Abstract
More than one-half of the ~50 human chemokines have been associated with or implicated in the pathogenesis of type 1 diabetes, yet their actual expression patterns in the islet environment of type 1 diabetic patients remain, at present, poorly defined. Here, we have integrated a human islet culture system, murine models of virus-induced and spontaneous type 1 diabetes, and the histopathological examination of pancreata from diabetic organ donors with the goal of providing a foundation for the informed selection of potential therapeutic targets within the chemokine/receptor family. Chemokine (C-C motif) ligand (CCL) 5 (CCL5), CCL8, CCL22, chemokine (C-X-C motif) ligand (CXCL) 9 (CXCL9), CXCL10, and chemokine (C-X3-C motif) ligand (CX3CL) 1 (CX3CL1) were the major chemokines transcribed (in an inducible nitric oxide synthase–dependent but not nuclear factor-κB–dependent fashion) and translated by human islet cells in response to in vitro inflammatory stimuli. CXCL10 was identified as the dominant chemokine expressed in vivo in the islet environment of prediabetic animals and type 1 diabetic patients, whereas CCL5, CCL8, CXCL9, and CX3CL1 proteins were present at lower levels in the islets of both species. Of importance, additional expression of the same chemokines in human acinar tissues emphasizes an underappreciated involvement of the exocrine pancreas in the natural course of type 1 diabetes that will require consideration for additional type 1 diabetes pathogenesis and immune intervention studies.
The molecular determinants in the control of initiation, coordination, and perpetuation of innate and adaptive immune responses that together precipitate β-cell destruction and eventual development of clinical type 1 diabetes constitute one of the principal topics of interest in contemporary diabetes research. Because the regulated spatio-temporal positioning of motile immune cells is a precondition for the targeted destruction of specific parenchymal tissues, the pathogenesis of type 1 diabetes is both dependent on factors that control immune cell trafficking and potentially susceptible to therapeutic modalities that interfere with the bioactivity of such factors. With at least 46 individual members, the human chemokine superfamily constitutes perhaps the single largest group of molecules involved in the recruitment of leukocytes to sites of inflammation (1–3), and multiple chemokines and chemokine receptors have emerged as pertinent contributors to the natural history of various autoimmune disorders, including type 1 diabetes; potential biomarkers; and possible drug targets (4–8). In fact, work conducted over the past 20 years has implicated more than one-half of all human and/or rodent chemokines in the pathogenesis of type 1 diabetes and/or its complications, although much of the work published to date on human type 1 diabetes and chemokines remains limited to genetic association studies and chemokine/receptor analyses in peripheral blood (9–23).
In several mouse models, however, the expression of individual islet-associated chemokines has been used as a foundation for the development of pathogenesis and intervention studies that directly implicate chemokines, such as CCL2, CCL3, CCL5, CCL22, and CXCL10, in type 1 diabetes development (14–23) and demonstrate that the chemokine/receptor blockade of CCL3, CCR5, CCL22 or CXCL10 (15–17,19,21), and Cxcl10 DNA vaccination (22) can ameliorate pathology and at least partially prevent the onset of type 1 diabetes. Although these studies support the potential utility of targeting the chemokine system in type 1 diabetes models, the disparate nature of the chemokines involved and the lack of complete success in preventing type 1 diabetes by chemokine-specific “monotherapies” suggest the need to consider therapeutic interference with more than one relevant chemokine/receptor. This point is illustrated in an elegant study by Martin et al. (13), who demonstrated the presence of multiple chemokine proteins (CCL1/3/4/21/22/24 and CXCL9/10) produced by islet-infiltrating cells, β-cells, and/or endothelial cells in prediabetic nonobese diabetic (NOD) mice and showed that β-cell–specific expression of the MHV68 protein M3, a “decoy receptor” that binds multiple chemokines, can in fact completely prevent spontaneous type 1 diabetes in the NOD model. Taken together, these findings emphasize the complex regulation of autoimmune responses by different chemokines as well as the possibilities and challenges associated with multiple therapeutic targets among members of the chemokine family.
In the current study, we have combined a human islet culture system, animal models, and the immunohistology of human pancreata to develop an unbiased approach for the identification of those chemokines that are in fact expressed in the context of type 1 diabetes pathogenesis and thus may constitute suitable targets for the development of therapeutic strategies.
RESEARCH DESIGN AND METHODS
Pancreatic tissue donors.
Pancreatic tissue sections were obtained through the Network for Pancreatic Organ Donors with Diabetes (nPOD) and included healthy control subjects (three donors negative for autoantibodies; case identification nos. 6112, 6115, and 6117) and type 1 diabetic donors at different disease stages (three donors with no evidence for other autoimmune disorders; case identification nos. 6036, 6052, and 6087). BMI for all patients was in the range of 17–26 kg/m2, and all other data are summarized in Supplementary Table 5. All experimental protocols were approved by the University of Colorado Institutional Review Board.
Procurement and culture of human islets.
Human islets were procured, processed, and cultured for 24 h in the absence or presence of recombinant human cytokines (interleukin [IL]-1β: 10 ng/mL [500 units/mL], interferon [IFN] γ: 25 ng/mL [500 units/mL], or tumor necrosis factor [TNF] α: 25 ng/mL [2,500 units/mL] or a cocktail mixture of IL-1β [2 ng/mL], IFNγ [10 ng/mL], and TNFα [10 ng/mL]), as described previously (24). Functionality of human islet preparations was assured by reversion of hyperglycemia following transplantation into streptozotocin-induced diabetic Rag2−/− B6 mice, as detailed elsewhere (24).
RNA isolation, microarray analyses, and quantitative real-time PCR.
Total RNA was extracted from groups of 8,000–10,000 human islets from four unrelated donors (two male and two female subjects, aged 26–46 years) or ~1,500 hand-picked murine islets (n = 3 × 3 pooled NOD pancreata per time point) using TRIzol reagent (Invitrogen), purified by RNeasy columns (QIAGEN), and RNA quality verified by capillary electrophoresis (Agilent-2100 Bioanalyzer; Agilent Technologies). Additional processing, hybridization to microarrays (Human Genome HG U133 Plus 2.0 chips; Affymetrix), quality control, and normalization by GC robust multi-array average (GC-RMA) analysis was conducted as reported previously (24,25). For quantitative real-time PCR (qRT-PCR), cDNA was prepared from total RNA (1 μg) using a iScript cDNA synthesis kit (Bio-Rad Laboratories), and samples equivalent to 0.1 μg of the original RNA sample were used as templates for amplification in the linear phase in a 5′-nuclease assay–based system using 6-carboxyfluorescein (FAM) dye–labeled TaqMan minor groove binder (MGB) probes (Applied Biosystems) on a 96-well ABI 7000 PCR system instrument. The hypoxanthine phosphoribosyl transferase (HPRT1) gene was selected for sample normalization on the basis of preliminary experiments with the ABI control plate.
