Abstract
To characterize the defects in β-cell function in subjects with impaired fasting glucose (IFG) and compare the results to impaired glucose tolerance (IGT) and normal glucose tolerance (NGT) subjects, β-cell glucose sensitivity and rate sensitivity during the oral glucose tolerance test were measured with the model by Mari in 172 Mexican Americans. A subgroup (n = 70) received a 2-h hyperglycemic clamp (+125 mg/dL), and first- and second-phase insulin secretion were quantitated. Compared with NGT, subjects with IFG and IGT manifested a decrease in β-cell glucose sensitivity; IFG subjects, but not IGT subjects, had decreased β-cell rate sensitivity. In IFG subjects, the defect in β-cell glucose sensitivity was time dependent, began to improve after 60 min, and was comparable to NGT after 90 min. The incremental area under the plasma C-peptide concentration curve during the first 12 min of the hyperglycemic clamp (ΔC-pep[AUC]0–12) was inversely related with the increase in FPG concentration (r = −36, r = 0.001), whereas ΔC-pep[AUC]15–120 positively correlated with FPG concentration (r = 0.29, r < 0.05). When adjusted for the prevailing level of insulin resistance, first-phase insulin secretion was markedly decreased in both IFG and IGT, whereas second-phase insulin secretion was decreased only in IGT. These results demonstrate distinct defects in β-cell function in IFG and IGT.
Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) are intermediate states in the transition in glucose tolerance from normal to overt diabetes. IFG was originally introduced by the American Diabetes Association to be analogous to IGT (1). Although subjects with isolated IFG and isolated IGT have a similarly increased risk for future type 2 diabetes, epidemiological studies have reported only partial overlap between the prevalence of the two states (2–7). In addition, the future risk of type 2 diabetes associated with IFG and IGT is additive. Thus, the risk of type 2 diabetes in subjects with IFG plus IGT is twofold greater compared with subjects with either state alone (2–7). Moreover, subjects with IFG and IGT differ not only by the fasting plasma glucose (FPG) and 2-h plasma glucose concentrations but also in the shape of the plasma glucose concentration curve following a glucose load, e.g., oral glucose tolerance test (OGTT) and mixed meal (8–10). The partial overlap between IFG and IGT, the additive type 2 diabetes risk, and the difference in plasma glucose profile following a glucose load suggest that distinct metabolic abnormalities underlie IFG and IGT.
Although subjects with IFG and IGT manifest both core defects that are characteristic of type 2 diabetes, i.e., insulin resistance and β-cell dysfunction, we (8–13) and others (14–23) have demonstrated that the metabolic abnormalities in IFG and IGT are distinct. Both IFG and IGT manifest insulin resistance, but the site of insulin resistance differs between the two states. Although subjects with IFG have severe hepatic insulin resistance with normal/near normal insulin sensitivity in skeletal muscle, individuals with IGT have severe insulin resistance in skeletal muscle with only a modest increase in hepatic insulin resistance. Both IFG and IGT individuals manifest β-cell dysfunction. The defect in β-cell function has been extensively studied in IGT (14–17,19,20,23). Studies with the hyperglycemic clamp have reported a marked decrease in both first- and second-phase insulin secretion in IGT subjects (15,16,23). Similarly, studies with the intravenous glucose tolerance test have demonstrated impaired acute insulin response in IGT (14,17,19,20). In addition, Ferrannini and colleagues (13,22) have demonstrated that the defect in β-cell function in IGT subjects can be explained by an intrinsic defect in β-cell glucose sensitivity to the ambient plasma glucose level. Similarly, studies with the graded intravenous glucose infusion technique have demonstrated impaired ability of IGT subjects to respond to a glucose stimulus (24). Despite the increased type 2 diabetes risk in IFG and the pivotal role of β-cell dysfunction in the development of type 2 diabetes, delineation of the defect(s) in β-cell function in IFG has received little attention, and conflicting results have been reported (17,19,23). The aim of the current study was to characterize the defect in β-cell function in subjects with IFG and compare the results to those in IGT and NGT.
