Abstract
During gastrulation, an embryo acquires the three primordial germ layers that will give rise to all of the tissues in the body. In amniote embryos, this process occurs via an epithelial to mesenchymal transition (EMT) of epiblast cells at the primitive streak. Although the primitive streak is vital to development, many aspects of how it forms and functions remain poorly understood. Using live imaging and immunohistochemistry, we have shown that the murine primitive streak arises in situ by progressive initiation of EMT beginning in the posterior epiblast, without large-scale movement or convergence and extension of epiblast cells. Loss of basal lamina (BL) is the first step of this EMT, and is strictly correlated with ingression of nascent mesoderm. This is the first description of dynamic cell behavior during primitive streak formation in the mouse embryo, and reveals mechanisms that are quite distinct from those observed in other amniote model systems.
Keywords: Primitive streak, Epithelial to mesenchymal transition, Gastrulation, Mouse embryo, Morphogenesis
Introduction
The primitive streak is critical for formation of the three primordial germ layers –endoderm, mesoderm, and ectoderm – in most amniote embryos. It does this via a spatially and temporally localized epithelial to mesenchymal transition (EMT) that produces mesoderm and definitive endoderm from the epithelial epiblast cell layer. Experiments in chick embryos have provided some insight into the morphogenetic cell movements that contribute to formation of the avian primitive streak. Prior to streak formation, avian epiblast cells initiate large-scale bilateral rotational movements, termed polonaise movements (Graeper, 1929), which bring streak precursor cells into position at the posterior of the embryo (Lawson and Schoenwolf, 2001), and are dependent on FGF signaling (Chuai et al., 2006). Fate mapping shows that this precursor population resides in a crescent shaped area of posterior epiblast overlying Koller’s sickle (Lawson and Schoenwolf, 2001), which is rearranged into the triangle-shaped incipient streak. The incipient streak then elongates by intercalation of epiblast cells (Lawson and Schoenwolf, 2001), which some studies have shown to be under the control of planar cell polarity (PCP) signaling (Voiculescu et al., 2007), while others have found it to be independent of this pathway (Chuai et al., 2006). Posterior epiblast cells converge upon the posterior midline, extending streak cells anteriorly to form the mature, rod shaped primitive streak.
Mesoderm formation results from EMT of epiblast cells within the primitive streak. This EMT is characterized by localized loss of basal lamina (BL) between the epiblast and underlying hypoblast, and by ingression and acquisition of migratory behavior by the resulting mesodermal cells. Loss of BL is tightly correlated with mesoderm ingression, and is thought to initiate the other events of EMT (Nakaya et al., 2008). RhoA activity has been shown to regulate BL integrity, and thereby EMT, but many other cellular and molecular mechanisms underlying primitive streak EMT in the chick are not well understood. Electron micrographs have shown that epiblast cells acquire a bottle shape within the streak (Nakaya and Sheng, 2009), strongly suggesting apical constriction as a method of ingression. The subsequent migration of mesoderm away from the streak has been more thoroughly studied (Yang et al., 2002; Hardy et al., 2008; Sweetman et al., 2008; Yang et al., 2008; Yue et al., 2008), however the intermediate steps between streak condensation and mesoderm migration are not well understood.
Much less is known about primitive streak formation and function in mammalian embryos. In addition to the difficulties of culturing and imaging at post-implantation stages, the shape of the embryo differs between species, making generalizations difficult. To date, the best-studied model in terms of the cellular mechanisms of streak formation has been the rabbit, in which the embryonic region is in the shape of a flat blastodisc, similar to the chick. At the same time, the mouse embryo has been the best-studied model for both the genetic regulation of gastrulation and the fate map of the epiblast (Lawson et al., 1991; Quinlan et al., 1995; Tam and Zhou, 1996; Tam et al., 1997; Tam et al., 1997; Kinder et al., 2001). Although the pre-streak epiblast of the rabbit embryo does not undergo polonaise movements per se, live imaging does reveal stereotyped cell movements termed “L turns” and “U turns” that may be the mammalian equivalent of this avian cell behavior (Halacheva et al., 2011). Dye-tracking studies suggest that rabbit epiblast cells undergo convergence and extension movements during primitive streak formation (Viebahn et al., 2002), which have been found to be the result of small scale cell intercalations termed “processional” movements (Halacheva et al., 2011). Electron microscopy has revealed apically constricted cells within both mouse and rabbit primitive streak (Tam et al., 1993; Viebahn et al., 1995), similar to the chick, but few other observations have been made regarding cell dynamics within the mammalian primitive streak. And although the study of transgenic mice has led to the identification of signaling pathways vital for streak formation, such as Wnt3, Nodal, and BMP4 (Winnier et al., 1995; Conlon, 1994; Liu et al., 1999; Brennan et al., 2001); the downstream cell biological processes by which these pathways induce the primitive streak are almost completely unknown. In the absence of evidence, what is known about the avian or rabbit primitive streak tends to be extrapolated to mouse embryos. But, because detailed imaging and cell tracking has not been performed in the posterior epiblast of the pre-gastrulation mouse embryo, the cell behaviors that precede and contribute to murine primitive streak formation have not been identified. Because of the vast differences in the geometry of chick, rabbit, and mouse embryos – chicks and rabbits develop as a flat blastodisc, mice as an egg cylinder – it is not unreasonable to suspect that morphogenesis of the streak proceeds differently in these systems.
Our evidence shows that the murine primitive streak does, in fact, form by mechanisms distinct from its avian and rabbit counterparts. Using live imaging of genetically labeled mouse embryos before, during, and after streak formation, we are able to observe and track the movements of individual cells over the course of development. We have found that the posterior epiblast does not display polonaise movements, nor does it undergo convergence and extension prior to streak formation. In addition, we have found no evidence for the streak being formed from a precursor population, as seen in the chick. Instead, we show that the mouse primitive streak forms in situ, without long range cell movements, and elongates by progressive initiation of EMT beginning in the posterior epiblast and proceeding anteriorly. This region is characterized by loss of the BL and production of mesoderm in a spatially and temporally progressive manner. Once the BL is lost in a given region, epiblast cells begin to constrict their apices and enter the underlying mesoderm layer by somal translocation. Although there is much yet to understand about murine gastrulation, this is the first detailed account of the dynamic morphogenetic mechanisms of primitive streak formation in the mouse.
Results
The posterior epiblast does not display polonaise movements or convergence and extension behavior
In order to directly observe cell behavior associated with primitive streak formation, live imaging was performed in the posterior epiblast of fluorescently labeled (mT/mG) pre-streak mouse embryos. Each time-lapse movie made consists of a full z-stack through the entire depth of one hemisphere of the embryo, and because the epiblast is a columnar epithelium, a z-slice at mid-cell height allows for visualization of almost every cell on the posterior side of the embryo. Because the mouse embryo is cylindrical in shape, we were unable to image all sides of the embryo simultaneously; hence this report only includes information regarding cell behavior on the embryo’s posterior side.
