Abstract
Establishing sufficient vascularization in scaffold remains a challenge for tissue-engineering. To improve angiogenesis, we incorporated vascular endothelial growth factor (VEGF) in collagen-coating over the porous polycaprolactone (PCL) scaffolds. The release kinetics of loaded VEGF from collagen-coated PCL (col-PCL) scaffolds was same as from scaffolds without the collagen. The bioactivity of VEGF delivered by the col-PCL scaffolds was confirmed by human umbilical vein endothelial cell (HUVEC) proliferation and chorioallantoic membrane (CAM) assay. The col-PCL scaffolds were implanted subcutaneously in mice for 7 and 14 days. At day 7, vascularization within scaffolds loaded with VEGF was superior to that in the scaffolds without VEGF. However, the vessel connectivity to host circulatory system was incomplete and restricted to the scaffold edges. At day 14, blood vessels in scaffolds reached density similar to the subcutaneous tissue and were perfusable throughout the implant thickness. Pre-washing the scaffolds with saline to remove the unbound growth factor decreased the initial burst release and sustained the VEGF-mediated angiogenesis in vivo. In conclusion, our study demonstrates that physically adsorbed VEGF stimulated angiogenesis in collagen-coated PCL scaffolds.
Keywords: Angiogenesis, scaffold, polycaprolactone, collagen, VEGF
1. INTRODUCTION
Tissue engineering utilizes scaffold, cells and growth factors to create microenvironment conducive for tissue regeneration. Adequate scaffold vascularization is necessary for the viability of transplanted cells and their integration with host tissue. Vascular endothelial growth factor (VEGF) is the most potent growth factor to promote formation of new blood vessels [1]. The tissue-engineering scaffolds serve as vehicles for sustained delivery of VEGF. The common methods used to localize VEGF in scaffolds are adsorption [2–6], encapsulation [7–9], entrapment [10, 11] or, covalent binding [12, 13]. The latter three strategies are more robust in achieving predictive drug release; however, they involve multiple steps during scaffold fabrication that may compromise protein’s bioactivity and receptor binding ability. On the other hand, adsorption of growth factor on prefabricated scaffold is a convenient, straightforward method that circumvents exposure to harsher conditions during scaffold preparation. Nevertheless, exposure to hydrophobic surfaces may cause protein unfolding and loss of bioactivity of the adsorbed VEGF. The interaction between the adsorbed protein and scaffold surface includes ionic bonds, hydrogen bonds, van der Waals and hydrophobic forces. Since these non-covalent interactions are rather weak, the adsorbed protein is more susceptible to release under in vivo physiological environment.
The three-dimensional scaffolds used in tissue-engineering include porous sponges, electrospun nanofibres or, cross-linked hydrogels depending on their intended application. In general, the interconnected structure in the macroporous scaffolds provides better diffusion of nutrients and infiltration of cells compared to the other scaffold types. Polycaprolactone (PCL) is a widely used polymer for fabrication of biodegradable scaffolds. Although synthetic materials, like PCL, allow good control over the mechanical and in vivo degradation properties, they lack the recognition sites for direct cell-interaction. In the native extracellular matrix (ECM), proteins such as collagen, fibrin, laminin and fibronectin, provide specific sites for cells adhesion. Investigators have shown that including various ECM proteins or, peptide sequences to synthetic scaffolds influences cell behavior. Given the high abundance of collagen in native tissues and presence of integrin binding sites for direct cellular interaction, we used collagen type I to modify the PCL surface, Moreover collagen type I is reported to facilitate migration of endothelial cells during in vitro angiogenesis [14, 15].
Prior studies have shown improved vascularization within scaffold upon delivery of various angiogenic growth factors. We evaluated the efficacy of collagen-coated PCL scaffold as vehicle for delivery of VEGF. In this study, our goal was to enhance in vivo angiogenesis by adsorption of VEGF in collagen-coated PCL scaffolds.