RESEARCH DESIGN AND METHODS
The participants were 172 subjects of Mexican-American descent who were part of the San Antonio Veterans Administration Genetic Epidemiology Study (VAGES) (9). In VAGES, Mexican-American subjects received a 75-g OGTT, and based on the OGTT, subjects were classified as having NGT, IFG, and IGT according to the American Diabetes Association criteria (1). This study reports on 172 subjects with NGT (n = 78), isolated IFG (n = 46), and isolated IGT (n = 48).
All subjects had normal liver, cardiopulmonary, and kidney function as determined by medical history, physical examination, screening blood tests, electrocardiogram, and urinalysis. No NGT, IFG, or IGT subject was taking any medication known to affect glucose tolerance. Body weight was stable (± 2 kg) for at least 3 months before the study in all subjects. The study protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio (UTHSCSA), and informed written consent was obtained from all subjects before their participation. All studies were performed at the General Clinical Research Center of UTHSCSA at 0800 following a 10–12-h overnight fast.
OGTT.
Before the start of the OGTT, a polyethylene catheter was placed into an anticubital vein, and blood samples were collected at −30, −15, 0, 15, 30, 45, 60, 75, 90, 105, and 120 min for the measurement of plasma glucose, insulin, and C-peptide concentrations. On the day of the OGTT, body weight, height, and waist circumference at the narrowest part of the torso were determined.
Hyperglycemic clamp.
All subjects were offered a hyperglycemic clamp, but only a subgroup agreed to participate in a 2-h hyperglycemic clamp. Before the start of the hyperglycemic clamp, a catheter was placed into an anticubital vein for the infusion of glucose. A second catheter was inserted retrogradely into a vein on the dorsum of the hand, and the hand was placed into a thermoregulated box heated to 70°C. After obtaining three baseline samples, plasma glucose concentration was raised and maintained at 125 mg/dL above fasting level for 120 min. Blood samples were collected at −20, −10, 0, 2, 4, 6, 8, 10, 12, 15, 30, 45, 60, 75, 90, 105, and 120 min for the measurement of plasma insulin and C-peptide concentrations. Plasma glucose concentration was measured every 2 min during the first 15 min and every 5 min thereafter, and the plasma glucose concentration was maintained at the desired hyperglycemic plateau with a variable infusion of 20% glucose.
Calculations.
Insulin secretory rate (ISR) during the OGTT was calculated by deconvolution of the plasma C-peptide concentration curve, and the ratio between the incremental area under the plasma insulin secretory rate and incremental area under the plasma glucose concentration was calculated as previously described (25). β-Cell glucose sensitivity, rate sensitivity, and the potentiation factor were calculated with the model by Mari (12,22). This model expresses glucose-stimulated insulin secretion (in pmol · min−1 · m−2) as the sum of two components: 1) β-cell glucose sensitivity and 2) rate sensitivity. First, β-cell glucose sensitivity represents the dependence of insulin secretion on the absolute glucose concentration at any time point during the OGTT and is characterized by a dose-response function relating the two variables. β-Cell glucose sensitivity is modulated by a potentiation factor that encompasses several glucose-dependent and glucose-independent potentiating mechanisms (e.g., prolonged exposure to hyperglycemia, nonglucose substrates, gastrointestinal hormones, neural modulation, and molecular/biochemical/enzymatic changes within the β-cell). In normal individuals, the potentiation factor typically increases from baseline to the end of the OGTT. Second, rate sensitivity represents the dependence of insulin secretion on the rate of change of plasma glucose and is represented by the first derivative of plasma glucose concentration against time. The rate sensitivity accounts for the observation that rapid changes in glucose concentration enhance insulin secretion.
To evaluate time dependency of β-cell glucose sensitivity during the OGTT, we divided the increment in ISR above baseline at each time point by the increment in plasma glucose concentration above the fasting level at the same time point (ΔISR/ΔG). This ratio represents the β-cell glucose sensitivity at each time point during the OGTT. We also calculated β-cell glucose sensitivity during the first hour of the OGTT as the mean β-cell glucose sensitivity 15, 30, 45, and 60 min and β-cell glucose sensitivity during the second hour of the OGTT as the mean β-cell glucose sensitivity at 75, 90, 105, and 120 min.