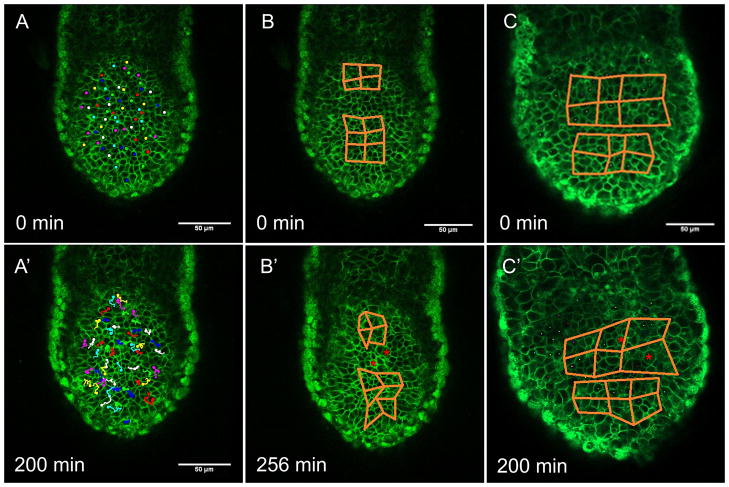
Manual cell tracking in timelapse movies of twelve individual pre- and peri- streak embryos revealed no concerted, long-range cell movements prior to gastrulation. In the example depicted in Figure 1A and A′, cell tracks over 200 minutes demonstrate limited movement within the epiblast, but do not resemble the large scale swirling patterns typical of the pre-streak chick embryo. The tracks of cells at the very posterior of the epiblast (near the top of Figure 1A′) do reflect slight convergence upon the midline, but this behavior is not global, and so does not resemble behavior of the avian pre-gastrulation epiblast. And while the occasional cell track does take the form of a “U” or “L”, this behavior is not widely observed, and so does not resemble the pre-gastrulation rabbit epiblast either. Results reported are a summary of behavior observed in all time-lapse movies of pre- or peri- streak stage embryos. While cell tracking was not identical for any two embryos, none showed any evidence for polonaise movements or other large-scale cell rearrangements.
Figure 1. The mouse epiblast does not exhibit polonaise movements or convergent extension behavior.
Still shots from timelapse movies of live peri-streak EGFP labeled mT/mG pre-streak embryos. A and A′. Cell tracking demonstrates that cells in the posterior epiblast do not undergo bilateral rotational movements, nor do they exhibit anterior displacement. B – C′. Distortion diagrams in the posterior epiblast (posterior identified by nascent mesoderm (red asterisks) in the later timepoints) illustrate that cells of the epiblast do not display convergent extension movements. Distal tip is down.
Pre-streak time-lapse movies were further analyzed using distortion diagrams to determine whether convergence and extension of the epiblast contributes to streak formation by elongation of a contiguous precursor population (Figure 1B and C). Distortion diagrams illustrate changes in relative cell positions over time, so diagrams that become longer and narrower are indicative of convergence and extension. In our time-lapse movies, distortion diagrams change shape as cells rearrange slightly within the epithelium over the course of 256 (Figure 1B and B′) or 200 minutes (Figure 1C and C′), but they do not form the longer and narrower arrangement that would indicate extensive cell intercalation. Although some epiblast cells may converge upon the posterior midline, they do not contribute to elongation of the tissue. These results are inconsistent with convergence and extension cell behavior as a mechanism for streak formation.
It is believed that polonaise movements in the chick serve to position streak precursor cells at the posterior of the embryo (Lawson and Schoenwolf, 2001; Chuai et al., 2006), and that convergence and extension behavior arranges these precursors in an elongated, linear array to establish the length of the streak (Lawson and Schoenwolf, 2001; Lawson and Schoenwolf, 2001; Voiculescu et al., 2007). Both of these cell movement patterns result in anterior displacement of midline epiblast cells in the chick embryo (Lawson and Schoenwolf, 2001; Chuai et al., 2006; Voiculescu et al., 2007). Cell tracking in twelve separate embryos, however, reveals no anterior displacement of cells within the posterior epiblast of the mouse (Figure 1A and 1A′). Although our analysis includes only cells on the posterior side of the egg cylinder, large scale movements such as convergence and extension and anterior displacement would certainly be apparent in the posterior epiblast if they contributed to streak formation. The fact that mouse embryos do not display polonaise movements, convergence and extension, or anterior displacement of posterior epiblast cells prior to gastrulation suggests that streak formation does not result from rearrangement or migration of a contiguous cell population, and that if there are any streak precursor cells present in the epiblast, they are already in position and develop in situ.
The primitive streak forms by progressive loss of BL and induction of EMT
Time-lapse imaging depicts an embryo (shown in figure 1) that is pre-streak at the beginning of the movie (Figure 1B), but has mesoderm 256 minutes later (Figure 1B′, red asterisks). Mesoderm is identified by cell morphology, which is verified by Brachyury (T) immuno-staining (supplemental Figure 1). These mesoderm cells appear from out of the posterior epiblast (see supplemental movie 1) with no significant rearrangement or migration of cells there. It is obvious that an EMT is underway here, but exactly when, where, and how it began is unclear. To track the progression of EMT, including changes to the BL, associated with primitive streak formation in the mouse, we performed immuno-fluorescent staining for mesenchymal and BL markers in serial transverse sections of embryos at various stages of streak development (Figure 2).
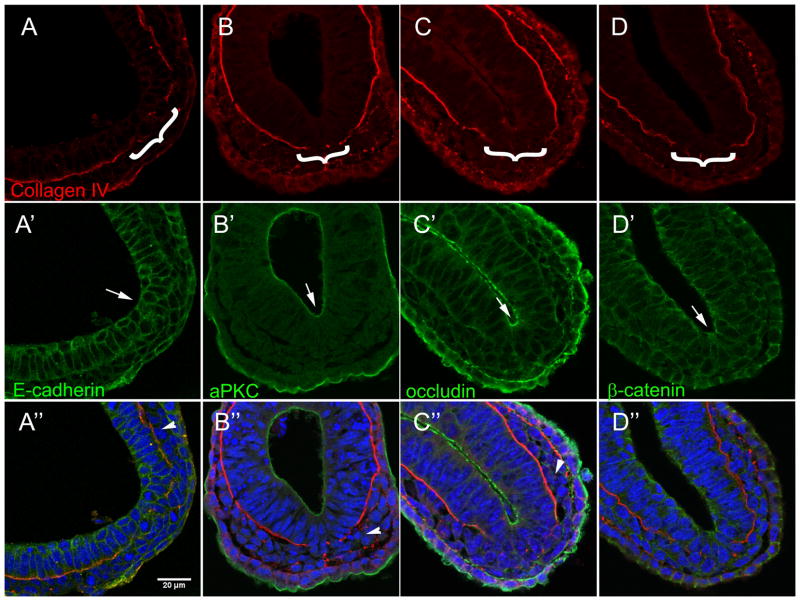
Figure 2. Primitive streak elongation involves progressive breakdown of basal lamina.
A – J. Serial 12 μm transverse sections of mid- to late-streak stage mouse embryos immuno-stained for Laminin (green A–B), Collagen IV (red C–F, green I–K′), and Brachyury (T) (red, I′-K′). Blue is DAPI staining. Posterior is to the right.
A – D. Mesodermal cells (red asterisks) are evident in proximal sections of mid-streak stage embryos (A and C), as is a break in the posterior basal lamina (arrows). More distal sections in the same embryos (B and D, respectively) display neither mesoderm nor loss of BL.
E – F. In a late-streak stage embryo, loss of basal lamina (arrows) and mesoderm (red asterisks) are evident in proximal (E) and distal (F) sections.
G – H. Embryos at early- (G) and late- (H) streak stage. Black lines indicate the approximate level of the indicated transverse sections. Arrowheads indicate distal extent of primitive streak.
