in explants obtained from SAT (Fig. 4G–L).
FIG. 4.
Ex vivo effects of LPI (1 μmol/L and 10 μmol/L) on fatty acid synthase (FASN) (A), acetyl CoA carboxylase (ACC) (B), PPARγ (C), leptin (LEP) (D), adiponectin (ADIPOQ) (E), and GPR55 (F) in VAT explants. Ex vivo effects of LPI on FASN (G), ACC (H), PPARG (I), LEP (J), ADIPOQ (K), and GPR55 (L) in SAT explants. R.U., relative units. *P < 0.05, **P < 0.01.
Effects of LPI on [Ca2+]i in cultured differentiated human adipocytes.
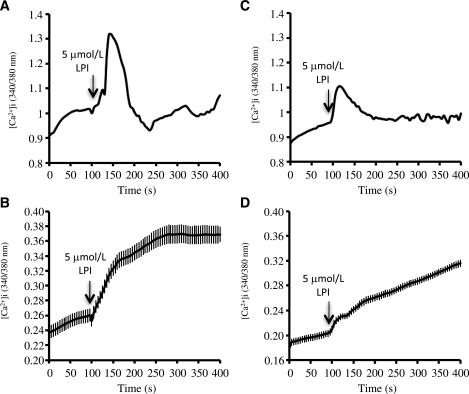
LPI has previously been shown to increase [Ca2+]i in HEK293 cells expressing GPR55 (15,16) as well as in rat pheochromocytoma PC12 cells (37). Given that increases in [Ca2+]i have been associated with lipogenesis in adipocytes (38), we next analyzed whether LPI was capable of enhancing [Ca2+]i in cultured differentiated adipocytes obtained from SVF of VAT and SAT of obese patients. For this purpose, after a 9-day differentiation period, VAT and SAT adipocytes were loaded with the calcium sensitive probe Fura 2-AM (Sigma Aldrich, St. Louis, MO), and [Ca2+]i was monitored over time (8–10 min) in the absence and presence of LPI. We found that exposure of cells to 5 μmol/L LPI induced a substantial rise in [Ca2+]i in 48.4% of differentiated VAT adipocytes (46 of 95 cells; n = 3 independent experiments) and in 24.4% of differentiated SAT cells (29 of 119 cells; n = 3 independent experiments) (Fig. 5A and C, respectively). In terms of response intensity, differentiated VAT adipocytes treated with 5 μmol/L LPI exhibited an increase in [Ca2+]i significantly higher than that observed in differentiated SAT adipocytes (43.18 ± 2.77% vs. 19.69 ± 1.93% above basal levels in VAT and SAT, respectively; P < 0.001) (Fig. 5B and D, respectively).
FIG. 5.
Representative profiles of the effects of LPI (5 μmol/L) on [Ca2+]i in cultured differentiated human adipocytes obtained from visceral (A) and subcutaneous (C) fat. Arrows indicate the time of addition of LPI. A Fura-2 dual-wavelength fluorescence imaging system was used to measure [Ca2+]i as described in research design and methods. Quantitation of [Ca2+]i dynamics in differentiated adipocytes of VAT (B) and SAT (D) responsive to LPI (46 of 95 cells and 29 of 119 cells for VAT and SAT, respectively). Three independent experiments were conducted for both SAT and VAT adipocytes.
Relative GPR55 mRNA expression in the liver.
In addition to its expression in adipose tissue, we also found that GPR55 is expressed in the liver. No significant differences in hepatic GPR55 gene expression levels between NGT, IGT, and type 2 diabetic obese patients were observed (Supplementary Fig. 2).
Effect of LPI on adipocyte differentiation of 3T3-L1 cells.
When 3T3-L1 cells were treated with LPI (1 and 10 μmol/L) during 10 days, we found no alteration in the Oil Red O staining in comparison with control cells (Supplementary Fig. 3). Thus, these results indicate that LPI may act differently in human and rodent adipocytes.
Relative GPR55 levels in WAT of obese animals.
Mice lacking leptin (Supplementary Fig. 4A) and rats fed a high-fat diet (45% by energy) (Supplementary Fig. 4B) showed significantly decreased GPR55 mRNA levels in WAT when compared with lean animals. Accordingly, GPR55 protein levels were also decreased in both obese models (Supplementary Figs. 4C and D). Therefore, these findings suggest that GPR55 is differentially regulated in humans and rodents.
