Abstract
Myogenic and angiotensin contractions of afferent arterioles generate reactive oxygen species. Resistance vessels express NOX-2 and -4. Angiotensin II activates p47phox/NOX-2 whereas it downregulates NOX-4. Therefore, we tested the hypothesis that p47phox enhances afferent arteriolar angiotensin contractions. Angiotensin II infusion in p47phox +/+, but not -/- mice, increased renal cortical nicotinamide adenine dinucleotide phosphate oxidase activity (7±1 to 12±1; P<0.01 vs 5±1 to 7±1; NS, 103 · RLU · min-1 · μg protein-1), mean arterial pressure (77±2 to 91±2; P<0.005 vs 74±2 to 77±1; NS, mmHg) and renal vascular resistance (7.5±0.4 to 10.1±0.7; P<0.01 vs 7.9±0.4 to 8.3±0.4 NS, mmHg/ml·min-1·gkwt-1). Afferent arterioles from p47phox -/- mice had a lesser myogenic response (3.1±0.4 vs 1.4±0.2 dynes·cm-1·mmHg-1; P<0.02) and a lesser (P<0.05) contraction to 10-6M angiotensin II (diameter change +/+: 9.3±0.2 to 3.4±0.6 μm vs -/-: 9.9±0.6 to 7.5±0.4 μm). Angiotensin and increased perfusion pressure generated significantly (P<0.05) more reactive oxygen species in p47phox +/+ than -/- arterioles. Angiotensin II infusion increased the maximum responsiveness of afferent arterioles from p47phox +/+ mice to 10-6 M angiotensin II yet decreased the response in p47phox -/- mice. The angiotensin infusion increased the sensitivity to angiotensin II only in p47phox +/+ mice. We conclude that p47phox is required to enhance renal nicotinamide adenine dinucleotide phosphate oxidase activity and basal afferent arteriolar myogenic and angiotensin II contractions and to switch afferent arteriolar tachyphylaxis to sensitization to angiotensin during a prolonged angiotensin infusion. These effects likely contribute to hypertension and renal vasoconstriction during infusion of angiotensin II.
Keywords: Nicotine adenine dinucleotide phosphate (NADPH) oxidase, hypertension, oxidative stress, reactive oxygen species (ROS)
INTRODUCTION
Blockade of angiotensin II (Ang II) reduced the blood pressure (BP) and renal vascular resistance (RVR) in many models of hypertension 1 and in humans with essential or renovascular hypertension 2. Afferent arteriolar contractions with Ang II increase the RVR and reduce the transmission of arterial pressure into the kidney, which may protect the glomeruli from potential barotrauma 3.
Ang II increases reactive oxygen species (ROS) in the afferent arteriole 4-6 and the kidney 7,8 which increase the vascular contractility and the renal vascular resistance (RVR) 4-6,9-18. ROS are implicated in Ang II responses since tempol 18 reduced the hypertension and the renal vasoconstriction of mice infused with Ang II at a slow pressor rate 7 which is considered a model of human essential hypertension 19.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase has been implicated in Ang II-induced increases in ROS 12,15,16,20,21. However, NADPH oxidase is a complex enzyme and at least two neutrophil oxidases (NOX-2 and -4) are expressed in rodent microvessels with different regulation 8,9,12,20 and activation by cytosolic subunits 20. NOX-2 is a prominent oxidase in small blood vessels and glomeruli where it interacts with p22phox in the membrane and p47phox, p67phox, p40phox and Rac2 from the cytosol 12. Ang II reduced the expression of NOX-4 8 but increased the afferent arteriolar mRNA expression for p22phox 4 and increased the vascular membrane association 12,22-24 and c-Src-dependent phosphorylation and activation of p47phox 13, which assembled with p22phox, NOX-2 and other cytosolic subunits to form a functional membrane oxidase 12. Whereas knockout of p47phox -/- attenuated the increase in ROS 9-11 and rate of rise of BP 14,25 with pressor infusions of Ang II and attenuated large vessel myogenic responses 14, prolonged Ang II infusion at a slow pressor rate did not change p47phox expression in the kidney 8,26 and p47phox -/- mice had a maintained 25, or even increased 9,10,14,27, basal level of superoxide (O2.-) generation in blood vessels, kidneys and vascular smooth muscle cells (VSMCs). Moreover, p47phox -/- mice had a normal basal BP 11,14,25,27. Thus, the role of p47phox in basal and Ang II-stimulated regulation of BP and RVR is not completely understood. We tested the hypothesis that p47phox is required for full afferent arteriolar contractions to Ang II and perfusion pressure (myogenic responses) and for increases in mean arterial pressure (MAP) and RVR in mice receiving a slow pressor (low dose) infusion of Ang II by contrasting responses in p47phox +/+ vs -/- mice.