2. MATERIALS AND METHODS
2.1. Scaffold Preparation
Porous PCL (Mw = 110,000) (Lactel Absorbable Polymers, Pelham, AL) scaffolds were prepared by solvent casting and particulate leaching technique [16 ]. Briefly, PCL (10% w/w) was dissolved in a mixed solution of chloroform and methanol (65 chloroform : 35 methanol w/w). Sieved sucrose particles of 212–300 μm diameter were added to the PCL solution. The ratio of sucrose:PCL was 96:4 (w/w). The blend was mixed briefly to form homogeneous slurry and was cast into Teflon molds with inner diameter of 8 mm and height of 2 mm. Scaffolds were freeze-dried, and the sucrose was leached out in deionized water. The scaffolds were sterilized in 70% ethanol for 30 minutes and washed several times with phosphate buffered saline (PBS).
Col-PCL scaffolds were prepared by uniform application of collagen type-I solution on prefabricated PCL scaffold. A total of 20 μg of collagen was coated per scaffold. In brief, bovine collagen type-I (Purecol, Advanced BioMatrix, Inc., San Diego, CA) was neutralized with 0.1N sodium hydroxide and diluted with PBS to obtain a 0.025% solution. The buffered collagen solution (pH 7.4) was kept on ice and applied to scaffolds in four aliquots of 20 μl each. The scaffolds were allowed to stand in laminar hood at room temperature for 15 minutes in between application of each aliquot.
The human rhVEGF165 (R&D Systems, Minneapolis, MN) was loaded by adding a desired quantity of VEGF to neutralized collagen solution before applying it over the scaffold. The pre-washed scaffolds were prepared by immersing VEGF loaded col-PCL in 1 ml of PBS at 4°C for overnight before implantation.
2.2 Release of VEGF
The in vitro release of VEGF from the scaffold was tracked by incorporating tracer amount of 125I-VEGF (PerkinElmer, Waltham, MA) to the bulk of unlabeled growth factor. The VEGF loaded scaffolds were immersed in incubation media maintained at 37°C. The incubation media (pH 7.4) was 1 ml of PBS or, 10% fetal bovine serum (FBS) in PBS. At designated time-points, 10 μl aliquot of media was withdrawn and measured for radioactivity using a Cobra Series Auto-Gamma Counter (Packard Instrument Co., Meriden, CT).
2.3 Bioactivity of VEGF
2.3.1 Human umbilical vein cell (HUVEC) proliferation assay
The in vitro bioactivity of scaffolds loaded with VEGF was tested by HUVEC proliferation assay. The HUVEC (Lonza, Basel, Switzerland) were cultured in EBM-2 basal media supplemented with EGM-2-SingleQuots (Lonza). The cells were maintained at 37° C in humidified incubator containing 10% carbon dioxide, and the culture medium was changed every 3 days. The cells were trypsinized upon reaching confluency and were re-plated for the next passage. For proliferation assay, the HUVEC (passage 3–6) were seeded in 6-well tissue culture plate (Corning Incorporated, Corning, NY) at density of 3000 cells/mm2. Following 4 hours of incubation, the cells were starved for 24 hours in EBM-2 basal media supplemented only with 0.5% FBS. After day 1, the cells were maintained in starvation media in absence of scaffold, in presence of col-PCL scaffold or, in presence of col-PCL scaffold loaded with 1μg of VEGF. On day 4, the cultured HUVEC were stained with live/dead stain (Invitrogen) and the cell density was visualized under a Zeiss Axioskop microscope (Thornwood, New York).
2.3.2 Chlorioallantoic membrane (CAM) assay
To assess the bioactivity of VEGF loaded scaffolds, open-shell chick CAM assay was performed [17]. In brief, fertilized white leghorn chicken eggs (E0) were purchased from AA Laboratory Eggs, Inc.Westminster, CA. The eggs were incubated horizontally at 37° C for 3 days with constant humidity, with the top side of the shell marked by pencil. On day 3, the eggs were wiped with 70% ethanol, pierced with 18G needle at the blunt end of the shell and incubated horizontally for 30 minutes at 37° C with pencil-mark on the top side. The eggs were opened in a sterile tissue culture hood without the blower running. The eggs were held horizontally with the pencil mark on top and opened into a 100 mm2 tissue culture petri dish (BD Biosciences). The embryos developing in petri dishes were incubated at 38° C with 98% humidity in aseptic conditions. The scaffolds were placed on 8 day old CAM away from the existing major blood vessels. After 48 hours, CAM was fixed in 10% formalin and the tissue surrounding the scaffold was visualized for development of new vessels.