Anti-inflammatory agents.
To evaluate specific signaling pathways for induced chemokine transcription, human islets were treated with 5 mmol/L acetylsalicylic acid, 7.5 mmol/L Bay-11-7085 (inhibitor of IκB phosphorylation), 1 mmol/L NG-monomethyl-arginine (inducible nitric oxide synthase [iNOS] pathway blocker), or 40 μmol/L 1-methyl-tryptophan (indoleamine 2,3 dioxygenase [IDO] blockade); all chemicals were obtained from Sigma.
Cell lines, vectors, transfection, chemokine antibodies, and flow cytometry.
Subcloning of human chemokine cDNA clones (Open Biosystems) into pIRES2-AcGFP1 vector (Clontech), transfection, and culture of human embryonic kidney 293 cells and staining with chemokine-specific antibodies for flow cytometric analysis were performed using previously published protocols (26). For the purpose of the current study, we approved the utility of the following human chemokine–specific antibodies: goat polyclonals αCCL5 (AF278-NA), αCCL8 (AF281-NA), αCXCL9 (AF392), αCXCL10 (AF266-NA), and αCX3CL1 (AF365) as well as polyclonal chicken IgY specific for CCL22 (AF336) (R&D Systems). A detailed characterization of all murine chemokine–specific antibodies used here is provided elsewhere (26).
Mice and virus.
C57BL/6J (B6) and Balb/c mice were purchased from The Jackson Laboratory, rat insulin promoter (RIP)-glycoprotein (GP) transgenic B6 mice expressing the lymphocytic choriomeningitis virus (LCMV) GP under control of the RIP (27) were a gift from Dr. M. von Herrath (La Jolla Institute for Allergy and Immunology, La Jolla, CA), and NOD mice were acquired from our in-house colony at the Barbara Davis Center. Origin, growth, and titration of the LCMV Armstrong clone 53b, obtained from Dr. M. Oldstone (Scripps Research Institute, La Jolla CA), has been described (28). Seven- to 8-week-old B6 and RIP-GP mice were infected with a single intraperitoneal dose of 105 pfu LCMV, and all procedures were performed in accordance with regulations set forth by the University of Colorado Institutional Animal Care and Use Committee.
Immunohistochemical detection of murine chemokines.
Pancreata obtained from NOD mice or LCMV-infected and uninfected B6 and RIP-GP mice were fixed in 10% formalin followed by dehydration in increasing concentrations of ethanol and overnight paraffin embedding, subsequent sectioning in 6-μm intervals, blocking with tyramide system amplification (TSA) buffer, and treatment with citrate buffer (0.01 mol/L) for antigen retrieval. Sections were stained for insulin (guinea pig polyclonal [1:100]; Dako A056401), glucagon (rabbit polyclonal [1:100]; Sigma SAB4501137), and chemokines (goat polyclonals, 10–15 μg/mL) overnight in a humidified chamber. Staining with secondary antibodies (1:200) was performed for 1 h using donkey α-rabbit-amino-methyl-coumarin-acetate (AMCA), α-guinea pig–Cy2, and α-goat-Cy3 F(ab’)2 fragments (Jackson ImmunoResearch). Images were acquired using an Olympus BX51 microscope and a Pixera Pro 150ES camera and were analyzed using Studio 3.0.1 software.
Immunohistochemical detection of human chemokines (islet cell cultures and nPOD sections).
Islets incubated in the presence and absence of cytokines were fixed in 4% formaldehyde, immobilized in a molten 4% agar block (45°C), and embedded for paraffin sectioning (6 μm). Sections were blocked with 5% normal goat serum and then reacted with human chemokine antibodies (10 μg/mL), guinea pig α-insulin (Dako North America), and mouse α-glucagon (Sigma) overnight at 4οC at concentrations recommended by the manufacturers. Secondary antibodies (α-guinea pig–Cy2, α-mouse-AMCA, and α-goat-Cy3 or α-chicken IgY-Cy3 F[ab’]2 fragments from Jackson ImmunoResearch) were used at 1:250. Images were acquired by Intelligent Imaging System software, using an Olympus 1 × 81 inverted motorized microscope equipped with Olympus DSU spinning disk confocal optics and a Hamamatsu ORCA IIER monochromatic charge-coupled device camera. Paraffin-embedded pancreatic sections were procured from the Network of Pancreatic Organ Donors With Diabetes (nPOD) (see above) and deparaffinized in Histoclear and serial changes of ethanol and hydrated with PBS. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 30 min, slides were blocked for 1 h in Tris-NaCl Buffer (TNB) buffer (Zymed) and incubated with mouse anti-human CD45 antibody (Richard Allen Scientific) for 2 h, and CD45-labeled cells were visualized by immunoperoxidase reaction with diaminobezidine (Dako). After rinsing and overnight incubation with guinea pig α-insulin (1:100), rabbit α-glucagon (1:100) (Dako), and chemokine antibodies (10 μg/mL), the slides were stained with species-specific F(ab’)2 fragments labeled with AMCA (α-guinea pig, 1:100), Cy2 (α-rabbit, 1:250), or Cy3 (α-goat or α-chicken IgY, 1:250) and cover slipped with a glycerol-based medium. A Leica SP5 laser-scanning laser confocal microscope running Leica software was used for imaging the pancreas. Negative controls included nondiabetic normal donor tissue sections, normal goat IgG (R&D Systems), and, in the case of CXCL10 stains, preabsorption of CXCL10 antibody with a 10 mol/L equivalent of recombinant human CXCL10 (Peprotech).
Statistical analyses.
Data handling, analysis, and graphic representation was performed using Prism 4.0 (GraphPad Software, San Diego, CA). All data summarized in bar diagrams are expressed as mean ± 1 SE; asterisks indicate statistical differences calculated by unpaired Student t test and adopt the following convention: *P < 0.05; **P < 0.01; and ***P < 0.001.
RESULTS
Cytokine-induced modulation of chemokine transcripts in human islet cells.