Experimental design
Male p47phox +/+ and -/- littermate mice aged 10-14 weeks were bred from ± founders and backcrossed at least 8 times to the C57BL/6 background 14. Since these mice are prone to infection, we followed the advice of our veterinarians that they be individually housed with precautions to minimize infection and with trimethoprim/sulfamethoxazole added to the drinking water for 7 days, followed by 3 days without antibiotics, as in a prior study 27. They were fed a normal mouse chow with a regular salt content of 0.4%.
Groups of p47phox+/+ and -/- mice (n=6-7) were anesthetized with isofluorane (1-2% in O2) 2 weeks before experiments. Radiotelemeters were inserted into a carotid artery 26. Basal recordings of MAP were made for 4 days after which mice were anesthetized with isofluorane for insertion of osmotic minipumps (Direct Corp, Glenn) to deliver Ang II (400 ng-1·kg-1·min-1) or vehicle (V) subcutaneously for 2 weeks 7,28. Thereafter, the mice were sacrificed and the kidney cortex was harvested. Cell membranes were separated to measure membrane-bound NADPH oxidase activity from lucigenin-enhanced chemiluminescence after addition of 200 μmol · l-1 of NADPH, as described 26. Other mice (n=5-7/group) were anesthetized with thiobarbital (Inactin 50 mg-1·kg-1) and ketamine (40 mg-1·kg-1) and prepared for renal clearance studies 26 12-14 days after Ang II or vehicle. The glomerular filtration rate (GFR) was the clearance of [3H]-inulin and the renal plasma flow was the clearance [14C]-paraaminohippurate (PAH) corrected for renal extraction. Renal blood flow (RBF) was RPF factored by 1-hematocrit and RVR was MAP factored by RBF.
Afferent arteriolar responses
After 12-14 days of Ang II or vehicle infusions, mice were anesthetized, the kidneys removed, and an afferent arteriole dissected and perfused 6. Contractions to bath addition of Ang II (10-12 to 10-6 M) were recorded. The myogenic responses were studied in other arterioles during ≈ 20 mmHg increases in renal perfusion pressure 29,30. The slope of active wall tension (difference between Ca2+-free and physiologic solution) against perfusion pressure defined the myogenic response. Only one arteriole was used per animal. The inner luminal diameter and medial thickness were measured at 60 mmHg perfusion pressure to compute the media: lumen ratio. The ROS generated in the afferent arterioles during incubation with 10-6 M Ang II or during increases in renal perfusion pressure from 40 to 80 mmHg was assessed from the ratio of ethidium to dihydroethidium fluorescence, as described and validated 6,30.
Statistical analysis
Values are presented as mean +/- SEM. Repeated measures ANOVA was used to test concentration-dependent changes in MAP or arteriolar diameter. A 2×2 ANOVA with interaction was used to assess the effects of genotype, Ang II and effects of genotype on the response to Ang II (interaction). Post hoc comparisons were performed using a Fisher test. Differences were considered to be statistically significant if P<0.05.
Ethics
The experiments were approved by the Georgetown University Animal Care and Use Committee. They conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals.