2.4 Scaffold Implantation
Eight-week-old C57BL/6 mice were purchased from Charles River Laboratories, Wilmington, MA. The animals were handled in compliance with the institutional regulations established and approved by the Animal Research Committee at the University of California, Los Angeles. For scaffold implantation, mice were anesthetized with isofluorane and a 1-cm long dorsal midline incision was made to create a subcutaneous pocket. The scaffold was inserted in the pocket about a centimeter away from the insicion, and the skin was sutured with 3-0 Vicryl (Ethicon, Somerville, NJ). One scaffold was implanted in each mouse. The animals were sacrificed at day 7 and 14 days post-implantation. The scaffolds, along with the adjacent subcutaneous tissue, were harvested and fixed promptly in 10% formalin overnight at 4 C.
2.5 Immunohistochemistry
The fixed samples were embedded in paraffin and sectioned at 6 μm. The de-paraffinized sections were stained with rat anti-mouse CD31 antibody and biotinylated horse anti-rat secondary antibody (Vector Laboratories, Burlingame, CA). The staining was visualized with DAB substrate (Vector Laboratories) at 100× magnification using a Leica DM IRB inverted microscope. The blood vessel density and area occupied by vessels in the anti-CD31 stained sections were measured using BioQuant Nova software (Bioquant Life Science, Nashville, TN).
For visualization of blood vessels by fluorescence immunostaining, the agarose-embedded samples were cut with a Vibratome (Ted Pella, Redding, CA) into 200-μm sections and stained with anti-CD31 antibody (BD Biosciences, Franklin Lakes, NJ), combined with indocarbocyanine (Cy3) labeled secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). The samples were visualized at 100× magnification on a Zeiss Axioskop microscope (Thornwood, New York).
2.6 Vessel Functionality
To assess vessel connectivity to the host circulation, 100 μl of tomato lectin (Vector Laboratories, Burlingame, CA) labeled with fluorescein isothiocyanate (FITC) was injected intravenously in the anesthetized mice. After 3 minutes, the mice were sacrificed, and the vasculature was perfused with 2% paraformaldehyde/PBS from a 22-gauge catheter inserted in the left ventricle. The fixative was allowed to exit from inferior vena cava. The harvested scaffolds and the adjacent subcutaneous tissue were fixed overnight and cut into 200 μm sections and stained with anti-CD31 antibody, followed by Cy3-labeled secondary antibody as described above. The samples were visualized using a Leica TCS-SP MP confocal microscope.
2.7 Statistics
The vessel area and vessel density at each time point were analyzed using ANOVA. To measure significant difference between groups, the post hoc testing of pairwise differences was performed using the Tukey's Studentized Range (HSD) test. The data were considered statistically significant when p<0.05.
3. RESULTS
3.1 In vitro release of VEGF
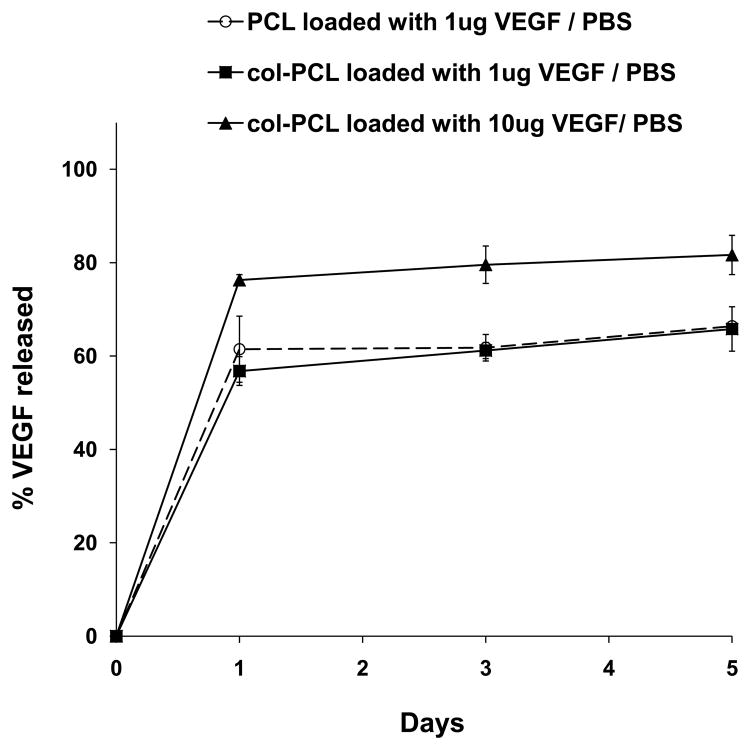
The in vitro release of VEGF from col-PCL and unmodified PCL scaffolds are shown in Figure 1. The kinetic study showed an initial burst release of loaded VEGF from scaffolds within first 24 hours of incubation in PBS. The release profile of VEGF from col-PCL was the same as the unmodified PCL. Increased loading dose resulted in higher fraction of the VEGF coming off into the media during the burst release. Loading 1 and 10 μg/scaffold of VEGF displayed burst release of about 600 ng and 8 μg, respectively, in PBS. After the initial burst release, there was a slow and sustained release of VEGF from col-PCL scaffolds during 5 days of incubation in PBS. The release rate from col-PCL scaffolds loaded with 1 and 10 μg was approximately 10 and 100 ng/day of VEGF, respectively.