DISCUSSION
The endocannabinoid system is overactive in WAT of obese patients, thereby contributing to excessive visceral fat accumulation and obesity-associated complications (4,6,39). GPR55 has been described as a putative receptor for atypical cannabinoids (9,40,41). However, its clinical implications remain largely unknown. Herein, we demonstrate that GPR55 is expressed in human SAT, VAT, and liver. Our findings, obtained in two independent cohorts, indicate that GPR55 mRNA levels are increased in VAT and SAT of obese subjects in comparison with lean volunteers. Moreover, VAT but not SAT GPR55 expression is positively associated with type 2 diabetes. Of interest, correlations between VAT GPR55 gene levels and weight, BMI, and percent body fat are stronger in women than in men. Consistently, plasma LPI levels are also increased in obese patients and positively correlated with weight, BMI, and percent body fat in women. Finally, we show that in explants obtained from VAT, LPI triggers the mRNA levels of genes promoting lipogenesis, and in cultured differentiated adipocytes obtained from VAT, LPI raises [Ca2+]i. However, we found that LPI evoked minor responses in adipose tissue explants or in cultured differentiated adipocytes obtained from SAT, suggesting that the LPI/GPR55 system is particularly important in VAT.
In rodents, GPR55 mRNA has been detected throughout the central nervous system (22) and in peripheral tissues (23). Our study is the first to show that GPR55 mRNA is expressed in human tissues, and its regulation is tissue specific because GPR55 expression in WAT, but not liver, is particularly upregulated in diabetic patients when compared with NGT obese individuals. Specifically, GPR55 is found at similar concentrations in both adipocytes and the SVF of fat tissue, indicating that this receptor is also present in other cell types in the SVF, such as mononuclear cells (monocytes, macrophages, and lymphocytes), among others. The current findings are consistent with previous reports demonstrating that GPR55 is located in human monocytes (17). In obese states, the SVF is largely infiltrated by mononuclear cells in comparison with normal conditions.
Although endocannabinoid levels are increased in obesity (39), data on CB1 gene expression in WAT is controversial because reduced (39), increased (42), or unchanged (43) expression between obese and lean subjects has been reported. In the current work, we detected higher CB1 mRNA levels in the adipose tissue of obese patients. Although the discrepancies for those differences are unknown, a plausible explanation might be the different populations used by the studies. Similar to CB1, CB2 and GPR55 mRNA levels in the VAT of obese subjects were also increased, indicating the lack of a negative feedback loop between circulating endocannabinoids and these three cannabinoid receptors. The similar pattern of expression of the three receptors in obese and diabetic patients also suggests that some of their metabolic functions might be overlapped or compensated. This is an important issue because specific drugs for each receptor also might act through the other two receptors when the drug is administered at high doses or during long-term treatments. As a matter of fact, a recent pharmacological study suggests that low concentrations of rimonabant specifically block CB1 receptors, but higher doses or long-term treatment also could be targeting GPR55 (44). Other reports carried out in HEK cells show that the endocannabinoids anandamide and 2-arachidonoylglycerol also have a low affinity by GPR55 (16,23,45). Thus, GPR55 activation could be contributing to the biological activity in situations wherein endocannabinoid levels are markedly increased.
Despite activation of GPR55 by cannabinoids, it is well accepted that LPI is its more potent endogenous ligand known up to date (15–18). Plasma LPI levels previously have been found to be increased in patients with ovarian cancer, thereby considering LPI as a biomarker for this disease (30,46,47). However, to our knowledge, no reports have studied the interaction between LPI levels and metabolism. Similar to GPR55 expression, we found that plasma LPI is increased in obesity, suggesting that the LPI/GPR55 system is overactive in obese states. It is well known that visceral fat accumulation represents a key pathophysiologic mechanism for the clustering of metabolic abnormalities, including insulin resistance and type 2 diabetes (48). We found that within obese patients, GPR55 gene levels in VAT and SAT are significantly increased in diabetic subjects, suggesting that this receptor may play a pivotal role in the development of insulin resistance and the pathogenesis of type 2 diabetes. GPR55 gene expression in VAT, but not SAT, was positively correlated with several anthropometric parameters, such as body weight and BMI, in the two cohorts examined. It is interesting that when men and women are analyzed separately, VAT GPR55 shows a stronger correlation with weight, BMI, and fat percentage in women. Marked sex differences have been reported with regard to degrees of insulin resistance, body composition, and energy balance (49). Our results showing that VAT GPR55 mRNA expression is correlated with weight, BMI, and percentage body fat in women suggest that estrogens might be relevant to the GPR55 signaling pathway. Of import, the correlation of plasma LPI levels to weight, BMI, and percentage body fat is almost identical to that of VAT GPR55 because those correlations were positive in women but not men. This sexual dimorphism in gene expression and circulating levels has been observed previously in other hormones with important metabolic implications, such as leptin gene expression (50) or circulating leptin concentrations (51).