First-phase insulin secretion during the hyperglycemic clamp was calculated as the incremental area under plasma C-peptide concentration between 0–12 min (ΔC-pep0–12) divided by the increment in plasma glucose concentration during the same time period. Second-phase insulin secretion during the hyperglycemic clamp was calculated as the increment in plasma C-peptide concentration between 15 and 120 min (ΔC-pep15–120) divided by the increment in plasma glucose concentration during the same time period. The glucose infusion rate (GIR) during the last 30 min of the hyperglycemic clamp divided by the mean plasma insulin (MPI) concentration during the same time period was used as an index of whole body insulin sensitivity. Previous studies have demonstrated that in comparable hyperglycemic clamp (+125 mg/dL), this index strongly correlates (r = 0.86) with whole body insulin-stimulated glucose disposal measured with the euglycemic hyperinsulinemic clamp (26). The insulin secretion/insulin resistance (disposition) index was calculated for the first and second phases of insulin secretion during the hyperglycemic clamp as ΔC-pep0–12 × GIR/MPI and ΔC-pep15–120 × GIR/MPI, respectively, and was used to evaluate β-cell function during the first and second phases of insulin secretion during the hyperglycemic clamp.
The incremental areas under the plasma glucose, insulin, and C-peptide concentration and the insulin secretory curves were calculated according the trapezoid rule.
Analytical techniques.
Plasma glucose was measured by the glucose oxidase reaction (Glucose Oxidase Analyzer; Beckman, Fullerton, CA). Plasma insulin and C-peptide concentrations were measured by radioimmunoassay (Linco Research, St. Louis, MO).
Statistical analysis.
Data are presented as the mean ± SE. Pearson correlation was used to assess the relationship between variables. To examine the predictors of first- and second-phase insulin secretion during the hyperglycemic clamp, we constructed a linear regression model with the first- and second-phase insulin secretion as the dependent variable and other variables as the independent variable. Similarly, the contribution of the first- and second-phase insulin secretion during the hyperglycemic clamp and glucose infusion rate, i.e., insulin sensitivity, to the incremental increase in plasma glucose concentration during the OGTT was assessed with a linear regression model with ΔG as the dependent variable and first- and second-phase insulin secretion and glucose infusion rate as the independent variable. For comparison between groups, Student t test was used. To compare the mean of more than two groups, ANOVA was used. Statistical significance was considered at P < 0.05.
RESULTS
The characteristics of the study participants are presented in Table 1. Subjects in the three groups had a similar BMI. NGT subjects were slightly younger than IFG and IGT subjects. As anticipated, there were more males with IFG.
TABLE 1.
Patient characteristics
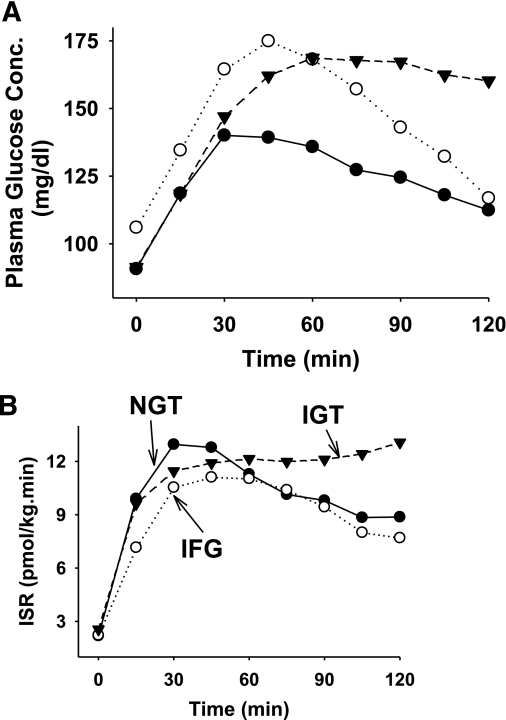
Figure 1 depicts the plasma glucose concentration and ISR during the OGTT in NGT, IFG, and IGT subjects. The absolute incremental area under the ISR curve during the OGTT (ΔISR0–120) was similar in NGT, IFG, and IGT (13.6 ± 0.6, 11.5 ± 0.9, and 14.9 ± 0.9, respectively; P = nonsignificant). However, the incremental area under the ISR curve during the first 30 min during the OGTT (ΔISR0–30) was significantly decreased in IFG subjects (2.1 ± 0.3) compared with IGT (2.8 ± 0.2) and NGT (3.2 ± 0.2) (P < 0.001) subjects. The incremental area under the ISR curve divided by the incremental area under the plasma glucose concentration (ΔISR/ΔG0–30) was markedly decreased in IFG (0.19 ± 0.02) and IGT (0.15 ± 0.01) subjects compared with NGT (0.38 ± 0.06) (P < 0.01).