Figure 1.
In vitro release of VEGF from scaffolds incubated in PBS. PCL and col-PCL scaffolds were loaded with 1 or 10 μg of VEGF. Values represent the mean and standard deviation from 3 samples.
3.2 Bioactivity of VEGF in col-PCL scaffold
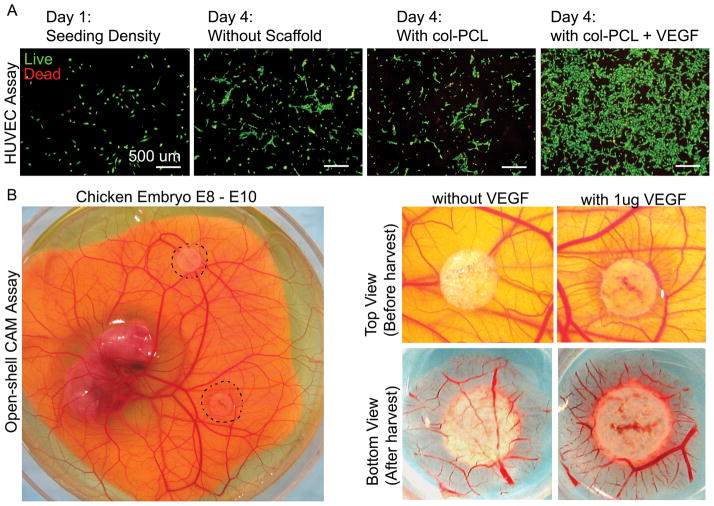
The in vitro bioactivity of VEGF delivered by col-PCL scaffolds was assessed qualitatively by HUVEC proliferation and CAM assay. After 72 hours of incubation, a higher density of HUVECs was observed in cultures with VEGF loaded col-PCL as compared to other groups without scaffold or, col-PCL alone (Figure 2A). Moreover, VEGF loaded scaffold incubated on chicken embryo CAM between E8–E10 displayed dense vessels a clear increase of vessels around the scaffold; the vessels converged radially to form the typical spoke-wheel like pattern (Figure 2B).
Figure 2.
In vitro bioactivity of VEGF loaded in col-PCL scaffold. HUVEC density was visualized by live-dead stain after 72 hours of culture in absence of scaffolds, in presence of col-PCL without VEGF or, in presence of col-PCL loaded with 1 μg of VEGF (A). Col-PCL loaded with 1 μg of VEGF and without VEGF were placed on chicken embryo CAM (E8) and assessed for blood vessel growth around scaffolds after 48 hours incubation (B).