Because both LPI and VAT GPR55 showed a clear upregulation in obese patients and were correlated with important metabolic parameters, we next sought to investigate the direct actions of this system on adipose tissue explants and isolated adipocytes. Our findings indicate that in explants obtained from VAT, LPI induced lipid storage by stimulating lipogenic genes and promoted adipocyte differentiation by increasing PPARγ expression. Although GPR55 was also present in SAT, the treatment of explants from SAT with LPI did not modify the expression of any of the genes studied. Consistent with the gene expression studies and the lipogenic action of [Ca2+]i in adipocytes (38), LPI elicited a significantly higher effect in [Ca2+]i in cultured differentiated adipocytes from VAT than in those from SAT, both in terms of percentage of responsive cells and the magnitude of the [Ca2+]i increase. Indeed, although the doses of LPI tested herein are similar to others used in previous works (32,33), they are higher than plasma concentrations observed in obese patients, but overall, these results indicate that LPI directly favors a condition of lipid deposition within adipocytes from VAT. Given the specific correlations between LPI/GPR55 and several metabolic parameters in women, further studies will be necessary to address if estrogens can interfere in the biological actions of LPI. In this sense, it is important to point out that body fat is differentially distributed in men and women, with men exhibiting more visceral fat than women, in whom subcutaneous fat predominates (49). The different distribution of fat between both sexes might have clear implications in the metabolic actions of the LPI/GPR55 system. It is also important to point out that LPI is esterified by the enzyme 1-acylglycerol-3-phosphate-O-acyltransferase 3 (AGPAT3), which is ubiquitously expressed in human tissues (52). Thus, it is tempting to speculate that the tissue-specific actions of LPI might be explained by the distinct grade of activity of this enzyme in those fat depots. It is also important to point out that the role of the LPI/GPR55 system in obesity appears to be species dependent because LPI did not modify adipogenecity in 3T3-L1 cells and GPR55 mRNA, and protein expression is decreased in obese rodent models compared with their lean controls. Further studies in rodents assessing LPI levels in different pathophysiological models are merited to precisely clarify this issue.
In addition to adipose tissue, we also detected the expression of GPR55 in the liver. However, we failed to detect significant changes in expression between the various subgroups of obese patients. Although further studies should elucidate the expression of this receptor in other tissues with metabolic functions, including the gastrointestinal tract, skeletal muscle, or pancreas, our current findings suggest that VAT GPR55 may be the most important one regarding energy homeostasis.
In summary, our findings obtained in two independent cohorts demonstrate that 1) GPR55 levels are increased in both VAT and SAT of obese subjects; 2) VAT GPR55 is positively correlated with weight, BMI, and percentage body fat, particularly in women; 3) hepatic GPR55 gene expression remains unchanged in obese and diabetic subjects; 4) circulating LPI levels are increased in obesity and correlated with weight, BMI, and fat percentage in women; and 5) LPI increases [Ca2+]i and the expression of lipogenic enzymes specifically in differentiated adipocytes from VAT. This work suggests that the LPI/GPR55 system is a new metabolic pathway of clinical relevance. Further studies are required to investigate the potential role of agonists/antagonists for GPR55 in obesity and insulin sensitivity.
ACKNOWLEDGMENTS
M.M.M. has received a grant from the Ministerio de Ciencia e Innovación (BFU2010-17116). C.D. has received grants from the Ministerio de Ciencia e Innovación (BFU2008-02001), the Xunta de Galicia (PGIDIT06PXIB208063PR), and the European Union (Health-F2-2008-223713: Reprobesity). J.M.F.-R. has received a grant from the Ministerio de Ciencia e Innovación (SAF2008-02073). G.F. has received a grant from the Instituto de Salud Carlos III (ISCIII; FIS PS09/02330). R.N. has received grants from the Ministerio de Ciencia e Innovación (RYC-2008-02219 and SAF2009-07049). The research leading to these results also has received funding from the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 245009. Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición is an initiative of the ISCIII.
No potential conflicts of interest relevant to this article were reported.