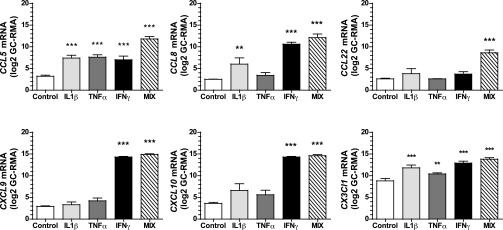
To delineate the complete spectrum of chemokine mRNA species transcribed by human islet cells in response to inflammatory stimuli, we used an established in vitro culture system in which islets purified from healthy organ donors were cultured in the absence versus the presence of IL-1β, TNFα, and/or IFNγ and subsequently subjected to gene expression microarray analysis (24,25). Adopting the chromosomal localization of chemokine genes as a basis for their genomic organization into four major clusters (growth-regulated oncogene [GRO] [CXCL1–8] and interferon γ-induced protein 10 (IP10) [CXCL9–11/13] clusters on chromosome 4; monocyte chemoattractant protein-1 (MCP) and macrophage inflammatory proteins (MIP) [CCL1–16/18/23] clusters on chromosome 17) (1) (Supplementary Fig. 1), the transcriptional profiles of islet cells stimulated by individual cytokines could be roughly divided into two groups: IL-1β and TNFα both induced a similar spectrum of major cluster genes (GRO: CXCL1–3/6, IP-10: CXCL9–11, and MCP/MIP: CCL2/3/5) as well as CCL20, whereas the action of IFNγ seemed to be more focused on the IP-10 group (CXCL9–11) and the three MCP/MIP genes, CCL5/7/8 (Supplementary Tables 1–3 and Supplementary Fig. 1). Upregulation of the latter MCP genes, CCL7/8, also was observed after IL-1β but not TNFα exposure, and the unclustered chemokine gene, CX3CL1, was readily induced by all cytokines (Supplementary Tables 1–3 and Supplementary Fig. 1). Islet treatment with a cocktail of all three cytokines (“Mix”) had a largely complementary, but in some cases also synergistic, effect, as shown by the significant induction of several additional chemokine mRNA species, including CXCL5, CXCL16, CCL4, and, most prominently, CCL22 (Supplementary Table 4). Finally, the only significant, if rather slight, downregulation of any chemokine gene pertained to the reduced IL8 (CXCL8) expression following TNFα treatment (Supplementary Table 2). For the remainder of our study, we selected six chemokines (CCL5/8/22, CXCL9/10, and CX3CL1) exhibiting >30-fold increased transcription after culture with the cytokine cocktail (Fig. 1) and verified the microarray data by means of qRT-PCR (Fig. 2). Please note that we have at present refrained from additional analyses of CCL3 and CXCL11 (Supplementary Table 4): the extent of cross-reactivities among probe sets and antibodies for CCL3, CCL3L1, CCL3L3, and/or the pseudogene, CCL3P1 (Supplementary Fig. 1 and Supplementary Tables 1–4), will require further clarification, and CXCL11 analyses cannot be performed in our corresponding murine studies (see below) because mice on the B6 background lack a functional Cxcl11 gene (29).
FIG. 1.
Chemokine transcripts induced in human islet cells in response to inflammatory stimuli (microarray analysis). Purified human islets obtained from healthy organ donors were cultured for 24 h in the absence (Control) or presence of individual recombinant human cytokines IL-1β, TNFα, or IFNγ or combinations thereof (MIX) prior to microarray analysis as described in RESEARCH DESIGN AND METHODS. The normalized intensity (log scale) from data obtained on the HG U133 Plus 2.0 Affymetrix chip is shown. Each data point is the mean ± SE of three to four observations. Cytokine cocktail (MIX)-induced expression by a factor of >30 was observed for CCL5, CCL8, CCL22, CX3CL1, CXCL9, and CXCL10 (asterisks indicate significant differences between control and cytokine-treated islets).
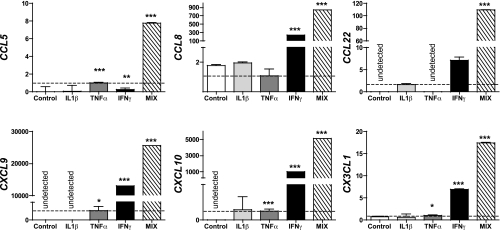
FIG. 2.
Chemokine transcripts induced in human islet cells in response to inflammatory stimuli (qRT-PCR analysis). Chemokine transcript expression in human islets cultured as described in the legend to Fig.1 and RESEARCH DESIGN AND METHODS was measured by qRT-PCR using a 5′-nuclease assay and FAM dye–labeled TaqMan MGB probes with two PCR primers. Endogenous HPRT1 was used for normalization. Data (mean ± SE; four donors) was quantified using the 2–ΔΔ CT method and expressed relative to an islet sample incubated in medium alone. For direct comparison, a value of 1.0 (dotted line) was assigned to TNFα-induced (CCL5, CXCL9/10, and CX3CL1) or IL-1β–induced (CCL22) chemokine transcripts. Asterisks indicate significant differences between control and cytokine-treated islets.
Regulation of cytokine-induced chemokine mRNA transcriptions by anti-inflammatory agents.
CCL5/8, CXCL9/10, and CX3CL1 are usually regarded as “inflammatory” chemokines, whereas CCL22 exerts both homeostatic and inflammatory functions (1). In agreement with this functional distinction, chemokine mRNA induction by islet cells exposed to the cytokine cocktail was significantly reduced in the presence of the nonsteroidal anti-inflammatory drug acetylsalicylic acid (Supplementary Fig. 2A). We previously reported that cytokine cocktail stimulation of human islets engages the nuclear factor (NF)-κB pathway in conjunction with both proinflammatory (iNOS) and immunomodulatory (IDO) factors (24,25) and, in support of the notion that NF-κB and iNOS signaling trigger β-cell apoptosis and dysfunction (30,31), documented the NF-κB–dependent regulation of genes involved in islet apoptosis and survival (25). Yet the induced transcription of chemokine mRNA species in human islet cells reported here was not affected by blockade of the NF-κB pathway with 7.5 mmol/L Bay-11-7085 (not shown). In contrast, inhibition of the iNOS pathway significantly compromised mRNA induction for all chemokines except CX3CL1 (Supplementary Fig. 2B). Finally, interference with tryptophan catabolism by blockade of IDO had only minor effects with a significant reduction observed only for CXCL9 (Supplementary Fig. 2B). Collectively, these results support the notion that the stimulation of human islets with cytokine cocktails adequately mimics the effects of inflammatory alterations and implicates in particular the iNOS rather than NF-κB pathway in the regulation of chemokine mRNA transcription.
Expression of chemokine proteins induced by cytokine-stimulated human islet cells.