FIG. 1.
Plasma glucose concentration (A) and insulin secretory rate (B) in subjects with IFG (n = 46), IGT (n = 48), and NGT (n = 78).
β-Cell glucose sensitivity, calculated with the Mari model, was significantly decreased (P < 0.01) in both IFG and IGT compared with NGT (Table 2 and Fig. 2A). β-Cell rate sensitivity was comparable in IGT and NGT, but was markedly decreased in IFG subjects (Table 2), and when related to the FPG in the entire group, it inversely related to the FPG (r = −0.34, P < 0.01). However, no significant correlation was observed between β-cell glucose sensitivity and 2-h plasma glucose concentration (r = 0.01, P = nonsignificant). The potentiation factor was significantly decreased in IGT compared with NGT, whereas it was significantly increased in IFG (Table 2). Although both IFG and IGT subjects had a decrease in β-cell glucose sensitivity, the time course of β-cell glucose sensitivity during the OGTT differed significantly between the two groups (Fig. 2B). In IGT subjects, β-cell glucose sensitivity was markedly decreased after 15 min and remained significantly lower than in NGT during the entire 120 min of the OGTT. β-Cell glucose sensitivity during the first hour of the OGTT (0–60 min) was 0.19 ± 0.01 and 0.30 ± 0.02 in IGT and NGT, respectively (P < 0.0001), and 0.14 ± 0.02 and 0.37 ± 0.04 during the second hour of the OGTT in IGT and NGT, respectively (P < 0.0001). In contrast, β-cell glucose sensitivity in IFG subjects was markedly decreased during the first 60 min of the OGTT (0.17 ± 0.02, P < 0.0001) but progressively increased with time, reaching a value comparable to NGT subjects at 90 min. β-Cell glucose sensitivity during the second hour of the OGTT was 0.34 ± 0.06, P = nonsignificant compared with NGT.
TABLE 2.
Model-derived β-cell parameters in NGT, IGT, and IFG subjects
FIG. 2.
A: β-Cell glucose sensitivity in IFG, IGT, and NGT subjects derived with the model by Mari. B: The ratio between the increment in insulin secretory rate above baseline and increment in plasma glucose concentration above the fasting level at each time point through the OGTT. *P < 0.05; **P < 0.01.
The characteristics of subjects who received the hyperglycemic clamp (32 NGT, 14 IGT, 24 IFG; age 39 ± 2, 42 ± 2, and 46 ± 2 years, respectively, and BMI 29.9 ± 0.9, 32.6 ± 0.9, and 30.9 ± 1.0, respectively) were similar to the entire group.
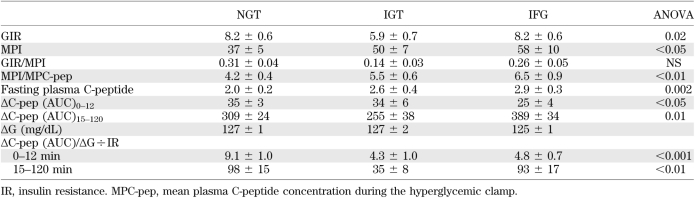
During hyperglycemic clamp, IFG subjects had lower first-phase insulin secretion (measured as ΔC-pep [AUC]0–12 or ΔISR[AUC]0–12) compared with NGT, whereas IGT subjects had a comparable ΔC-pep (AUC)0–12 (Table 3). In contrast, subjects with IGT had a significant decrease in the second-phase insulin secretion (ΔC-pep [AUC]15–120) compared with NGT subjects (Table 3), whereas ΔC-pep (AUC)15–120 was significantly increased in IFG subjects. The glucose infusion rate divided by the mean plasma insulin concentration during the hyperglycemic clamp (0–120), an index of insulin sensitivity (26), was markedly reduced by 54% in subjects with IGT (P < 0.01) and only modestly reduced in IFG subjects (Table 3). Thus, the insulin secretion/insulin resistance (disposition) index for first-phase (0–12 min) insulin secretion during the hyperglycemic clamp was markedly decreased in both IFG and IGT compared with NGT subjects (by 47 and 52%, respectively, P < 0.01). However, second-phase (15–120 min) insulin secretion/insulin resistance index was markedly decreased only in IGT subjects (by 64%, P < 0.01).