3.3 Angiogenesis in implanted scaffolds
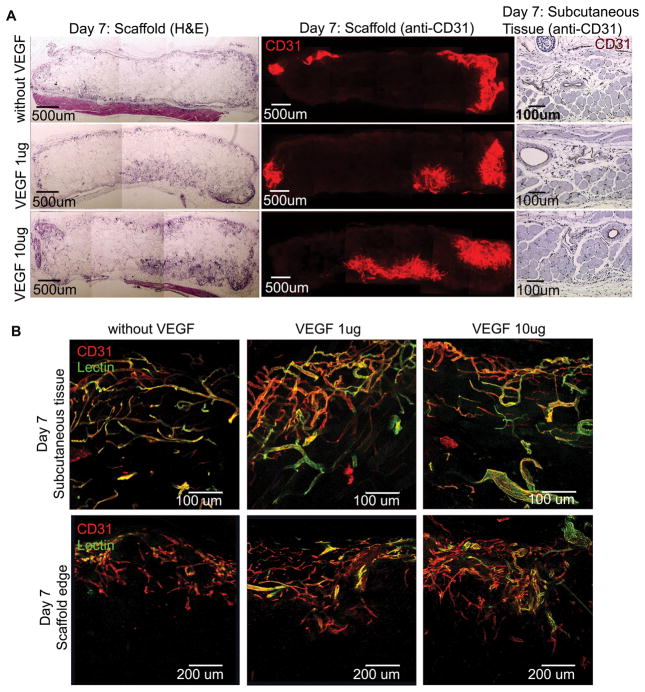
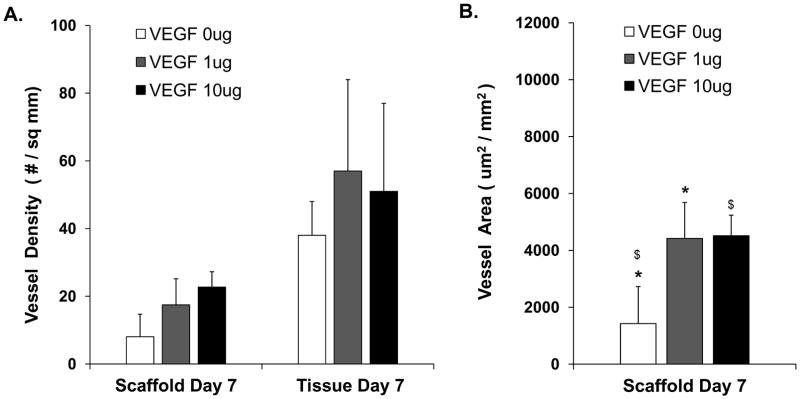
The scaffolds implanted subcutaneously in mice were retrieved after 7 and 14 days. At day 7, cross sections showed cellular infiltration in the periphery of the implants (Figure 3A). There was increased ingrowth of CD-31 positive vessels in col-PCL scaffolds loaded with VEGF compared to the scaffolds without VEGF. The connectivity of vessels to the host circulatory system was assessed by intravenous injection of endothelium-binding fluorescent lectin. All vessels in the surrounding tissue were lectin-perfused. However, the vessel-perfusability inside scaffold was negligible and confined to the margins (Figure 3B). The total vessel density and vessel area was quantified from 6 μm sections using DAB based anti-CD31 stain (Figure 4). Col-PCL scaffolds loaded with 1 μg and 10 μg of VEGF possessed significantly higher vessel area compared to scaffolds without VEGF. Nonetheless, vessel density in all the scaffolds was significantly lower than the surrounding tissue.
Figure 3.
Angiogenesis in scaffolds harvested at day 7. Col-PCL and adjacent subcutaneous tissue sections were stained with H&E and anti-CD31 antibody (A). Functionality of vessels was evaluated by endothelium-binding FITC- lectin injected intravenously before sample harvest (B).
Figure 4.
Quantification of average vessel density in col-PCL scaffolds and adjacent tissue harvested at day 7, and stained with anti-CD31 antibody (A). Vessel area was quantified in col-PCL scaffolds harvested at day 7 (B). Values represent the mean and standard deviation from 3 samples. (*, $) p<0.05.