I – J. Immuno-staining for T confirms the presence of mesoderm (arrows) in proximal sections (I, I′) and absence of mesoderm in distal sections (J, J′)
K. Optical sagittal section of a mid-streak stage embryo whole mount immuno-stained for Collagen IV (green) and T (red). Arrows indicate mesoderm cells as confirmed by T staining (K′), arrowhead indicates the distal extent of BL loss.
In embryos at mid-streak stage, the BL is lost and the mesoderm layer has begun to form in the proximal/ posterior region of the embryo (Figures 2A and C, arrows). Again, mesoderm is identified by morphology in sections, but is confirmed by Brachyury immuno-staining (Figure 2I′). More distal/ anterior sections, however, reveal that there is no loss of BL and no mesoderm 70, 36, and 48μm further anterior, respectively (Figures 2B, D, and J). This pattern of BL loss and production of mesoderm is also apparent in whole embryos (Figure K, K′). In the optical sagittal section shown, mesoderm cells (arrows, as indicated by immuno-staining for Brachyury) are observed only in the posterior portion of the embryo where the BL has been lost, and not further anterior where the BL is still intact (arrowhead). These observations indicate not only that the loss of BL at the streak is tightly correlated with mesoderm production (because one is not seen without the other), but also that both BL loss and mesoderm ingression occur in a progressive manner from posterior to anterior. This indicates that EMT is initiated in progressively more anterior sites, thereby expanding the length of the primitive streak.
This progressive initiation of EMT is responsible for the formation of the primitive streak all the way to the distal tip of the embryo. Although it is generally accepted that the streak does not reached its full absolute length until about E7.5, the streak reaches its full relative length significantly earlier, at mid- late- streak stage (Downs and Davies, 1993). In our late-streak stage embryo, the BL is lost all the way from the extraembryonic border (Figure 2E) to the distal tip (Figure 2F) on the posterior side, indicating full relative length. The streak then achieves its full length at E7.5 by growing as the embryo grows.
Because loss of epithelial junctional and polarity proteins are important steps of EMT (Baum et al., 2008), the expression of epithelial markers was further characterized in late-streak stage embryos (Figure 3). The apical polarity protein aPKC (Figure 3B′, arrow) and tight junction protein Occludin (Figure 3C′, arrow), in addition to adherens junction components E-cadherin (Figure 3A′, arrow) and β-catenin (Figure 3D′, arrow), are all maintained in the epiblast cells of the streak region after the basal lamina is lost (Figure 3A–D, brackets). This indicates that loss of BL precedes other steps of the EMT process during streak formation, but also that loss of basal lamina is not an immediate trigger for loss of epithelial phenotype in all cells at the primitive streak.
Figure 3. Loss of basal lamina precedes other steps of EMT within the streak.
Transverse sections of late-streak stage embryos. The primitive streak in each embryo (brackets) is identified by a break in Collagen IV staining (red, A–D). Arrows indicate Immuno-fluorescent staining for E-cadherin (green, A′), aPKC (green, B′), Occludin (green, C′), and β-catenin (green, D′) in the epiblast at the site of the primitive streak. Arrowheads indicate lack of staining in the mesoderm layer. Blue in merged images is DAPI. Posterior is to the bottom right.
Together, our data from immunohistochemistry of embryos and embryo sections and timelapse confocal microscopy suggest that the primitive streak forms and expands by progressive initiation of EMT in the epiblast from posterior to anterior. Loss of BL is not only the first step of this EMT, but the only step that correlates with anterior expansion of the streak.
Epiblast cells enter the primitive streak by apical constriction and somal translocation
Electron microscopic studies of chick, mouse, rabbit, and sea urchin embryos reveal that prospective mesenchymal cells often take on a bottle shaped morphology as they leave an epithelium (Katow, 1980; Tam et al., 1993; Viebahn et al., 1995; Nakaya and Sheng, 2009). Time-lapse movies of sea urchin embryos reveal that this apical constriction is the mechanism by which primary mesenchyme cells ingress (Wu et al., 2007), and it is a likely mechanism for ingression within the primitive streak of amniotes.
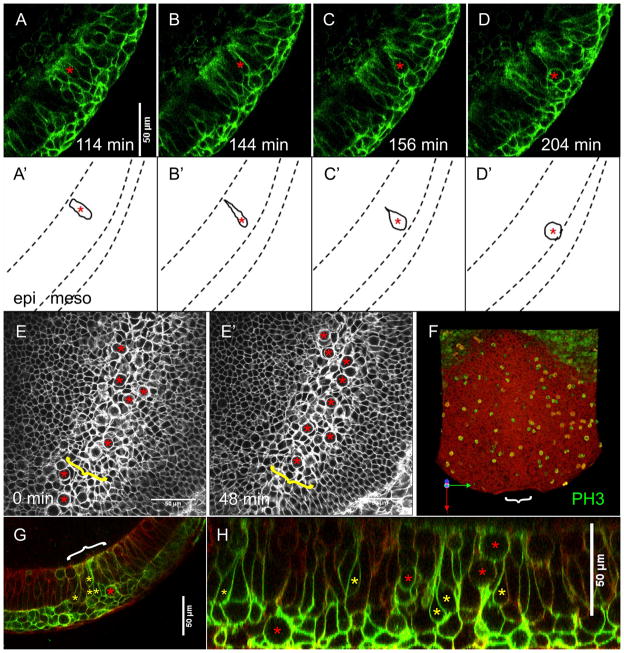
Time-lapse movies of live mT/mG E7.5 embryos demonstrate apical constriction and somal translocation within the murine primitive streak directly. In a time-lapse movie of the primitive streak in optical sagittal section at early-bud stage (Figure 4A -D), the highlighted cell can be observed traversing the streak and entering the mesoderm layer over the course of 90 minutes (asterisks). This cell changes from columnar (Figure 4A, A′), to bottle-shaped within the epiblast (Figure 4B, B′), then finally becomes rounded as it enters the mesoderm layer (Figure 4D, D′). This cell behavior was observed in many cells in four individual movies of the primitive streak in optical sagittal section, providing direct evidence for apical constriction and subsequent translocation as a mechanism of ingression within the murine streak.
Figure 4. Epiblast cells enter the primitive streak by apical constriction and somal translocation.
A – D. Still shots from a timelapse movie of a live mT/mG:TCre early-bud stage embryo in optical sagittal section. Asterisks mark a cell undergoing apical constriction as it ingresses at the streak. A′ – D′ are representations of still shots A - D to demonstrate changes in the shape of this cell over time. Dashed lines represent borders between germ layers. Epi = epiblast, meso = mesoderm. Mesoderm/ basal surface of epiblast is down.
E, E′. Timelapse movie of a live mT/mG no-bud stage embryo viewed en face at the level of the epiblast. The primitive streak (yellow brackets) contains many large, round cells (red asterisks).
F. 3 dimensional reconstruction of a whole early head-fold stage embryo immuno-stained for phosphorylated Histone 3 staining (green), as viewed from the inside / epiblast. White bracket marks position of the primitive streak. Distal / anterior is down in E - F.
G - H. An optical transverse section (G) and optical sagittal section (H) of a live mT/mG:T Cre no-bud stage embryo show bottle shaped cells (yellow asterisks) and rounded cells (red asterisks) within the primitive streak (white bracket). Mesoderm/ basal surface of epiblast is down.