TABLE 3.
Metabolic parameters in NGT, IGT, and IFG subjects during the hyperglycemic clamp
When all subjects were pooled into one group, the increase in FPG concentration was associated with opposite changes in the first- and second-phase insulin secretion during the hyperglycemic clamp. Whereas the incremental area under the plasma C-peptide concentration curve for the first-phase insulin secretion (ΔC-pep [AUC]0–12) precipitously decreased with the increase in FPG concentration, r = 0.36. P < 0.001 (Fig. 3A), ΔC-pep(AUC)15–120 for second-phase insulin secretion progressively increased with the increase in FPG concentration r = 0.29, P < 0.05 (Fig. 3B). Similarly, the insulin secretion/insulin resistance index for both first- (0–12 min) and second- (15–120 min) phase was inversely related to the 2-h plasma glucose concentration (r = −37, P < 0.01 and r = −0.51, P < 0.0001, respectively). However, only the insulin secretion/insulin resistance index for first-phase insulin secretion inversely correlated with the FPG concentration (r = −0.41, P < 0.001), whereas insulin secretion/insulin resistance index for second-phase insulin secretion tended to be positively correlated with the FPG concentration (r = 0.14, P = nonsignificant).
FIG. 3.
Relationship between incremental area under the plasma C-peptide curve during the first phase (0–12 min, left) and second phase (15–120 min, right) of the hyperglycemic clamp. Triangles represent NGT subjects, open circles represent IGT subjects, and closed circles represent IFG subjects.
We used linear regression analysis to evaluate predictors of the first- and second-phase insulin secretions during the hyperglycemic clamp. Only FPG, β-cell glucose sensitivity, β-cell rate sensitivity, and glucose infusion rate were significant predictors of insulin secretion during the hyperglycemic clamp. FPG concentration, β-cell glucose sensitivity, and GIR were significant predictors of first-phase (0–12 min) insulin secretion, and a regression model that includes these three parameters could explain 50% of the variance in the first-phase insulin secretion (Table 4). Of note, when β-cell glucose sensitivity derived with the Mari model was replaced with β-cell glucose sensitivity during the first hour of the OGTT (0–60 min), β-cell rate sensitivity was no longer a predictor of the first-phase insulin secretion measured with the hyperglycemic clamp (Table 4). FPG concentration, β-cell glucose sensitivity during the first hour of the OGTT (0–60 min), and the potentiation factor were significant predictors of second-phase insulin secretion (Table 4), and a regression model that included the three parameters explained 33% of the variability in second-phase insulin secretion.
TABLE 4.
Determinants of first- and second-phase insulin secretion during the hyperglycemic clamp
To examine the contribution of first- and second-phase insulin secretion during the hyperglycemic clamp and whole body insulin sensitivity to the incremental area under the plasma glucose concentration curve during the OGTT, we constructed a linear regression model with the incremental area under the plasma glucose concentration curve as the dependent variable and first-phase insulin secretion, second-phase insulin secretion, and glucose infusion rate during the hyperglycemic clamp as independent variables (Table 5). Only first-phase insulin secretion significantly contributed to the incremental area under the plasma glucose concentration during the first hour ΔG0–60, and it explained 19% of the variance in ΔG0–60, whereas second-phase insulin secretion and the glucose infusion rate were significant predictors of ΔG60–120 and explained 27% of the variance in ΔG60–120 (Table 5).
TABLE 5.