RESULTS
The mice were healthy and had similar body and kidney weight (supplement table S1; please see http://hyper.ahajournals.org). Afferent arterioles from p47phox +/+ mice had readily detectable levels of p47phox mRNA whereas it was absent from arterioles of p47phox -/- mice (supplement figure S1; please see http://hyper.ahajournals.org)
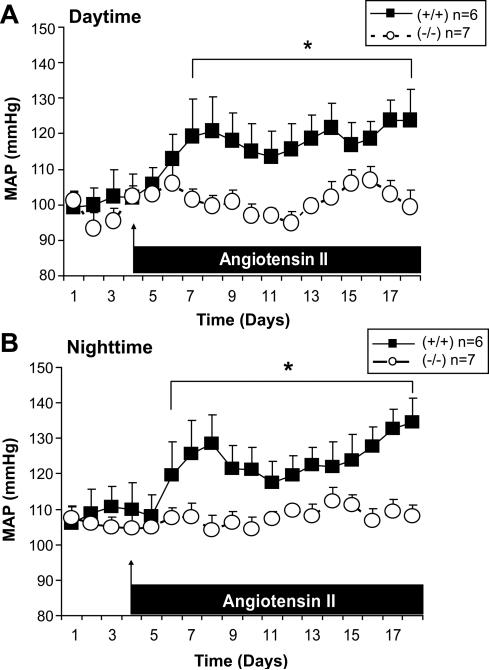
The basal levels of telemetric MAP were not different between strains whereas the MAP measured during the daytime (Figure 1, panel A) or nighttime (Figure 1. panel B) increased after 2-3 days of Ang II infusion in p47phox +/+ mice, but did not change with Ang II in p47phox -/- mice.
Figure 1.
Mean ± SEM values for telemetric mean arterial pressure of p47phox +/+ (solid squares and continuous lines; n = 6) and p47phox -/-(open circles and broken lines n = 7) mice before and during angiotensin II (400 ng · kg-1 · min-1 · sc) during the daytime (asleep) in panel A and the nighttime (awake) in panel B. Comparing groups: *, P<0.05.
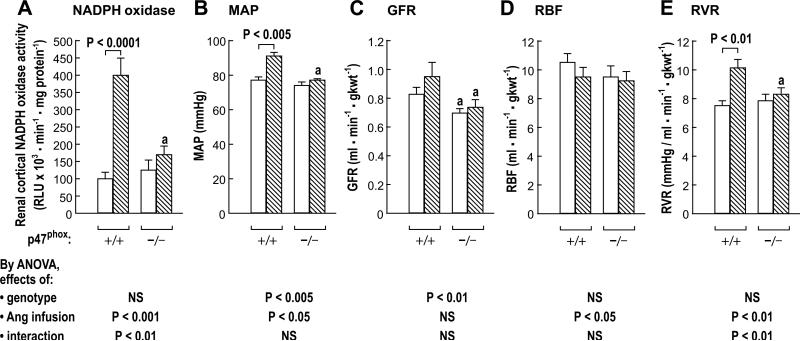
The basal values of NADPH oxidase, MAP, RBF and RVR of vehicle-infused mice under anesthesia were similar in the two strains (Figure 2) but the GFR was lower in p47phox -/- mice (P<0.05). Two weeks of Ang II infusion in p47phox +/+ mice increased the NADPH oxidase (100±5 to 400±50; P<0.0001), the MAP (77±2 to 91±2 mmHg; P<0.005) and the RVR (7.5±0.4 to 10.1±0.7 mmHg/ml·min-1·gkwt-1; P<0.01). However, Ang II infusion in p47phox -/- mice did not change these variables.
Figure 2.
Mean ± SEM values (n = 6-9 per group) for mice studied under anesthesia showing renal cortical NADPH oxidase activity (panel A), arterial pressure (panel B), glomerular filtration rate (panel C), renal blood flow (panel D), and renal vascular resistance (panel E) in groups of p47phox +/+ and -/- mice given a vehicle (open boxes) or Ang II (400 · kg-1 · min-1 sc) for 12 days. Compared to equivalent p47phox +/+ mice: a, P<0.05.