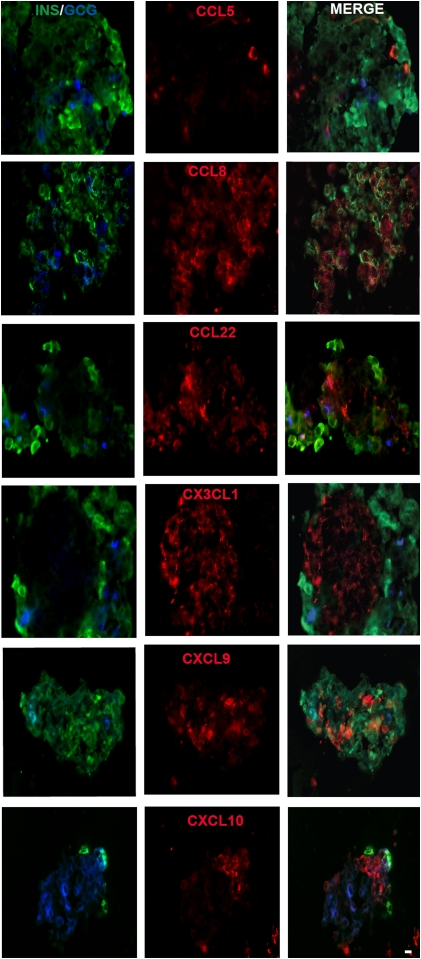
At present, the immunohistochemical (IHC) detection of human chemokine proteins at large is limited to approximately one-half of all chemokines for which approved antibody reagents are readily available. We therefore made use of a recently described flow cytometry–based strategy to select, test, and validate suitable chemokine-specific antibodies for use in IHC (26). As shown in Supplementary Fig. 3A, we identified a panel of polyclonal antibodies specific for CCL8 and CCL22 not previously validated for flow cytometry/IHC, confirmed the utility of a CCL5 antibody previously characterized by us (26), and demonstrated that the selected CXCL9/10 and CX3CL1 antibodies are indeed specific for their cognate chemokines. We note, however, that the CXCL10-specific antibody (also used in two recent publications for pancreatic in situ CXCL10 staining [32,33]) is partially cross-reactive with CXCL9 (Supplementary Fig. 3A), thus emphasizing the importance of evaluating CXCL10 and CXCL9 expression in parallel (the CXCL9-specific antibody, in contrast, does not cross-recognize CXCL10). Although our procedure used for islet cell isolation, cytokine stimulation, and paraffin embedding distorts some of their anatomic organization, we found that CCL5/8/22, as well as CXCL9/10 and CX3CL1, were readily translated in our in vitro culture system (Fig. 3).
FIG. 3.
Immunofluorescent localization of chemokines in cultured human islets. Islets were cultured for 24 h in MIX, fixed with 4% paraformaldehyde (PFA), embedded in paraffin, sectioned, and stained by the immunofluorescent procedure. Insulin (Cy2), glucagon (AMCA), and chemokine (Cy3) immunofluorescent reactivity are shown. Please note that a certain loss of insulin-staining intensity typically occurs as a result of the nature of our islet isolation and culture procedure; however, the integrity of β-cell function was verified in vivo as detailed in RESEARCH DESIGN AND METHODS. The figure is representative of two experiments performed with two different donor islets. INS/GCG, insulin/glucagon; MERGE, merged images. Scale bar: 15 μm. (A high-quality digital representation of this figure is available in the online issue.)
In situ chemokine protein expression in the murine RIP-GP model of virus-induced type 1 diabetes.
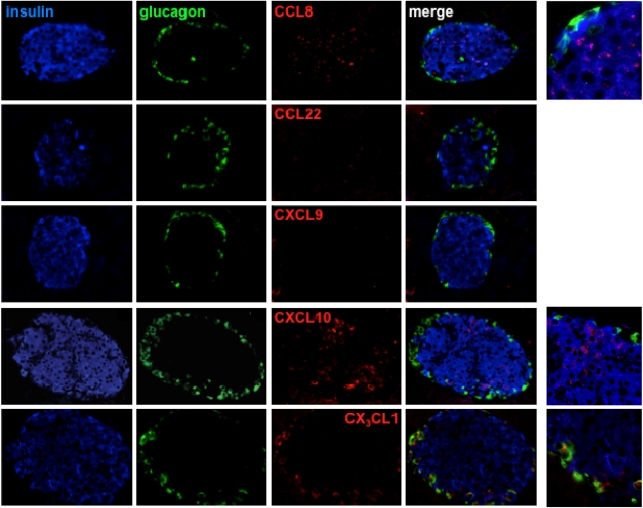
In an attempt to correlate our observations made in the human in vitro culture system with a suitable in vivo model, we used the RIP-GP model (i.e., transgenic mice that express the GP of LCMV under the control of the RIP specifically in pancreatic β-cells). In the absence of LCMV infection, these mice exhibit a normal phenotype, and none of the above chemokines, evaluated with specific antibodies characterized in detail elsewhere (26), were detected in the islets (Supplementary Fig. 4). However, after challenge with LCMV, these mice reliably develop type 1 diabetes within 2–3 weeks as a result of the generation of an LCMV-GP–specific CD8+T-cell response and progressive destruction of the transgene-expressing β-cells (27). In the prediabetic phase (day 7 after infection), we observed little, if any, expression of CCL5, CXCL9, and CCL22, although some CCL5 produced by α-cells was detectable at earlier time points (not shown). In contrast, β-cell–produced CCL8 and CXCL10, as well as α-cell–associated CX3CL1, were readily discernible in the RIP-GP islets (Fig. 4). The unexpected anatomic location of CX3CL1 is of particular interest because practically all LCMV-specific CD8+ effector T cells express the corresponding chemokine receptor (CX3CR1, not shown) and thus may account for the frequently observed positioning of T cells in the islet periphery (peri-islitis).
FIG. 4.
Chemokine expression in the RIP-GP model of virus-induced type 1 diabetes. RIP-GP mice were infected with LCMV, and their pancreata were harvested 7 days later and processed for immunohistological analysis as detailed in RESEARCH DESIGN AND METHODS. Note the minimal or absent expression of CCL22 and CXCL9, the preferential expression of CCL8 and CXCL10 by β-cells, as well as CX3CL1 production by α-cells; the right-hand column features magnified sections of merged CCL8, CXCL10, and CX3CL1 stains. (A high-quality digital representation of this figure is available in the online issue.)
Chemokine expression in the murine NOD model of spontaneous type 1 diabetes.
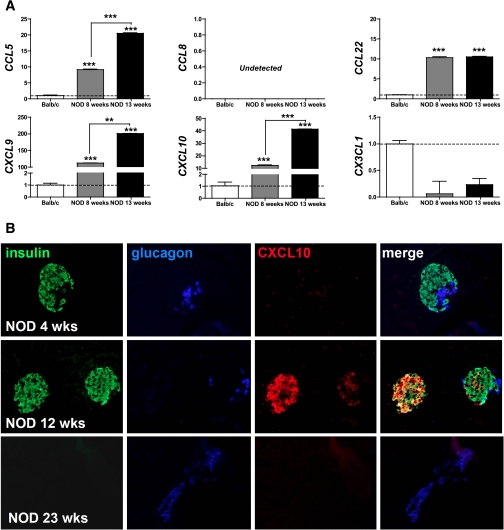
To provide an expanded in vivo context for chemokines produced in the course of type 1 diabetes development, we further examined chemokine mRNA and protein expression in the NOD mouse model. Here, the expression of Ccl5, Cxcl9/10, and Cx3cl1 mRNA species quantified in purified NOD islets increased in an age-dependent fashion, whereas Ccl22 message already was significantly elevated in young NOD compared with Balb/c control mice, and Ccl8 was not detected in any islet cell preparations (Fig. 5A). Similar to the RIP-GP model, CXCL10 was the most prominent islet-expressed chemokine (Fig. 5B and not shown): very weakly expressed in the pancreata of prediabetic NOD mice (4 weeks of age), β-cell–associated CXCL10 expression increased progressively up to ~12 weeks of age, subsequently declined, and became virtually undetectable by the age of 23 weeks.