These changes in cell shape can also be observed en face when viewing optical planes at the level of the epiblast, and distinguish the primitive streak from the surrounding epiblast (Figures 4E and E′, yellow brackets). Lateral epiblast cells have narrow basal surfaces due to their columnar shape, and form a honey comb like arrangement. Many cells within the streak, however, have a large, round, bulbous appearance (Figures 4E and E′, red asterisks). When viewed over time (Figure 4E′), one can see that these cells are dynamic as they pass through the plane of view from epiblast to mesoderm cell layers (also see supplemental movie 2). The characteristic round shapes are bulging somas, the result of apical constriction in ingressing cells. All of our time-lapse movies consist of full Z stacks at every time point, allowing us to follow individual cells through the stack and over time from the epiblast into the mesoderm as they traverse the streak (data not shown). This movie (of a no-bud stage embryo) also shows that while the streak is a narrow region (Figures 4E and E′, yellow brackets), not all of the cells within it ingress at the same time. Cells leave the epithelium from different places within the streak at different times (Figures 4A and A′, red asterisks), while the intervening regions of epiblast remain intact. Eleven individual movies of the primitive streak viewed en face demonstrate this same cell behavior.
Immuno-fluorescent staining for epithelial markers in embryo sections corroborates this observation. In late-streak stage embryos (Figure 3), aPKC (Figure 3B′), Occludin (Figure 3C′), β-catenin (Figure 3D′) and E-cadherin (Figure 3A′) were all observed in the epiblast immediately overlying the primitive streak, even though the basal lamina was absent and mesoderm ingression had begun. These markers are not, however, (with the exception of β-catenin) expressed in migrating mesodermal cells (Figure 3A”-D”, arrowheads). This indicates that epiblast cells maintain an intact and organized epithelium even at the site of the primitive streak, a region defined by lack of BL, and that tight junctions and polarity are lost on a cell-by-cell basis as individual cells leave the epithelium and ingress through the streak.
When mT/mG mice are crossed to T (Brachyury) cre mice, the resulting embryos are red with green primitive streaks and streak derivatives. Optical sections of these live embryos at no-bud stage (Figures 4G and H) further support apical constriction as a mechanism of ingression. A population of cells within the streak display a bottle shaped morphology with their expanded somas bulging into the underlying mesoderm layer (Figures 4G and H, yellow asterisks). These optical streak sections depict not only bottle shaped cells, but also several rounded somas at different stages of ingression within the streak (red asterisks).
This bulbous appearance is, however, also common to epithelial cells undergoing mitosis. Cells withdraw their basal sides and round up on their apical surface prior to division (Gardner and Cockroft, 1998). To ensure that the bulbous morphology observed within the streak is in fact due to apical constriction and is not the result of cell division, whole embryos (n=9) and embryo sections (n=4 embryos) were immuno-stained for phosphorylated Histone H3 (PH3) (Figure 4F, and data not shown). In embryos with fully elongated streaks (embryo shown is early head-fold stage), no increase of mitoses was observed within the streak (Figures 4F, white bracket) compared to the lateral epiblast. This was confirmed by staining in serial transverse sections (data not shown). Cell divisions are certainly common within the epiblast, but are not concentrated within the streak. This means that while some of the basally rounded cells we observe are likely undergoing mitosis, cell divisions are not largely responsible for the changes in cell morphology observed within the streak.
Cells of the lateral epiblast are drawn into the primitive streak as cells ingress
Fate mapping studies have shown that much of the epiblast will traverse the primitive streak to form mesoderm and definitive endoderm, while those cells that do not will become ectoderm (Lawson et al., 1991; Quinlan et al., 1995; Tam et al., 1997). How new cells are recruited to the streak after others have passed through it, however, has remained a topic of speculation. To determine how lateral epiblast cells are recruited to the streak, we have examined time-lapse movies that allow for direct observation of lateral epiblast cells and their interactions with the primitive streak.
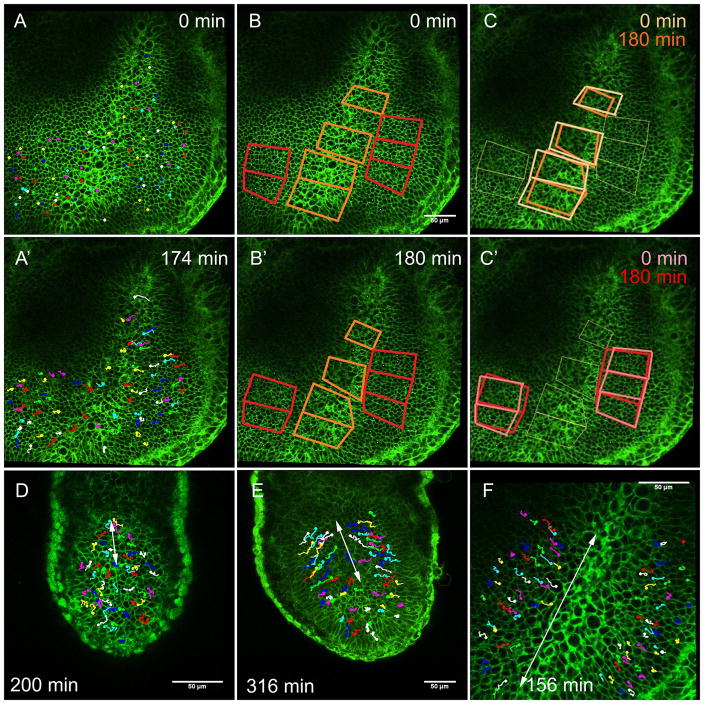
Cells were tracked at the level of the epiblast within four individual movies of mT/mG embryos with fully elongated streaks, and results reported hold true for each. In the no-bud stage embryo shown in Figure 5, epiblast cells can be seen tracking medially, toward the primitive streak (Figures 5A and A′). Distortion diagrams of these same cells reveal that while cells are converging toward the posterior midline, they do not contribute to streak elongation. The orange diagrams, which represent cells within the primitive streak, have not rearranged into a longer and narrower array (Figures 5B and B′). Diagrams at the end of the movie were then compared to their original position at the beginning of the movie for both cells within the streak (Figure 5C) and those of the lateral epiblast (Figure 5C′). Diagrams overlying the primitive streak become substantially smaller in all dimensions over the course of the movie (Figure 5C, compare orange to peach diagrams), a change in size that is indicative of cells leaving these fields as they ingress through the streak. In fact, the number of cells in the combined primitive streak fields underwent a significant 17% reduction over 180 minutes (p<.05, repeated measures ANOVA). Fields of lateral epiblast cells, on the other hand, do not change significantly in size and only exhibit a 4% reduction in cell number (p>.05) over 180 minutes (Figure 5C′, compare red to pink diagrams). Fields of lateral epiblast also converge slightly upon the midline, which matches the cell tracking data (Figure 5A) and is consistent with lateral epiblast advancing toward the streak as cells leave the epiblast.
Figure 5. Cells from the lateral epiblast replenish the primitive streak as cells ingress.
A – C′. Still shots from a timelapse movie of a live mT/mG no-bud stage embryo. A. Cell tracking of the posterior epiblast over time (A′). B. Distortion diagrams at the end of the movie (B′) and at the movie’s beginning (B), indicate no convergence and extension. Relationships between cells within the streak are represented by orange diagrams, lateral epiblast by red diagrams. C. Diagrams within the streak are smaller at the end of the movie (dark orange, C) than at the beginning of the movie (light orange, C). Diagrams within the lateral epiblast do not change significantly in size from the movie’s beginning (pink, C′) to its end (red, C′). Distal / anterior is down.