The basal afferent arteriolar diameter and the media: lumen ratio were similar in both strains and were unaffected by Ang II infusion (supplement table S2a; please see http://hyper.ahajournals.org).
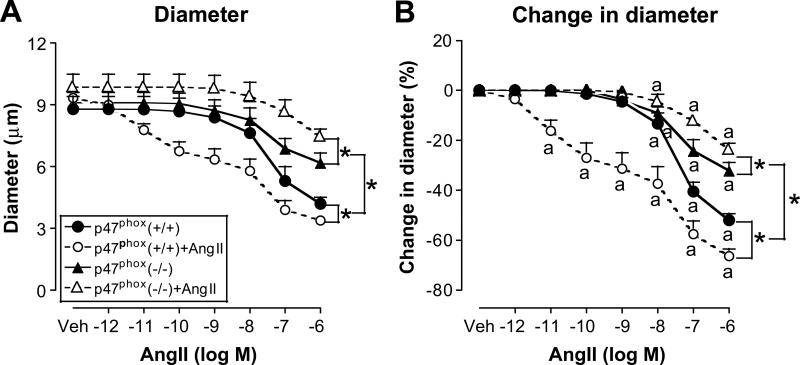
Afferent arterioles isolated from vehicle-infused mice of both strains had graded contractions with Ang II (Figure 3). After vehicle infusion, afferent arterioles from p47phox -/- mice, compared to p47phox +/+ mice, had a 40% reduced maximum response (supplement table 2b; please see http://hyper.ahajournals.org). Two weeks of Ang II infusion in p47phox +/+ mice increased the Ang II sensitivity, whether assessed from the lowest Ang II concentration to cause a contraction (from 10-8 M during vehicle to 10-11 M with Ang II infusion) (Figure 3B) or from the ED50 (supplement table S2b; please see http://hyper.ahajournals.org). In contrast, two weeks of Ang II infusion in p47phox -/- mice actually reduced the maximum response to 10-6 M Ang II (Figure 3 and supplement table 2b; please see http://hyper.ahajournals.org) without changing the Ang II sensitivity.
Figure 3.
Mean ± SEM values (n = 4 - 7) for afferent arteriolar diameter (Panel A) and changes in diameter from baseline (Panel B) during bath addition of angiotensin II to p47phox +/+ mice infused with vehicle (solid circles and continuous lines) or angiotensin II (open circles and broken lines) or p47phox -/- mice infused with vehicle (solid triangles and continuous lines) or angiotensin II (open triangles and broken lines). Significance of difference between groups: * P<0.05. Significance of difference from vehicle: a, P<0.05.
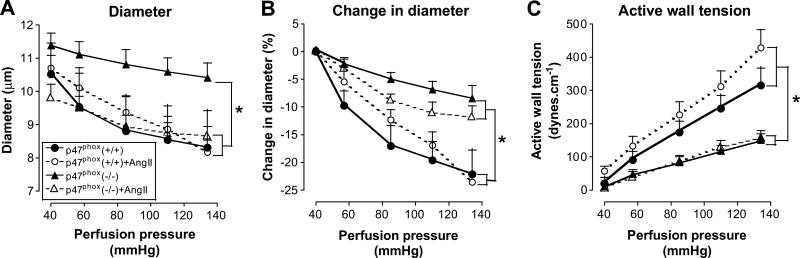
An increase in perfusion pressure of afferent arterioles above 40mmHg from vehicle-infused p47phox +/+ mice led to a graded contraction (Figure 4A and B). There were no changes in passive wall tension assessed in the absence of calcium but an increase in active wall tension (Figure 4C). This myogenic response was reduced by 48% in arterioles from p47phox -/- mice (supplement table S2a; please see http://hyper.ahajournals.org). Ang II infusion did not change myogenic responses is either strain.
Figure 4.