Determinants of the increment in plasma glucose concentration during the OGTT
DISCUSSION
The results of the current study demonstrate that, although both IFG and IGT are characterized by β-cell dysfunction, the defects in insulin secretion in IFG and IGT are very distinct. Subjects with IFG have a severe impairment in first-phase insulin secretion in response to both oral (ΔISR0–30) and intravenous (0–12 min) glucose stimuli. In contrast, ΔISR60–120 during the OGTT and second-phase insulin secretion during the hyperglycemic clamp in IFG subjects were comparable to values in NGT subjects (Table 3). These results are consistent with previous studies (14,17,19,20), which demonstrated an impairment in the acute insulin response to intravenous glucose administration in IFG subjects and extended them to demonstrate that the defect in early insulin secretion is also present following oral glucose administration and is limited to the early (0–30 min) β-cell response to glucose stimulus. Unlike first-phase (ISR0–12) insulin secretion, both second-phase insulin secretion (ISR15–120) during the hyperglycemic clamp and late insulin secretion (60–120 min) during the OGTT in IFG subjects were comparable to that in NGT subjects. Moreover, the rise in FPG concentration was correlated with the increase in second-phase insulin secretion (Fig. 3). In marked contrast, IGT subjects manifested severe defects in β-cell function during both the first and second phases of insulin secretion during both the OGTT and hyperglycemic clamp. This is evident by the marked decrease in the insulin secretion/insulin resistance (disposition) index during both the OGTT and hyperglycemic clamp (Table 3), and both first- and second-phase insulin secretion strongly and inversely correlated with 2-h plasma glucose concentration. These results demonstrate that the defects in β-cell function associated with the increase in the FPG concentration, i.e., in IFG subjects, are very distinct from those associated with the increase in 2-h plasma glucose concentration, i.e., in IGT subjects.
Both IFG and IGT manifest decreased β-cell glucose sensitivity to the oral glucose stimulus compared with NGT. Since β-cell glucose sensitivity represents the ability of the β-cell to respond to a hyperglycemic stimulus, this observation indicates a “blindness” of the β-cell to the glucose stimulus in IFG and IGT compared with NGT individuals. However, the time course of decreased β-cell glucose sensitivity differs between the two states. Whereas the impairment in β-cell glucose sensitivity persisted through the 2-h of the OGTT in IGT, the defect was transient in IFG subjects. The restoration of β-cell glucose sensitivity after 60 min and the increase in potentiation factor in IFG subjects explain the near-normal second-phase insulin secretion response observed during the OGTT and hyperglycemic clamp. Moreover, the impaired rate sensitivity and early defect in β-cell glucose sensitivity (0–30 min) explain the severe defect in first-phase insulin secretion in IFG subjects. The transient defect in β-cell glucose sensitivity, the defect in β-cell rate sensitivity, the impaired early phase insulin secretion during the OGTT, and the impaired first-phase insulin secretory response during the hyperglycemic clamp collectively indicate the presence of a β-cell defect in the ability of IFG subjects to respond to the change in plasma glucose concentration. However, the ability of the β-cell to respond to a prolonged hyperglycemic stimulus is maintained in IFG. This is in marked contrast to IGT subjects who manifest persistent “blindness to glucose”, i.e., impaired β-cell glucose sensitivity for the entire duration of 2-h oral glucose stimulus (13,22). Although the molecular/biochemical abnormalities in the β-cell responsible for these defects remain unknown, these results collectively indicate that the β-cell defect in IFG and IGT subjects is very distinct, and both states represent separate entities of β-cell dysfunction. Moreover, the distinct abnormalities in the β-cell response to the glucose stimulus contribute to the distinct shape of the curve of the plasma glucose concentration during the OGTT in IFG and IGT (see below).
The increase in fasting plasma glucose concentration was correlated with both first- (negatively) and second- (positively) phase insulin secretion (Table 4). Previous studies have reported that a small rise in the plasma glucose concentration has a detrimental effect on first-phase insulin secretion (14,17,19,20,25). Conversely, improving glycemic control in type 2 diabetic subjects with insulin therapy improves β-cell function (27), and experimental studies in animals (28–30) have reported that a small rise (16 mg/dL) in FPG concentration is associated with a marked inhibition of glucose-stimulated insulin secretion, whereas correction of the hyperglycemia with phlorizin restored normal β-cell function (28). Future studies will be required to determine whether the defects in the first phase of insulin secretion, rate sensitivity, and β-cell glucose sensitivity in IFG subjects represent a primary defect in the β-cell (e.g., genetic) or are acquired secondary to the increase in fasting plasma glucose concentration, i.e., glucotoxicity, or other environmental factors.