J.M.M.-N., V.C., L.W., R.V.-M., J.G.-A., and M.R.P. researched data and contributed to discussion. A.D.-A., F.R., R.G., W.R.R., and M.I. researched data. R.A.R., M.M.M., C.D., J.M.F.-R., and G.F. reviewed and edited the manuscript. R.N. wrote the manuscript.
R.N. serves as the guarantor.
The authors gratefully acknowledge the valuable collaboration of all the members of the Multidisciplinary Obesity Team of the Clínica Universidad de Navarra and thank Lorraine Scobbie and Gary Duthie (Rowett Institute of Nutrition and Health) for their excellent technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0649/-/DC1.
REFERENCES
- 1.Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 2007;132:2103–2115 [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev 2003;24:278–301 [DOI] [PubMed] [Google Scholar]
- 3.Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 2006;91:3171–3180 [DOI] [PubMed] [Google Scholar]
- 4.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 2006;27:73–100 [DOI] [PubMed] [Google Scholar]
- 5.Blüher M, Engeli S, Klöting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006;55:3053–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 2008;51:1356–1367 [DOI] [PubMed] [Google Scholar]
- 7.Cota D. Role of the endocannabinoid system in energy balance regulation and obesity. Front Horm Res 2008;36:135–145 [DOI] [PubMed] [Google Scholar]
- 8.Pagotto U, Cervino C, Vicennati V, Marsicano G, Lutz B, Pasquali R. How many sites of action for endocannabinoids to control energy metabolism? Int J Obes (Lond) 2006;30(Suppl. 1):S39–S43 [DOI] [PubMed] [Google Scholar]
- 9.Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci 2009;30:156–163 [DOI] [PubMed] [Google Scholar]
- 10.Pertwee RG. GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol 2007;152:984–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petitet F, Donlan M, Michel A. GPR55 as a new cannabinoid receptor: still a long way to prove it. Chem Biol Drug Des 2006;67:252–253 [DOI] [PubMed] [Google Scholar]
- 12.Ross RA. L-α-lysophosphatidylinositol meets GPR55: a deadly relationship. Trends Pharmacol Sci 2011;32:265–269 [DOI] [PubMed] [Google Scholar]
- 13.Okuno T, Yokomizo T. What is the natural ligand of GPR55? J Biochem 2011;149:495–497 [DOI] [PubMed] [Google Scholar]
- 14.Bondarenko AI, Malli R, Graier WF. The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+-activated large-conductance K+ channels in endothelial cells. Pflugers Arch 2011;461:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun 2007;362:928–934 [DOI] [PubMed] [Google Scholar]
- 16.Henstridge CM, Balenga NA, Ford LA, Ross RA, Waldhoer M, Irving AJ. The GPR55 ligand L-alpha-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. FASEB J 2009;23:183–193 [DOI] [PubMed] [Google Scholar]
- 17.Whyte LS, Ryberg E, Sims NA, et al. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc Natl Acad Sci U S A 2009;106:16511–16516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka S, Kimura S, Toshida T, Ota R, Yamashita A, Sugiura T. Lysophosphatidylinositol induces rapid phosphorylation of p38 mitogen-activated protein kinase and activating transcription factor 2 in HEK293 cells expressing GPR55 and IM-9 lymphoblastoid cells. J Biochem 2010;147:671–678 [DOI] [PubMed] [Google Scholar]
- 19.Billah MM, Lapetina EG. Formation of lysophosphatidylinositol in platelets stimulated with thrombin or ionophore A23187. J Biol Chem 1982;257:5196–5200 [PubMed] [Google Scholar]
- 20.Kobayashi T, Kishimoto M, Okuyama H. Phospholipases involved in lysophosphatidylinositol metabolism in rat brain. J Lipid Mediat Cell Signal 1996;14:33–37 [DOI] [PubMed] [Google Scholar]
- 21.Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta 2008;1781:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawzdargo M, Nguyen T, Lee DK, et al. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res 1999;64:193–198 [DOI] [PubMed] [Google Scholar]
- 23.Ryberg E, Larsson N, Sjögren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 2007;152:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staton PC, Hatcher JP, Walker DJ, et al. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 2008;139:225–236 [DOI] [PubMed] [Google Scholar]
- 25.Ginde SR, Geliebter A, Rubiano F, et al. Air displacement plethysmography: validation in overweight and obese subjects. Obes Res 2005;13:1232–1237 [DOI] [PubMed] [Google Scholar]
- 26.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 27.Catalán V, Gómez-Ambrosi J, Ramirez B, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg 2007;17:1464–1474 [DOI] [PubMed] [Google Scholar]