Mean ± SEM values (n= 4 - 7) for afferent arteriolar diameter (panel A), change in diameter (panel B) and active wall tension (panel C) during increases in renal perfusion pressure. Data are shown for vessels from p47phox +/+ mice infused with vehicle (solid circles and continuous lines) or angiotensin II (open circles and broken lines) or p47phox -/- mice infused with vehicle (solid triangles and continuous lines) or angiotensin II (open triangles and broken lines). Significance of difference between groups: * P<0.05.
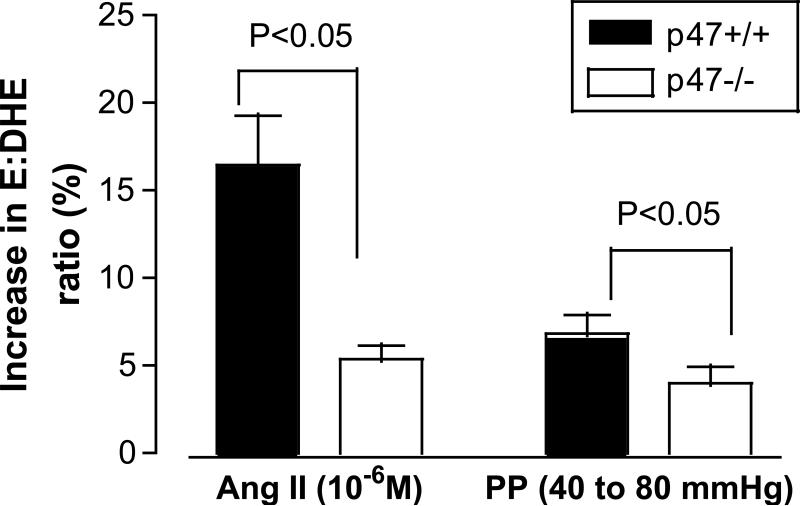
Ang II (10-6 M) or increasing perfusion pressure (40 to 80 mmHg) both increased afferent arteriolar ROS more in afferent arterioles from p47phox +/+ than -/- mice (Figure 5).
Figure 5.
Mean ± SEM values (n= 5 to 7) for increases in ethidium: dihydroethidium ratio of afferent arterioles from p47phox +/+ mice (solid black) and p47phox -/- mice (open box). Data are shown for changes with 10-6 M angiotensin II or increased perfusion from 40-80 mmHg.
DISCUSSION
The main new findings were the p47phox -/- mice had a normal basal renal cortical NADPH oxidase activity, MAP and RVR under anesthesia and a normal conscious MAP during the daytime or nighttime but a lower GFR. However, unlike the p47phox +/+ mice, the NADPH oxidase activity, MAP and RVR of p47phox -/- mice failed to increase during a slow pressor infusion of Ang II. Afferent arterioles from p47phox -/- mice had a 38% reduced maximum responsiveness to Ang II and a 48% reduced myogenic response. Ang II and increased perfusion pressure increased afferent arteriolar ROS, but these responses were reduced in p47phox -/- mice. Whereas Ang II infusion enhanced the maximum Ang II responsiveness of afferent arterioles from p47phox +/+ mice by 26%, and enhanced their sensitivity 1,000-fold, Ang II infusion in p47phox -/- mice actually reduced the maximum response of their arterioles to Ang II by 26% and did not change their sensitivity.
The normal basal NADPH oxidase activity in the kidney cortex from p47phox -/- mice confirms a prior study 27. Basal O2.- generation from cultured VSMCs 9,11 and from aortas of p47phox -/- mice has been unchanged 14 or increased 10,27. Apparently, p47phox is not required for basal O2.- generation. The source of the p47phox-independent basal O2.- generation in this study was not established but likely was from the NOX-4 component of NADPH oxidase since it does not require p47phox for activity, and in VSMCs, NOX-4 is the major source of basal ROS but does not contribute to Ang II-stimulated ROS generation 31.
Grote et al reported a higher basal MAP in young p47phox -/- mice that they attributed to an enhanced aortic angiotensin converting enzyme (ACE) activity, although ACE gene expression in the kidney was unchanged 27. However, the absence of any rise in MAP with Ang II infusion in the p47phox -/- mice in our study suggests that excessive Ang II generation by ACE likely did not account for altered responses of p47phox -/- mice in our study. Moreover, we did not confirm the difference in basal MAP between p47phox +/+ and -/- mice.