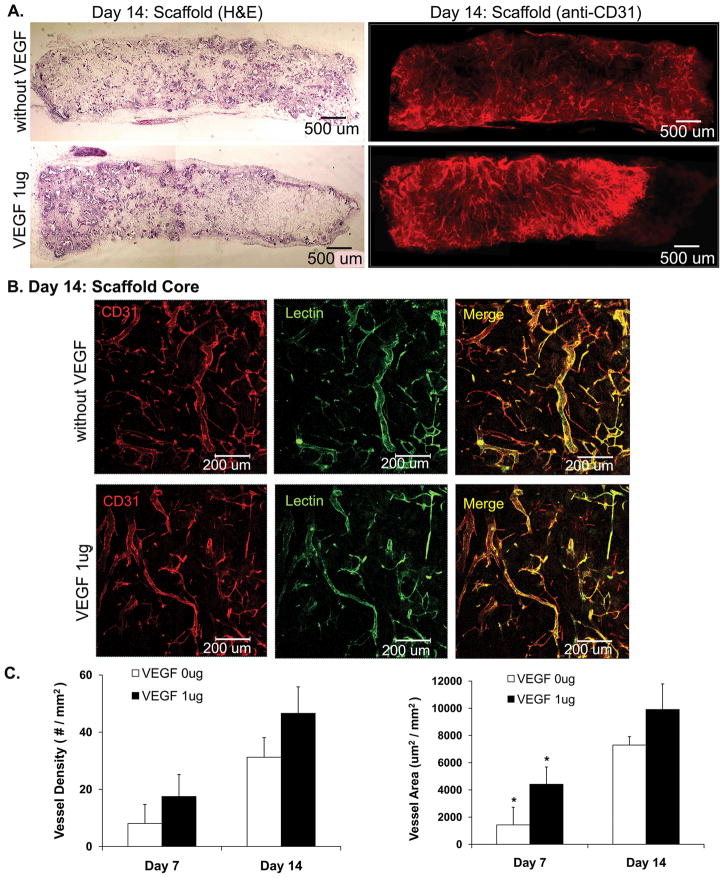
At day 14, col-PCL scaffolds possessed blood vessels running all across the entire thickness of 2 mm within the scaffold (Figure 5). Moreover, all blood vessels were double positive for anti-CD31 and lectin perfusion. The total vessel density and vessel area in the VEGF-loaded scaffolds was not significantly different than the scaffolds without VEGF.
Figure 5.
Angiogenesis in col-PCL scaffolds harvested at day 14. Scaffold sections were stained with H&E and anti-CD31 antibody (A). Functionality of vessels was evaluated by endothelium-binding FITC- lectin injected intravenously before sample harvest (B). Average vessel density and vessel area was quantified in sections of col- PCL scaffolds stained with anti-CD31 antibody (C). Values represent the mean and standard deviation from 3 samples. (*) p<0.05.
3.4 Prewashed col-PCL scaffolds
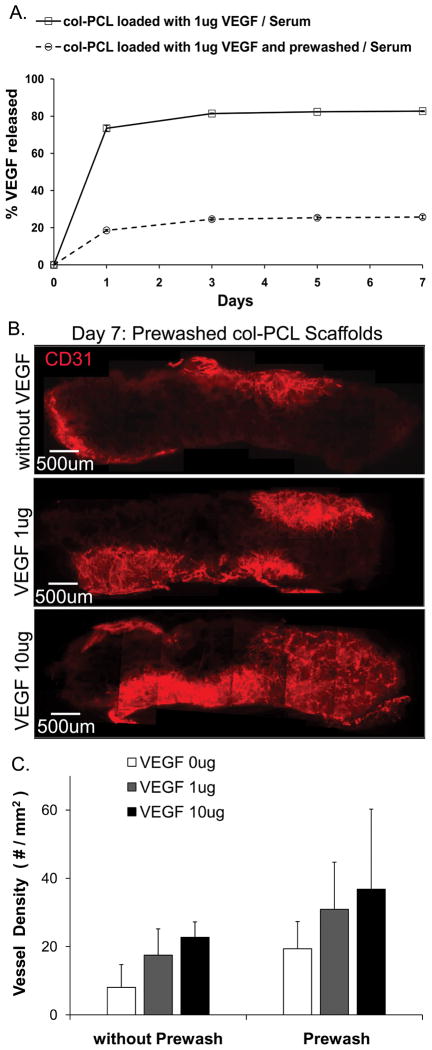
Prewashed scaffolds were prepared by immersing VEGF loaded col-PCL in PBS to remove the unbound fraction of growth factor. The release profile of VEGF from the prewashed and unwashed col-PCL in presence of 10% serum is shown in Figure 6. Prewashed and unwashed scaffolds exhibited initial burst release of 200 ng and 750 ng of VEGF, respectively. Prewashing the scaffolds significantly reduced the initial burst release of VEGF in the incubation media. The prewashed and unwashed col-PCL scaffolds were implanted subcutaneously in mice. There was no significant difference in the vessel density between prewashed and unwashed col-PCL scaffolds after 7 days in vivo.