D – F. Still shots from timelapse movies of live mT/mG embryos with cell tracking in the epiblast. White arrows indicate regions of medial cell movement in the epiblast. D. In an early-streak stage embryo, cells converge upon the streak only in the posterior third of the epiblast. E. In a mid-streak stage embryo, cells converge upon the streak in the posterior two-thirds of the epiblast. F. In a no-bud stage embryo, epiblast cells converge upon the streak along its entire length. Distal / anterior is down.
This level of coordinated cell movement is expected of an intact epithelial sheet, and is not surprising, considering the high levels of E-cadherin and Occludin expressed between cells within the epiblast (Figure 3). Even within the primitive streak (Figure 3A, A′ and C, C′; brackets, arrows), the apical ends of epiblast cells express high levels of these junction proteins and maintain an organized epithelial sheet. A similar phenomenon has been reported in sea urchin, whereby neighboring cells will reorganize their cell-cell contacts to maintain tissue integrity even as a primary mesenchyme cell ingresses (Katow, 1980). These data support a simple model for the replenishment of cells at the streak: lateral epiblast cells are pulled passively via their epithelial integrity (cell-cell contacts with neighbors) toward a region where cells are continually leaving the epithelium.
Because cell tracking toward the streak is associated with cells ingressing there, we can assume that a region of the epiblast displaying medial cell movement is adjacent to a region of active cell ingression, i.e. the primitive streak. In an early-streak stage embryo (Figure 5D) only cells in the posterior third of the epiblast track medially (indicated by white arrow), reflecting the length of the streak at that time in development. In a mid-streak stage embryo (Figure 5E), cells in the posterior two-thirds of the epiblast track medially (5E, arrow), indicating an anterior expansion of the area of ingression, and a longer primitive streak. Finally, in a no-bud stage embryo (Figure 5F), cells within the entire epiblast track medially (5F, arrow). Taken together with the following: 1) Mesoderm ingression at the streak occurs only where the basal lamina is lost (Figure 2), and 2) Epiblast cells move medially to replenish cells that have ingressed at the streak (Figure 5A,C); these live imaging data further support our model of streak expansion by progressive initiation of EMT.
Discussion
Morphogenesis of the primitive streak
This study has identified many of the morphogenetic mechanisms that contribute to primitive streak formation in mouse embryos. Our results suggest that there are no gross cell rearrangements that sculpt the streak from a pre-designated population, but rather the streak develops in situ by initiation of EMT within the posterior epiblast. Loss of basal lamina defines the area of this EMT, and therefore, the region of the primitive streak. In this sense, the streak can be thought of not as a structure made up of cells, but rather as a conduit through which cells pass. This is not to say that there are not cells that reside within the streak, as there is certainly evidence for such a population (Bonnerot and Nicolas, 1993; Cambray and Wilson, 2007). But in terms of streak elongation, there is no evidence for a structure comprised of cells that pushes anteriorly as it elongates (as anterior displacement of cells is not observed at the site of the streak), only a region that expands – a hole in the BL that becomes larger. This EMT is initiated progressively from posterior to anterior, incorporating ever more anterior cells and producing mesoderm in ever more anterior regions, thus “elongating” the streak to the distal tip of the embryonic cylinder
When considered in comparison to the much better defined processes of primitive streak formation in the chick and rabbit, our results highlight substantial differences in the mechanisms of formation and elongation, but substantial similarity in the process and progression of EMT. In the chick and rabbit embryos, the primitive streak is a visibly distinct structure made up of cells that condense and elongate to shape the structure (Lawson and Schoenwolf, 2001; Lawson and Schoenwolf, 2001), (Viebahn et al., 1995). This is achieved through polonaise movements in the chick (Graeper, 1929; Cui et al., 2005), and “U” and “L” turns in the rabbit (Halacheva et al., 2011), as well as convergence and extension movements that elongate and distribute the streak precursors along the anterior/posterior axis as a distinct thickened population (Idkowiak et al., 2004; Voiculescu et al., 2007). In contrast, we find that none of these epiblast cell movements occur during primitive streak formation in the mouse, and indeed no morphologically distinct structure forms prior to the onset of EMT. The murine primitive streak forms when and where the EMT that produces mesoderm occurs. This means that the murine streak develops in situ, and does not involve rearrangement of a designated population of “streak cells”. It is possible that these differences in the cellular mechanism for establishing the primitive streak between chick, rabbit, and mouse are related to the geometry of the embryo. Cells in the more anterior regions of the chick or rabbit epiblast, which are flat blastodiscs, have much further to travel than those in the analogous part of the mouse epiblast, which is a cup-shaped cylinder.
The convergence and extension movements within the pre-streak epiblast in the chick have been reported to depend on PCP signaling (Voiculescu et al., 2007), although others have found no role for PCP signaling (Chuai et al., 2006). In the mouse, primitive streak defects have not been observed in embryos mutant for most PCP genes (Kibar et al., 2001; Lu et al., 2004; Yen et al., 2009; Curtin et al., 2003; Murdoch et al., 2003), with the exception of embryos lacking Wnt5a, which display a shortened primitive streak at E7.75 (Yamaguchi et al., 1999). Because this particular defect is not seen until at least 24 hours after streak initiation, it suggests that Wnt5a does not play a role in the formation or initial elongation of the streak to the distal tip of the embryo (which is complete by around E6.75 [Figure 2]), but rather in the subsequent phase of streak growth that is associated with expansion of the embryo overall. These results, then, are consistent with our observations that the mouse epiblast does not undergo convergence and extension prior to streak formation.
EMT at the primitive streak
The EMT that occurs at the primitive streak appears to have several steps that are completed in sequence, and are associated with a series of behavioral changes. Loss of basal lamina is the first step of this transition, which defines the location of the primitive streak and provides cells the ability to ingress, although most will not immediately do so. Indeed, many cells within the streak retain their epithelial junctions and polarity within the BL gap, maintaining epithelial integrity across the entire epiblast. Occludin and aPKC are then lost from the apical surface of epiblast cells as they ingress at the streak, a process involving apical constriction and translocation of these cells into the underlying mesoderm layer. Once located in the mesoderm layer, cells then lose their E-cadherin as they migrate away from the site of the streak. It is not until these cells have taken on this migratory behavior, and E-cadherin has been down-regulated, that many would consider the EMT to be complete. Not all cells within the streak will undergo these steps at the same time, ensuring both epithelial integrity and a steady stream of mesoderm from the streak.
Each of these steps of EMT (BL loss, ingression, and migration) appears to be distinct, and likely under the control of separate molecular mechanisms. For example, mouse embryos mutant for Wnt3 or Nodal (among others) do not have loss of BL and do not initiate streak formation at all (Winnier et al., 1995; Conlon, 1994; Liu et al., 1999; Brennan et al., 2001). In mouse embryos mutant for the transcription factor Snail, BL is lost and cells do ingress at the streak, but these cells re-epithelialize, suggesting a failure of EMT (Carver et al., 2001). In mouse embryos mutant for FGF signaling components, cells ingress at the streak and adopt a mesenchymal morphology, but are unable to migrate away from the streak, seemingly due to a failure of E-cadherin down-regulation (Sun et al., 1999; Ciruna and Rossant, 2001; Garcia-Garcia and Anderson, 2003). These examples illustrate that loss of BL, ingression, and migration are distinct processes during mouse gastrulation, each being just one step of the complete EMT.