Unexpectedly, the p47phox -/- mice had lower GFRs in the basal state and during Ang II infusion. This contrasts with the effects of tempol to increase the GFR of mice during a slow pressor infusion of Ang II 7. Since both tempol 7 and p47phox knockout prevented Ang II-induced oxidative stress, it suggests that the effects of p47phox -/- on the GFR may be dissociated from its antioxidant actions. We detected prominent expression of p47phox in the podocytes of rats 32 where it could regulate the GFR although the mechanism is unclear. There were no differences in basal MAP between p47phox +/+ and -/- to explain the lower basal GFR in the p47phox -/- mice.
Previous studies in dogs and rats have reported that Ang II infusion did not change 33, or diminish, the myogenic components of renal autoregualtion 34-37. This is the first direct study in isolated afferent arterioles. We detected no effect of two weeks of Ang II infusion on myogenic responses. This difference from the prior studies may relate to interactions between myogenic responses and tubuloglomerular feedback (TGF) that were conducted in whole kidneys since TGF responses are enhanced by Ang II infusion 38 and this can impair myogenic responses 39. The present study removed any confounding effects of TGF by studying isolated arterioles.
Our finding that Ang II infusion increased the Ang II responsiveness of afferent arterioles from p47phox +/+ mice confirms prior studies in rabbits 4 where the enhanced response was accompanied by a significant increase in the mRNA expression for p22phox, but a significant reduction in mRNA expression for ATI-receptors, in the afferent arterioles. This suggests that enhanced responsiveness to Ang II in afferent arterioles of rodents infused with Ang II is due to activation of NADPH oxidase by p22phox and phosphorylated p47phox that enhances ROS generation. In the absence of this enhancing effect of ROS, the effects of Ang II to downregulate AT1-receptors may become apparent as tachyphylaxis and an impaired response to Ang II.
Since oxidative stress determined BP in many rodent models 18, our findings of an unchanged basal level of NADPH oxidase activity in the kidneys may explain the unchanged basal MAP and RVR in p47phox -/- mice. Ang II infusion increased the excretion of lipid peroxidation products 7, ROS generation in kidneys 7 and afferent arterioles of normal mice 6. Indeed, NADPH oxidase is a major source of O2.- in VSMCs 15, kidneys of rats infused with Ang II at a slow pressor rate 8 and the NADPH oxidase activity increased four-fold with Ang II infusion in p47phox +/+ mice in this study. Since the NADPH oxidase activity was unchanged in the renal cortex of p47phox -/- mice infused with Ang II, we concluded that p47phox was required for NADPH oxidase activation in the kidneys by Ang II. This extends prior reports of a reduced generation of O2.- in VSMCs or aortas from p47phox -/- mice during stimulation with arachidonic acid 27, Ang II 10,11,14,25, platelet derived growth factor or phorbol myristate acetate 11. In another study, p47phox -/- mice had a preserved, albeit delayed, increase in MAP in response to a direct pressor infusion of Ang II 14. This suggests that O2.- derived from p47phox-dependent NADPH oxidase sets the sensitivity of the BP response to Ang II, whereas other actions of Ang II contribute to its maximal response. This is consistent with the substantial increase in sensitivity of afferent arterioles from p47phox +/+ mice to Ang II produced by an Ang II infusion, which was entirely lacking in those from p47phox -/- mice.
Perspectives
Studies from gene deleted mice have highlighted the importance of ROS in the regulation of BP. SOD-1 -/- mice had an enhanced slow pressor response to Ang II, their afferent arterioles generated more ROS and they had a 10,000–fold increased sensitivity to Ang II 6. The present studies demonstrated the essential role of p47phox in O2.- generation and in the hypertension and renal vasoconstriction with a low dose infusion of Ang II. Oxidative stress underlies many adverse cardiovascular and renal effects of Ang II 17,18,40. Renal vasoconstriction is implicated in the development of hypertension 41. Thus, p47phox is an attractive target to prevent hypertension and some of its complications.