Figure 6.
In vitro release of VEGF from prewashed col-PCL and un-washed col-PCL scaffolds was assessed by incubation in PBS supplemented with 10% serum (A). Values represent the mean and standard deviation from 3 samples. Blood vessels in prewashed col-PCL scaffolds harvested at day 7 were stained with anti-CD31 antibody (B). Average vessel density was quantified in scaffold sections stained with anti-CD31 antibody (C). Values represent the mean and standard deviation from 3 samples.
4. DISCUSSION
Localized delivery of VEGF by tissue engineering scaffolds prevents the systemic implications associated with bolus administration, such as hypotension, tachycardia and reduced cardiac output [18, 19]. The objective of this study is to stimulate angiogenesis in the porous PCL scaffold by delivery of VEGF. Physical adsorption of VEGF to various scaffolding surfaces, such as alginate [2], calcium-phosphate ceramics [3–5], poly(L-lactide-co-glycolide) [6], improved angiogenesis in vivo.
The adsorbed VEGF is released from scaffold surface in two phases - an initial burst due to weakly-bound protein fraction, followed by a sustained release of the protein bound for longer duration due to collective effect of weak interactions. This investigation attempts to dissect the respective contribution of burst and sustained release phases. The VEGF released during the initial burst would be cleared rapidly from implantation site by fluid circulation and degraded, considering the short half-life of VEGF in plasma [20]. We test the hypothesis that the VEGF fraction released slowly from the scaffold is the major contributor of angiogenesis. The scaffolds were prewashed with PBS to eliminate the sudden burst release phase. As anticipated, prewashed scaffolds exhibited significantly reduced burst, from 80% to 20% of loaded VEGF, under serum conditions mimicking the actual physiological environment.
Our group has previously shown that the collagen type I coating formed fibrils over the PCL surface [21]. Collagen coating makes the polymer surface hydrophilic and promotes cell adhesion. Although both collagen type I fibrils and VEGF (pI ~ 8–8.5) are positively charged at pH 7.4 [22–24], we found that inclusion of collagen to PCL scaffold did not influence the in vitro release of VEGF in PBS.
Our results showed cellular and vascular infiltration in col-PCL scaffolds as early as day 7, which intensified remarkably at day 14. Although, VEGF induced early angiogenesis, there was no significant difference in vessel density between the scaffolds with VEGF and without VEGF at day 14. The possible explanation for this lowers signal / noise ratio could be that scaffold implantation triggers release of various endogenous angiogenic factors associated with inflammation during wound healing and foreign body response.
An important finding of our study was that prewashing scaffolds did not diminish the angiogenic response. Based on this result, we inferred that the initial burst release of VEGF has not significant contribution towards promoting angiogenesis in scaffold. Our observation corroborates with the existing literature that suggests VEGF bound to matrix plays crucial role in inducing angiogenesis [17]. Additionally, prewashing scaffolds before implantation reduced the actual VEGF dose delivered to the subject, minimizing the possible systemic side effects.
In conclusion, the adsorption of VEGF induced early angiogenesis in collagen-coated PCL scaffolds. Our findings would contribute to optimize VEGF delivery for enhancing angiogenesis in tissue-engineering scaffolds.
Acknowledgments
We are grateful to Dr. Luisa Iruela-Arispe for her help with the CAM assay. This work was funded by the National Institutes of Health R01 DK083319 and the Fubon Foundation.
Footnotes
Conflict of Interest
No benefit of any kind will be received either directly or indirectly by the author(s).