Loss of E-cadherin is important in this distinction between complete EMT and ingression, as it appears to be vital for the acquisition of migratory behavior, but not ingression. Importantly, blocking E-cadherin function in explanted mouse epiblast is sufficient to cause EMT (Burdsal et al., 1993), which taken together with the afore mentioned examples of Snail and FGF mouse mutants, suggests that loss of E-cadherin is necessary and sufficient for completion of EMT at the murine streak. In the chick embryo, however, while treatment with FGF inhibitors does prevent mesoderm migration away from the site of the streak, it does not affect E-cadherin expression (Hardy et al., 2011). Some investigators have found that overexpressing Snail in chick embryos results in down-regulation of E-cadherin and precocious EMT (Acloque et al., 2011), while others have found the opposite to be true (Hardy et al., 2011), perhaps suggesting subtle differences in the role of E-cadherin in EMT during gastrulation in mouse and chick.
Elongation of the primitive streak
Initiation of EMT at the primitive streak occurs progressively from posterior to anterior, and it is the expansion of the region of EMT that accounts for elongation of the streak. Instructions for cells in the epiblast to undergo this transition must also then be passed from posterior to anterior. What this signal is and how it is passed is not known. However, likely candidates for this signal(s) have been identified through study of transgenic mice, and include BMP4, Wnt3, Nodal, or a combination thereof (Conlon et al., 1994; Winnier et al., 1995; Liu et al., 1999; Brennan et al., 2001). There are several possible mechanisms by which these molecules could induce and elongate a streak: 1) Early signaling events could establish a stable pre-pattern determining which cells will undergo EMT and when; 2) A population of cells in the most posterior portion of the embryo could induce EMT in ever anterior regions by production of a diffusible signal; or 3) A population of “instigator” cells in the proximal posterior epiblast might initiate a feed-forward mechanism, whereby each tier of cells not only initiates EMT, but then subsequently induces its anterior neighbors both to undergo EMT and to be competent to induce a new tier of cells. Interaction between the extraembryonic ectoderm and the proximal posterior epiblast between E5.5 and E6.0 is required for formation of the primitive streak in the mouse, demonstrating the presence of an initiating signal (Rodriguez et al., 2005; Georgiades and Rossant, 2006). The function of this signal in regulating streak elongation, however, is not known. It has been shown in the chick that a diffusible signal from the region of Köller’s sickle is responsible for streak induction (Khaner and Eyal-Giladi, 1986; Callebaut et al., 2003), and also that a combination of signals at the marginal zone is necessary for posterior streak induction (Skromne and Stern, 2001), but this does not rule out the possibility of a feed-forward mechanism for elongation.
Signaling within the primitive streak
Beyond the identity of the signaling pathways responsible for streak induction, it is still not known how downstream effectors of such signaling cause a loss of BL and a switch to a mesenchymal phenotype. It has been shown in the chick embryo that loss of basal lamina is the first step of EMT at the streak, and that causing ectopic BL loss is sufficient to induce mesoderm ingression (Nakaya et al., 2008). This may also be the case in mouse embryos. Embryoid bodies made from mouse embryonic stem cells that lack the Laminin γ1 chain, and are therefore unable to form basement membranes, do not epithelialize and have increased expression levels of mesodermal and EMT markers (Fujiwara et al., 2007). And in FLRT3 mutant mouse embryos, holes develop in the visceral endoderm and in its associated BL, resulting in ectopic EMT at these sites (Egea et al., 2008). In the chick, loss of BL was shown to be the result of microtubule instability, caused by loss of RhoA activity at the basal side of epiblast cells (Nakaya et al., 2008). Regulation of RhoA activity is also important for proper EMT within the mouse primitive streak (Fuse et al., 2004). The upstream signaling responsible for changes in RhoA localization and activity, however, has yet to be identified.
Ingression within the primitive streak
This study has demonstrated that once EMT has been initiated at the primitive streak, cells ingress by apical constriction and somal translocation. Remarkably, the integrity of the epiblast epithelial sheet is maintained during the ingression process. This not only contributes to structural integrity of the embryo, but is also likely responsible for the replenishment of cells at the streak. As cells within the streak constrict their apices, it pulls neighboring cells closer to the midline, resulting in what appears to be a passive advancement of the lateral epiblast toward the streak (although we cannot rule out the possibility of some other contributing force). Although epiblast cells converge upon the streak during ingression, the overall shape of the embryo is not affected. One explanation would be that the ever increasing cell number and volume of the epiblast, resulting from the large number of cell divisions that occur there (figure 4F), counteract any physical forces produced at the primitive streak that might cause mass tissue distortion. This pattern of epiblast movement also reflects the progressive nature of EMT at the streak. Because cells are drawn toward the midline where cells are ingressing at the streak, the region of EMT can be identified by the behavior of the lateral epiblast cells. Indeed, the area of epiblast that displays medial movement expands in coordination with streak elongation.
This report is among the first to elucidate morphogenetic events involved in murine primitive streak formation. There is much yet to understand about the formation and function of the primitive streak, however, knowing that the mechanisms of gastrulation vary among amniotes will allow developmental biologists to proceed with the understanding that what is true of the chick and rabbit may not hold true for the mouse. A comparative approach between these species, and reptile embryos as well, could provide us with a better understanding of the evolutionary origins of the primitive streak and perhaps other EMTs.
Experimental Procedures
Animals
Animal use protocols were reviewed and approved by the University of Virginia Institutional Animal Care and Use Committee, and were in compliance with PHS and USDA guidelines for laboratory animal welfare. Mice of strain Gt(ROSA)26Sortm4(ACTB-tdTomato,-Egfp)Luo/J, ((Muzumdar et al., 2007) designated mT/mG) were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained as a homozygous stock. These mice carry a tandem array with a floxed gene for a membrane-targeted Tomato fluorescent protein (mT) followed by a gene for membrane-targeted Egfp (mG). To create embryos with membrane-bound EGFP in all cells, mT/mG animals were crossed to mice of the C57BL/6-Tg(Zp3-cre)93Knw/J strain, also obtained from Jackson Laboratory. This is an oocyte specific cre, which produces second generation offspring that are labeled entirely green. To create embryos with membrane-bound EGFP in the primitive streak and its derivatives, mT/mG animals were crossed to T (Brachyury) cre animals (Perantoni et al., 2005). Wildtype (WT) mice of mixed background were also used to produce un-labeled embryos for immunohistochemical analysis.
Crosses were set up between mT/mG females and either mT/mG: ZP3 cre or T cre males, or between WT females and males. The morning a plug was identified was designated 0.5 days post-coitum (dpc). Progeny were dissected at 6.5 or 7.5 dpc in ice cold medium. Embryos were staged according to Downs and Davies (Downs and Davies, 1993). For time-lapse imaging, mT/mG embryos were examined for EGFP expression, and EGFP-positive embryos were then staged and oriented on glass-bottom culture dishes with their posterior sides facing down. They were secured in this position with silicone grease and nitex mesh and cultured in 50% rat serum, 50% whole embryo culture medium on a stage heater at 37°C with 5% CO2. For sectional analysis, WT embryos were fixed and processed as described below.