The results of the current study help to explain the shape of the plasma glucose concentration following glucose ingestion in IFG subjects. The decrease in the first-phase insulin secretion contributes to the excessive rise in plasma glucose concentration during the first hour of the OGTT (ΔG0–60) (Table 5), and normal insulin sensitivity and normal second-phase insulin secretion are important determinants of the return in plasma glucose concentration to baseline fasting level during the second hour of the OGTT (ΔG60–120) (Table 5). Because first-phase insulin secretion plays an important role in priming the liver and inhibiting endogenous glucose production during the OGTT or a meal (31), the impairment in early phase insulin secretion in subjects with IFG would be expected to result in less inhibition of endogenous glucose production, and this would contribute to the excessive rise in plasma glucose concentration during the first 60 min of the OGTT in IFG.
Despite the excessive early (0–60 min) rise in plasma glucose concentration during the OGTT (9,10,18) and the defects in β-cell glucose sensitivity and rate sensitivity, subjects with IFG return their 2-h plasma glucose concentration to the baseline fasting glucose level. This can be explained by 1) the time-related improvement in β-cell glucose sensitivity during the 60–120 min time period; 2) the increase in potentiation factor (Table 2); and 3) normal to near-normal muscle insulin sensitivity (9). In marked contrast, the plasma glucose concentration during the last hour (60–120 min) of the OGTT in IGT subjects fails to decline whatsoever because of 1) the failure of the β-cell glucose sensitivity to improve with time (Fig. 2); 2) the decrease in potentiation factor; and 3) severe resistance to the action of insulin, as manifested both by the reduced Matsuda index of insulin sensitivity and the reduced GIR/mean plasma insulin concentration during the hyperglycemic clamp. Thus, the markedly elevated 2-h plasma glucose concentration during the OGTT in IGT versus IFG individuals is explained by the greater severity of the two basic core defects, insulin resistance and β-cell dysfunction, that characterize type 2 diabetes.
Only Mexican Americans participated in the current study. Because previous studies have demonstrated that the contribution of β-cell dysfunction to the deterioration in glucose tolerance could be ethnic dependent (32), validation of the results of the current study in other ethnic groups will help generalize the results.
In summary, the results of the current study demonstrate that the increase in FGP concentration in IFG subjects is associated with β-cell defects, which are distinct from the defects associated with the increase in 2-h plasma glucose concentration in IGT subjects. It follows that interventions aimed to halting/reverting β-cell failure should be individualized to each state.
ACKNOWLEDGMENTS
This work was supported by American Heart Association Grant 10SDG4470014 to M.A.A.-G. M.K. is supported by the Turkish Diabetes, Obesity, and Nutrition Association; the Turkish Diabetes Foundation; and the University of Abant Izzet Baysal.
C.J., D.W., and L.N. contributed to data generation. A.M. and M.K. performed the data analysis. R.A.D. reviewed the manuscript and contributed to discussion. M.A.A.-G. contributed to data generation and analysis and wrote the manuscript. M.A.A.-G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the nurses, James King, John Kincaid, Rose Kaminski-Graham, and Norma Diaz (Bartter Research Unit, Audie L. Murphy Veterans Affairs Hospital), for assistance in performing the OGTT studies and for their excellent care of the patients throughout the study. Lorrie Albarado (Diabetes Division, UTHSCSA) provided expert secretarial assistance in manuscript preparation.
Footnotes
See accompanying commentary, p. 270.