References
- 1.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 2.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122–2131. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 3.Lode A, Wolf-Brandstetter C, Reinstorf A, Bernhardt A, König U, Pompe W, Gelinsky M. Calcium phosphate bone cements, functionalized with VEGF: release kinetics and biological activity. J Biomed Mater Res A. 2007;81:474–483. doi: 10.1002/jbm.a.31024. [DOI] [PubMed] [Google Scholar]
- 4.Wernike E, Montjovent MO, Liu Y, Wismeijer D, Hunziker EB, Siebenrock KA, Hofstetter W, Klenke FM. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 5.Moioli EK, Clark PA, Chen M, Dennis JE, Erickson HP, Gerson SL, Mao JJ. Synergistic actions of hematopoietic and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues. PLoS One. 2008;3:e3922. doi: 10.1371/journal.pone.0003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindhorst D, Tavassol F, von See C, Schumann P, Laschke MW, Harder Y, Bormann KH, Essig H, Kokemüller H, Kampmann A, Voss A, Mülhaupt R, Menger MD, Gellrich NC, Rücker M. Effects of VEGF loading on scaffold-confined vascularization. J Biomed Mater Res A. 2010;95:783–792. doi: 10.1002/jbm.a.32902. [DOI] [PubMed] [Google Scholar]
- 7.Borselli C, Ungaro F, Oliviero O, d’Angelo I, Quaglia F, La Rotonda MI, Netti PA. Bioactivation of collagen matrices through sustained VEGF release from PLGA microspheres. J Biomed Mater Res A. 2010;92:94–102. doi: 10.1002/jbm.a.32332. [DOI] [PubMed] [Google Scholar]
- 8.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79:176–184. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, Cuthbertson A. Development of poly-(d,l-lactide-coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Control Release. 2001;72:13–24. doi: 10.1016/s0168-3659(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 10.Peattie RA, Rieke ER, Hewett EM, Fisher RJ, Shu XZ, Prestwich GD. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials. 2006;27:1868–1875. doi: 10.1016/j.biomaterials.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 11.Yeo Y, Geng W, Ito T, Kohane DS, Burdick JA, Radisic M. Photocrosslinkable hydrogel for myocyte cell culture and injection. J Biomed Mater Res B Appl Biomater. 2007;81:312–322. doi: 10.1002/jbm.b.30667. [DOI] [PubMed] [Google Scholar]
- 12.Chiu LLY, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 13.Zisch AH, Schenk U, Schense JC, Sakiyama-Elbert SE, Hubbell JA. Covalently conjugated VEGF--fibrin matrices for endothelialization. J Control Release. 2001;72:101–113. doi: 10.1016/s0168-3659(01)00266-8. [DOI] [PubMed] [Google Scholar]
- 14.Iruela-Arispe ML, Diglio CA, Sage EH. Modulation of extracellular matrix proteins by endothelial cells undergoing angiogenesis in vitro. Arterioscler Thromb. 1991;11:805–815. doi: 10.1161/01.atv.11.4.805. [DOI] [PubMed] [Google Scholar]
- 15.Iruela-Arispe ML, Hasselaar P, Sage H. Differential expression of extracellular proteins is correlated with angiogenesis in vitro. Lab Invest. 1991;64:174–186. [PubMed] [Google Scholar]
- 16.Singh S, Wu BM, Dunn JC. The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin. Biomaterials. 2011;32:2059–2069. doi: 10.1016/j.biomaterials.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang R, Thomas GR, Bunting S, Ko A, Ferrara N, Keyt B, Ross J, Jin H. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol. 1996;27:838–844. doi: 10.1097/00005344-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Bunting S, Ko A, Keyt BA, Modi NB, Zioncheck TF, Ferrara N, Jin H. Substantially attenuated hemodynamic responses to Escherichia coli-derived vascular endothelial growth factor given by intravenous infusion compared with bolus injection. J Pharmacol Exp Ther. 1998;284:103–110. [PubMed] [Google Scholar]
- 20.Eppler SM, Combs DL, Henry TD, Lopez JJ, Ellis SG, Yi JH, Annex BH, McCluskey ER, Zioncheck TF. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72:20–32. doi: 10.1067/mcp.2002.126179. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Chen TT, Iruela-Arispe ML, Wu BM, Dunn JC. Modulation of protein delivery from modular polymer scaffolds. Biomaterials. 2007;28:1862–1870. doi: 10.1016/j.biomaterials.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Asadi A, Monroe MR, Douglas EP. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater Sci Eng C. 2009;29:1643–1649. [Google Scholar]
- 23.Mertz EL, Leikin S. Interactions of inorganic phosphate and sulfate anions with collagen. Biochemistry. 2004;43:14901–14912. doi: 10.1021/bi048788b. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara N, Leung DW, Cachianes G, Winer J, Henzel WJ. Purification and cloning of vascular endothelial growth factor secreted by pituitary folliculostellate cells. Methods Enzymol. 1991;198:391–405. doi: 10.1016/0076-6879(91)98040-d. [DOI] [PubMed] [Google Scholar]