Immunohistochemistry
For sectional analysis, embryos were fixed for 15 minutes in 4% paraformaldehyde, washed in 1x PBS, infiltrated with a solution of cold water fish gelatin (15% cold water fish gelatin, 15% sucrose in PBS (Fagotto, 1994)), embedded, frozen in liquid nitrogen, and serially sectioned in the transverse plane at 12 μm thickness. During immuno-staining for Occludin or aPKC, antigen retrieval was performed on sections in citrate buffer pH6.0 at 95 C for 20 minutes using a microwave oven. Sections without antigen retrieval were re-hydrated in 1x PBS, and all sections were then blocked in 10% FBS / 5% BSA in PBS for at least one hour. Sections were then incubated with antibodies against type IV collagen (Milipore AB769), laminin (Sigma L8271),β-catenin (Abcam ab2982), E-cadherin (Sigma U3254), aPKC (Santa Cruz sc-216), Occludin (Invitrogen 71-1500), Brachyury (R&D AF2085), or phosphorylated histone H3 (Cell Signaling 9706) in blocking solution at least one hour at room temperature or overnight at 4C. Sections were washed with 0.5% Tween-20 in PBS and incubated with Alexa 546 anti-goat, Alexa 488 anti-mouse, Alexa 488 anti-rabbit, or Alexa 488 anti-rat antibodies (all from Invitrogen) at least one hour at room temperature or overnight at 4C. Sections were then washed with PBS tween, incubated 5 minutes in 300nM DAPI, rinsed with PBS, and mounted for confocal microscopy analysis. For whole mount immunofluorescence, embryos were fixed as described, then permeabalized for 10 minutes in 0.1% Triton-100 in PBS prior to antibody incubation.
Microscopy
Live fluorescent embryos were positioned posterior side down on an inverted microscope to collect a Z stack (generally with a 2μm step size) through the full depth of the primitive streak region at every time-point (generally every 4 to 6 minutes). Imaging of live and immunostained embryos was performed using either a BioRad Radiance 2100 or a Zeiss 510 Meta laser scanning confocal microscope. The BioRad system consists of a Nikon TE300 epifluorescent microscope with a 100W Hg Arc Lamp; an Argon ion laser; a HeNe 543 nm laser; and a 10W Verdi pumped, tunable (model 900 Mira, www.coherentinc.com) modelocked ultrafast (78 MHz) pulsed (<150 femtosecond) laser coupled to a BioRad Radiance2100 confocal /multiphoton scanning system (www.carlzeiss.com). The system is equipped with laser spectrum analyzer (Model E201; www.istcorp.com) to monitor the excitation wavelength and power meter to measure the laser power at the specimen plane (Model SSIM-VIS & IR; www.coherentinc.com). The system is also equipped with an external detector and four internal detectors for fluorescence imaging. A Plan Fluor 20x NA 0.75 multiimmersion or PlanApo 40x NA 1.4 oil objective lens LaserSharp2000 software was used to acquire confocal and images using the internal detectors. The Zeiss system consisted of a Zeiss Axiovert 200M epifluorescent motorized microscope with a 100W Hg arc lamp and halogen lamp, 45mW argon laser (458, 488, 514 nm), a 10mW DPS (561 nm) laser, and a diode 633 nm laser. The Axiovert 200M is coupled to a Zeiss510 confocal-multiphoton-spectral imaging system (www.zeiss.de), and the system is controlled using the LSM software (version 4.0). A plan-apochromat 25x water NA 0.8 objective lens was used for the studies described here.
Image Analysis
Data were analyzed using Volocity and Image J software. Volocity was used to view and analyze sectional data. Image J was used to view time-lapse movies and to create Z projections of generally one to three 2μm optical Z-sections in order to best visualize the tissue layer of interest. Image J was then used to align time-lapse movies, and to perform manual cell tracking and distortion diagram analysis. Image J was also used to re-slice Z stacks to create optical transverse and sagittal sections. Statistics were performed using Graphpad Prism 5 software.
Supplementary Material
A – C. Images of three different optical Z planes within an early-streak stage mT/mG embryo: A shows morphology of the epiblast layer, B shows morphology of the mesoderm layer, C shows morphology of the endoderm layer. Red is immuno-staining for T (Brachyury) to confirm mesoderm. Some cells within the nascent mesoderm layer demonstrate a round, bulbous appearance (arrowheads), which is the result of apical constriction as cells ingress at the streak.
Timelapse movie of a live e6.5 mT/mG embryo. This embryo is pre-streak at the beginning of the movie, but is early streak (i.e. has mesoderm) by the movie’s end.
Timelapse movie of a live no-bud stage mT/mG embryo. Primitive streak is seen en face at the level of the epiblast. Large, bulbous cells can be seen passing through the plane of view at varied times and locations as they ingress through the streak.
Acknowledgments
We would like to thank Dr. Ray Keller for critical reading of the manuscript and advice on data analysis, and the faculty and staff of the W. M. Keck Center for Cellular Imaging for advice and assistance with imaging. This work was supported by grants to AES from the National Science Foundation (IOS-1051294) and from the National Institute for Child Health and Human Development (RO1 HD034807), as well as private donations from Drs. Tom and Jean Sutherland. MW was supported by a training grant from the National Institutes of Health (T32 GM008136).
References
- Acloque H, Ocana OH, Matheu A, Rizzoti K, Wise C, Lovell-Badge R, Nieto MA. Reciprocal Repression between Sox3 and Snail Transcription Factors Defines Embryonic Territories at Gastrulation. Dev Cell. 2011;21:546–558. doi: 10.1016/j.devcel.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Bonnerot C, Nicolas JF. Clonal analysis in the intact mouse embryo by intragenic homologous recombination. C R Acad Sci III. 1993;316:1207–1217. [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Burdsal CA, Damsky CH, Pedersen RA. The role of E-cadherin and integrins in mesoderm differentiation and migration at the mammalian primitive streak. Development. 1993;118:829–844. doi: 10.1242/dev.118.3.829. [DOI] [PubMed] [Google Scholar]
- Callebaut M, Van Nueten E, Bortier H, Harrisson F. Positional information by Rauber’s sickle and a new look at the mechanisms of primitive streak initiation in avian blastoderms. J Morphol. 2003;255:315–327. doi: 10.1002/jmor.10065. [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–2840. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuai M, Zeng W, Yang X, Boychenko V, Glazier JA, Weijer CJ. Cell movement during chick primitive streak formation. Dev Biol. 2006;296:137–149. doi: 10.1016/j.ydbio.2006.04.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Cui C, Yang X, Chuai M, Glazier JA, Weijer CJ. Analysis of tissue flow patterns during primitive streak formation in the chick embryo. Dev Biol. 2005;284:37–47. doi: 10.1016/j.ydbio.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, Nolan PM, Steel KP, Brown SD, Gray IC, Murdoch JN. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- Egea J, Erlacher C, Montanez E, Burtscher I, Yamagishi S, Hess M, Hampel F, Sanchez R, Rodriguez-Manzaneque MT, Bosl MR, Fassler R, Lickert H, Klein R. Genetic ablation of FLRT3 reveals a novel morphogenetic function for the anterior visceral endoderm in suppressing mesoderm differentiation. Genes & Development. 2008;22:3349–3362. doi: 10.1101/gad.486708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Gumbiner BM. Beta-catenin localization during Xenopus embryogenesis: accumulation at tissue and somite boundaries. Development. 1994;120:3667–3679. doi: 10.1242/dev.120.12.3667. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hayashi Y, Sanzen N, Kobayashi R, Weber CN, Emoto T, Futaki S, Niwa H, Murray P, Edgar D, Sekiguchi K. Regulation of mesodermal differentiation of mouse embryonic stem cells by basement membranes. J Biol Chem. 2007;282:29701–29711. doi: 10.1074/jbc.M611452200. [DOI] [PubMed] [Google Scholar]