FIG. 5.
Chemokine expression in the NOD model of spontaneous type 1 diabetes. A: Chemokine mRNA transcript expression was quantified in islets isolated from Balb/c (n = 6) as well as 8- and 13-week-old female NOD mice (n = 6) by qRT-PCR. Data were quantified using the 2–Δ Δ CT method expressed as means ± SD (n = 3) and normalized to housekeeping Hprt and Balb/c samples (calibrator). Relative chemokine mRNA expression is displayed in relation to Balb/c samples (dotted line); asterisks indicate significant differences between control and cytokine-treated islets. B: CXCL10 production by pancreatic β-cells as a function of female NOD age was determined as detailed in RESEARCH DESIGN AND METHODS. Note the weak CXCL10 staining in acinar tissues in 4-week-old NOD mice, preferential colocalization with insulin in 12-week-old NOD mice, and complete absence of CXCL10 at 23 weeks of age. (A high-quality digital representation of this figure is available in the online issue.)
In situ chemokine protein expression in human type 1 diabetes.
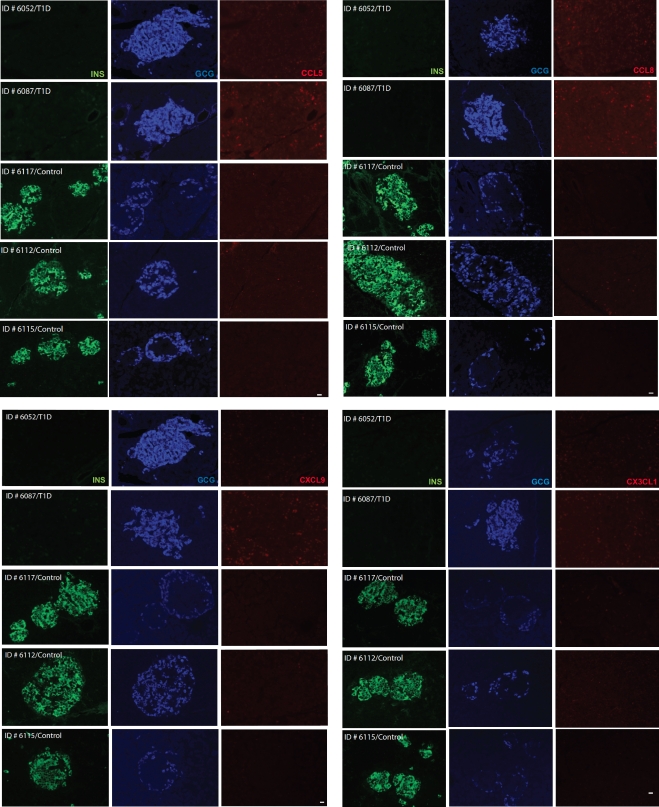
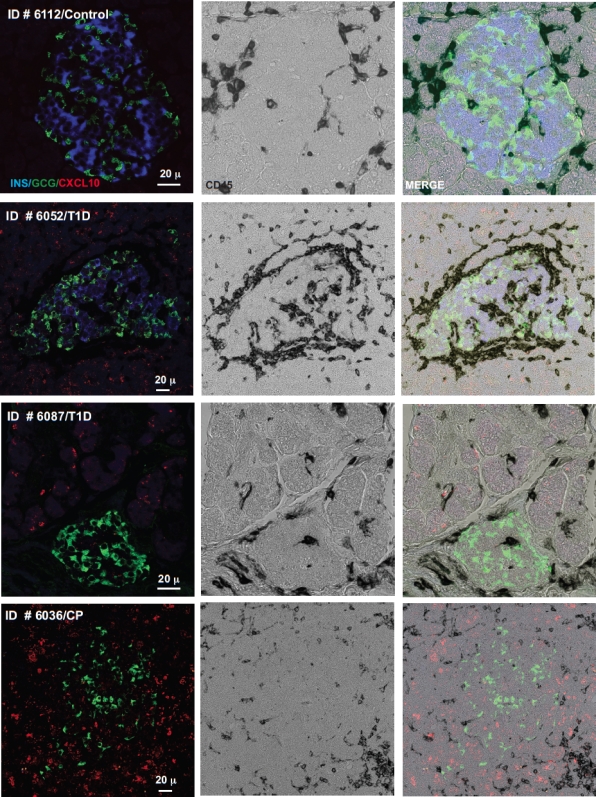
Collectively, the good correspondence between different experimental approaches (in vitro vs. in vivo), readouts (mRNA vs. protein), and species under investigation (man vs. mouse) indicates that chemokines may in fact be involved in the coordination of immune responses in the islet environment of type 1 diabetic patients. We therefore directly evaluated chemokine expression in the pancreata of diabetic tissue donors and healthy control subjects. Although no expression of CCL5, CCL8, and CXCL9 could be detected in healthy control subjects, weak staining was observed in the islets of diabetic donors and, unexpectedly, also to some extent in the surrounding acinar tissue (Fig. 6). In contrast, CX3CL1 only was marginally expressed in the pancreas of one diabetic donor (Fig. 6), and analyses of CCL22 expression were compromised by elevated nonspecific background staining (not shown). The above pattern of islet and acinar cell–associated chemokine expression, however, was most evident for CXCL10, and the inclusion of a sample from a type 1 diabetic patient with active pancreatitis demonstrating particularly pronounced CXCL10 expression in the exocrine pancreas further corroborates these staining patterns (Fig. 7). Thus, CXCL10 not only emerges as the most prominent chemokine induced in the pancreata of type 1 diabetic patients, but its expression in acinar cells also suggests the existence of an accompanying inflammation of the exocrine tissue, even in the absence of corresponding clinical evidence for acute pancreatitis.
FIG. 6.
Chemokine expression in type 1 diabetic (T1D) and healthy control (Control) pancreata. Pancreatic sections from healthy control subjects (case identification nos. 6117, 6112, and 6115) and type 1 diabetic donors (case identification nos. 6052 and 6087) were acquired through the nPOD program and stained for insulin, glucagon, and chemokines as detailed in RESEARCH DESIGN AND METHODS. Note the presence of some CCL5, CCL8, and CXCL9 in diabetic donors but their absence in healthy control samples. Only very faint CX3CL1 staining was observed in one of the type 1 diabetic samples (identification no. 6087). Scale bar: 20 μm. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 7.