Supplementary Material
Acknowledgements
We thank Ms. Glenda Baker for preparing and editing the manuscript.
Sources of Funding:
This study was supported by research grants to Christopher S. Wilcox and William J. Welch from NIDDK (DK-036079 and DK-049870) and to Christopher S. Wilcox, William J. Welch, Anton Wellstein, and Kathryn Sandberg from the NHLBI (HL-68686) and by funds from the George E. Schreiner Chair of Nephrology.
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hall JE, Mizelle HL, Woods LL. The renin-angiotensin system and long-term regulation of arterial pressure. J Hypertens. 1986;4:387–397. doi: 10.1097/00004872-198608000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Hollenberg NK. ACE Inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, and renin antagonists. In: Wilcox CS, editor. Therapy in Nephrology and Hypertension. 3rd ed. Saunders / Elsevier Inc.; Philadelphia, PA: 2008. pp. 601–609. [Google Scholar]
- 3.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertens. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego L, Umans J, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to Ang II of afferent arterioles from rabbits infused with Ang II. J Am Soc Nephrol. 2003;14:2783–2789. doi: 10.1097/01.asn.0000090747.59919.d2. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane-prostanoid receptors and endothelium. Circ Res. 2004;94:1436–1442. doi: 10.1161/01.RES.0000129578.76799.75. [DOI] [PubMed] [Google Scholar]
- 6.Carlstrom M, Lai EY, Ma Z, Steege A, Patzak A, Eriksson UJ, Lundberg JO, Wilcox CS, Persson AE. Superoxide dismutase 1 limits renal microvascular remodeling and attenuates arteriole and blood pressure responses to angiotensin II via modulation of nitric oxide bioavailability. Hypertens. 2010;56:907–913. doi: 10.1161/HYPERTENSIONAHA.110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 8.Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of Ang II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase and SOD expression. Am J Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- 9.Chose O, Sansilvestri-Morel P, Badier-Commander C, Bernhardt F, Fabiani JN, Rupin A, Verbeuren TJ. Distinct role of nox1, nox2, and p47phox in unstimulated versus angiotensin II-induced NADPH oxidase activity in human venous smooth muscle cells. J Cardiovasc Pharmacol. 2008;51:131–139. doi: 10.1097/FJC.0b013e31815d781d. [DOI] [PubMed] [Google Scholar]
- 10.Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM. Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2- production, vascular tone, and mitogen-activated protein kinase activation. Circ. 2004;109:1307–1313. doi: 10.1161/01.CIR.0000118463.23388.B9. [DOI] [PubMed] [Google Scholar]
- 11.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circ. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 14.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertens. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 16.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circ. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharm Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lever AF. The fast and slow developing pressor effect of angiotensin II. In: Robertson JIS, Nicholls MG, editors. The Renin-Angiotensin System. Gower Medical Publishing; London, U.K.: 1993. pp. 28.1–28.9. [Google Scholar]
- 20.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxidants and Redox Signaling. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 21.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertens. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 22.Papparella I, Ceolotto G, Lenzini L, Mazzoni M, Franco L, Sartori M, Ciccariello L, Semplicini A. Angiotensin II-induced over-activation of p47phox in fibroblasts from hypertensives: which role in the enhanced ERK1/2 responsiveness to angiotensin II? J Hypertens. 2005;23:793–800. doi: 10.1097/01.hjh.0000163148.97459.9d. [DOI] [PubMed] [Google Scholar]
- 23.Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun. 2005;328:183–188. doi: 10.1016/j.bbrc.2004.12.152. [DOI] [PubMed] [Google Scholar]