- Fuse T, Kanai Y, Kanai-Azuma M, Suzuki M, Nakamura K, Mori H, Hayashi Y, Mishina M. Conditional activation of RhoA suppresses the epithelial to mesenchymal transition at the primitive streak during mouse gastrulation. Biochem Biophys Res Commun. 2004;318:665–672. doi: 10.1016/j.bbrc.2004.04.076. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Anderson KV. Essential role of glycosaminoglycans in Fgf signaling during mouse gastrulation. Cell. 2003;114:727–737. doi: 10.1016/s0092-8674(03)00715-3. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Cockroft DL. Complete dissipation of coherent clonal growth occurs before gastrulation in mouse epiblast. Development. 1998;125:2397–2402. doi: 10.1242/dev.125.13.2397. [DOI] [PubMed] [Google Scholar]
- Georgiades P, Rossant J. Ets2 is necessary in trophoblast for normal embryonic anteroposterior axis development. Development. 2006;133:1059–1068. doi: 10.1242/dev.02277. [DOI] [PubMed] [Google Scholar]
- Graeper L. Die primitiventwicklung des huehnchens nach stereokinematographischen untersuchungen kontrolliert durch vitale farbmarkierung und verglichen mit der entwicklung andere wirbeltiere. Willhelm Roux’ Arch Entwicklungsmech. 1929;116:382–429. doi: 10.1007/BF02145235. [DOI] [PubMed] [Google Scholar]
- Halacheva V, Fuchs M, Donitz J, Reupke T, Puschel B, Viebahn C. Planar cell movements and oriented cell division during early primitive streak formation in the mammalian embryo. Dev Dyn. 2011;240:1905–1916. doi: 10.1002/dvdy.22687. [DOI] [PubMed] [Google Scholar]
- Hardy KM, Garriock RJ, Yatskievych TA, D’Agostino SL, Antin PB, Krieg PA. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11 gene, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy KM, Yatskievych TA, Konieczka J, Bobbs AS, Antin PB. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev Biol. 2011;11:20. doi: 10.1186/1471-213X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idkowiak J, Weisheit G, Plitzner J, Viebahn C. Hypoblast controls mesoderm generation and axial patterning in the gastrulating rabbit embryo. Dev Genes Evol. 2004;214:591–605. doi: 10.1007/s00427-004-0436-y. [DOI] [PubMed] [Google Scholar]
- Katow HS, Michael Ultrastructure of primary mesenchyme cell ingression in the sea urchin Lytechinus pictus. The Journal of Experimental Zoology. 1980;213:231–246. [Google Scholar]
- Khaner O, Eyal-Giladi H. The embryo-forming potency of the posterior marginal zone in stages X through XII of the chick. Dev Biol. 1986;115:275–281. doi: 10.1016/0012-1606(86)90248-4. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kinder SJ, Tsang TE, Wakamiya M, Sasaki H, Behringer RR, Nagy A, Tam PP. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development. 2001;128:3623–3634. doi: 10.1242/dev.128.18.3623. [DOI] [PubMed] [Google Scholar]
- Lawson A, Schoenwolf GC. Cell populations and morphogenetic movements underlying formation of the avian primitive streak and organizer. Genesis. 2001;29:188–195. doi: 10.1002/gene.1023. [DOI] [PubMed] [Google Scholar]
- Lawson A, Schoenwolf GC. New insights into critical events of avian gastrulation. Anat Rec. 2001;262:238–252. doi: 10.1002/1097-0185(20010301)262:3<238::AID-AR1041>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Sheng G. An amicable separation: Chick’s way of doing EMT. Cell Adh Migr. 2009:3. doi: 10.4161/cam.3.2.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–775. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005;132:3859–3871. doi: 10.1242/dev.01945. [DOI] [PubMed] [Google Scholar]
- Quinlan GA, Williams EA, Tan SS, Tam PP. Neuroectodermal fate of epiblast cells in the distal region of the mouse egg cylinder: implication for body plan organization during early embryogenesis. Development. 1995;121:87–98. doi: 10.1242/dev.121.1.87. [DOI] [PubMed] [Google Scholar]
- Rodriguez TA, Srinivas S, Clements MP, Smith JC, Beddington RS. Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm. Development. 2005;132:2513–2520. doi: 10.1242/dev.01847. [DOI] [PubMed] [Google Scholar]
- Skromne I, Stern CD. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development. 2001;128:2915–2927. doi: 10.1242/dev.128.15.2915. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman D, Wagstaff L, Cooper O, Weijer C, Munsterberg A. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Dev Biol. 2008;8:63. doi: 10.1186/1471-213X-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124:1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- Tam PP, Steiner KA, Zhou SX, Quinlan GA. Lineage and functional analyses of the mouse organizer. Cold Spring Harb Symp Quant Biol. 1997;62:135–144. [PubMed] [Google Scholar]
- Tam PP, Williams EA, Chan WY. Gastrulation in the mouse embryo: ultrastructural and molecular aspects of germ layer morphogenesis. Microsc Res Tech. 1993;26:301–328. doi: 10.1002/jemt.1070260405. [DOI] [PubMed] [Google Scholar]
- Tam PP, Zhou SX. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev Biol. 1996;178:124–132. doi: 10.1006/dbio.1996.0203. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Mayer B, Miething A. Morphology of incipient mesoderm formation in the rabbit embryo: a light- and retrospective electron-microscopic study. Acta Anat (Basel) 1995;154:99–110. doi: 10.1159/000147756. [DOI] [PubMed] [Google Scholar]
- Viebahn C, Stortz C, Mitchell SA, Blum M. Low proliferative and high migratory activity in the area of Brachyury expressing mesoderm progenitor cells in the gastrulating rabbit embryo. Development. 2002;129:2355–2365. doi: 10.1242/dev.129.10.2355. [DOI] [PubMed] [Google Scholar]
- Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 2007;449:1049–1052. doi: 10.1038/nature06211. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wu SY, Ferkowicz M, McClay DR. Ingression of primary mesenchyme cells of the sea urchin embryo: a precisely timed epithelial mesenchymal transition. Birth Defects Res C Embryo Today. 2007;81:241–252. doi: 10.1002/bdrc.20113. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yang X, Chrisman H, Weijer CJ. PDGF signalling controls the migration of mesoderm cells during chick gastrulation by regulating N-cadherin expression. Development. 2008;135:3521–3530. doi: 10.1242/dev.023416. [DOI] [PubMed] [Google Scholar]
- Yang X, Dormann D, Munsterberg AE, Weijer CJ. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev Cell. 2002;3:425–437. doi: 10.1016/s1534-5807(02)00256-3. [DOI] [PubMed] [Google Scholar]
- Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. PTK7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009;136:2039–48. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Wagstaff L, Yang X, Weijer C, Munsterberg A. Wnt3a-mediated chemorepulsion controls movement patterns of cardiac progenitors and requires RhoA function. Development. 2008;135:1029–1037. doi: 10.1242/dev.015321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A – C. Images of three different optical Z planes within an early-streak stage mT/mG embryo: A shows morphology of the epiblast layer, B shows morphology of the mesoderm layer, C shows morphology of the endoderm layer. Red is immuno-staining for T (Brachyury) to confirm mesoderm. Some cells within the nascent mesoderm layer demonstrate a round, bulbous appearance (arrowheads), which is the result of apical constriction as cells ingress at the streak.
Timelapse movie of a live e6.5 mT/mG embryo. This embryo is pre-streak at the beginning of the movie, but is early streak (i.e. has mesoderm) by the movie’s end.
Timelapse movie of a live no-bud stage mT/mG embryo. Primitive streak is seen en face at the level of the epiblast. Large, bulbous cells can be seen passing through the plane of view at varied times and locations as they ingress through the streak.