In situ CXCL10 expression in type 1 diabetic (T1D) and healthy control (Control) pancreata. Combined insulin, glucagon, CD45, and CXCL10 stains were performed as detailed in RESEARCH DESIGN AND METHODS. Note the absence of CXCL10 staining in the healthy control subjects (case identification no. 6112) but ready presence in type 1 diabetic samples (identification nos. 6087 and 6052), sometimes in close association with infiltrating leukocytes (CD45+). To confirm the pattern of exocrine CXCL10 staining, we included a sample from an additional diabetic donor with clinically confirmed pancreatitis (identification no. 6036). (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
Type 1 diabetes is a progressive autoimmune disease that, according to most recent perspectives, is accompanied by a previously underappreciated pronounced inflammatory component (8). The historical description of multiple individual chemokines as mediators of generalized inflammatory alterations in this pathophysiological context is consistent with this notion, yet it also poses a notable challenge for the identification of specific chemokines and/or their receptors that may constitute suitable targets for the development of therapeutic interventions. Here, we have used an experimental approach that integrates in vitro studies of human islets, animal models, and pancreatic donor samples from recent-onset type 1 diabetic patients to delineate in situ chemokine protein expression patterns with the overreaching goal of focusing ongoing and future investigations on a defined group of pathogenetically relevant chemokines. Our observations may therefore assist in the selection of a defined group of chemokine/receptors for additional mechanistic and therapeutic studies.
The potential functional relevance of the six chemokines described here (CCL5/8/22, CXCL9/10, and CX3CL1) is in part supported by previous work: CCL5, which binds to the chemokine receptors CCR1/3/5 may contribute to type 1 diabetes development, as evidenced by reduced CCR5 expression in NOD mice protected from type 1 diabetes by IL-4 treatment (15) and the reduced disease incidence in NOD mice treated with a CCR5-blocking antibody (16). Nevertheless, genetic association studies have failed to establish a significant correlation between type 1 diabetes protection and a naturally occurring deletion of CCR5 (CCR5-Δ32) (34,35). Here, the promiscuous receptor binding of CCL5, as well as the use of CCR5 by several other chemokines (CCL3/4/8/12), likely contributes to the experimental challenges, and it is noteworthy that CCL8, identified in the current study and not previously associated with type 1 diabetes, binds to the same range of chemokine receptors as does CCL5 (CCR1/3/5). In addition, CCL8 binds CCR2, polymorphisms of which may contribute to type 1 diabetes susceptibility (35), and the major CCR2 ligand CCL2 causes spontaneous type 1 diabetes when transgenically expressed in β-cells of otherwise diabetes-resistant mice (14). A pathogenic role for CCL22 was postulated based on reduced insulitis and diabetes frequencies in NOD mice treated with a neutralizing αCCL22 antibody (17), yet more recent observations suggest that adenoviral-mediated CCL22 expression may actually prevent type 1 diabetes in NOD mice by recruitment of CCR4-bearing TREGs (36). In the RIP-GP model, we observed, at best, marginal CCL22 expression, and corresponding protein expression analysis in human pancreata remained inconclusive owing to technical difficulties. Thus, additional studies will be required to ascertain the precise relevance of CCL22 and its sole receptor, CCR4, in the pathogenesis of type 1 diabetes. The upregulation of CX3CL1 mRNA in cytokine-treated human islets as well as in rodent models has been noted previously (9,16), but to date no protein expression analyses have been reported in the specific context of type 1 diabetes. Our observation of CX3CL1 protein expression in human islet cultures, its peculiar association with α-cells in the RIP-GP model, and at least some expression in the pancreas of a type 1 diabetic donor together warrant additional investigation, in particular in light of the fact that the corresponding CX3CR1 receptor is highly expressed by subsets of monocytes (37) and diabetogenic CD8+ effector T cells in the RIP-GP model (not shown) as well as the unique therapeutic potential of targeting the CX3CL1/CX3CR1 axis in a variety of experimental settings (38). Finally, the emergence of CXCL10 as the most prominent chemokine in our comprehensive screen confirms its likely relevance in the natural history of type 1 diabetes and supports four very recent publications that have specifically interrogated islet-associated CXCL10 protein expression in “fulminant” as well as recent-onset type 1 diabetes (32,33,39,40).
The recent work on CXCL10, arguably the best-studied chemokine in the field of type 1 diabetes, also illustrates certain limitations and unresolved questions as to its mechanism of action and utility as a target for therapeutic interventions. Early work conducted in the RIP-GP model of virus-induced type 1 diabetes documented the initial protection of mice deficient for the CXCL9/10/11 receptor CXCR3, yet by 40 days after LCMV challenge, the study end point, ~50% of the CXCR3-deficient mice had become diabetic as defined by blood glucose levels of >33 mmol/L, a threshold value that is somewhat higher than the commonly used limit of 14–17 mmol/L for the diagnosis of type 1 diabetes in the RIP-LCMV model (18). Furthermore, very recent work with a pharmacological CXCR3 antagonist documented a rather limited impact on type 1 diabetes development in the same model (41), and β-cell–specific CXCL10 expression in the absence of concurrent inflammation promoted spontaneous insulitis but not overt type 1 diabetes (20). Thus, it seems that a fine-tuned balance between localized CXCL10 expression and accompanying inflammatory alterations determines the specific pathogenic relevance of CXCL10 and its utility as a drug target (5). The precise mechanism by which islet-associated CXCL10 contributes to type 1 diabetes development also remains a topic of debate: although the aforementioned studies provide evidence for the recruitment of CXCR3-bearing autoreactive CD8+ T cells (18–20), a contention that is also supported by the presence of islet-infiltrating CXCR3+ cells in the proximity of CXCL10-producing cells in the pancreata of human type 1 diabetic patients (32,33,39), other work suggests that partial type 1 diabetes protection as a result of CXCL10 neutralization or DNA vaccination primarily operates through enhanced proliferation of β-cells without affecting immune cell infiltration (21,22). As to the other CXCR3-binding chemokines, the rather weak (18), or practically absent (present study), expression of CXCL9 in the RIP-GP model, combined with the failure of CXCL9 blockade to prevent type 1 diabetes (19), would make this chemokine less attractive for additional study; however, we note the presence of some CXCL9 in the pancreata of type 1 diabetic patients that contrasts its complete absence in healthy control subjects. Collectively, our findings emphasize a prominent role for CXCL10 in human type 1 diabetes and further suggest that any optimized therapeutic targeting of the chemokine/receptor system likely will have to include additional chemokine/receptors, such as those discussed above.
The overall remarkable correspondence between different experimental systems, readouts, and species under investigation in our study also retrospectively validates the human islet culture system, and we have used this model system here to interrogate basic pathways of inflammation-induced chemokine mRNA regulation in human islet cells. The complexity of the β-cell responses, function, and fate following an immune attack or cytokine exposure is governed by diverse gene regulatory networks (42), and our previous work has emphasized the interconnected nature of the NF-κB, iNOS, and IDO pathways (24,25). While crosstalk between these signaling pathways and IL-1β/TNFα/IFNγ–induced synergy in human islets also was observed at the level of chemokine gene transcription, their regulation primarily was dependent on iNOS (IL-1β/TNFα induced) rather than NF-κB signaling cascades. Though unexpected, this observation is in accordance with recent data that CXCL10 transcription in human islets also can be downregulated with Janus kinase inhibitors (through IFNγ action) or higher doses of NF-κB inhibitors (43). In our hands, however, higher doses of NF-κB inhibitors also modulated gene expression of many housekeeping genes, including HPRT1 (not shown).