- 24.Ebrahimian T, Li MW, Lemarie CA, Simeone SM, Pagano PJ, Gaestel M, Paradis P, Wassmann S, Schiffrin EL. Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin II-induced inflammation and hypertension. regulation of oxidative stress. Hypertens. 2011;57:245–254. doi: 10.1161/HYPERTENSIONAHA.110.159889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr., Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circ. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welch WJ, Chabrashvili T, Solis G, Chen Y, Gill P, Aslam S, Wang X, Ji H, Sandberg K, Jose P, Wilcox CS. Role of extracellular superoxide dismutase in the mouse angiotensin slow pressor response. Hypertens. 2006;48:934–941. doi: 10.1161/01.HYP.0000242928.57344.92. [DOI] [PubMed] [Google Scholar]
- 27.Grote K, Ortmann M, Salguero G, Doerries C, Landmesser U, Luchtefeld M, Brandes RP, Gwinner W, Tschernig T, Brabant EG, Klos A, Schaefer A, Drexler H, Schieffer B. Critical role for p47phox in renin-angiotensin system activation and blood pressure regulation. Cardiovasc Res. 2006;71:596–605. doi: 10.1016/j.cardiores.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Kawada N, Solis G, Ivey N, Connors S, Dennehy K, Modlinger P, Hamel R, Kawada JT, Imai E, Langenbach R, Welch W, Wilcox CS. Cyclooxygenase-1-deficient mice have high sleep-to-wake blood pressure ratios and renal vasoconstriction. Hypertens. 2005;45:1131–1138. doi: 10.1161/01.HYP.0000166141.69081.80. [DOI] [PubMed] [Google Scholar]
- 29.Lai E, Onozato ML, Solis G, Aslam S, Welch WJ, Wilcox CS. Myogenic responses of mouse isolated perfused renal afferent arterioles: effects of salt intake and reduced renal mass. Hypertens. 2010;55:983–989. doi: 10.1161/HYPERTENSIONAHA.109.149120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai E, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertens. 2011;58:650–656. doi: 10.1161/HYPERTENSIONAHA.111.170472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chabrashvili T, Tojo A, Onozato M, Kitiyakara C, Quinn MT, Fujita T, Welch WJ, Wilcox CS. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertens. 2002;39:269–274. doi: 10.1161/hy0202.103264. [DOI] [PubMed] [Google Scholar]
- 33.Just A, Ehmke H, Wittmann U, Kirchheim HR. Role of angiotensin II in dynamic renal blood flow autoregulation of the conscious dog. J Physiol. 2002;538:167–177. doi: 10.1113/jphysiol.2001.012593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeed A, DiBona GF, Marcussen N, Guron G. High-NaCl intake impairs dynamic autoregulation of renal blood flow in ANG II-infused rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1142–R1149. doi: 10.1152/ajpregu.00326.2010. [DOI] [PubMed] [Google Scholar]
- 35.Casellas D, Bouriquet N, Moore LC. Branching patterns and autoregulatory responses of juxtamedullary afferent arterioles. Am J Physiol. 1997;272:F416–F421. doi: 10.1152/ajprenal.1997.272.3.F416. [DOI] [PubMed] [Google Scholar]
- 36.Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol. 1999;10:S178–S183. [PubMed] [Google Scholar]
- 37.Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, Pollock JS, Imig JD, Inscho EW. Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertens. 2005;46:562–568. doi: 10.1161/01.HYP.0000179584.39937.41. [DOI] [PubMed] [Google Scholar]
- 38.Welch WJ, Wilcox CS. Feedback responses during sequential inhibition of angiotensin and thromboxane. Am J Physiol. 1990;258:F457–F466. doi: 10.1152/ajprenal.1990.258.3.F457. [DOI] [PubMed] [Google Scholar]
- 39.Just A, Arendshorst WJ. Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R619–R631. doi: 10.1152/ajpregu.00766.2002. [DOI] [PubMed] [Google Scholar]
- 40.Griendling KK, FitzGerald G. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circ. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 41.Norrelund H, Christensen KL, Samani NJ, Kimber P, Mulvany MJ, Korsgaard N. Early narrowed afferent arteriole is a contributor to the development of hypertension. Hypertens. 1994;24:301–308. doi: 10.1161/01.hyp.24.3.301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.