REFERENCES
- 1.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 2.Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract 2007;78:305–312 [DOI] [PubMed] [Google Scholar]
- 3.Unwin N, Shaw J, Zimmet P, Alberti KGMM. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 4.Dankner R, Abdul-Ghani MA, Gerber Y, Chetrit A, Wainstein J, Raz I. Predicting the 20-year diabetes incidence rate. Diabetes Metab Res Rev 2007;23:551–558 [DOI] [PubMed] [Google Scholar]
- 5.Shaw JE, Zimmet PZ, de Courten M, et al. Impaired fasting glucose or impaired glucose tolerance. What best predicts future diabetes in Mauritius? Diabetes Care 1999;22:399–402 [DOI] [PubMed] [Google Scholar]
- 6.Gabir MM, Hanson R, Dabelea D, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care 2000;23:1113–1118 [DOI] [PubMed] [Google Scholar]
- 7.de Vegt F, Dekker JM, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. The 1997 American Diabetes Association criteria versus the 1985 World Health Organization criteria for the diagnosis of abnormal glucose tolerance: poor agreement in the Hoorn Study. Diabetes Care 1998;21:1686–1690 [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep 2009;9:193–199 [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29:1130–1139 [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Sabbah M, Kher J, Minuchin O, Vardi P, Raz I. Different contributions of insulin resistance and beta-cell dysfunction in overweight Israeli Arabs with IFG and IGT. Diabetes Metab Res Rev 2006;22:126–130 [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Gastaldelli A, Miyazaki Y, et al. Predominant role of reduced beta-cell sensitivity to glucose over insulin resistance in impaired glucose tolerance. Diabetologia 2003;46:1211–1219 [DOI] [PubMed] [Google Scholar]
- 14.Festa A, D’Agostino R, Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM. Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 2004;53:1549–1555 [DOI] [PubMed] [Google Scholar]
- 15.van Haeften TW, Pimenta W, Mitrakou A, et al. Disturbances in beta-cell function in impaired fasting glycemia. Diabetes 2002;51(Suppl. 1):S265–S270 [DOI] [PubMed] [Google Scholar]
- 16.Pimenta WP, Santos ML, Cruz NS, Aragon FF, Padovani CR, Gerich JE. Brazilian individuals with impaired glucose tolerance are characterized by impaired insulin secretion. Diabetes Metab 2002;28:468–476 [PubMed] [Google Scholar]
- 17.Godsland IF, Jeffs JA, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia 2004;47:1157–1166 [DOI] [PubMed]
- 18.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T; Impaired Glucose Tolerance for Atherosclerosis and Diabetes study Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care 2003;26:868–874 [DOI] [PubMed] [Google Scholar]
- 19.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 1999;48:2197–2203 [DOI] [PubMed] [Google Scholar]
- 20.Wasada T, Kuroki H, Katsumori K, Arii H, Sato A, Aoki K. Who are more insulin resistant, people with IFG or people with IGT? Diabetologia 2004;47:758–759 [DOI] [PubMed] [Google Scholar]
- 21.Osei K, Gaillard T, Schuster DP. Pathogenetic mechanisms of impaired glucose tolerance and type II diabetes in African-Americans. The significance of insulin secretion, insulin sensitivity, and glucose effectiveness. Diabetes Care 1997;20:396–404 [DOI] [PubMed] [Google Scholar]
- 22.Mari A, Tura A, Gastaldelli A, Ferrannini E. Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 2002;51(Suppl. 1):S221–S226 [DOI] [PubMed] [Google Scholar]
- 23.Meyer C, Pimenta W, Woerle HJ, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 2006;29:1909–1914 [DOI] [PubMed] [Google Scholar]
- 24.Ehrmann DA, Breda E, Cavaghan MK, et al. Insulin secretory responses to rising and falling glucose concentrations are delayed in subjects with impaired glucose tolerance. Diabetologia 2002;45:509–517 [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani MA, Matsuda M, Jani R, et al. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab 2008;295:E401–E406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 27.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes 1985;34:222–234 [DOI] [PubMed] [Google Scholar]
- 28.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer J, Sturis J, Katschinski M, Arnold R, Göke B, Byrne MM. Acute hyperglycemia alters the ability of the normal beta-cell to sense and respond to glucose. Am J Physiol Endocrinol Metab 2002;282:E917–E922 [DOI] [PubMed] [Google Scholar]
- 30.Leahy JL, Bonner-Weir S, Weir GC. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest 1988;81:1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luzi L, DeFronzo RA. Effect of loss of first-phase insulin secretion on hepatic glucose production and tissue glucose disposal in humans. Am J Physiol 1989;257:E241–E246 [DOI] [PubMed] [Google Scholar]
- 32.Abdul-Ghani MA, Matsuda M, Sabbah M, et al. The relative contributions of insulin resistance and beta cell failure to the transition from normal to impaired glucose tolerance varies in different ethnic groups. Diab Met Syn Res Rev 2007;1:105–112 [Google Scholar]