- 28.Gómez-Ambrosi J, Salvador J, Rotellar F, et al. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes Surg 2006;16:262–269 [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Ambrosi J, Catalán V, Ramírez B, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab 2007;92:3719–3727 [DOI] [PubMed] [Google Scholar]
- 30.Sutphen R, Xu Y, Wilbanks GD, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev 2004;13:1185–1191 [PubMed] [Google Scholar]
- 31.Moreno-Navarrete JM, Ortega FJ, Rodríguez-Hermosa JI, et al. OCT1 Expression in adipocytes could contribute to increased metformin action in obese subjects. Diabetes 2011;60:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bondarenko A, Waldeck-Weiermair M, Naghdi S, Poteser M, Malli R, Graier WF. GPR55-dependent and -independent ion signalling in response to lysophosphatidylinositol in endothelial cells. Br J Pharmacol 2010;161:308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapur A, Zhao P, Sharir H, et al. Atypical responsiveness of the orphan receptor GPR55 to cannabinoid ligands. J Biol Chem 2009;284:29817–29827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez A, Gómez-Ambrosi J, Catalán V, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009;33:541–552 [DOI] [PubMed] [Google Scholar]
- 35.Catalán V, Gómez-Ambrosi J, Rotellar F, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res 2007;39:495–500 [DOI] [PubMed] [Google Scholar]
- 36.Velásquez DA, Martínez G, Romero A, et al. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 2011;60:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma MT, Yeo JF, Farooqui AA, Zhang J, Chen P, Ong WY. Differential effects of lysophospholipids on exocytosis in rat PC12 cells. J Neural Transm 2010;117:301–308 [DOI] [PubMed] [Google Scholar]
- 38.Gericke MT, Kosacka J, Koch D, et al. Receptors for NPY and PACAP differ in expression and activity during adipogenesis in the murine 3T3-L1 fibroblast cell line. Br J Pharmacol 2009;157:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engeli S, Böhnke J, Feldpausch M, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 2005;54:2838–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown AJ. Novel cannabinoid receptors. Br J Pharmacol 2007;152:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johns DG, Behm DJ, Walker DJ, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol 2007;152:825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003;63:908–914 [DOI] [PubMed] [Google Scholar]
- 43.Löfgren P, Sjölin E, Wåhlen K, Hoffstedt J. Human adipose tissue cannabinoid receptor 1 gene expression is not related to fat cell function or adiponectin level. J Clin Endocrinol Metab 2007;92:1555–1559 [DOI] [PubMed] [Google Scholar]
- 44.Henstridge CM, Balenga NA, Schröder R, et al. GPR55 ligands promote receptor coupling to multiple signalling pathways. Br J Pharmacol 2010;160:604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A 2008;105:2699–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Y, Chen Y, Kennedy AW, Belinson J, Xu Y. Evaluation of plasma lysophospholipids for diagnostic significance using electrospray ionization mass spectrometry (ESI-MS) analyses. Ann N Y Acad Sci 2000;905:242–259 [DOI] [PubMed] [Google Scholar]
- 47.Shen Z, Wu M, Elson P, et al. Fatty acid composition of lysophosphatidic acid and lysophosphatidylinositol in plasma from patients with ovarian cancer and other gynecological diseases. Gynecol Oncol 2001;83:25–30 [DOI] [PubMed] [Google Scholar]
- 48.Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006;38:52–63 [DOI] [PubMed] [Google Scholar]
- 49.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 2009;30:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perica’s J, Oliver P, Guitard R, Pico C, Palou A. Sexual dimorphism in age-related changes in UCP2 and leptin gene expression in subcutaneous adipose tissue in humans. J Nutr Biochem 2001;12:444–449 [DOI] [PubMed] [Google Scholar]
- 51.Rosenbaum M, Pietrobelli A, Vasselli JR, Heymsfield SB, Leibel RL. Sexual dimorphism in circulating leptin concentrations is not accounted for by differences in adipose tissue distribution. Int J Obes Relat Metab Disord 2001;25:1365–1371 [DOI] [PubMed] [Google Scholar]
- 52.Prasad SS, Garg A, Agarwal AK. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: localization of AGPAT5 to mitochondria. J Lipid Res 2011;52:451–462 [DOI] [PMC free article] [PubMed] [Google Scholar]