Finally, perhaps the most surprising observation made in the current study is the significant extent of exocrine pancreas inflammation, as evidenced by chemokine expression in the human type 1 diabetic samples. Again, CXCL10 dominated this overall expression pattern, but the presence of other chemokines, such as CCL5/8 and CXCL9 and, to a lesser degree, CX3CL1, further supported the impression of inflammatory alterations in the exocrine pancreas of type 1 diabetic donors. Although these findings are in line with the notion that inflammation constitutes a major component of type 1 diabetes (8,44), the precise relationship between type 1 diabetes and exocrine pancreas inflammation (pancreatitis) has received very little attention to date, notwithstanding the fact that diabetes can be caused under rare circumstances by chronic pancreatitis (45), fulminant type 1 diabetes is associated with some lymphocytic infiltrates in the exocrine pancreas (46–48), and autoimmune pancreatitis by now is a recognized pathophysiological entity (49). Both acute and chronic pancreatitis, however, also are characterized by an activation of the chemokine system (50,51), and the spectrum of chemokines induced in chronic pancreatitis (CCL2–5, CXCL9–11, and CX3CL1 [51,52]) indeed resembles the pancreatic chemokine signatures of type 1 diabetic patients reported here. Of interest, CXCL10 blockade in a model of murine leukemia virus–induced chronic autoimmune pancreatitis had some therapeutic effects because it reduced mononuclear cell infiltration and ameliorated pancreatic pathology (53). As for the “pancreatitic” alterations in type 1 diabetic patients, recent gene expression studies observed the preferential induction of chemokines and other inflammatory mediators in whole pancreata compared with purified islets obtained from the same type 1 diabetic donors and indeed suggest a concomitant inflammatory involvement of exocrine tissues, particularly in early-onset type 1 diabetes (54). Thus, the chemokine expression patterns in islets and exocrine tissue in the recent-onset type 1 diabetic samples evaluated here point toward common pathophysiological pathways that may jointly influence both the natural disease course of type 1 diabetes as well as the efficacy of immunomodulatory therapeutic regimens that target specific components of the chemokine/receptor system.
ACKNOWLEDGMENTS
This work was supported by Juvenile Diabetes Research Foundation (JDRF) Grants CDA-2-2007-240 and IG-5-2010-387 (to D.H.), K01-DK-080193 (to S.A.S.), the JDRF nPOD (to S.A.S. and D.H.), and Diabetes and Endocrinology Research Center Grant P30-DK-057516; the microarray core was supported in part by the Colorado Clinical and Translational Science Institute.
S.K.H. is an Associate Professor of Medicine at the University of Freiburg, Germany, as well as an employee of Merck/Merck, Sharp & Dohme (Leader Global Clinical Trial Operations Europe I). No other potential conflicts of interest relevant to this article were reported.
S.A.S. and D.H. researched data; wrote, reviewed, and edited the manuscript; and are the guarantors for this article. C.E.L., F.V., T.T.N., J.A.W., A.B., and J.E. researched data, contributed to the discussion, and reviewed and edited the manuscript. S.K.H. contributed to the discussion and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 2012 Annual Meeting of the nPOD, Miami, Florida, 15–17 January 2012.
The authors thank Drs. J.C. Hutton and G. Eisenbarth of the Barbara Davis Center for Childhood Diabetes for support with the preparation of the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0853/-/DC1.
REFERENCES
- 1.Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 2006;7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 2004;22:891–928 [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev 2003;195:58–71 [DOI] [PubMed] [Google Scholar]
- 4.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev 2007;28:492–520 [DOI] [PubMed] [Google Scholar]
- 5.Christen U. Chemokines as drug targets in type 1 diabetes. Endocr Metab Immune Disord Drug Targets 2007;7:7–12 [DOI] [PubMed] [Google Scholar]
- 6.Smyth DJ, Plagnol V, Walker NM, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med 2008;359:2767–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–467 [DOI] [PubMed] [Google Scholar]
- 8.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 9.Overbergh L, Gysemans C, Mathieu C. Quantification of chemokines by real-time reverse transcriptase PCR: applications in type 1 diabetes. Expert Rev Mol Diagn 2006;6:51–64 [DOI] [PubMed] [Google Scholar]
- 10.Shehadeh N, Pollack S, Wildbaum G, et al. Selective autoantibody production against CCL3 is associated with human type 1 diabetes mellitus and serves as a novel biomarker for its diagnosis. J Immunol 2009;182:8104–8109 [DOI] [PubMed] [Google Scholar]
- 11.Hanifi-Moghaddam P, Kappler S, Seissler J, et al. Altered chemokine levels in individuals at risk of type 1 diabetes mellitus. Diabet Med 2006;23:156–163 [DOI] [PubMed] [Google Scholar]
- 12.Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol 2007;179:5760–5767 [DOI] [PubMed] [Google Scholar]
- 13.Martin AP, Grisotto MG, Canasto-Chibuque C, et al. Islet expression of M3 uncovers a key role for chemokines in the development and recruitment of diabetogenic cells in NOD mice. Diabetes 2008;57:387–394 [DOI] [PubMed] [Google Scholar]
- 14.Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes 2008;57:3025–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cameron MJ, Arreaza GA, Grattan M, et al. Differential expression of CC chemokines and the CCR5 receptor in the pancreas is associated with progression to type I diabetes. J Immunol 2000;165:1102–1110 [DOI] [PubMed] [Google Scholar]
- 16.Carvalho-Pinto C, García MI, Gómez L, et al. Leukocyte attraction through the CCR5 receptor controls progress from insulitis to diabetes in non-obese diabetic mice. Eur J Immunol 2004;34:548–557 [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Cleary MM, Fox HS, Chantry D, Sarvetnick N. CCR4-bearing T cells participate in autoimmune diabetes. J Clin Invest 2002;110:1675–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frigerio S, Junt T, Lu B, et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med 2002;8:1414–1420 [DOI] [PubMed] [Google Scholar]
- 19.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol 2003;171:6838–6845 [DOI] [PubMed] [Google Scholar]
- 20.Rhode A, Pauza ME, Barral AM, et al. Islet-specific expression of CXCL10 causes spontaneous islet infiltration and accelerates diabetes development. J Immunol 2005;175:3516–3524 [DOI] [PubMed] [Google Scholar]
- 21.Morimoto J, Yoneyama H, Shimada A, et al. CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis. J Immunol 2004;173:7017–7024 [DOI] [PubMed] [Google Scholar]
- 22.Shigihara T, Shimada A, Oikawa Y, et al. CXCL10 DNA vaccination prevents spontaneous diabetes through enhanced beta cell proliferation in NOD mice. J Immunol 2005;175:8401–8408 [DOI] [PubMed] [Google Scholar]
- 23.Shigihara T, Oikawa Y, Kanazawa Y, et al. Significance of serum CXCL10/IP-10 level in type 1 diabetes. J Autoimmun 2006;26:66–71 [DOI] [PubMed] [Google Scholar]
- 24.Sarkar SA, Wong R, Hackl SI, et al. Induction of indoleamine 2,3-dioxygenase by interferon-γ in human islets. Diabetes 2007;56:72–79 [DOI] [PubMed] [Google Scholar]
- 25.Sarkar SA, Kutlu B, Velmurugan K, et al. Cytokine-mediated induction of anti-apoptotic genes that are linked to nuclear factor kappa-B (NF-kappaB) signalling in human islets and in a mouse beta cell line. Diabetologia 2009;52:1092–1101 [DOI] [PubMed] [Google Scholar]
- 26.Eberlein J, Nguyen TT, Victorino F, Golden-Mason L, Rosen HR, Homann D. Comprehensive assessment of chemokine expression profiles by flow cytometry. J Clin Invest 2010;120:907–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homann D, Jahreis A, Wolfe T, et al. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity 2002;16:403–415 [DOI] [PubMed] [Google Scholar]
- 28.Homann D, Tishon A, Berger DP, Weigle WO, von Herrath MG, Oldstone MB. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J Virol 1998;72:9208–9216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierro F, Biben C, Martínez-Muñoz L, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA 2007;104:14759–14764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortis F, Pirot P, Naamane N, et al. Induction of nuclear factor-kappaB and its downstream genes by TNF-alpha and IL-1beta has a pro-apoptotic role in pancreatic beta cells. Diabetologia 2008;51:1213–1225 [DOI] [PubMed] [Google Scholar]
- 31.Li F, Mahato RI. iNOS gene silencing prevents inflammatory cytokine-induced beta-cell apoptosis. Mol Pharm 2008;5:407–417 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka S, Nishida Y, Aida K, et al. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated β-cell failure in fulminant type 1 diabetes. Diabetes 2009;58:2285–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uno S, Imagawa A, Saisho K, et al. Expression of chemokines, CXC chemokine ligand 10 (CXCL10) and CXCR3 in the inflamed islets of patients with recent-onset autoimmune type 1 diabetes. Endocr J 2010;57:991–996 [DOI] [PubMed] [Google Scholar]
- 34.Buhler MM, Craig M, Donaghue KC, et al. CCR5 genotyping in an Australian and New Zealand type 1 diabetes cohort. Autoimmunity 2002;35:457–461 [DOI] [PubMed] [Google Scholar]
- 35.Szalai C, Császár A, Czinner A, et al. Chemokine receptor CCR2 and CCR5 polymorphisms in children with insulin-dependent diabetes mellitus. Pediatr Res 1999;46:82–84 [DOI] [PubMed] [Google Scholar]
- 36.Montane J, Bischoff L, Soukhatcheva G, et al. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J Clin Invest 2011;121:3024–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82 [DOI] [PubMed] [Google Scholar]
- 38.D’Haese JG, Demir IE, Friess H, Ceyhan GO. Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets 2010;14:207–219 [DOI] [PubMed] [Google Scholar]
- 39.Roep BO, Kleijwegt FS, van Halteren AG, et al. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol 2010;159:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulthess FT, Paroni F, Sauter NS, et al. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab 2009;9:125–139 [DOI] [PubMed] [Google Scholar]
- 41.Christen S, Holdener M, Beerli C, et al. Small molecule CXCR3 antagonist NIBR2130 has only a limited impact on type 1 diabetes in a virus-induced mouse model. Clin Exp Immunol 2011;165:318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eizirik DL, Moore F, Flamez D, Ortis F. Use of a systems biology approach to understand pancreatic beta-cell death in Type 1 diabetes. Biochem Soc Trans 2008;36:321–327 [DOI] [PubMed] [Google Scholar]
- 43.Pugazhenthi U, Velmurugan K, Tran A, Mahaffey G, Pugazhenthi S. Anti-inflammatory action of exendin-4 in human islets is enhanced by phosphodiesterase inhibitors: potential therapeutic benefits in diabetic patients. Diabetologia 2010;53:2357–2368 [DOI] [PubMed] [Google Scholar]
- 44.Foxman EF, Iwasaki A. Genome-virome interactions: examining the role of common viral infections in complex disease. Nat Rev Microbiol 2011;9:254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelopoulos N, Dervenis C, Goula A, et al. Endocrine pancreatic insufficiency in chronic pancreatitis. Pancreatology 2005;5:122–131 [DOI] [PubMed] [Google Scholar]
- 46.Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y; Osaka IDDM Study Group A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med 2000;342:301–307 [DOI] [PubMed] [Google Scholar]
- 47.Tanaka S, Kobayashi T, Momotsu T. A novel subtype of type 1 diabetes mellitus. N Engl J Med 2000;342:1835–1837 [PubMed] [Google Scholar]
- 48.Aida K, Nishida Y, Tanaka S, et al. RIG-I- and MDA5-initiated innate immunity linked with adaptive immunity accelerates β-cell death in fulminant type 1 diabetes. Diabetes 2011;60:884–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lara LP, Chari ST. Autoimmune pancreatitis. Curr Gastroenterol Rep 2005;7:101–106 [DOI] [PubMed] [Google Scholar]
- 50.Shanmugam MK, Bhatia M. The role of pro-inflammatory molecules and pharmacological agents in acute pancreatitis and sepsis. Inflamm Allergy Drug Targets 2010;9:20–31 [DOI] [PubMed] [Google Scholar]
- 51.Ito T. Can measurement of chemokines become useful biological and functional markers of early-stage chronic pancreatitis? J Gastroenterol 2007;42(Suppl. 17):72–77 [DOI] [PubMed] [Google Scholar]
- 52.Singh L, Bakshi DK, Majumdar S, Vasishta RK, Arora SK, Wig JD. Expression of interferon-gamma- inducible protein-10 and its receptor CXCR3 in chronic pancreatitis. Pancreatology 2007;7:479–490 [DOI] [PubMed] [Google Scholar]
- 53.Kawauchi Y, Suzuki K, Watanabe S, et al. Role of IP-10/CXCL10 in the progression of pancreatitis-like injury in mice after murine retroviral infection. Am J Physiol Gastrointest Liver Physiol 2006;291:G345–G354 [DOI] [PubMed] [Google Scholar]
- 54.Planas R, Pujol-Borrell R, Vives-Pi M. Global gene expression changes in type 1 diabetes: insights into autoimmune response in the target organ and in the periphery. Immunol Lett 2010;133:55–61 [DOI] [PubMed] [Google Scholar]